Abstract
Objective:
To explore the value of nuclear magnetic resonance (NMR) and functional assessments for follow-up of ambulatory and nonambulatory patients with Duchenne muscular dystrophy (DMD).
Methods:
Twenty-five 53-skippable patients with DMD were included in this study; 15 were nonambulatory at baseline. All patients underwent clinical and functional assessments every 6 months using the Motor Function Measure (MFM), hand grip and key pinch strength, MoviPlate, and NMR spectroscopy and imaging studies.
Results:
Upper limb distal strength decreased in nonambulatory patients over the period of 1 year; ambulatory patients showed improvement during the same period. The same applied for several NMRS indices, such as phosphocreatine/adenosine triphosphate, which decreased in older patients but increased in younger ambulatory patients. Fat infiltration in the upper limbs increased linearly with age. Almost all NMR and functional assessment results correlated.
Conclusions:
Our results underscore complementarity of functional and NMR assessments in patients with DMD. Sensitivity to change of various indices may differ according to disease stage.
As therapeutic strategies are developed in Duchenne muscular dystrophy (DMD), the need for robust outcome measures to assess the effects of these interventions through the different stages of the disease is increasingly crucial. Currently, the 6-Minute Walk Test (6MWT) is the most commonly used primary outcome measure for assessing the efficacy of therapeutic agents. Therefore, most clinical drug studies are conducted in ambulatory patients. The extension of efficacy data to nonambulatory patients, in whom muscular tissue is more damaged, remains challenging. Given the potential side effects and the very high cost of innovative therapies, evaluation of efficacy in nonambulatory patients is essential.
New upper limb muscle strength and motor ability assessments have recently been developed.1–6 Other approaches to define surrogate endpoints, such as biomarkers or nuclear magnetic resonance (NMR) imaging (NMRI) and spectroscopy (NMRS), have also been described. Resting-state NMR is equally valuable in examination of upper and lower limbs. Despite pioneering NMRS work in forearm muscles of patients with DMD,7 NMR data have since been primarily acquired in pelvic muscles or lower limbs in ambulant patients.8–20 We recently reported the feasibility of using NMR for assessing the upper limb in nonambulatory patients.21
Our main objective in the present study was to establish the baseline values for NMR and functional variables and at a 1-year follow-up time point period for ambulatory and nonambulatory patients with DMD. Secondary aims were to explore the possible relationships between functional and NMR variables and their respective and complementary responsiveness to disease evolution over time.
METHODS
Participants and study design.
Within the framework of a large gene therapy program, and with the ultimate aim of initiating a phase I–II adeno-associated virus 8-U7 clinical trial,22 we launched a natural history study of patients potentially eligible for treatment by induction of skipping of exon 53 of the dystrophin gene, in order to establish long-term pretreatment values in patients with DMD who are likely to take part in the protocol. The main inclusion criteria were the following: confirmation of a mutation theoretically treatable by exon 53 skipping, aged between 6 and 20 years, and weight above 15 kg. Exclusion criteria were the following: inability to sit upright in a wheelchair for at least 1 hour, severe intellectual impairment preventing full understanding of tests, recent upper limb surgery or trauma, or known immunodeficiency. All patients underwent clinical and functional assessments every 6 months using the Motor Function Measure (MFM; http://www.motor-function-measure.org) and the MyoSet devices specifically developed and validated for weak patients4,5,23 (MyoGrip and MyoPinch for hand grip and key pinch strength and MoviPlate for function). Patients were assessed by NMRS and NMRI annually. The duration of the study is planned for 4 years. Here we report the results of the first year (3 visits).
Standard protocol approvals, registrations, and patient consents.
All patients (or legal guardians for patients younger than 18 years) gave written informed consent prior to participation in this study. The local ethics committee approved the study protocol (CPP-Ile de France VI; La Pitié-Salpêtrière, protocol 88-10; clinicaltrials.gov, NCT01385917). Patients were invited to participate through the French Registry for DMD and collaborating neuromuscular centers in Belgium, Switzerland, and Romania.
Strength and functional assessments.
Assessments took place in a quiet muscle evaluation laboratory. We performed tests with patients seated on a chair or in their wheelchair facing a height-adjustable table with their forearm placed on the table or on the wheelchair tray. Before each test, we described the task to patients, demonstrated the movement required, and instructed the patient on maintaining correct practice. If standard upper limb position prescribed by the protocol could not be maintained because of patient contractures, an alternative position was allowed. The patients performed strength tests and then functional tests.
For the MyoSet tests, at least 2 attempts were recorded for each tool and side. We conducted a third and possibly fourth trial if the score for the second attempt was higher than that of the first one, or if the difference between trials was greater than 10%. We used a dedicated custom-made quality control software program to record signals generated using MyoGrip, MyoPinch, and MoviPlate. The side chosen to be tested first was based upon whether the patient had an even or odd patient registry number. We defined the dominant hand as the hand that the patient wrote with (or the one with which the patient previously wrote) and used verbal encouragement when conducting MyoSet. Patients were given a 1-minute rest period between trials for the same device and a 3-minute rest period when changing devices.
NMRI/NMRS acquisitions.
Patient examinations took place in a 3T-60 cm Siemens (Munich, Germany) TRIO with the arm resting alongside the body, as previously detailed.21 We examined each arm in separate sessions on the same day and repeated the examinations 1 year later. Each session consisted of quantitative NMRI and phosphorous NMRS, each lasting approximately 20 minutes, with the patient repositioned between the 2 examinations. We acquired quantitative NMRI measurements of (1) muscle water relaxation time T2 in 3 slices in the forearm (echo times 8.7–147.9 ms, repetition time [TR] 4 seconds, field of view 104 × 128 mm2); (2) fat fraction by 3D, 3-point Dixon imaging covering the same regions, TR 10 ms, 166 × 480 × 128 mm3; and (3) phosphorous NMRS to measure phosphate metabolites using an 11-cm-diameter surface coil predominantly facing flexor muscles (nonlocalized, 500 μs hard pulse excitation [TR 4 seconds, number of excitations 64–128, bandwidth = 3,000 Hz]).
We used 3 NMRI indices in the study: % fat signal (%F), muscle water T2, deconvoluted from fat,24 and T2 heterogeneity (coefficient of variation in a region of interest comprising all flexor muscles of the forearm). We also used 7 NMRS indices: the weighted average pH and 6 metabolic ratios combining adenosine triphosphate (ATP), phosphocreatine (PCr), phosphomonoesters (PME) and phosphodiesters (PDE), and 2 pools of inorganic phosphate (cytosolic [Pia] and an anomalous alkaline pool present in dystrophic muscle [Pib]).25
Statistical analyses.
We used nonparametric tests for analysis of non-Gaussian distribution of most variables and classified patients at inclusion according to ambulation status. We classified ambulatory patients as having the ability to walk 10 meters independently without technical or human aid. Descriptive statistics are presented for both groups of patients as median and first and third quartiles of the distribution of the variables (Q1; Q3). We pooled data from dominant and nondominant sides because differences between sides were not statistically different in 12 of 14 variables (tested with a Wilcoxon test). We tested 1-year differences using a Wilcoxon test. We tested for differences between variables from ambulatory and nonambulatory patients at inclusion using a Mann-Whitney test. For correlation analyses between variables, we treated data from V1 and V3 and dominant and nondominant sides as independent observations and Spearman rho correlation coefficients were computed. We did not perform a statistical analysis to check for the effect of steroids on the various outcome measures as steroid users were not evenly distributed over the various ages of the patients: the large majority of the steroid users were young ambulatory patients. We conducted all analyses using SPSS v.19 statistical software (SPSS Inc., Chicago, IL, USA). A p < 0.05 was considered significant.
RESULTS
Population description.
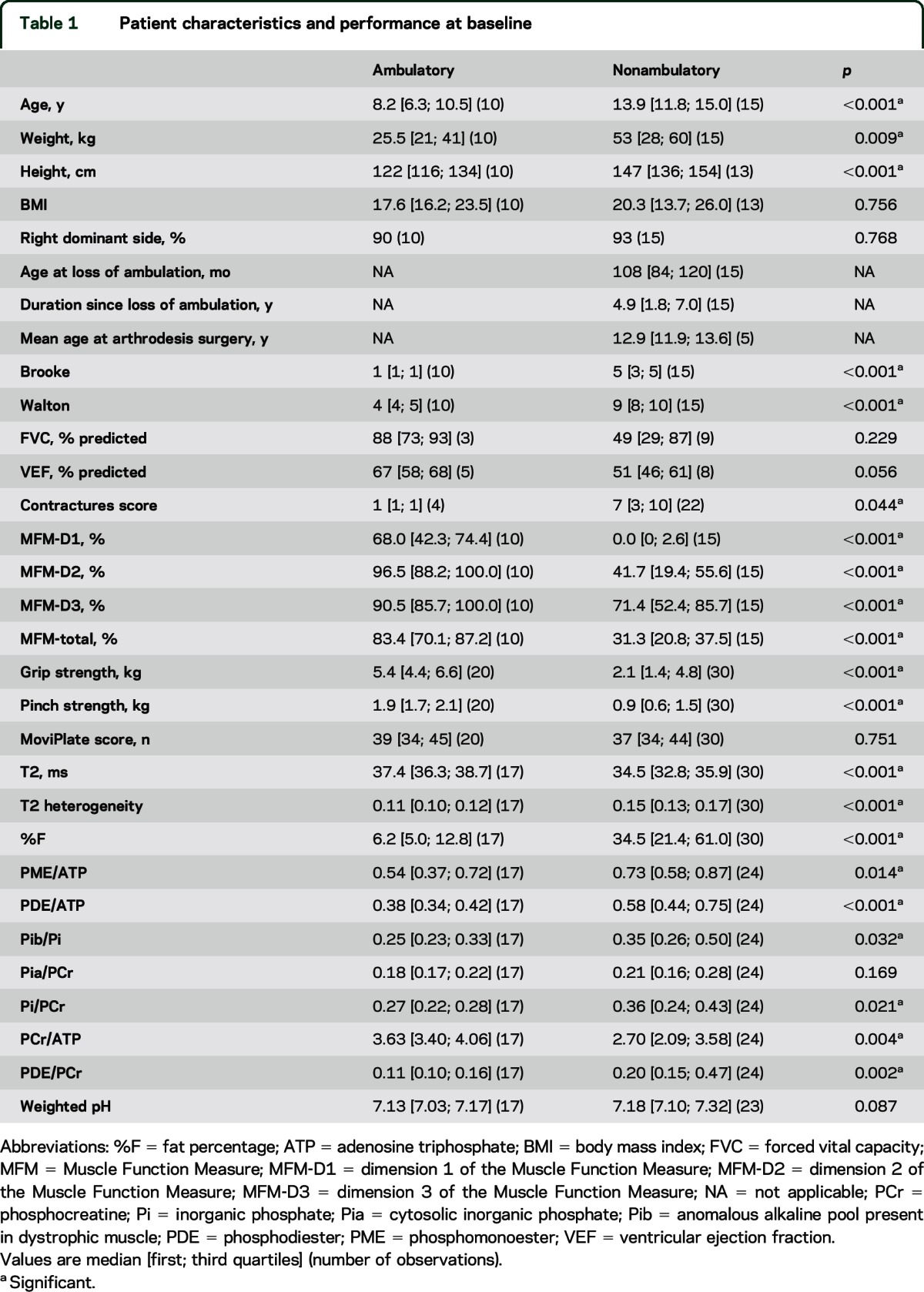
Twenty-five patients with DMD were included in the study; 15 were nonambulatory at baseline. One patient became nonambulatory between the first and second visits. Characteristics of both ambulant and nonambulant groups are presented in table 1. We observed 6 different deletions in the dystrophin gene among the patients (del45-52 in 8 patients, del47-52 in 1 patient, del48-52 in 5 patients, del49-52 in 3 patients, del50-52 in 5 patients, del52 in 3 patients).
Table 1.
Patient characteristics and performance at baseline
Baseline data.
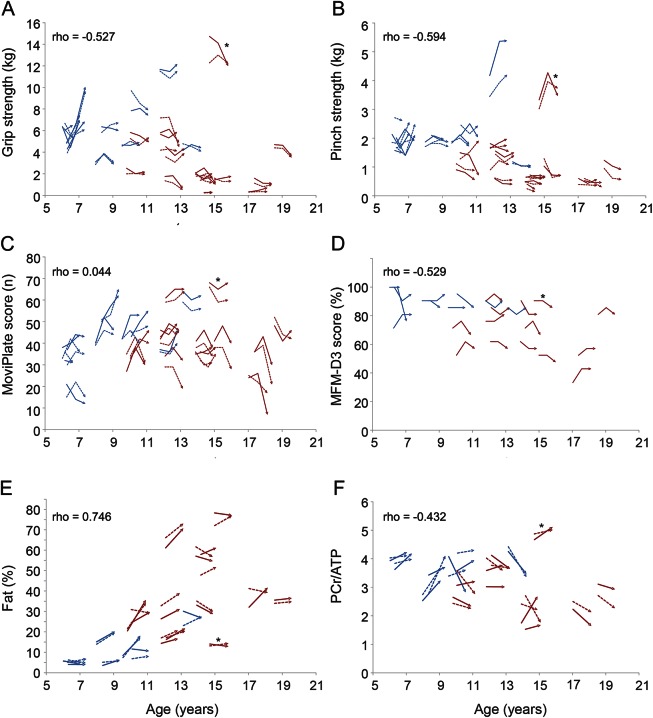
Data for functional and NMR variables at baseline are presented in table 1 for both patient groups. Most variables differed between ambulatory and nonambulatory patients, underlining the more severe phenotype of the latter. Hand grip strength, pinch strength, and MFM scores (and each of its dimensions) decreased with increasing age (all p < 0.001) (figure 1). This was not the case for the MoviPlate results (p = 0.670), which decreased only for the oldest and therefore weakest patients. One nonambulatory patient who showed more preserved strength relative to his age was considered an outlier. All selected NMR variables except Pi/ATP also correlated with age with rho values ranging from 0.237 (p = 0.038) for Pia/PCr to 0.746 (p < 0.001) for %F; T2 (rho = 0.457; p < 0.001) showed normalization with increasing age, but all other measures worsened, as reported previously.21
Figure 1. Correlations between various functional and nuclear magnetic resonance variables and age.
Variables plotted against age: grip strength (A), pinch strength (B), MoviPlate score (C), dimension 3 of the Muscle Function Measure (MFM-D3) (D), % fat (E), and phosphocreatine/adenosine triphosphate (F). Spearman correlations. Ambulatory patients represented in blue; nonambulatory patients in red. *The patient with more preserved strength, considered an outlier.
Changes over 1 year.
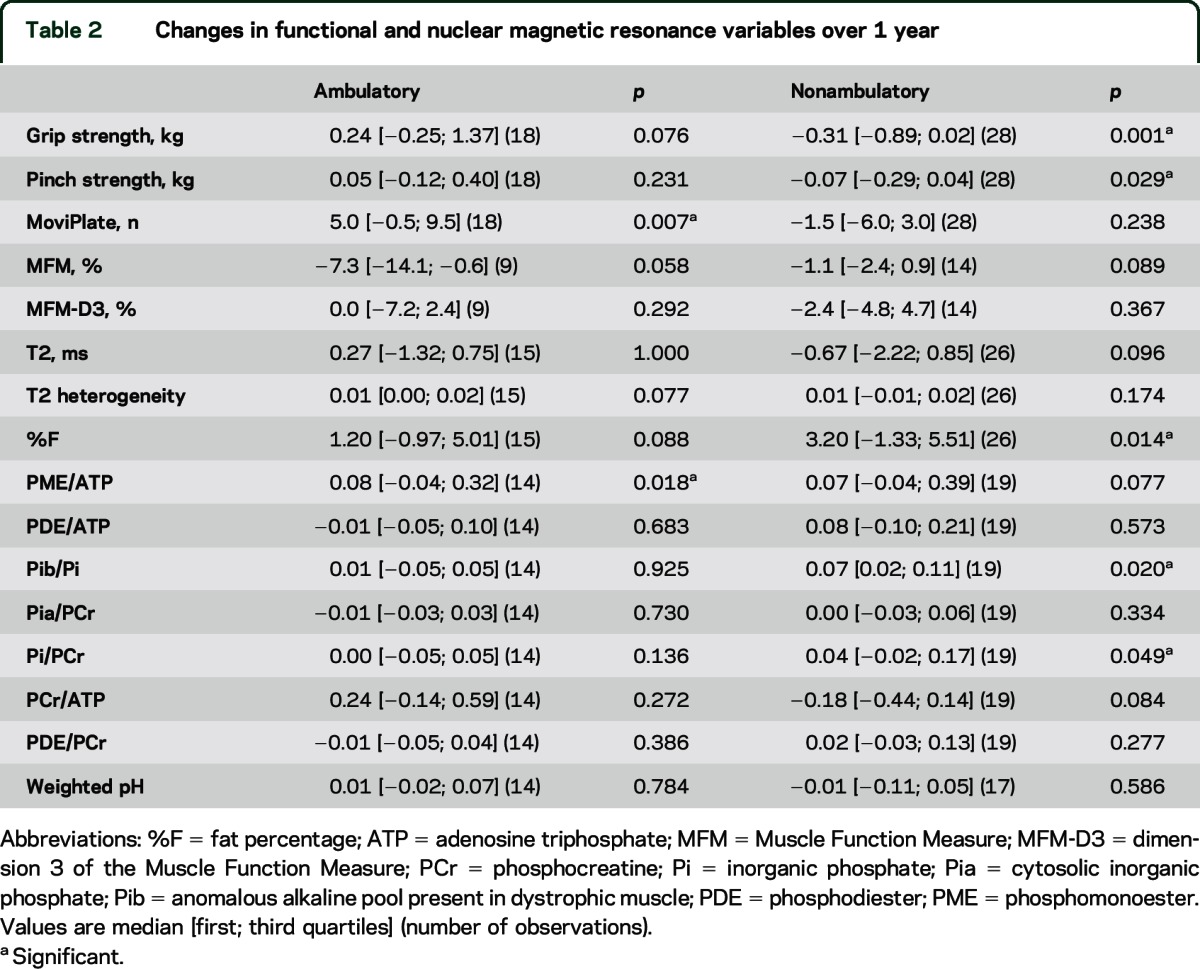
Changes over 1 year contrasted between ambulatory and nonambulatory patients (table 2). For instance, in functional upper limb measurements, ambulatory patients showed improved MoviPlate performance score and no change in grip and pinch, while nonambulatory patients showed a loss of grip and pinch strength and no change in MoviPlate score. For NMR indices, no variables but one (PME/ATP) changed in ambulatory patients. %F, Pib/Pi, and Pi/PCr increased in nonambulatory patients.
Table 2.
Changes in functional and nuclear magnetic resonance variables over 1 year
Correlations between function and NMR variables.
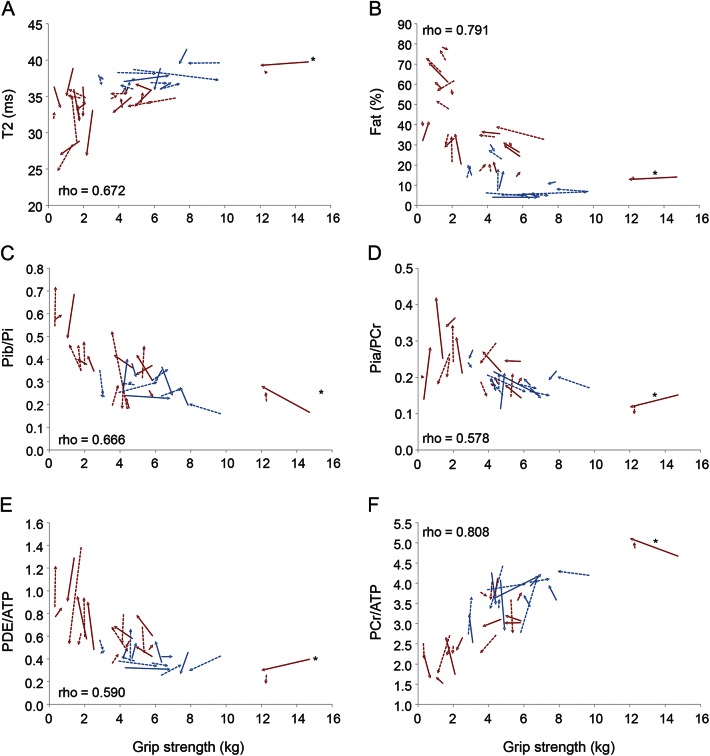
At baseline, almost all NMR and functional variables were correlated (table 3). Most correlations were not linear. Figure 2 illustrates the relationships between grip strength and selected NMR variables, as measured in the flexor muscles of the forearm, and demonstrates the sensitivity of the various indices to disease stage. The patient, noted above as an outlier for functional performance, fell within the correlation curve with regard to NMR variables.
Table 3.
Correlations between functional and nuclear magnetic resonance variables
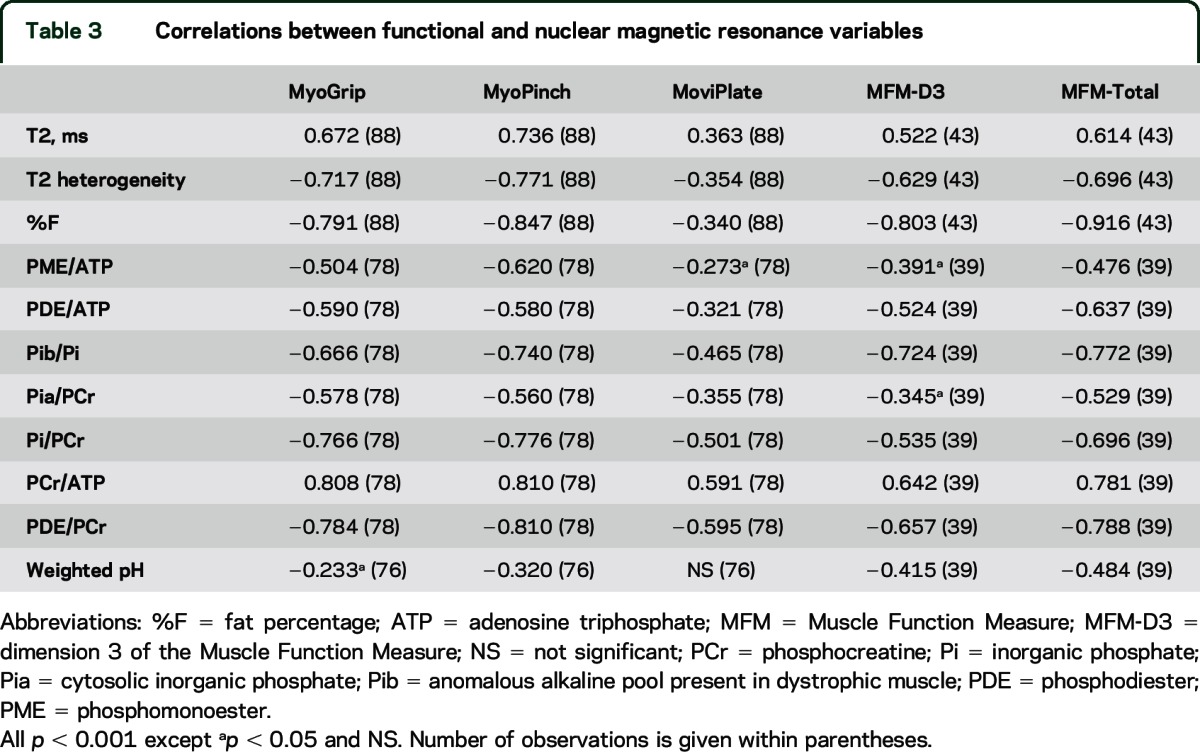
Figure 2. Correlations between grip strength and nuclear magnetic resonance variables.
Variables plotted against grip strength: T2 (A), % fat (B), anomalous alkaline pool present in dystrophic muscle (Pib)/inorganic phosphate (Pi) (C), cytosolic inorganic phosphate (Pia)/phosphocreatine (PCr) (D), phosphodiester (PDE)/adenosine triphosphate (ATP) (E), and PCr/ATP (F). Spearman correlations. Ambulatory patients represented in blue; nonambulatory patients in red. *The patient with more preserved strength, considered an outlier.
DISCUSSION
In this longitudinal study, we assessed and analyzed upper limb function and strength of patients with DMD using both functional tests and NMRI and NMRS. We have followed ambulatory and nonambulatory patients over a 1-year period in a study planned to last 4 years. We demonstrated that evolution of the disease as monitored by NMR and functional assessments of the upper limb is very different in nonambulatory and in ambulatory patients; the exception is %F, which increased as disease progressed.
One strength of this study is its inclusion as part of gene therapy program; data collection was strictly controlled and monitored, and the same evaluators performed all data acquisition and analysis. In addition, the study evaluated a relatively homogenous population from a genetic point of view, since all patients present with deletions potentially treatable by induction of skipping of exon 53 of the dystrophin gene. One limitation is potential generalization of such results to the overall DMD population. Although patients potentially treatable by exon 53 skipping tend to have more severe symptoms than the overall DMD population,26 evolution over a 1-year period is similar to that of the general DMD population (data not shown). Several possible reasons could underlie the more severe phenotype of these patients: they may be smaller in stature compared to other patients with DMD, may have a smaller number of revertant fibers, and may have a weaker response to steroids. These assumptions need to be explored further.
DMD is characterized by a progressive loss of muscle fibers, which are gradually replaced by fat and connective tissue. The disease typically develops from proximal to more distal muscles. Muscles of the hands are thus less affected throughout the disease course.27 Few studies have focused on the upper limbs of patients with DMD, despite the importance of maintaining functional independence as long as possible.28 Grip and pinch strength correlate with physical disability in patients with DMD older than 10 years.5,27 Before 10 years of age, growth and maturation seem to partly compensate for disease progression,27 as depicted here by an improvement in both strength (grip and pinch) and function (MoviPlate) of ambulatory patients over the 1-year observation period. The increase in MoviPlate score in younger nonambulatory patients, as previously reported,4 suggests that compensation strategies are still developing at this stage, until the weakness overrides the potential for adaptation. A possible training effect cannot be ruled out, however. One method to minimize the confounding factors of growth and maturation would be to express strength values as a percentage of predicted values based on normative data. Stature is a major predictive factor of muscle strength,29 but methods for an accurate estimate of stature may be challenging because height or femur length may not be easy to obtain in nonambulatory or retracted patients. This is not the case with hand circumference, which can be easily measured and which has been shown to provide a good estimate of stature and a good prediction of grip strength.30 Strength normalized by stature could be used in the future to provide markers of disease activity that are independent of growth and maturation. Similarly, in NMR evaluations, PCr/ATP increased in ambulatory patients, corresponding to a likely maturation process, but decreased in nonambulatory patients, especially in the older patients, corresponding to loss of metabolically active tissue.21
Various NMR indices reflect different aspects of muscle anomalies and their interpretation has been detailed in a dog model of DMD.25,31 Overall, NMR alterations observed here in the upper limb of patients correlated with those observed in the lower limbs of younger patients. Fat infiltration correlated with clinical assessments such as timed walking/running or rising from supine tests, manual muscle testing, MFM, or Brooke scales.8,9,11–13,15,19,20,32,33 %F in lower limb has been reported previously to be predictive of ambulation loss and linearly correlated with MFM9 and strength,19 whereas metabolic factors did not correlate linearly. We reproduced these findings in upper limbs of nonambulatory patients. A likely explanation is that %F increases linearly with age, whereas many of the metabolic NMR measures evolve principally at a more specific stage of disease according to the different muscle groups. This particular issue needs further investigation.
T2 of muscle water reflects inflammation, edema, or the presence of cell lesions and increases in dystrophic muscle.31,34–36 It does not follow the same disease progression as fat infiltration13,34; instead, T2 of muscle water appears to be highest in earlier stages of the disease.15 In a study on corticosteroid therapy follow-up in young patients with DMD with very low fat infiltration,35 investigators used NMRI and 1H NMRS to measure fat fraction and T2 of water with the 6MWT, timed tests, and quantitative force measurements of the lower limb. Though no direct comparison was given, the 6MWT did not discriminate between treated and untreated patients, nor did plantar flexion strength. In contrast, timed tests, knee extension strength, %F, and water T2 all discriminated between treated and untreated patients. Moreover, investigators observed reduced T2 after 3 months of therapy, suggesting reduced inflammation, with differences in %F only after 1 year.
Spectroscopy findings in the upper limbs also reproduced previous findings in lower limb studies,7,37–40 such as the reduction in PCr/ATP related to loss of functional muscle tissue, the increase in membrane metabolite phosphomonoesters and phosphodiesters related to membrane disruption, and the increase in diesters possibly related to preferential glycolytic fiber destruction. Additionally, we report an increased level of Pib, presumably an anomalous pool of Pi, with poor pH regulation.25 The Pib/Pi pool increases, as does average pH, with disease progression, whereas Pia/PCr increases as cell energy wasting increases. We also propose an arbitrary but sensitive to disease progression index for PDE/PCr that combines PDE/ATP and PCr/ATP, which respectively increases and decreases with disease progression.21 All metabolic indices correlated with functional measurements and deteriorated as strength and function diminished, though possibly not linearly.
Overall, even though all variables except the MoviPlate score strongly discriminated between ambulatory and nonambulatory patients, none of the upper limb clinical assessments showed marked progression over 1 year in the subgroup of ambulatory patients. Contrary to what has been described previously in a similar population, the change in MFM over 1 year did not achieve significance (p = 0.058). This is possibly related to the limited number of patients in the study. In NMR indices, there was no difference over 1 year in upper limbs of ambulatory patients. Despite the excellent correlations between NMR and functional measures at baseline, there were almost no correlations in how measures for individual patients evolved over 1 year. This was not surprising as none of the patients changed dramatically over the course of the year and the nonlinear associations observed suggest that whereas some variables in an individual may change, others may not, depending on his clinical status.
Despite slow progression, the metabolic NMRS indices, the %F, and the distal strength of patients with DMD were initially abnormal compared to age-matched healthy children.21 Some variables (grip and pinch strength, %F, and Pib/Pi) demonstrated a 1-year change in nonambulatory patients and are thus good comparators for this population. Outcome measures are often presented as competing, but here we aimed to show complementarity. Correlation of the variables is somewhat dependent on clinical status. This is hardly surprising, given the very different nature of measured outcomes, ranging from strength to cellular metabolism. For instance, grip strength is clearly more sensitive to change in PDE/ATP when grip strength is higher than approximately 4 kg, but the opposite holds true when the patient becomes weaker. In addition, combining approaches may help to better understand performance of apparent outliers and to validate a measure that may appear to be false. This is illustrated in this study by the patient who clearly outperformed peers in distal strength, but presented as normal when related to NMR indices.
Thus, the question of how a single outcome is clinically meaningful alone is probably less important than the question of how several outcomes can depict the changes experienced by the patients in a complementary way in order to conveniently describe the evolution of pathophysiologic features and clinical status throughout life. Correct choice and stratification of outcome measures in primary, secondary, or tertiary outcomes in nonambulatory patients should take into account the stage of the disease. In nonambulatory patients with early stage DMD, grip and pinch strength combined with T2 measures could constitute a good choice. Percentage of fat in the upper limb muscles is useful throughout the late ambulatory and the nonambulatory period, as it appeared to highly correlate with functional assessments in these patients.
ACKNOWLEDGMENT
The authors thank the patients and their families for participation in the study; and Simone Birnbaum for reading the manuscript and improving the text.
GLOSSARY
- %F
fat percentage
- 6MWT
6-Minute Walk Test
- ATP
adenosine triphosphate
- DMD
Duchenne muscular dystrophy
- MFM
Motor Function Measure
- NMR
nuclear magnetic resonance
- NMRI
nuclear magnetic resonance imaging
- NMRS
nuclear magnetic resonance spectroscopy
- PCr
phosphocreatine
- Pi
inorganic phosphate
- Pia
cytosolic inorganic phosphate
- Pib
anomalous alkaline pool present in dystrophic muscle
- PDE
phosphodiester
- PME
phosphomonoester
- TR
repetition time
AUTHOR CONTRIBUTIONS
Designing the study: L.S., J.-Y.H., P.C. Drafting the manuscript: J.-Y.H., C.W. Revising and approving the manuscript: all authors. Functional data acquisition: V.D., G.O., A.C., C.L. Clinical evaluation and follow-up of patients: L.S., R.C., T.G., A.G.L. NMR data acquisition, processing, and analysis: C.W., N.A. Statistical analyses: A.M. Data interpretation: J.-Y.H., C.W., A.M., M.A., P.G.C., L.S. Study supervision: J.-Y.H., P.G.C., L.S. Study guarantor: LS.
STUDY FUNDING
The natural history study was sponsored by Genethon. This project was supported by Association Française contre les Myopathies (AFM) and Advanced Diagnostics for New Therapeutic Approaches (ADNA), a program dedicated to personalized medicine, coordinated by Institut Mérieux and supported by research and innovation aid from the French public agency, OSEO. The funders were not involved in data collection or interpretation or preparation of the paper.
DISCLOSURE
J. Hogrel is coinventor of the MyoGrip, MyoPinch, and MoviPlate and receives research support from the European Community and the Association Française contre les Myopathies (AFM). C. Wary reports no disclosures. A. Moraux is coinventor of the MyoPinch. N. Azzabou, V. Decostre, and G. Ollivier report no disclosures relevant to the manuscript. A. Canal is coinventor of the MoviPlate. C. Lilien, I. Ledoux, M. Annoussamy, N. Reguiba, T. Gidaro, A. Le Moing, and R. Cardas report no disclosures relevant to the manuscript. T. Voit serves on scientific advisory boards for Prosensa/Biomarin and is coinventor of the MoviPlate. P. Carlier receives support from the European Community and the Association Française contre les Myopathies. L. Servais is coinventor of the MoviPlate, has received consulting fees from Roche, Sarepta, Biomarin, and aTyrPharma, receives support from the European Community and the Association Française contre les Myopathies, and is coordinating natural history studies funded by Valerion and Roche. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Han JJ, Kurillo G, Abresch RT, De Bie E, Nicorici A, Bajcsy R. Upper extremity 3-dimensional reachable workspace analysis in dystrophinopathy using Kinect. Muscle Nerve 2015;52:344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowes LP, Alfano LN, Crawfis R, et al. Reliability and validity of active-seated: an outcome in dystrophinopathy. Muscle Nerve 2015;52:356–362. [DOI] [PubMed] [Google Scholar]
- 3.Mayhew A, Mazzone ES, Eagle M, et al. Development of the performance of the upper limb module for Duchenne muscular dystrophy. Dev Med Child Neurol 2013;55:1038–1045. [DOI] [PubMed] [Google Scholar]
- 4.Seferian AM, Moraux A, Annoussamy M, et al. Upper limb strength and function changes during a one-year follow-up in non-ambulant patients with Duchenne muscular dystrophy: an observational multicenter trial. PLoS One 2015;10:e0113999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Servais L, Deconinck N, Moraux A, et al. Innovative methods to assess upper limb strength and function in non-ambulant Duchenne patients. Neuromuscul Disord 2013;23:139–148. [DOI] [PubMed] [Google Scholar]
- 6.Vuillerot C, Girardot F, Payan C, et al. Monitoring changes and predicting loss of ambulation in Duchenne muscular dystrophy with the Motor Function Measure. Dev Med Child Neurol 2010;52:60–65. [DOI] [PubMed] [Google Scholar]
- 7.Newman RJ, Bore PJ, Chan L, et al. Nuclear magnetic-resonance studies of forearm muscle in Duchenne dystrophy. Br Med J 1982;284:1072–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akima H, Lott D, Senesac C, et al. Relationships of thigh muscle contractile and non-contractile tissue with function, strength, and age in boys with Duchenne muscular dystrophy. Neuromuscul Disord 2012;22:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischmann A, Hafner P, Gloor M, et al. Quantitative MRI and loss of free ambulation in Duchenne muscular dystrophy. J Neurol 2013;260:969–974. [DOI] [PubMed] [Google Scholar]
- 10.Forbes SC, Walter GA, Rooney WD, et al. Skeletal muscles of ambulant children with Duchenne muscular dystrophy: validation of multicenter study of evaluation with MR imaging and MR spectroscopy. Radiology 2013;269:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaeta M, Messina S, Mileto A, et al. Muscle fat-fraction and mapping in Duchenne muscular dystrophy: evaluation of disease distribution and correlation with clinical assessments: preliminary experience. Skeletal Radiol 2012;41:955–961. [DOI] [PubMed] [Google Scholar]
- 12.Kim HK, Laor T, Horn PS, Racadio JM, Wong B, Dardzinski BJ. T2 mapping in Duchenne muscular dystrophy: distribution of disease activity and correlation with clinical assessments. Radiology 2010;255:899–908. [DOI] [PubMed] [Google Scholar]
- 13.Kim HK, Merrow AC, Shiraj S, Wong BL, Horn PS, Laor T. Analysis of fatty infiltration and inflammation of the pelvic and thigh muscles in boys with Duchenne muscular dystrophy (DMD): grading of disease involvement on MR imaging and correlation with clinical assessments. Pediatr Radiol 2013;43:1327–1335. [DOI] [PubMed] [Google Scholar]
- 14.Kinali M, Arechavala-Gomeza V, Cirak S, et al. Muscle histology vs MRI in Duchenne muscular dystrophy. Neurology 2012;76:346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marden FA, Connolly AM, Siegel MJ, Rubin DA. Compositional analysis of muscle in boys with Duchenne muscular dystrophy using MR imaging. Skeletal Radiol 2005;34:140–148. [DOI] [PubMed] [Google Scholar]
- 16.Mathur S, Lott DJ, Senesac C, et al. Age-related differences in lower-limb muscle cross-sectional area and torque production in boys with Duchenne muscular dystrophy. Arch Phys Med Rehabil 2010;91:1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senesac CR, Lott DJ, Forbes SC, et al. Longitudinal evaluation of muscle composition using magnetic resonance in 4 boys with Duchenne muscular dystrophy: case Series. Phys Ther 2015;95:978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wokke BH, Bos C, Reijnierse M, et al. Comparison of Dixon and T1-weighted MR methods to assess the degree of fat infiltration in Duchenne muscular dystrophy patients. J Magn Reson Imaging 2013;38:619–624. [DOI] [PubMed] [Google Scholar]
- 19.Wokke BH, van den Bergen JC, Versluis MJ, et al. Quantitative MRI and strength measurements in the assessment of muscle quality in Duchenne muscular dystrophy. Neuromuscul Disord 2014;24:409–416. [DOI] [PubMed] [Google Scholar]
- 20.Wren TA, Bluml S, Tseng-Ong L, Gilsanz V. Three-point technique of fat quantification of muscle tissue as a marker of disease progression in Duchenne muscular dystrophy: preliminary study. AJR Am J Roentgenol 2008;190:W8–W12. [DOI] [PubMed] [Google Scholar]
- 21.Wary C, Azzabou N, Giraudeau C, et al. Quantitative NMRI and NMRS identify augmented disease progression after loss of ambulation in forearms of boys with Duchenne muscular dystrophy. NMR Biomed 2015;28:1150–1162. [DOI] [PubMed] [Google Scholar]
- 22.Le Guiner C, Montus M, Servais L, et al. Forelimb treatment in a large cohort of dystrophic dogs supports delivery of a recombinant AAV for exon skipping in Duchenne patients. Mol Ther 2014;22:1923–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seferian AM, Moraux A, Canal A, et al. Upper limb evaluation and one-year follow up of non-ambulant patients with spinal muscular atrophy: an observational multicenter trial. PLoS One 2015;10:e0121799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azzabou N, Loureiro de Sousa P, Caldas E, Carlier PG. Validation of a generic approach to muscle water T2 determination at 3T in fat-infiltrated skeletal muscle. J Magn Reson Imaging 2015;41:645–653. [DOI] [PubMed] [Google Scholar]
- 25.Wary C, Naulet T, Thibaud JL, Monnet A, Blot S, Carlier PG. Splitting of Pi and other 31P NMR anomalies of skeletal muscle metabolites in canine muscular dystrophy. NMR Biomed 2012;25:1160–1169. [DOI] [PubMed] [Google Scholar]
- 26.Servais L, Montus M, Le Guiner C, et al. Non-ambulant Duchenne patients theoretically treatable by exon 53 skipping have severe phenotype. J Neuromuscul Dis 2015;2:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattar FL, Sobreira C. Hand weakness in Duchenne muscular dystrophy and its relation to physical disability. Neuromuscul Disord 2008;18:193–198. [DOI] [PubMed] [Google Scholar]
- 28.Wagner MB, Vignos PJ, Carlozzi C, Hull AL. Assessment of hand function in Duchenne muscular-dystrophy. Arch Phys Med Rehab 1993;74:801–804. [DOI] [PubMed] [Google Scholar]
- 29.Hogrel JY, Decostre V, Alberti C, et al. Stature is an essential predictor of muscle strength in children. BMC Musculoskelet Disord 2012;13:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hogrel JY. Grip strength measured by high precision dynamometry in healthy subjects from 5 to 80 years. BMC Musculoskelet Disord 2015;16:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thibaud JL, Azzabou N, Barthelemy I, et al. Comprehensive longitudinal characterization of canine muscular dystrophy by serial NMR imaging of GRMD dogs. Neuromuscul Disord 2012;22(suppl 2):S85–S99. [DOI] [PubMed] [Google Scholar]
- 32.Torriani M, Townsend E, Thomas BJ, Bredella MA, Ghomi RH, Tseng BS. Lower leg muscle involvement in Duchenne muscular dystrophy: an MR imaging and spectroscopy study. Skeletal Radiol 2012;41:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willcocks RJ, Arpan IA, Forbes SC, et al. Longitudinal measurements of MRI-T2 in boys with Duchenne muscular dystrophy: effects of age and disease progression. Neuromuscul Disord 2014;24:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arpan I, Forbes SC, Lott DJ, et al. T(2) mapping provides multiple approaches for the characterization of muscle involvement in neuromuscular diseases: a cross-sectional study of lower leg muscles in 5-15-year-old boys with Duchenne muscular dystrophy. NMR Biomed 2013;26:320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arpan I, Willcocks RJ, Forbes SC, et al. Examination of effects of corticosteroids on skeletal muscles of boys with DMD using MRI and MRS. Neurology 2014;83:974–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vulin A, Barthelemy I, Goyenvalle A, et al. Muscle function recovery in golden retriever muscular dystrophy after AAV1-U7 exon skipping. Mol Ther 2012;20:2120–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banerjee B, Sharma U, Balasubramanian K, Kalaivani M, Kalra V, Jagannathan NR. Effect of creatine monohydrate in improving cellular energetics and muscle strength in ambulatory Duchenne muscular dystrophy patients: a randomized, placebo-controlled 31P MRS study. Magn Reson Imaging 2010;28:698–707. [DOI] [PubMed] [Google Scholar]
- 38.Barbiroli B, Funicello R, Iotti S, Montagna P, Ferlini A, Zaniol P. 31P-NMR spectroscopy of skeletal muscle in Becker dystrophy and DMD/BMD carriers: altered rate of phosphate transport. J Neurol Sci 1992;109:188–195. [DOI] [PubMed] [Google Scholar]
- 39.Griffiths RD, Cady EB, Edwards RH, Wilkie DR. Muscle energy metabolism in Duchenne dystrophy studied by 31P-NMR: controlled trials show no effect of allopurinol or ribose. Muscle Nerve 1985;8:760–767. [DOI] [PubMed] [Google Scholar]
- 40.Younkin DP, Berman P, Sladky J, Chee C, Bank W, Chance B. 31P NMR studies in Duchenne muscular dystrophy: age-related metabolic changes. Neurology 1987;37:165–169. [DOI] [PubMed] [Google Scholar]