Abstract
Objective:
To describe retrospectively the clinical associations of immunoglobulin G (IgG) targeting metabotropic glutamate receptor 1 (mGluR1-IgG).
Methods:
Specimens of 9 patients evaluated on a service basis in the Mayo Clinic Neuroimmunology Laboratory by tissue-based immunofluorescence assay (IFA) yielded a robust, synaptic immunostaining pattern consistent with mGluR1-IgG (serum, 9; CSF, 2 available). Transfected HEK293 cell-based assay (CBA) confirmed mGluR1 specificity in all 11 specimens. A further 2 patients were detected in Germany primarily by CBA.
Results:
The median symptom onset age for the 11 patients was 58 years (range 33–81 years); 6 were male. All 9 Mayo Clinic patients had subacute onset of cerebellar ataxia, 4 had dysgeusia, 1 had psychiatric symptoms, and 1 had cognitive impairment. All were evaluated for malignancy, but only 1 was affected (cutaneous T-cell lymphoma). One developed ataxia post–herpes zoster infection. Head MRIs were generally atrophic or normal-appearing, and CSF was inflammatory in just 1 of 5 tested, though mGluR1-IgG was detected in both specimens submitted. Five patients improved (attributable to immunotherapy in 4, spontaneously in 1), 3 stabilized (attributable to immunotherapy in 2, cancer therapy in 1), and 1 progressively declined (untreated). The 2 German patients had ataxia, but fulfilled multiple sclerosis diagnostic criteria (1 relapsing-remitting, 1 progressive). However, both had histories of hematologic malignancy (acute lymphocytic leukemia and mantle cell lymphoma), and had mGluR1-IgG detected in serum by CBA (weakly positive on tissue-based IFA).
Conclusions:
mGluR1 autoimmunity represents a treatable form of cerebellar ataxia. Dysgeusia may be a diagnostic clue. Paraneoplastic, parainfectious, or idiopathic causes may occur.
Metabotropic glutamate receptor 1 (mGluR1) is a G-protein-coupled receptor, activation of which facilitates long-term depression of parallel fiber–Purkinje cell synapses critical for cerebellar motor learning.1 mGluR1–immunoglobulin G (IgG) autoantibody is a biomarker of autoimmune cerebellar ataxia, reported in a paraneoplastic context (usually lymphoma) or without neoplasm detected.2–5 Given the limited literature, we report 11 patients (with archived specimens and recorded histories) in whom mGluR1 autoimmunity was encountered, in order to determine the disease spectrum.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was carried out with the approval of institutional review boards and written informed consents were obtained.
Patients and assays.
Nine patients were identified in the Mayo Clinic Neuroimmunology Laboratory by 2 mechanisms. All specimens were clinically submitted for paraneoplastic antibody evaluation. (1) Four patients were identified by reviewing archived descriptions of mouse tissue-based immunofluorescence assays (IFAs) for 1,074 patients with unclassified neural antibody staining (1996–2013, serum or CSF). Thirty-two patients had descriptions resembling the reported mGluR1-IgG staining pattern; their specimens were re-evaluated by IFA (screening dilutions: serum, 1:240; CSF, 1:2).2 Of the 32 patients, 4 had the mGluR1-IgG-characteristic antibody staining (figure), which appears most prominent in the cerebellar molecular layer, dentate gyrus, and thalamus. (2) A further 5 patients detected prospectively (2013–2014, of 80,000 tested) also had the mGluR1-IgG-characteristic staining by IFA. mGluR1 specificity was confirmed molecularly in each available specimen of the 9 patients (9 sera [screened at 1:10] and 2 CSF [screened undiluted]) by CBA (on HEK293 cells transfected with mGluR1 complementary DNA [full-length, human, untagged], figure). Control cells were mock-transfected with empty vector. The remaining 28 patients from the first group were mGluR1-IgG-negative by CBA. Four patients were neurologically evaluated at Mayo Clinic; the remainder had histories submitted from outside. As controls, 61 sera (from healthy subjects) and 27 CSF specimens (from patients with normal-pressure hydrocephalus) were tested by both assays.
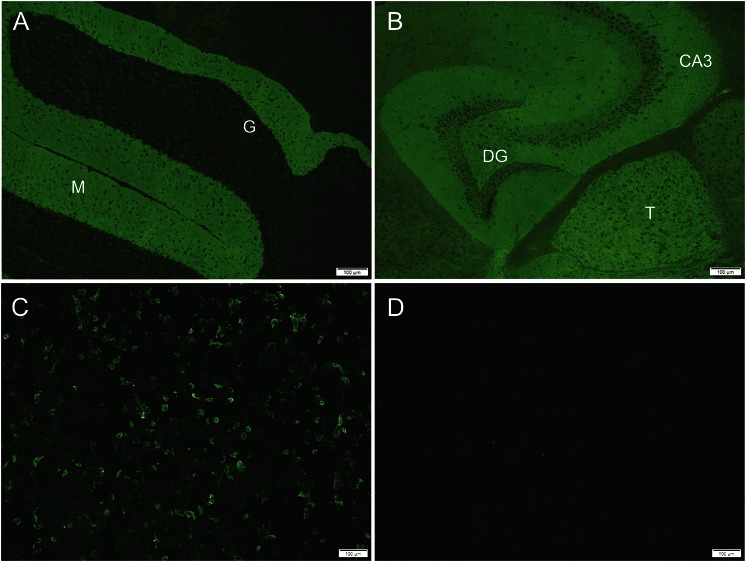
Figure. Metabotropic glutamate receptor 1 (mGluR1) antibody tissue- and cell-based assays.
Indirect immunofluorescence assays, tissue-based (A, cerebellum; B, cerebrum) and cell-based (C, mGluR1-transfected; D, mock-transfected) demonstrate mGluR1–immunoglobulin G (IgG) in patient serum. The synaptic CNS pattern of mGluR1-IgG immunoreactivity is most prominent in the molecular layer (M) of cerebellum, thalamus (T), and hippocampus (CA3 region and dentate gyrus [DG]). Granular layer of cerebellum (G) is dark. Serum is reactive with mGluR1-transfected cells (C), but not mock-transfected cells (D).
Two additional patients (of >10,000 tested over 3 months) were detected in Euroimmun's diagnostic service laboratory, Germany, by screening with broad IFA and CBA mosaics.6
RESULTS
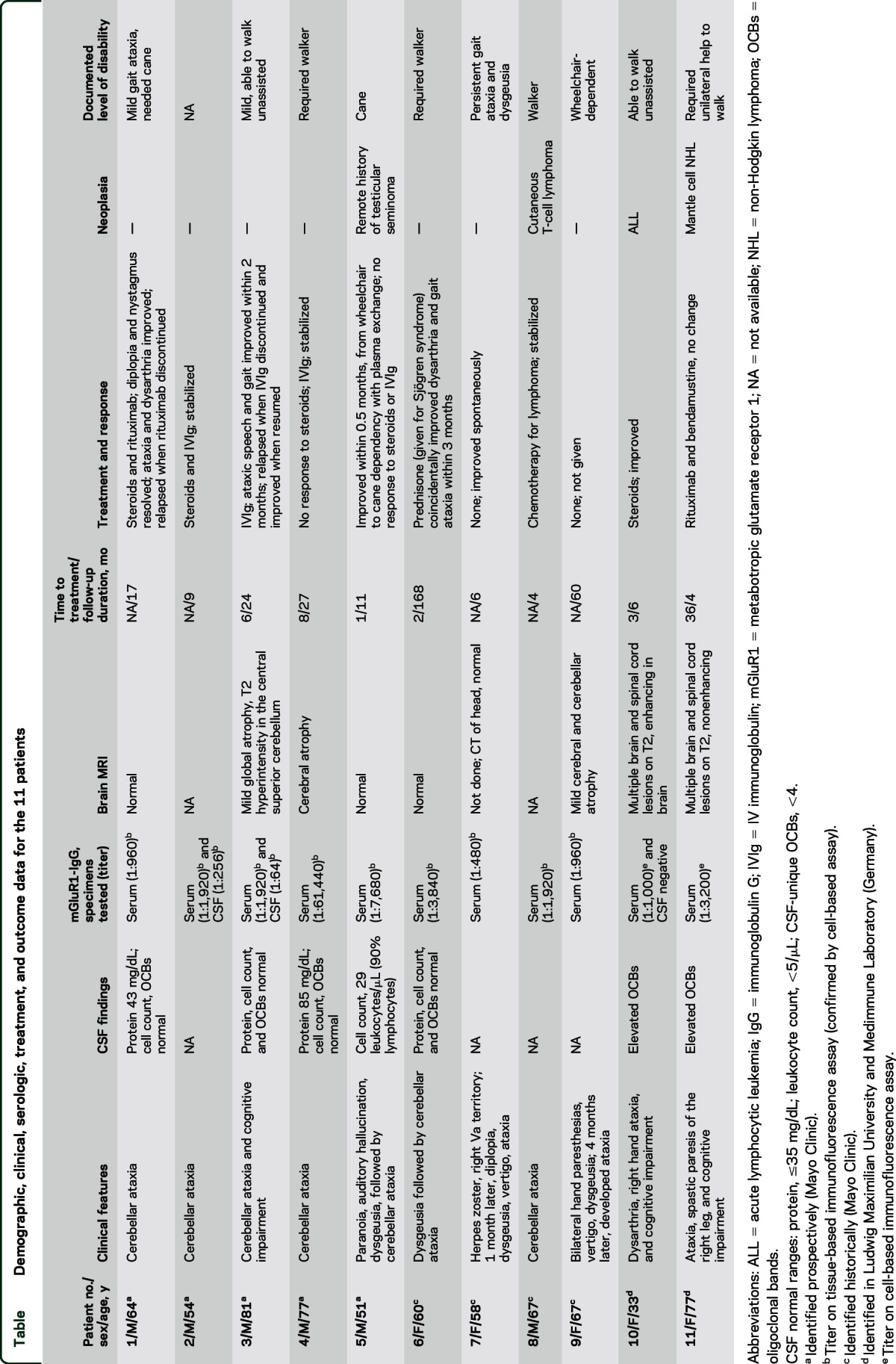
The median symptom onset age for the 11 patients was 58 years (range 33–81 years); 6 were male (table). The median follow-up period was 11 months (range 4–168 months).
Table.
Demographic, clinical, serologic, treatment, and outcome data for the 11 patients
Mayo Clinic patients.
All control specimens tested negative by both assays. Among the 9 positive Mayo patients, median mGluR1-IgG antibody value in serum was 1:1,920 (range 1:480–1:61,440; normal value, ≤120). Neurologic symptoms evolved subacutely (over 4–8 weeks) in all (table) and affected cerebellar function in all (gait and limb incoordination and slurred speech). Three patients had diplopia and 2 had vertigo. Six patients had extracerebellar symptoms: ageusia or dysgeusia in 4, preceding ataxia in 2; patient 3 had cognitive symptoms, peripheral neuropathy, and ataxia (and was vitamin B12 deficient); patient 6 had ataxia that initially stabilized, but relapsed 9 years later, accompanied by seizures; patient 5 had paranoia and auditory hallucinations preceding ataxia.
One patient had herpes zoster virus infection affecting the right Vth cranial nerve cutaneous distribution (A segment) 1 month prior to developing ataxia. Three patients had coexisting autoimmune diseases (hypothyroidism in 1, Sjögren syndrome in 1, pernicious anemia in 1). One patient had circular, raised gray-blue skin lesions on his back and face; biopsy revealed T-cell lymphoma. The remaining 8 patients had normal oncologic evaluations (CT with or without PET-CT of trunk in 8, bone marrow biopsy in 2).
MRI head was abnormal in 3 of 7 patients: mild cerebellar atrophy in 2 and mild cerebral and cerebellar atrophy and T2 hyperintensity in 1. One or more coexisting neural autoantibodies were detected in 4 patients (50%) without additional neurological correlates: α-3 ganglionic AChR antibody (2 patients), muscle AChR binding antibody (1 patient), and striational antibody (1 patient).
Six patients received immunotherapy: 4 improved neurologically (attributable to corticosteroids in 1, plasma exchange in 1, IV immunoglobulin [IVIg] in 1, and steroids and rituximab in 1) and 2 patients stabilized (both received steroids and IVIg sequentially). Improvements occurred over a median of 2 months (range 0.5–3). The patient with T-cell lymphoma received chemotherapy only and stabilized neurologically. Final ambulatory outcomes, available for 6 patients, were as follows: independent (n = 2), cane-dependent (n = 3), and walker-dependent (n = 1). One untreated patient spontaneously improved and the other progressed to wheelchair dependency over 5 years.
German patients.
Both patients (10 and 11) were women, with clinical, radiologic, and CSF features of demyelinating disease with a history of hematologic malignancy (table). They were screened for neural antibodies because paraneoplastic neurologic autoimmunity was suspected. Both had mGluR1-IgG detected in serum by CBA (titers, 1:1,000 and 1:3,200, respectively), but had weak IFA staining only (titers, 1:10 and 1:32, respectively). CSF became available in patient 10 during treatment (negative on both assays) and was not available in patient 11. Neither patient had aquaporin-4 antibodies or myelin oligodendrocyte glycoprotein antibodies detected.
Patient 10, aged 33 years, presented with dysarthria, right-hand ataxia, and cognitive impairment, which improved with corticosteroid treatment. She had been cured of acute lymphocytic leukemia 23 years earlier, without recurrence at neurologic presentation. MRI (brain and spine) and CSF findings (abnormal supernumerary oligoclonal bands numbers) indicated fulfillment of multiple sclerosis (MS) diagnostic criteria (relapsing-remitting).
Patient 11, age 72 years, had a new diagnosis of mantle cell lymphoma (treated with bendamustine and rituximab). She had abducens nerve palsy at age 52 years. At age 55 years, MRI (brain and spine) and CSF findings (abnormal supernumerary oligoclonal bands numbers) indicated fulfillment of MS diagnostic criteria (primary progressive).7 From age 69 years, she had progressive gait and appendicular ataxia, spastic right foot paresis, and cognitive impairment. A first cousin had MS.
DISCUSSION
The 9 Mayo Clinic mGluR-IgG autoimmune cases described share characteristics similar to the 5 previously published: a cerebellar degeneration–predominant phenotype in all and lymphoma encountered in a minority.2–5 Notably, 4 of our 9 patients reported dysgeusia. mGluR1 localization also extends to circumvallate and foliate papillae of the posterior mammalian tongue, a region likely responsible for umami taste.8 Consistent with the presence of mGluR1 within the hippocampal formation, limbic symptoms (psychiatric symptoms, seizures, and memory loss) occasionally coexisted with ataxia.2
Neurologic improvements with immunotherapy were anticipated based on previously published reports and experimental animal data supportive of mGluR1 antibody pathogenicity.9 Though limited by retrospective study designs, 3 of our cases, and 1 previous case, where times to treatment were documented, received immunotherapy within 6 months of symptom onset and improved. One of our cases, and 1 previous case, received treatment 8–12 months after symptom onset and did not improve. Progressive neurologic decline may have been attributable to lack of treatment in 1 of our patients. Though statistical analyses were not applicable, early immunotherapy seems preferable based on our experience with other autoimmune CNS disorders.10
In contrast to the established literature, neoplasm was only encountered in 1 of 9 patients who had cutaneous T-cell lymphoma (1 of 2 neoplasms reported in 1 previous case).5 Lymphoma and prostate adenocarcinoma aside, other neoplasms may merit exclusion because mGluR1 receptors are known to be expressed in melanoma and breast adenocarcinoma.11,12 One patient likely had zoster-induced parainfectious neurologic autoimmunity, a phenomenon reported in patients with NMDA receptor encephalitis with preceding herpes simplex encephalitis.13
The neurologic significance of mGluR1-IgG seropositivity detected in serum, primarily by CBA, in 2 German patients with MS findings was unclear. These findings may have occurred in the hematologic neoplastic context without neurologic significance (also reported for other cancer-neural autoantibody associations), or may have been a nonspecific finding similar to that observed in patients with NMDA receptor antibody detected in serum by CBA but not IFA.14–16 We cannot exclude the occurrence of paraneoplastic MS mimics or coexisting autoimmune ataxia and MS.
Paraneoplastic mGluR1 autoimmunity, which is potentially treatable, should be considered in patients with subacute-onset ataxia. Preceding or accompanying taste loss and limbic symptoms may serve as diagnostic clues.
ACKNOWLEDGMENT
The authors thank Dr. Jerome B. Posner (Memorial Sloan Kettering Cancer Center), Dr. Ty L. Schwertfeger (Neurology Consultants Wichita, KS), Dr. Jodi Hawes (Duke University), and Dr. Laur Birlea (University of Colorado) for providing clinical information and Amy Ennis and Jade Zbacnik for technical support.
GLOSSARY
- IgG
immunoglobulin G
- IFA
immunofluorescence assay
- IVIg
IV immunoglobulin
- mGluR1
metabotropic glutamate receptor 1
- MS
multiple sclerosis
AUTHOR CONTRIBUTIONS
A.S.L.-C.: data collection and analysis, drafting of manuscript. L.K. and C.P. supplied critical reagents and critical revision of manuscript. S.R.H.: data collection and analysis, critical revision of manuscript. T.K.: data collection and analysis, critical revision of manuscript. S.J.P.: data interpretation, critical revision of manuscript. A.M.: study concept and design, data collection, analysis, and interpretation, critical revision of manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
A.S. Lopez-Chiriboga reports no disclosures relevant to the manuscript. L. Komorowski is an employee of Euroimmun Inc., which manufactures and markets assay kits for detecting mGluR1 antibody. T. Kümpfel reports no disclosures relevant to the manuscript. C. Probst is an employee of Euroimmun Inc., which manufactures and markets assay kits for detecting mGluR1 antibody. S.R. Hinson reports no disclosures relevant to the manuscript. S. Pittock has received no royalties to date but may accrue revenue for patents relating to AQP4 antibodies for diagnosis of neuromyelitis optica and AQP4 autoantibody as a cancer marker. He receives research support from the Guthy-Jackson Charitable Foundation, Alexion Pharmaceuticals, Inc., and the NIH (RO1 NS065829). A. McKeon receives research support from and has consulted for Medimmune, Inc., but has not received personal compensation. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Benarroch EE. Metabotropic glutamate receptors: synaptic modulators and therapeutic targets for neurologic disease. Neurology 2008;70:964–968. [DOI] [PubMed] [Google Scholar]
- 2.Sillevis Smitt P, Kinoshita A, De Leeuw B, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med 2000;342:21–27. [DOI] [PubMed] [Google Scholar]
- 3.Marignier R, Chenevier F, Rogemond V, et al. Metabotropic glutamate receptor type 1 autoantibody-associated cerebellitis: a primary autoimmune disease? Arch Neurol 2010;67:627–630. [DOI] [PubMed] [Google Scholar]
- 4.Lancaster E, Martinez-Hernandez E, Titulaer MJ, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology 2011;77:1698–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iorio R, Damato V, Mirabella M, et al. Cerebellar degeneration associated with mGluR1 autoantibodies as a paraneoplastic manifestation of prostate adenocarcinoma. J Neuroimmunol 2013;263:155–158. [DOI] [PubMed] [Google Scholar]
- 6.Scharf M, Miske R, Heidenreich F, et al. Neuronal Na+/K+ ATPase is an autoantibody target in paraneoplastic neurologic syndrome. Neurology 2015;84:1673–1679. [DOI] [PubMed] [Google Scholar]
- 7.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.San Gabriel A, Maekawa T, Uneyama H, Torii K. Metabotropic glutamate receptor type 1 in taste tissue. Am J Clin Nutr 2009;90:743S–746S. [DOI] [PubMed] [Google Scholar]
- 9.Coesmans M, Smitt PA, Linden DJ, et al. Mechanisms underlying cerebellar motor deficits due to mGluR1-autoantibodies. Ann Neurol 2003;53:325–336. [DOI] [PubMed] [Google Scholar]
- 10.Flanagan EP, McKeon A, Lennon VA, et al. Autoimmune dementia: clinical course and predictors of immunotherapy response. Mayo Clin Proc 2010;85:881–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollock PM, Cohen-Solal K, Sood R, et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet 2003;34:108–112. [DOI] [PubMed] [Google Scholar]
- 12.Teh JL, Shah R, La Cava S, et al. Metabotropic glutamate receptor 1 disrupts mammary acinar architecture and initiates malignant transformation of mammary epithelial cells. Breast Cancer Res Treat 2015;151:57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leypoldt F, Titulaer MJ, Aguilar E, et al. Herpes simplex virus-1 encephalitis can trigger anti-NMDA receptor encephalitis: case report. Neurology 2013;81:1637–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horta ES, Lennon VA, Lachance DH, et al. Neural autoantibody clusters aid diagnosis of cancer. Clin Cancer Res 2014;20:3862–3869. [DOI] [PubMed] [Google Scholar]
- 15.Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol 2013;13:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruse JL, Lapid MI, Lennon VA, et al. Psychiatric autoimmunity: N-methyl-D-aspartate receptor IgG and beyond. Psychosomatics 2015;56:227–241. [DOI] [PubMed] [Google Scholar]