Abstract
Objective:
To examine frequencies and relationships of 5 common neuropathologic abnormalities identified at autopsy with late-life cognitive impairment and dementia in 2 different autopsy panels.
Methods:
The Nun Study (NS) and the Honolulu-Asia Aging Study (HAAS) are population-based investigations of brain aging that included repeated cognitive assessments and comprehensive brain autopsies. The neuropathologic abnormalities assessed were Alzheimer disease (AD) neuropathologic changes, neocortical Lewy bodies (LBs), hippocampal sclerosis, microinfarcts, and low brain weight. Associations with screening tests for cognitive impairment were examined.
Results:
Neuropathologic abnormalities occurred at levels ranging from 9.7% to 43%, and were independently associated with cognitive impairment in both studies. Neocortical LBs and AD changes were more frequent among the predominantly Caucasian NS women, while microinfarcts were more common in the Japanese American HAAS men. Comorbidity was usual and very strongly associated with cognitive impairment. Apparent cognitive resilience (no cognitive impairment despite Braak stage V) was strongly associated with minimal or no comorbid abnormalities, with fewer neocortical AD lesions, and weakly with longer interval between final testing and autopsy.
Conclusions:
Total burden of comorbid neuropathologic abnormalities, rather than any single lesion type, was the most relevant determinant of cognitive impairment in both cohorts, often despite clinical diagnosis of only AD. These findings emphasize challenges to dementia pathogenesis and intervention research and to accurate diagnoses during life.
A 1997 Nun Study (NS) report demonstrated a powerful effect on cognitive function of comorbid brain infarcts and Alzheimer disease (AD) neuropathologic changes, reporting an odds ratio (OR) of 20.7 for dementia in participants with both high levels of AD neuropathologic changes and cerebral infarcts.1 A 2002 report of 285 autopsied participants in the Honolulu-Asia Aging Study (HAAS) provided a comprehensive description of 4 major neuropathologic abnormalities, each robustly associated with late-life cognitive impairment: AD neuropathologic changes, neocortical Lewy bodies (LBs), hippocampal sclerosis (HS), and microinfarcts.2 Subsequent HAAS analyses demonstrated that generalized brain atrophy may occur independently, as a fifth common abnormality associated with cognitive impairment.3,4 Extensive analyses using autopsy and clinical data have further documented both the multiplicity of neuropathologic abnormalities associated with late-life dementia and the complex relationships between them.5–12 Indeed, the high frequency of neuropathologic comorbidity in older persons with dementia has become widely appreciated around the globe.13–21 In 2013, the first 22 brain autopsies from the Alzheimer's Disease Neuroimaging Initiative (ADNI) were reported.22 All had been diagnosed during life with AD dementia or AD-type mild cognitive impairment. Of these, all but 4 had comorbid abnormalities that might have contributed to cognitive impairment. In 2015, it was shown that the presence of multiple pathologic diagnoses occurred more frequently in those with dementia compared with those without dementia in 183 participants of the 90+ Study.23
Despite support for the significance of comorbid neuropathologic abnormalities in dementia in older adults, a large-scale comprehensive examination of their frequencies and overall neurocognitive effects has not yet been reported. Here we address this gap by parallel analyses in 2 distinct population-based brain autopsy panels, the predominantly Caucasian NS women and the Japanese American HAAS men.
METHODS
Standard protocol approvals, registrations, and patient consents.
The NS and HAAS are population-based studies of health and functioning in late life.1,2,4 Some data used for this report were included in previous publications.5,6,14,24,25 Histories, cohorts, and operations are described in e-Methods on the Neurology® Web site at Neurology.org. The NS was reviewed and approved by Universities of Kentucky and Minnesota institutional review boards (IRBs). Participating School Sisters of Notre Dame had agreed to autopsy prior to death, with final authorizations provided by the Provincial Leader; an autopsy was completed for >90% of NS participants. The HAAS was reviewed and approved by the Kuakini Hospital IRB. A consent form was signed by participants at every cycle (12 from 1965 to 2012), including consent for use of information and materials for research purposes by researchers and their colleagues, including NIH-associated, with no ending of this permission. HAAS autopsy acquisition procedure required notification of a participant's death by family member, hospital, medical examiner's office, or other source, with permission for autopsy and research use of information provided by next of kin. Autopsies were completed for 25% of HAAS participants, as previously reported.4
Cognitive function.
NS primary cognitive testing was done annually with the Consortium to Establish a Registry for Alzheimer's Disease neuropsychological battery, which includes the Mini-Mental State Examination (MMSE).26,27 HAAS participants were screened at each examination with the Cognitive Abilities and Screening Instrument (CASI).28 For NS participants, we considered no or mild cognitive impairment for a final MMSE score of 22 or greater, moderate cognitive impairment as 17–21, and severe cognitive impairment as <17. For HAAS participants, we considered no or mild cognitive impairment for a final CASI score of 74 or greater, moderate cognitive impairment as 60–73.9, and severe cognitive impairment as <60. The endpoint was a 3-level definition of cognitive impairment (no or mild, moderate, or severe) represented as 0, +, or ++, respectively. Analyses comparing cognitive impairment in a merged NS/HAAS dataset employed a HAAS MMSE score calculated from MMSE items included in the CASI (MMSEc) for that specific purpose28 (see e-Methods, figure e-1, and tables e-1 through e-4).
Neuropathologic evaluations.
Frequencies and correlates of individual neuropathologic abnormalities were examined at 3 levels of severity for each. We have shown previously that relative risk for dementia from standardized AD neuropathologic change, cerebral microinfarcts, and cortical LBs are similar, supporting analogous contributions of these disease processes to dementia in community settings14,29; similar analyses support analogous contributions from HS and low brain weight.4,30 Thus, index values of 1, 0.4, or 0 were assigned for severe, moderate, or negligible, respectively, based on prior analyses relating lesion measures to cognitive decline and dementia.2,4,31 Choosing 0.4 as the intermediate level allowed us to distinguish the influence of cases with a single severe lesion type (comorbid index 1.0) from those with 2 or 3 moderate lesions (comorbid indices 0.8 and 1.2, respectively).
Severe AD pathology (ADindex = 1.0) was defined as Braak stage V or VI, moderate (ADindex = 0.4) as Braak stage IV, and negligible (ADindex = 0) as Braak stage <IV.32 Severe LB pathology (LBindex = 1.0) was based on a McKeith score of 7 or higher (corresponding to a total unilateral frontal, parietal, and temporal cortex count <50),33 moderate (LBindex = 0.4) on a McKeith score of 2–6 (typically with LBs in the insula, anterior cingulate, or amygdala, with fewer than 50 counted in frontal, parietal, and temporal neocortex sections), and negligible (LBindex = 0) on no LBs observed or LBs limited to the pigmented brainstem nuclei. Severe HS (HSindex = 0) was based on bilateral occurrence, moderate (HSindex = 0.4) on unilateral occurrence, and negligible (HSindex = 0) on evidence of the abnormality.6,34 Severe microinfarcts (MINFindex = 1.0) required more than 3 microinfarcts counted on available neocortical and striatal sections, moderate (MINFindex = 0.4) required 2 or 3 microinfarcts, and negligible (MINFindex = 0) meant one or none were found. Severe low brain weight was defined as <1,030 g for NS and <1,135 g for HAAS, moderate was 1,030–1,099 g for NS and 1,135–1,199 g for HAAS, and negligibly low was defined as at least 1,100 g for NS and 1,200 g for HAAS. The brain weight cutpoints corresponded to the ∼25th and ∼50th percentiles for each cohort. A summary comorbid index was computed by adding the 5 individual severity indices. Possible values were 0, 0.4, 0.8, 1.0, 1.2, 1.4, 1.6, 1.8, 2, 2.2, 2.4, 2.6, 2.8, 3.2, 3.4, 3.6, 3.8, 4.0, 4.4, and 5.0.
Statistical analysis.
Analyses utilized SAS version 9.3 statistical package and were independently validated using R statistical programming language. Ordinal logistic regression analysis was used to model the relationship between cognitive status prior to death and autopsy brain lesion scores. ORs of cognitive impairment (0, +, or ++) were assessed as functions of lesion distribution using the cumulative logit model. This method provides intercepts, p values, and single ORs for the independent variables, summarizing their associations with the endpoint across 3 levels of cognitive test performance (see http://www.ats.ucla.edu/stat/sas/dae/ologit.htm and http://support.sas.com/kb/24/315.html). Analogous linear regression using MMSE as the continuous dependent variable was fully confirmatory.
Another ordinal logistic regression model was reported estimating proportional ORs for 5 levels of coprevalent lesion index score as independent predictor variables modeling dichotomized comorbidity index categories of 0 (reference), 0.4–0.8, 1–1.8, 2–2.4, and 2.6–4.4. Proportional odds assumptions were not violated based on the proportional odds score test (all p values > 0.05). Spearman correlation coefficients were used to assess the association of neuropathologic lesion severity scores with each other and with last MMSE or CASI score.
RESULTS
Study participants.
Descriptions of autopsied NS women and HAAS men are shown in table 1. In addition to obvious sex, ethnicity, and cultural differences, years of schooling completed by NS women was greater than among HAAS men (p < 0.0001). Frequency distributions of final test scores in the 2 cohorts (evident in table 1 and figure e-1) demonstrate higher cognitive test scores in the Sisters, especially in the no or mild range. Interval elapsed between final cognitive testing and death was substantially shorter for NS women, reflecting their annual examinations and prearranged autopsies. Age at death was slightly older in the NS (p < 0.0001). Severe cognitive impairment (assessed by MMSE or MMSEc) was not significantly different.
Table 1.
Characteristics of autopsied NS and HAAS participants
Prevalence and allied cognitive impairment for participant brains stratified by 5 neuropathologic abnormalities considered individually.
As shown in table 2, prevalence of severe AD changes (index 1.0) was slightly higher among NS women (p = 0.04), controlling for APOE ε4 positivity and age, while HAAS men had a substantially higher prevalence of microinfarcts (p < 0.0001), controlling for age at death. Neocortical LB severity was greater in the NS (p < 0.0001). The proportions with moderate or severe cognitive impairment generally increased with neuropathologic severities in both cohorts.
Table 2.
Prevalence of 5 neuropathologic abnormalities identified at autopsy showing the percent distributions of no or mild (0), moderate (+), or severe (++) cognitive impairment at 3 levels of severity for each pathologic abnormality
Associations with cognitive impairment for 5 neuropathologic abnormalities considered together in a single logistic model.
Table 3 shows parallel logistic regression modeling in the 2 cohorts, examining strengths of association with cognitive impairment for the 5 lesion types considered simultaneously, each thereby assessed while controlling for the others. This ensures a relatively unbiased assessment of the unique (unshared) contribution of each type of abnormality to the endpoint, with proportional attribution to each when one or more occur in the same brain. In general, the results demonstrate independent influences of the 5 neuropathologic abnormalities, and greater effect of greater severity.
Table 3.
OR of impaired cognitive performance as a function of demographic variables and brain lesion severities at autopsy in the NS and the HAAS
ORs from logistic models are multiplicative, meaning that the presence of multiple lesion types substantially increases risk. While sample sizes preclude a definitive assessment of the multiplicative proportional odds assumption, stepwise regression using the larger HAAS sample failed to implicate nonmultiplicative effects, except for risk in the presence of both AD neuropathologic changes and microinfarcts, where a supramultiplicative effect was suggested (p < 0.05), indicating even greater risk than predicted when a combination of neuropathologic abnormalities was observed. The irregular associational patterns apparent for HS reflect instability attributable to the lower frequency of this lesion type, and possibly to unilateral vs bilateral differences.
We carried out separate linear regression analyses at 5 Braak stage levels (<III, III, IV, V, VI) in the merged NS/HAAS dataset using the MMSE (or MMSEc) as the dependent variable (table e-2). Significant associations with neocortical neurofibrillary tangles (NFTs) and a weaker association with neocortical neuritic plaque counts were limited to Braak V and VI subsets. Very strong associations with non-AD coprevalent neuropathologic abnormalities were evident at all Braak levels. Cohort membership had no apparent relationship with impairment in any of the subsets once these other independent variables were considered.
In both cohorts, we observed nearly all possible combinations of the 5 neuropathologic abnormalities at frequencies approximating those expected by chance, given their mutual independence (correlation matrices in table e-5). Examples of comorbid lesion heterogeneity are described and enumerated in tables e-4 and e-6, with special consideration of single abnormalities and comorbid admixtures that included AD abnormalities. Successively lower final MMSE scores were observed with increasing comorbidity, with or without contribution by AD lesions.
Associations among the neuropathologic abnormalities.
Spearman correlation matrices (table e-5) demonstrated that the 5 neuropathologic abnormalities were largely independent in their occurrence in both cohorts. Five substantial exceptions were evident: (1) HSindex was associated with ADindex in both cohorts. (2) ADindex was associated with lower brain weight in the NS but not HAAS. (3) ADindex was associated with LBindex in the NS but not HAAS. (4) MINFindex was associated with low brain weight in the HAAS but not NS. (5) HSindex was associated with low brain weight in the HAAS but not NS.
Associations of cognitive impairment with comorbid neuropathologic abnormalities.
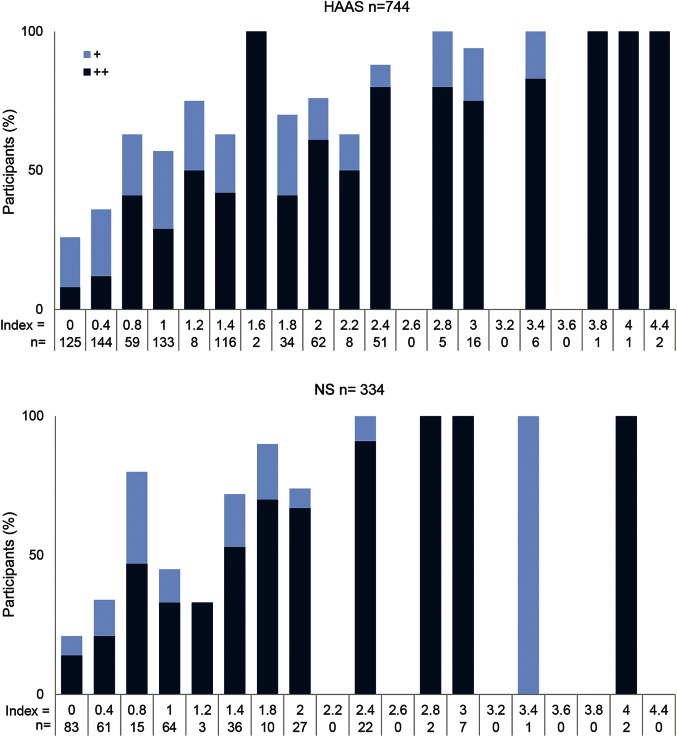
A comorbidity index was computed by summing the 5 individual indices. Figure 1 shows the percentage of participants with moderate or severe cognitive impairment at each observed level of the comorbidity index. The shaded components of the bars indicate the percentage with moderate (gray) or severe (black) cognitive impairment. This illustrates an increase in cognitive impairment with increasing lesion comorbidity in both cohorts. Severe cognitive impairment was observed in 33% of NS women and 35% of HAAS men with a single, severe lesion type (comorbidity index = 1). In both autopsy panels, the proportion of participants who had severe cognitive impairment at final testing approached or reached 100% at comorbidity index values between 2 and 3.
Figure 1. Relationship of comorbidity index to cognitive impairment.
Percent of Honolulu-Asia Aging Study (HAAS) (top) and Nun Study (NS) (bottom) autopsied participants with moderate (+, light blue) or severe (++, dark blue) cognitive impairment in each strata based on the lesion comorbidity index. No or mild cognitive impairment makes up the balance of cases to reach 100%. The bottom rows indicate the number of participants in each stratum.
Strength of the association of neuropathologic comorbidity with cognitive impairment.
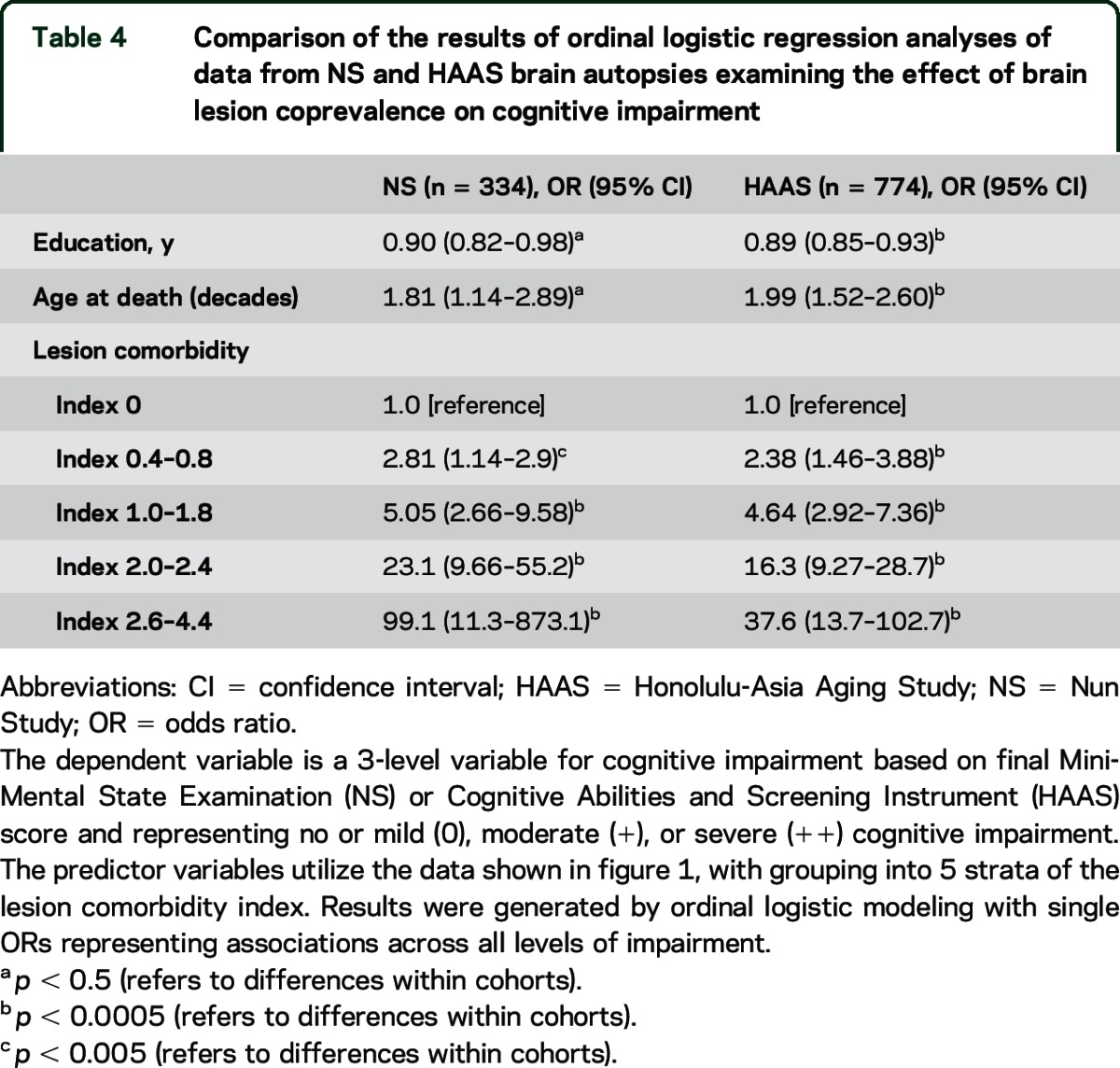
Table 4 presents the results of parallel logistic regression analyses in the 2 panels. The full comorbidity index (20 levels) was collapsed to 5 levels for these models. A dramatic association of increasing cognitive impairment with increasing lesion comorbidity is apparent. The very high ORs observed with high comorbidity indices correspond to expectations based on the multiplicative model apparent in table 3.
Table 4.
Comparison of the results of ordinal logistic regression analyses of data from NS and HAAS brain autopsies examining the effect of brain lesion coprevalence on cognitive impairment
Relationship of interval between testing and death with apparent cognitive resilience.
Substantial levels of AD neuropathologic abnormalities are sometimes found in participants whose cognitive function had been normal. This sort of clinical-pathologic disconcordance has been labeled cognitive resilience. A prolonged interval between cognitive assessments and autopsy could certainly obscure cognitive decline, generating apparent cognitive resilience, as could variables such as the higher education levels found in NS participants.
We examined the frequency and predictors of apparent cognitive resilience in parallel analyses in the NS and HAAS, choosing cases where Braak stage V was assigned in order to focus on the occurrence of severe AD pathology that was nonetheless frequently accompanied by no or mild cognitive impairment. Apparent cognitive resilience in our analysis was defined as no or mild cognitive impairment recognized at final testing in an individual assigned Braak stage V at autopsy.
A total of 44 NS participants received Braak stage V. Average interval between final contact and death was 1.05 years (SD 0.54, range 0.38–2.2) in the 17 (39%) with apparent cognitive resilience and 0.77 years (SD 0.77, range 0.03–1.9) in the 27 who had been cognitively impaired. Multivariate logistic regression analyses identified no significant association of apparent cognitive resilience with the interval, but marginal or statistically significant associations with younger age (p = 0.05), with lesser average neocortical neuritic plaque counts (p = 0.06), and with few or no comorbid neuropathologic abnormalities (p < 0.01).
A total of 121 HAAS participants were assigned Braak stage V. Average interval between final contact and death varied widely, with 38 autopsies within the first year, 32 within the second, 14 within the third, 16 within the fourth, and 21, 4 or more years after final cognitive testing. Levels of apparent cognitive resilience in each of these years were 26%, 31%, 21%, 37%, and 52%, respectively. These percentages indicate a dramatic increase in apparent cognitive resilience after an interval of 3 years. Having few or no comorbid non-AD lesions was strongly associated with apparent cognitive resilience among participants autopsied within 3 years of final testing (p = 0.003). No associations of apparent cognitive resilience were observed with APOE ε4 positivity, APOE ε2 positivity, neocortical NFT counts, or neocortical neuritic plaque counts.
DISCUSSION
Our observations highlight the significance of comorbid neuropathologic abnormalities. We observed substantial differences in strength of association with cognitive impairment for the common dementia-related neuropathologic abnormalities considered individually (as shown in tables 2 and 3) as compared with a much higher likelihood of impairment when multiple types of lesion occur in the same individual (as shown in table 4). Our results are best interpreted as reflecting a summation of geometrically measured influences of independent abnormalities in the same individual. For example, if a brain shows 2 lesion types (i.e., 2 distinct disease processes), each of which triples the likelihood of severe cognitive impairment, one would expect a 9-fold increase in severe cognitive impairment, compared with brains having negligible levels of both. While not of great importance for most younger persons, high levels of comorbidity may be fundamental to late-life frailty and to aging itself.35 Although the frequencies and distributions of comorbidity patterns in both autopsy panels approximate what would be expected by chance given their individual prevalence levels and mutual independence, higher comorbidity indices were far more common in participants who had been impaired. Moreover, the effect of the total comorbidity burden was unrelated to the types of lesion involved, including those of AD. Although AD lesions were more common than the other abnormalities taken individually, most of the impaired participants had lesion mixtures that included few or no AD abnormalities (shown in table e-4).
Such comorbidity might confound clinical research and the assembling of representative or homogeneous cohorts, as needed for meaningful clinical trials. For example, even though HAAS clinical diagnoses of pure AD were reached with rigorous application of National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria (not shown), more than 75% had a low Braak stage (<IV) or substantial levels of neocortical Lewy bodies, microinfarcts, or HS. Interestingly, 75% is near that recently reported for autopsied ADNI participants diagnosed with AD during life.22
The prevalence of Braak stage V or VI and associated cognitive impairment (shown in tables 2 and 3) were both somewhat lower in HAAS participants. Subsequent analyses suggested that this might be at least partially attributable to the Braak stage failing to fully represent the contributions of neocortical NFT and neuritic amyloid plaque counts, which were present in slightly higher levels in the somewhat older NS participants (shown in table e-3).
Apparent cognitive resilience (no or mild cognitive impairment despite Braak stage V) was strongly associated with minimal or no comorbid abnormalities and fewer neocortical AD lesions, and weakly with longer interval between final testing and autopsy. This sort of cognition-neuropathologic discordance could be due to the development of impairment during a protracted interval between final testing and death. Evidence for this idea was observed in HAAS when death occurred 4 or more years after final testing, but was not observed in NS analyses with shorter intervals. Further analyses suggested that younger age and absence of comorbid lesions may contribute to apparent cognitive resilience.
Weaknesses of our study include the range of intervals between final cognitive assessment and death. This interval was relatively short among NS participants, but longer in HAAS. In addition, we focused only on final MMSE or CASI score without considering more extensive cognitive assessments, and without considering prior scores or decline trajectory. Any of these could influence recognized associations with comorbidity patterns and neuropathology–cognition relationships, as previously shown.11,12 Neither cohort was fully representative of the US population, and neither incorporated information from regular medical care. NS participants had a high average education level, which could affect MMSE scores and thus our definition of cognitive impairment. However, despite differences in sex, ethnicity, and life experiences in these 2 distinct cohorts, the similarities observed in cognitive decline and brain abnormalities support the generalizability of our findings.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the School Sisters of Notre Dame and the men of the HAAS for participation and the late Dr. William Markesbery for his contributions to both studies and to the field of neuropathologic evaluation.
GLOSSARY
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- CASI
Cognitive Abilities and Screening Instrument
- HAAS
Honolulu-Asia Aging Study
- HS
hippocampal sclerosis
- IRB
institutional review board
- LB
Lewy bodies
- MMSE
Mini-Mental State Examination
- MMSEc
Mini-Mental State Examination items included in the Cognitive Abilities and Screening Instrument
- NFT
neurofibrillary tangle
- NS
Nun Study
- OR
odds ratio
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. White: study concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision. Dr. Edland: analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Hemmy: acquisition of data, critical revision of the manuscript for important intellectual content, study supervision. Dr. K. Montine: critical revision of the manuscript for important intellectual content. Dr. Zarow: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Sonnen: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Uyehara-Lock: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Gelber: critical revision of the manuscript for important intellectual content. Dr. Ross: critical revision of the manuscript for important intellectual content. Dr. Petrovitch: critical revision of the manuscript for important intellectual content. Dr. Masaki: critical revision of the manuscript for important intellectual content. Dr. Lim: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Launer: study concept and design, critical revision of the manuscript for important intellectual content. Dr. T. Montine: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content.
STUDY FUNDING
Supported by NIH grants U01 AG046871 and R03 AG046614; the Intramural Research Program of the NIH, National Institute on Aging, ALZ Assn ZEN-12-239028; the Chia-Ling Chang Fund of the Hawaii Community Foundation; the Office of Research and Development, Department of Veterans Affairs Pacific Islands Health Care System; and the Nancy and Buster Alvord Endowment.
DISCLOSURE
L. White reports financial support from NIH grants U01-AG046871, U01-AG019349, and U01-AG017155, and from an Alzheimer's Association Zenith fellowship award. S. Edland reports financial support from NIH grants P50-AG005131, U01-AG046871, R03-AG046614, and R03-AG047580, and from Janssen Research & Development. L. Hemmy reports financial support from NIH grants U01-AG046871, R01-AG039396, and R01-AG038651. Previous financial support includes the NIH, the Alzheimer's Association, AHRQ, HRSA, GE Healthcare, and J7J/Janssen. K. Montine, C. Zarow, J. Sonnen, and J. Uyehara-Lock report no disclosures relevant to the manuscript. R. Gelber reports financial support from NIH grants U01-AG017155 and U01-AG019349. G. Ross receives research support from the National Institute of Neurological Disorders and Stroke, the Department of Defense, and the Parkinson's Disease Foundation. He receives salary from the Department of Veterans Affairs. H. Petrovitch reports no disclosures relevant to the manuscript. K. Masaki reports financial support from NIH grants R01-AG038707, R01-AG027060, and R01-HL71561. Previous financial support (more than 2 years ago) related to this manuscript included NIH grants U01-AG019349 and R01-AG017155. Dr. Masaki reports no financial conflicts with commercial enterprises. K. Lim and L. Launer report no disclosures relevant to the manuscript. T. Montine receives financial support from NIH grants P50-AG005136, P50-NS062684, R01-AG031892, R01-AG048232, U01-AG006781, U01-AG046161, U01-AG046871, and U01-AG32984. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease: The Nun Study. JAMA 1997;277:813–817. [PubMed] [Google Scholar]
- 2.White L, Petrovitch H, Hardman J, et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann NY Acad Sci 2002;977:9–23. [DOI] [PubMed] [Google Scholar]
- 3.Launer LJ, Hughes TM, White LR. Microinfarcts, brain atrophy, and cognitive function: the Honolulu-Asia Aging Study autopsy study. Ann Neurol 2011;70:774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gelber RP, Launer LJ, White LR. The Honolulu-Asia Aging Study: epidemiologic and neuropathologic research on cognitive impairment. Curr Alzheimer Res 2012;9:664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SantaCruz KS, Sonnen JA, Pezhouh MK, Desrosiers MF, Nelson PT, Tyas SL. Alzheimer disease pathology in subjects without dementia in 2 studies of aging: the Nun Study and the Adult Changes in Thought Study. J Neuropathol Exp Neurol 2011;70:832–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson PT, Schmitt FA, Lin Y, et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain 2011;134:1506–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neltner JH, Abner EL, Baker S, et al. Arteriolosclerosis that affects multiple brain regions is linked to hippocampal sclerosis of ageing. Brain 2014;137:255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longstreth WT, Jr, Sonnen JA, Koepsell TD, Kukull WA, Larson EB, Montine TJ. Associations between microinfarcts and other macroscopic vascular findings on neuropathologic examination in 2 databases. Alzheimer Dis Assoc Disord 2009;23:291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke 2011;42:722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson K, Stephan BC, Ince PG, Brayne C, Matthews FE, Esiri MM. The neuropathology of vascular disease in the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Curr Alzheimer Res 2012;9:687–696. [DOI] [PubMed] [Google Scholar]
- 11.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology 2004;62:1148–1155. [DOI] [PubMed] [Google Scholar]
- 12.Boyle PA, Yu L, Wilson RS, Schneider JA, Bennett DA. Relation of neuropathology with cognitive decline among older persons without dementia. Front Aging Neurosci 2013;5:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montine TJ, Sonnen JA, Montine KS, Crane PK, Larson EB. Adult changes in thought study: dementia is an individually varying convergent syndrome with prevalent clinically silent diseases that may be modified by some commonly used therapeutics. Curr Alzheimer Res 2012;9:718–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonnen JA, Santa Cruz K, Hemmy LS, et al. Ecology of the aging human brain. Arch Neurol 2011;68:1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 2003;62:1087–1095. [DOI] [PubMed] [Google Scholar]
- 16.Uchino A, Takao M, Hatsuta H, et al. Incidence and extent of TDP-43 accumulation in aging human brain. Acta Neuropathol Commun 2015;3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis 2009;18:691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brayne C, Richardson K, Matthews FE, et al. Neuropathological correlates of dementia in over-80-year-old brain donors from the population-based Cambridge city over-75s cohort (CC75C) study. J Alzheimers Dis 2009;18:645–658. [DOI] [PubMed] [Google Scholar]
- 19.Jellinger KA, Attems J. Challenges of multimorbidity of the aging brain: a critical update. J Neural Transm 2015;122:505–521. [DOI] [PubMed] [Google Scholar]
- 20.Giannakopoulos P, Gold G, Kovari E, et al. Assessing the cognitive impact of Alzheimer disease pathology and vascular burden in the aging brain: the Geneva experience. Acta Neuropathol 2007;113:1–12. [DOI] [PubMed] [Google Scholar]
- 21.Boyle PA, Wilson RS, Yu L, et al. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol 2013;74:478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toledo JB, Cairns NJ, Da X, et al. Clinical and multimodal biomarker correlates of ADNI neuropathological findings. Acta Neuropathol Commun 2013;1:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: the 90+ Study. Neurology 2015;85:535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gelber RP, Petrovitch H, Masaki KH, Ross GW, White LR. Coffee intake in midlife and risk of dementia and its neuropathologic correlates. J Alzheimers Dis 2011;23:607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gelber RP, Petrovitch H, Masaki KH, et al. Lifestyle and the risk of dementia in Japanese-American men. J Am Geriatr Soc 2012;60:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD): part II: standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–486. [DOI] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 28.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr 1994;6:45–58; discussion 62. [DOI] [PubMed] [Google Scholar]
- 29.Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol 2007;62:406–413. [DOI] [PubMed] [Google Scholar]
- 30.Brenowitz WD, Monsell SE, Schmitt FA, Kukull WA, Nelson PT. Hippocampal sclerosis of aging is a key Alzheimer's disease mimic: clinical-pathologic correlations and comparisons with both Alzheimer's disease and non-tauopathic frontotemporal lobar degeneration. J Alzheimers Dis 2014;39:691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White LR. Evidence of important, common disease processes contributing to late-life dementia that have not yet been recognized, characterized, or even named. J Alzheimers Dis 2012;28:481–483. [DOI] [PubMed] [Google Scholar]
- 32.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 33.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996;47:1113–1124. [DOI] [PubMed] [Google Scholar]
- 34.Zarow C, Weiner MW, Ellis WG, Chui HC. Prevalence, laterality, and comorbidity of hippocampal sclerosis in an autopsy sample. Brain Behav 2012;2:435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armstrong JJ, Andrew MK, Mitnitski A, Launer LJ, White LR, Rockwood K. Social vulnerability and survival across levels of frailty in the Honolulu-Asia Aging Study. Age Ageing 2015;44:709–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.