Abstract
Uveitis, which occurs in association with systemic immunological diseases, presents a considerable medical challenge due to incomplete understanding of its pathogenesis. The signals that initiate T cells to target the eye, which may be of infectious or non-infectious origin, are poorly understood. Experimental autoimmune uveoretinitis (EAU) develops in mice immunized with the endogenous retinal protein interphotoceptor retinoid binding protein (IRBP) in the presence of the adjuvant CFA. EAU manifests as posterior ocular inflammation consisting of vasculitis, granulomas, retinal damage and invasion of self-reactive T cells, which are key clinical features of human uveitis. Our studies uncover Card9 as a critical genetic determinant for EAU. Card9 was responsible for Th17 polarization and Th17-associated antigen-specific responses, but not Th1-associated responses. Nonetheless, Card9 expression was essential for accumulation of both lineages within the eye. Consistent with its recently identified role as an intracellular signaling mediator for C-type lectin receptors (CLRs), a Card9-dependent transcriptional response in the neuroretina was observed involving genes encoding the CLRs Dectin-1, Dectin-2 and Mincle. Genetic deletion of these individual CLRs revealed an essential role for Mincle. Mincle activation was sufficient to generate the EAU phenotype, and this required activation of both Syk and Card9. In contrast, Dectin-1 contributed minimally and a possible repressive role was shown for Dectin-2. These findings extend our understanding of CLRs in autoimmune uveitis. The newly identified role of Mincle and Syk/Card9-coupled signaling axis in autoimmune uveitis could provide novel targets for treatment of patients with ocular inflammatory disease.
INTRODUCTION
There is a need to define underlying mechanisms of uveitis (intraocular inflammation), a leading cause of blindness in the Western world (1, 2). Uveitis encompasses a heterogeneous group of disorders that arise from infectious as well as non-infectious etiologies. Uveitis presents in many forms that can affect different tissue(s) of the eye, be chronic or acute, and affect one eye or both. Most non-infectious uveitis cases occur in the context of systemic immunological disorders such as inflammatory bowel disease (IBD), Crohn’s disease, multiple sclerosis as well as many forms of rheumatic diseases such as ankylosing spondylitis (AS) (2). Intriguingly, even in some of these “non-infectious” uveitis cases the pathology is granulomatous-like, which has historically been described as “mycobacterial-infection-like pathology” (3), and which is usually associated with an infectious etiology. Unfortunately, the cellular and molecular mechanisms by which the eye is targeted for disease are not completely understood, thereby hampering the identification of novel safe and effective therapies.
As an immune-privileged site, the eye maintains tight control of inflammation in order to prevent irreversible tissue damage and vision loss. Experimental autoimmune uveoretinitis (EAU) is a prototypic animal model used to study mechanisms by which a T cell-mediated disease specifically targets the eye. Peripheral immunization with interphotoreceptor retinoid-binding protein (IRBP), an immune-privileged retinal antigen, results in many clinicopathological features of human uveitis such as: break in immune tolerance, polarization of autoreactive Th cell subsets, breakdown of the blood-retina barrier, leukocyte infiltration, damage to ocular tissues (e.g. photoreceptor destruction), and visual morbidity (3-5). Much of the uveitis research has focused on the significance of acquired CD4+ T cell pathways, where the importance of Th1/Th17-driven responses has been established. The Th17 effector population is considered the main pathogenic driver in EAU (6), but both T cell subsets can conceivably influence disease outcome (7, 8).
The innate immune signals that precede and shape autoreactive T cell responses in uveitis are poorly defined, but the prevailing paradigm proposes that initiating events arise from host-microbial interactions. The innate immune system senses microbial triggers by means of germ-line encoded receptors known as pattern recognition receptors (PRRs), which include toll-like receptors (TLRs), NOD-like receptors (NLRs), RIG-I-like receptors (RLRs) and C-type lectin receptors (CLRs). Collectively, these distinct families of PRRs are capable of detecting and distinguishing diverse types of microorganisms including bacteria, viruses or fungi. Their transcriptional activation of cytokine/chemokine subsets is intended to form the optimal response(s) against the specific type of microbe (9). Studies of innate immune receptors in EAU have focused primarily on the prototypical family of PRRs, the TLRs (10). However, mice deficient for TLRs 2, 3, 4 or 9 (as well as double-knockout (KO) mice) develop EAU comparable to their WT counterparts (11, 12); suggesting a redundant or non-essential role. The intracellular adaptor molecule MyD88, which is essential for TLR as well as IL-1R signaling, is however a requisite. This is not surprising given that IL-1R-deficiency results in the same resistant phenotype as MyD88 KO mice (11), thereby identifying IL-1 as an important, non-redundant signal in EAU. Consequently, the question as to which innate receptor pathway is responsible for orchestration of ocular autoimmunity remains unanswered.
The CLRs are an integral PRR system in host defense against fungal infection as well as some bacterial infections. The most well-studied CLRs have been Dectin-1 along with Dectin-2 and Mincle, which are a subgroup of CLRs that signal through the adaptor molecule caspase-associated recruitment domain adaptor 9 (Card9) (13). Card9 is expressed predominantly in myeloid cells such as dendritic cells and macrophages (14), and its activation involves the upstream kinases Syk and PKC-delta. Activation of the Syk/Card9-coupled signaling axis is important for induction of cytokines such as IL-6, IL-1β and IL-23 and optimal expansion of the Th17 effector population (15). As such, Card9 expression is vital for host immunity against fungal and Mycobacterium tuberculosis (M. tuberculosis, Mtb) infection (16, 17). A precise role for Card9 in autoimmune disease has not yet been described, but the juxtaposition of Card9 with microbial sensing and Th17-associated inflammatory diseases such as uveitis is extremely compelling. Conceivably, aberrant regulation of Card9, either through genetic mutation (eg polymorphism) or activation by environmental triggers, could contribute to pathological immune activation and uveitis development.
The goal of this study was to evaluate the functional contribution of Card9 in EAU and to elucidate the innate immune receptor(s) that influence pathogenesis of uveitis. Taken together, data presented support the central role of a Syk/Card9-coupled signaling axis in induction of ocular autoimmunity; specifically, that which is initiated predominantly upon engagement of Mincle. These findings extend our understanding of PRRs beyond TLRs to include the CLR system in ocular autoimmune disease.
MATERIALS AND METHODS
Mice
Mice lacking genes for Card9 (Card9), Mincle (Clec4e), Dectin-2 (Clec4n), and Dectin-1 (Clec7a) have been previously described (18-21) and were backcrossed onto the C57BL/6J background (Jackson Laboratory, Bar Harbor, ME) for more than 10 generations. Littermate controls were used to generate WT and homozygous KO mice for experiments; all of which were bred in-house under specific pathogen-free conditions at the VA Portland Health Care System in Portland, OR. Mice were used in experiments between the ages of 8-12 wks and were gender-matched. Studies were performed in compliance with the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals, and were carried out in adherence to institutional protocols approved at the VA Portland Health Care System.
Induction of EAU and histopathology scoring
Mice were immunized by s.c. injections in both flanks and at the base of the tail with 150 μg purified bovine IRBP (22) + 300 μg IRBP1-20 peptide (synthesized by Anaspec, Fremont, CA) mixture emulsified in CFA (Sigma, St. Louis, MO) that was supplemented with 2.5 mg/ml M. tuberculosis (strain H37RA; Difco). On the day of immunization, mice also received an i.p. injection of 0.5 μg Bordetella pertussis toxin (PTX; List Biological Laboratories). For adjuvant replacement studies (Figs. 5 and 6), mice were immunized as described above, but in the absence of CFA. Instead, IFA (Sigma) was supplemented to a final concentration of 2.5 mg/ml trehalose-6,6'-dibehenate (TDB, a synthetic analogue of mycobacterial cord factor; InvivoGen) or the bacterial products peptidoglycan (PGN; InvivoGen) or muramyl dipeptide (MDP; Bachem, Torrance, CA). For pharmacological inhibition of Syk, mice were i.p. injected with 5 mg/kg piceatannol (InvivoGen) on days 0, 4, 8, 12, and 16 in relation to immunization.
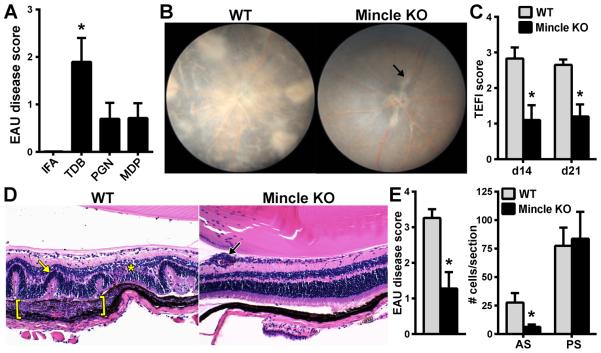
Figure 5. Activation of Mincle by TDB is responsible for induction of EAU.
IFA was reconstituted to 2.5 mg/ml with individual mycobacterial-associated components: TDB (synthetic cord factor, trehalose-6-6’-dibehenate), PGN (peptidoglycan), MDP (muramyl dipeptide). Adjuvant with the indicated substitutions were used for immunization of WT mice and uveitis was evaluated d21 post-immunization (A); *p<0.05 vs PGN or MDP. WT vs Mincle KO mice were evaluated for EAU induced in the presence of TDB. Clinical uveitis was significantly reduced in Mincle KO compared to WT mice (B, C). Histology showed little inflammation in Mincle KO mice compared to WT (D) with overall reduction in retinal disease severity (E) but not in cellular infiltrate of the vitreous. Black arrow = vasculitis, Yellow arrow=retinal fold, Asterisk=granuloma, Bracket = Subretinal granulomatous inflammation *p<0.05 vs WT mice (n=10-14 total mice/group; combined from 2 independent experiments).
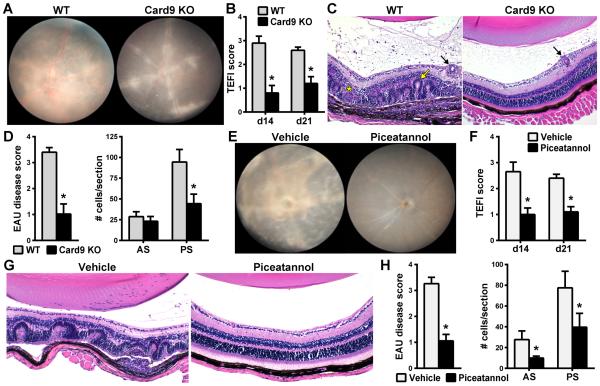
Figure 6. The Syk/Card9 coupled axis is responsible for mechanisms by which Mincle induces EAU.
WT and Card9 KO mice were immunized with adjuvant containing TDB and evaluated clinically by fundoscopy (A,B) and histologically (C,D) ). (E-H) The reduced susceptibility of Card9 KO mice to EAU was reproduced in WT mice by pharmacologic inhibition of the upstream kinase Syk with systemically administered piceatannol beginning on day of immunization (d0) and continuing every 4 days until day 16. Eyes were evaluated by fundus exam (E,F) and by histopathology scoring and quantification of cells infiltrated into the anterior and posterior chambers (G,H) (AS = anterior segment; PS = posterior segment) on d21. *P<0.05 vs. WT or vehicle treatment (n=12-14/group; combined data of 2 separate experiments).
Eyes collected at the indicated time points were fixed in 10% neutral-buffered formalin and embedded in paraffin for tissue sectioning. Sections (7 μm) were cut through the pupillary-optic nerve axis at four different depths and three H&E-stained sections from each depth were prepared. Uveitis severity was evaluated by masked observers using defined grading criteria ranging from 0 (no disease) to 4 (maximum disease) (22) based on extent of retinal cell damage, retinal detachment, vasculitis, granulomatous-like lesions, hemorrhages, perivascular exudates, and optic disc infiltration. Leukocyte infiltration into the aqueous humor of the anterior segment and the vitreous body of the posterior segment were quantified as the mean number of cells per section per eye. Incidence was calculated based on an animal having achieved an EAU score ≥ 1 in at least one eye.
Topical endoscopic fundus imaging (TEFI) and clinical scoring
Mice were anesthetized with isoflurane inhalation and pupils were dilated with topical 2.5% phenylephrine hydrochloride (Bausch & Lomb) and 1% tropicamide (Alcon). Genteal® gel (Novartis) was applied over the cornea. Fundus images were captured (adapted from (23)) with a system consisting of: a tripod-mounted Nikon D90 camera, 60 mm F/2.8 D lens (Nikon), Storz 481C halogen light (Karl Storz), and Storz 1218 tele-otosope (Karl Storz). Clinical disease severity was assessed in masked fashion using a previously defined scoring system (22, 24) ranging from 0 (no disease) to 4 (maximal disease) based on the extent of vasculitis, infiltrative lesions, optic disc inflammation, retinal hemorrhages, and retinal detachment.
Evaluation of leukocyte populations in the eye by flow cytometry
Eyes were enucleated and the intraocular lens removed. Previously described methods (7) were adapted to prepare single cell suspensions. In brief, finely minced eye tissue was pooled and digested with Collagenase D (1 mg/ml, Roche) for 40 min at 37°C with intermittent shaking, sequentially passed through a 70 μm and 40 μm filter, washed and suspended in FACS buffer. Cells were stained with vital fluorescent dye (Viability Dye eFluor® 506; eBioscience, San Diego, CA, USA) and incubated with Fc block (3 μg/ml, BD Pharmingen) for 30 min on ice. Cells were then incubated for 30 min on ice with the following fluorescently-labeled antibodies (all from BD Biosciences): CD45, CD3, CD4, CD8, and for exclusion (CD11b, B220, Gr1, CD11c). Cells were fixed with 1% paraformaldehyde and analyzed on a BD LSRFortessa (BD Biosciences). Dead cells were excluded based on viability dye staining so that only live cell events were collected. FCS Express (De Novo Software) was used for analysis, allowing compensations for spectral overlaps within each sample. Gating used defined criteria based on control samples stained with corresponding isotype controls (I.C.). Live cells were gated from forward and side scatter plots in identical fashion for each group after which frequencies and numbers of leukocyte subpopulations were determined from CD45+ gated cells. For intracellular cytokine staining, ocular cell suspensions were seeded (1×106 per well) in 96-well V-bottom polypropylene plates (Corning Inc., Corning, NY) in HL-1 medium (Lonza, Basel, Switzerland) and stimulated with PMA, ionomycin, and brefeldin A as described below.
Antigen-stimulation assay and intracellular cytokine staining
Assays were performed as previously described with modification (22, 25). Single cell suspensions were prepared from spleens on d21 post-immunization as described. Cells (5 × 105/ml) were plated in HL-1 media (Lonza) containing 2mM glutamine and 100 U/ml penicillin-strepotmycin (Invitrogen Life Technologies). After 48 h stimulation with 20 μg/ml whole IRBP protein, cells were stimulated in the presence of 20 ng/ml PMA (LC Labs), 200 ng/ml ionomycin (LC Labs, Woburn, MA), and brefeldin A (BD GolgiPlug; BD Biosciences) for 4h at 37°C prior to fixation/permeabilization using BD Cytofix/CytopermTM Fixation/Permeabilization solution Kit (BD Biosciences). Cells were stained with fluorescently-conjugated antibodies to CD45, CD4, CD8, IFNγ, and IL-17A. Flow cytometry with a BD LSRFortessa (BD Biosciences) was used to collect a total of 300,000 CD45+ events. Dead cells were excluded based on Viability Dye eFluor® 506 (eBioscience) staining and live CD45+CD4+ gated cells were further evaluated for the frequency of IFNγ, IL-17A, or double-positive cells. Data were analyzed with FCS Express (De Novo Software) based on compensation and gating criteria described above.
ELISA
To measure cytokine production of IRBP-responsive T cells, single cell suspensions were prepared as described above and were stimulated for 48h with 20 μg/ml whole IRBP protein; after which the supernatants were collected for analysis of cytokine levels. Concentration of cytokines (IFNγ, IL-17A, IL-10, IL-12p40, IP-10, KC, TNFα, IL-6, and IL-2) was measured using a Luminex® multiplex assay (Millipore, Billerica, MA, USA) on Model L100IS (Luminex, Austin, TX, USA) and analyzed with BeadView™ (Upstate Cell Signaling Solutions, Lake Placid, NY, USA) as described (25).
Proliferation assay
IRBP-specific lymphocyte proliferation was measured by [3H]-thymidine incorporation using previously described methods (26). Cells (5 × 105/ml) were prepared as described above for antigen-stimulation assays and seeded into 96-well plates in 200 μl complete medium in the presence of IRBP (concentration ranging from 0 to 200 μg/ml) for 72 h. Cells were pulsed with 0.5 uCi [3H]-thymidine for the last 18 h of culture, and [3H]-thymidine uptake was determined by liquid scintillation spectrometry (Wallac model, WinSpectral).
Multiplex quantitative real-time PCR
Total RNA was extracted from pooled neuroretinas (n=6 mice/group) using RNeasy kit (Qiagen) and cDNA was synthesized (Reverse Transcription Kit; Applied Biosystems). Transcript analysis was performed using multiplex quantitative real-time PCR (RT2Profiler™ PCR Gene Expression Assay kit, SABiosciences) and Applied Biosystems PRISM Sequence Detection system. Controls were performed to confirm the lack of contaminating genomic DNA. Levels of target gene expression were determined by standard comparative ΔΔCt method and normalized to the average of 5 housekeeping genes within the same sample (Gusb, Hprt, Hsp90ab1, Gapdh, Actb), all of which were expressed at similar levels. Array results are representative of 4 independently performed experiments (each sample contained pooled RNA from 6 mice or 24 total mice/genotype).
Statistical analysis
Complex data sets were compared by ANOVA with Tukey-Kramer HSD or Newman-Keuls post-hoc tests for multiple comparisons. Single data points were analyzed by Student’s two-tailed t-test. The Mann-Whitney U test (two-tailed) was used for analysis of nonparametric data (i.e. clinical and histopathology scores) using Prism (GraphPad Software, Inc.). All experiments were performed up to 3 times independently. Results are presented as mean ± SEM. P<0.05 were considered statistically significant.
RESULTS
Card9 is required for the inflammatory pathology in EAU
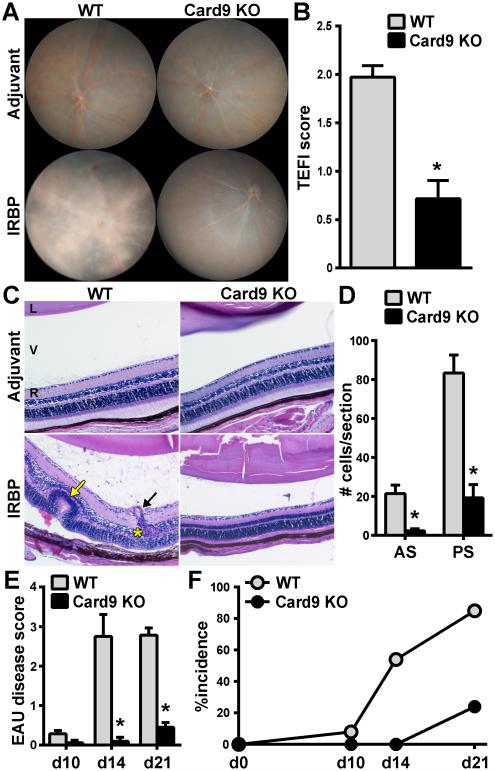
To address the impact of Card9 expression on development of autoimmunity targeted to the eye, WT and Card9 KO mice were immunized with the endogenous retinal protein IRBP. Under standard induction protocols, WT mice developed ocular inflammation characterized by papilledema, retinal vasculitis (i.e. “cuffing” of retinal vessels), and retinal lesions (clinical TEFI images; Fig. 1A). In contrast, inflammation in Card9 KO mice was markedly reduced (Fig. 1A); albeit some mild inflammation was detectable around the optic nerve and blood vessels (0.5 scores; Fig 1B). Accompanying histological changes noted in uveitic WT mice, but that were absent in Card9 KO mice included retinal structural damage (i.e. “folding”), perivascular exudates, granulomatous-like lymphoid aggregates within the retina, chorioretinal infiltrates, subretinal hemorrhage, and photoreceptor layer damage (Fig. 1C). Cellular infiltration of the vitreous and aqueous, other clinical parameters of uveitis, was quantified from histological sections as number of cells within the vitreous body of the posterior segment (PS) and aqueous humor of the anterior segment (AS)(Fig. 1D). In contrast, Card9 KO mice showed significant reduction in immune cell infiltration within both eye segments. Inflammation was detectable in WT mice by 14 day post-immunization (dpi) and reached “peak” pathological disease and incidence within 21 dpi (Fig. 1E, F). In comparison, Card9 KO mice had delayed onset and significantly less disease. Clinical and histological evaluation of adjuvant-injected controls of either genotype appeared normal akin to naïve mice and were scored as 0 throughout all experiments (Fig. 1A, C-adjuvant controls and Supplementary Fig. S1). These findings uncover the central importance of Card9 as a key regulator in the onset and severity of EAU.
Figure 1. Card9 is required for the inflammatory pathology in EAU.
EAU was induced in WT and Card9 KO mice and the onset and severity of uveitis was evaluated by clinical fundoscopy exam (A,B) and histopathology (C-E). (A) Representative fundus images of the posterior pole showing extensive inflammation (e.g. diffuse retinal lesions and vascular cuffing) in WT compared to Card9 KO eyes. (B) Clinical severity of retinal inflammation evaluated by fundoscopy. (C) H&E-stained sections of eye harvested on d21 post-immunization (i.e. peak of disease) show pathological features within this disease including retinal folding (yellow arrow), granulomatous formation (yellow asterisk) and vasculitis (black arrow). Images acquired at 200X magnification. D) Quantification from histological sections of the number of cells infiltrated into the anterior and posterior segments. EAU disease was graded pathologically as a function of time (E), and used to calculate incidence over time (F). ; *P<0.05 vs WT response (n=10-16 mice/group from 3 independent experiments). L, lens; V, vitreous; R, retina.
Card9 promotes polarization of Th17 cellular responses to IRBP
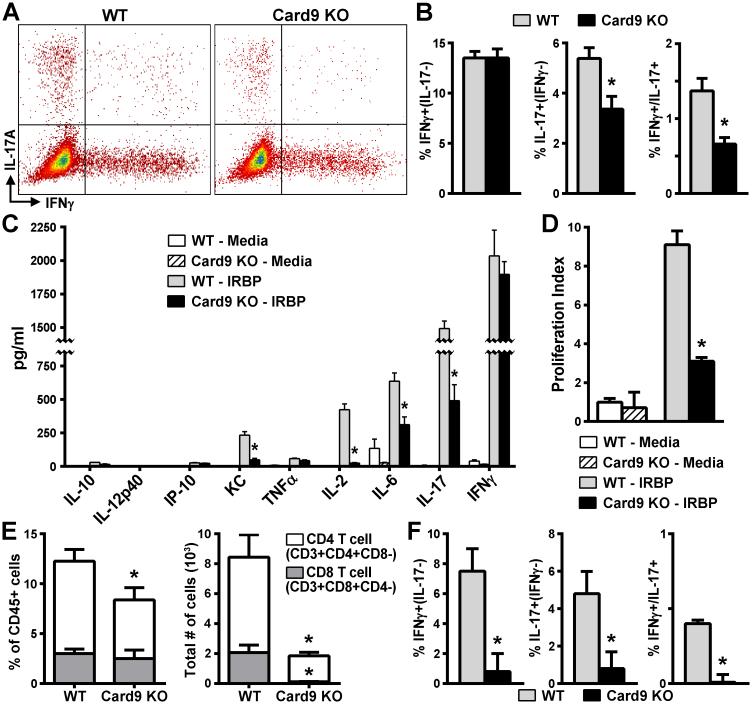
We next evaluated whether Card9 expression influenced development of Th1 vs. Th17 T cell effector responses in EAU. Flow cytometric analysis of peripheral lymphoid tissue did not reveal any major influence of Card9 expression on the ratios of CD4+ versus CD8+ T cells in media controls (Fig. S2). However, in vitro-stimulated splenocyte cultures showed decreased antigen-specific expansion of CD4+ cells in Card9 KO mice compared to WT mice. Consistent with this observation, intracellular cytokine staining for IFNγ and IL-17A (indicative of Th1 vs. Th17 populations, respectively) after re-stimulation with the IRBP ex vivo demonstrated the expected increased frequencies of both Th1 and Th17 CD4+ subsets of WT antigen-specific cells (Fig. 2A, B). IRBP-primed Card9 KO cells exhibited a significant reduction in the IL-17A+ Th17 effector cells as well as double-positive IL-17A+/IFNγ+ cells compared to WT. Card9-deficiency did not affect the IFNγ+Th1 CD4+ population, indicating specificity for Card9 in control of Th17 differentiation rather than a global impairment of effector T cell development during EAU.
Figure 2. Card9 promotes polarization of the Th17 cellular response to IRBP.
In vitro-stimulated cultures prepared from Card9 KO and WT mice at d21 post-immunization were analyzed for antigen-specific responses. Flow cytometric analysis (dot plot, panel A) showing frequency of IFNγ+, IL-17+, or double-positive cells among IRBP-stimulated CD4+ cell effector populations that were quantified (B; n=6-10 mice/experiment, experiment performed 3 times). C) Cytokine concentration in culture supernatants was determined at 48 h post IRBP-stimulation by multiplex ELISA (Luminex® assay) (n=8-10 mice/group; data are average of 3 independently performed experiments). D) Proliferative responses of IRBP-specific T cells from WT or Card9 KO mice were assessed 72 h later by 3H-thymidine incorporation (n = 6 mice/group, representative of 3-independently performed experiments). Values are C.P.M. plotted as fold induction to media controls. Panels E-F) Flow cytometric analysis of T cell populations within eyes of mice d21 post-immunization that were evaluated for composition and number of CD45+ gated cells (E; n=10 mice/group, experiment performed 3 times). CD4+ T subsets were further evaluated by intracellular cytokine staining for IFNγ+, IL-17+, or double-positive cells (F) (n=10 mice/groups, experiment performed 3 times). *p<0.05 vs WT response.
Further analysis of the role for Card9 in antigen-specific T cell responses was evaluated by multiplex ELISA assay. IRBP-specific induction of IL-17A was regulated by Card9 whereas production of IFNγ remained unaltered in Card9 KO cells (Fig. 2C). Production of other Th17-associated response cytokines such as KC and IL-6 were also significantly higher in IRBP-primed WT cells compared to Card9 KO cells (Fig. 2C). Such altered cytokine profiles observed in Card9 KO cells was associated with an impaired IRBP-induced proliferation in lymphocytes as evaluated by [3H]-thymidine incorporation assay (Fig. 2D). The reduction in proliferative response would be consistent with the observed diminished IL-2 production in IRBP-primed Card9 KO cells (Fig. 2C).
To equate the peripheral antigen-specific T cell responses involving Card9 to the in situ phenotype, we examined the T cell populations within the eye (Fig. 2E-F). Flow cytometric analysis of whole eyes revealed that both CD3+ T cell populations (CD4+/− and CD8+/−) were present in uveitic WT eyes following immunization. Comparison of cell composition (i.e. % of gated CD45+ expressing cells) revealed a diminished proportion of CD4+ cells in the Card9 KO mice (Fig. 2E). The overall magnitude of the ocular T cell response was also reduced in immunized Card9 KO mice (Fig. 2E), as were the frequencies of both Th1 and Th17 cells (Fig. 2F). This data would suggest that even though the generation of IRBP-responsive Th1 cells in peripheral lymphoid tissue was not altered by Card9-deficiency, trafficking of both Th1 and Th17 into eye tissue was significantly impaired in Card9 KO mice. Flow cytometry analysis of circulating leukocytes after IRBP-immunization showed no changes in T cell numbers or percentages in Card9 KO mice (data not shown) thereby supporting a role for Card9 in promoting a more pathogenic Th17 cell response directed to the eye.
The ocular autoimmune response reflects the Card9 transcriptional profile in the retina
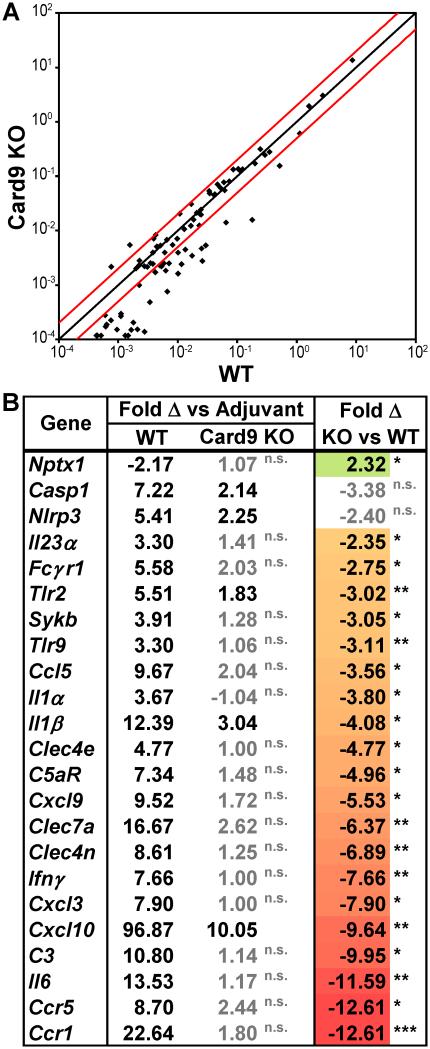
To gain insight into polarizing factors controlled by Card9, we examined the early or acute phase of ocular inflammatory responses within the eye. Using multiplex quantitative qtPCR analysis, we evaluated the response within neuroretinas harvested during the early, acute phase of EAU (i.e. 10 dpi—a time prior to onset of uveitis; score = 0.17±0.06; Fig. 1E). Data revealed substantial changes in gene expression in the neuroretina that were Card9-dependent, with most genes underrepresented in Card9 KOs compared to WT response (Fig. 3A). Differences between WT and Card9 KO mice were noted particularly amongst some chemokines involved in T-cell recruitment (e.g. Ccl5/Rantes, Cxcl9, Cxcl10) and other transcripts associated with T cell responses (including Ccr5, Cxcr3, Ccr1, IFNγ, IL-6, IL-23) (Fig. 3B). Consistent with its known role in host defense against fungal and mycobacterial infections, a Card9-dependent transcriptional profile was observed that involved CLRs and downstream pathways including Sykb and IL-1β/inflammasome responses (Casp1, Nlrp3, Il1b). Notably, genes encoding the CLRs Dectin-1, Dectin-2 and Mincle (Clec7a, Clec4n, Clec4e, respectively) were induced in the neuroretina of WT vs. Card9 KO mice (Fig. 3B). Given that this time (d10) precedes clinical observation of peripheral infiltrate, one might infer that changes in expression of these myeloid genes are indicative of macrophage/microglia responses over neutrophils. Of note, we did observe some genes within the retina that retained some expression in Card9 KOs such as Casp1, Nlrp3, and Il-1b, which fall within the inflammasome pathway. This could suggest residual Card9-independent transcriptional response within the retina that is initiated by resident retinal cells vs myeloid infiltrate.
Figure 3. Card9 controls acute transcriptional responses related to CLR-immune pathways within the retina.
Multiplex qt-PCR analysis of genes differentially expressed in the retina dissected on d10 (i.e. prior to onset of disease; see Fig.1F) from WT vs Card9 KO mice that were immunized with IRBP or with adjuvant alone (n=6 mice pooled/group). Array results are representative of 4 independently performed experiments (each of 6 pooled mice or 24 total mice/genotype). mRNA expression levels are indicated as fold change of IRBP-immunized response as compared to adjuvant controls within each genotype. A) Scatter plot demonstrating pattern of individual genes. The black line indicates fold change of 1, while red lines indicate boundaries of 2-fold change after normalization for gene regulation in response to adjuvant-injected mice. B) Selected genes that achieved statistical difference between IRBP vs adjuvant controls in WT mice (and met our greater than 2-fold criteria) compared to Card9 KO response are shown in the first 2 columns (significance indicated in black text; non-significant response (“n.s.”) in grey text. Genes are further ranked according to the extent to which Card9 is responsible for this response (far right panel). Expression was considered statistically significant when *p<0.05; **p<0.01; ***p<0.001.
Differential roles for the CLRs Dectin-1, Dectin-2 and Mincle in the pathogenesis of EAU
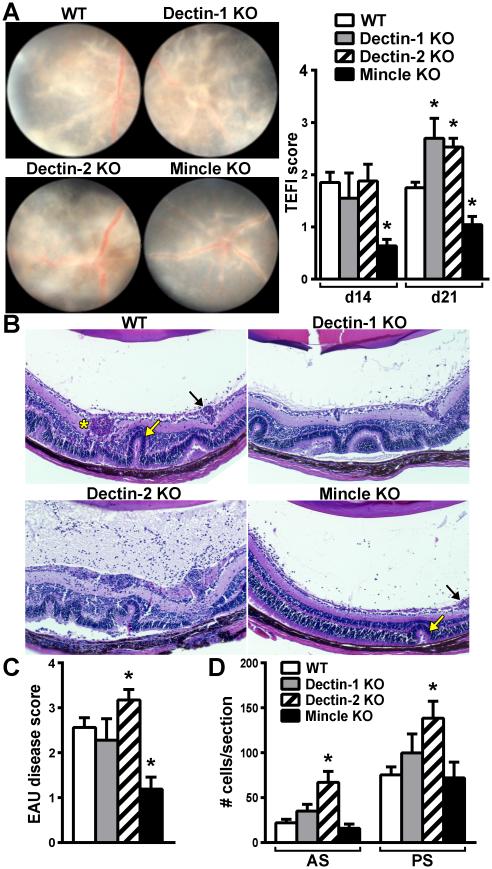
The data thus far support a Card9-dependent pathway as a core mechanism in the induction of EAU, which we further theorized to be initiated by proximal CLR activation. To address this hypothesis, we used a genetic approach to test whether deficiency in any one of the prototypical CLRs identified above (Fig. 3, Dectin-1, Dectin-2, or Mincle) that couple with the Syk/Card9-signaling pathway would replicate the Card9 KO-associated resistance to EAU. To elucidate the CLR(s) responsible for the development of autoimmune disease in the eye, EAU was induced in WT and single CLR KO mice (Fig. 4). Adjuvant-injected control mice did not develop any discernible histopathological or clinical signs of disease in any genotype (n=10 mice scored/genotype) and retinas appeared normal and healthy (Fig. S3). Negligible differences were observed between immunized WT and Dectin-1 KO mice; both clinical exams (Fig. 4A) and histopathological scores (Fig. B, C) showed similar degrees of uveitis. Somewhat surprisingly, Dectin-2 KO mice had exacerbated clinical uveitis compared to WT mice (Fig. 4A), which was corroborated histologically (Fig. 4B-D). This identifies Dectin-2 as having a potential novel, counter-regulatory role in EAU.
Figure 4. Differential roles for the CLRs Dectin-1, Dectin-2 and Mincle in the pathogenesis of EAU.
To determine the specific CLR(s) responsible for development of uveitis, the onset and severity of EAU was evaluated in mice deficient in Dectin-1, Dectin-2, or Mincle by clinical fundoscopy (A) and by histology (B-D). Stained sections from eyes 21d post-immunization were scored (C) and quantified for numbers of infiltrated cells in the anterior and posterior chambers (D) (AS = anterior segment; PS = posterior segment). *P<0.05 vs. WT response (n=12-14/group; combined data of 2-separate experiments). Black arrow = vasculitis, Yellow arrow = retinal fold, Asterisk=granuloma.
In contrast, both the clinical and histopathological severity of EAU was significantly reduced in Mincle KO mice (Fig. 4A-C), thereby indicating Mincle as the principal CLR in inductive mechanisms of EAU over that of Dectin-1 or Dectin-2. Intriguingly, Mincle-deficiency did not result in the near-to-complete abrogation of EAU as was observed in Card9 KO mice, suggesting an additional Mincle-independent (but Card9-dependent) mechanism for EAU induction. The residual Mincle-independent disease that we noted involved primarily vasculitis as visualized by fundus exam (Fig. 4A) and by histology (Fig. 4B, black arrow). Histologically, occasional focal retinal folds (Fig. 4B, yellow arrow) along with some accumulation of cells within the vitreous body of the posterior segment and aqueous humor of the anterior segment were also noted (Fig. 4D). Collectively, these data reveal differential functions for CLRs in uveitis and support a key pathogenic role for Mincle as the driving component of induction of EAU.
Activation of Mincle is important for induction of EAU and requires Syk/Card9 coupled signaling axis
Mincle (Clec4e) is a recently discovered CLR that is less well-characterized than Dectin-1 or Dectin-2, but is nonetheless important in fungal infection and perhaps to an even greater extent in mycobacterial infection. It is known that Mincle recognizes the glycolipid trehalose-6,6-dimycolate (TDM, also called cord factor) (27), a component of the mycobacterial cell wall. To relate the functional consequences of Mincle deficiency to its potential for induction of uveitis, WT mice were immunized with IRBP emulsified with incomplete Freund’s adjuvant (IFA), which is devoid of mycobacterium M. tuberculosis, but which was re-supplemented with the synthetic Mincle agonist, trehalose-6-6’-dibehenate (TDB), or other mycobacterial-associated immune stimulants (Fig. 5A). As expected, a lack of microbial stimuli in the case of immunization with IFA alone did not result in development of EAU even in the presence of autoantigen (i.e. IRBP). Reconstitution of the IRBP emulsion with TDB however did restore the EAU disease phenotype. As with mice immunized with CFA, mice immunized with TDB developed a uveitic disease that affected both anterior and posterior segments (Fig.4D, 5D); albeit some uveitis pathology differences were noted. Most notably, the granulomatous inflammation was much more severe in TDB-immunized WT mice, which extended through the retina to the retinal pigmented epithelium and choroid, causing extensive photoreceptor destruction and even subretinal hemorrhaging (Fig 5D). Peptidoglycan (PGN) and its byproduct muramyl dipeptide (MDP) have long been considered potent immunostimulants through activation of TLR2 and NOD2; however we found that animals immunized with IRBP emulsified with either compound did not reproduce the full EAU phenotype and were significantly less potent vs. TDB (Fig. 5A).
To further evaluate the specificity of receptor actions of TDB and Mincle, the ability of TDB to induce EAU was tested in Mincle KO mice (Fig. 5B-E). In contrast to induction of EAU in WT mice with TDB, Mincle KO mice demonstrated a near to complete reversion of disease as indicated by significant reduction in clinical severity of EAU (Fig. 2B, C) as well as significant abrogation in EAU histologically (Fig. 5D, E). Somewhat consistent with the phenotype of Mincle KOs presented above (Fig. 4B, D), Mincle deficiency did not appear to influence cell accumulation in the posterior segment. Residual vessel damage and vasculitis was also noted (Fig. 5B, D) in Mincle KO mice. These data underscore both the sufficiency and necessity for Mincle in induction of EAU, while also suggesting a minor, Mincle-independent mechanism in EAU induction.
Finally, we further evaluated the Syk/Card9-coupled signaling axis as a mechanism by which TDB/Mincle interactions elicit EAU. Given that Card9 expression was essential for EAU induction, we evaluated its necessity for EAU induced by TDB (Fig. 6A-D). Card9-deficient animals immunized with IRBP emulsified in TDB were resistant to induction of EAU as evidenced by reduced disease as evaluated clinically (Fig. 6A, B) as well as histologically (Fig. 6C, D). Adjuvant control groups (i.e. those injected with emulsified TDB alone, without IRBP) did not develop ocular pathology (data not shown). Syk is a tyrosine kinase recruited by ITAM motifs on a subgroup of CLRs including Mincle, which couples with Fc receptor γ chain (FcRγ) for its activation, thereby leading to Card9-dependent responses (28). To complement the observations made in the Card9 KO mice, we pharmacologically targeted the Syk using piceatannol, a small molecule inhibitor of Syk. Piceatannol-treated mice showed reduced uveitis compared to those treated with vehicle when evaluated both clinically (Fig. E, F) and histologically (Fig. 6G, H). Taken together, these data demonstrate that activation of Mincle by TDB requires a Syk/Card9-coupled mechanism for the induction of EAU.
DISCUSSION
Despite the progress made in understanding genetic and immunological mechanisms involved in T cell-mediated diseases such as uveitis, our understanding of the early signals initiating the disease process remains incomplete. Numerous studies have examined the contribution of TLRs in ocular inflammatory responses, yet investigation of other PRR families has been limited. This study delineates the relative roles of proximal CLRs Dectin-1, Dectin-2 and Mincle in an experimental T cell-mediated autoimmune disease of the eye. We demonstrated a Syk/Card9 coupled pathway that is central for induction of Th17-associated responses and pathogenesis of EAU, which is initiated predominantly upon engagement of the CLR Mincle.
Infectious triggers have long been considered an initiating factor in uveitis; even in those cases without an active infection, the chronicity of the disease is thought to involve continuous priming of new effectors that perpetuate pathogenic processes. Our studies highlight an essential, non-redundant role for Syk/Card9 signaling in induction of EAU, which places this pathway at the juncture of microbial detection and autoimmune disease. This observation holds clinical importance, as CARD9 is highly associated with inflammatory diseases such as Crohn’s disease inflammatory bowel disease and AS (29, 30). In the prevailing context that disease arises from genetic-environmental interactions, it seems conceivable that aberrant regulation of CARD9, either through genetic mutation or activation by microbial infection (or both), could cause pathological immune development such as in uveitis. Our studies here demonstrating the importance of Card9-mediated signals in development of the Th17 response in EAU would be in agreement with the known role of the Th17 response in host defense against fungal infections.
The protection of Card9 KO mice against EAU correlated with reduced frequency of IRBP-specific Th17 vs. Th1 cells, antigen-specific induction of Th17-related cytokines (IL-17A, IL-6 and KC), and proliferative capacity. Changes in the transcriptional response within the retina revealed the importance of Card9 in controlling the degree of ocular pathology as evidenced by early (d10) changes in cytokines/chemokine and complement genes associated with host defense against fungal infection. For example, Ccl5 was regulated in a Card9-dependent fashion within the retina and CCL5 (RANTES) has been found to activate CCR5 on T cells to promote EAU (31). The anaphylaxins C3a and C5a, whose induction was also regulated by Card9, were recently shown to contribute to EAU through promotion of the Th17 response (32). Taken together, these data identify an important role for Card9 in induction of genetic responses and pathways relevant to the Th17 cell response, which is an underlying mechanism of EAU. The transcriptional data derived from retinal tissue might also point towards early myeloid cell responses that could also contribute to perpetuation of tissue damage within the eye in addition to merely T cell responses.
Intriguingly, the peripheral-associated Th1 response was retained in Card9 KO mice. Both antigen-specific induction of IFNγ and frequency of Th1 cells (intracellular cytokine staining) were unaltered in Card9 KO mice, thereby revealing a specific function for Card9 in pathogenic Th17-driven mechanisms in EAU. Admittedly, such conclusions are based on using IFNγ and IL-17 as the principal cytokines for identification of these effector populations. We cannot rule out the possibility that other cytokines are controlled by Card9 that would also influence the overall pathogenicity of Th17 vs. Th1 cells. Intriguingly, retention of Th1 antigen-specific responses would be reminiscent of mycobacterial infection, since the Th1 response is important in host defense. The essential role for Card9 in control of mycobacterial infection has also been recently demonstrated by Dorhoi et al (16) who noted that Card9 KO cells maintain their ability to generate other cytokines (e.g. MCP-1 or G-CSF). Clearly, the mere presence of peripheral IRBP-reactive Th1 cells does not equate to disease, thereby suggesting that Card9 likely controls the uveitogenic potential of Th1 cells in some yet-to-be determined way that minimizes their capacity to cause disease.
Despite such peripheral nuances, both Th1 and Th17 populations were found to be reduced in eyes of Card9 KO mice, likely owing to altered T cell trafficking mechanisms, an observation that would suggest that the ocular Th1 response may be secondary to the Th17 response in initiation of EAU (as has been recently described in EAE, an animal model for multiple sclerosis (33). One could also reason that since gene expression data (Fig. 3) showed reduced expression of CXCL10, an IFNγ-dependent chemokine that binds to CXCR3, in Card9 KO retina, recruitment into the eye of Th1 as well as other cells could be affected. This could consequently result in reduced disease development in Card9 KO animals. Alternatively, Card9 may promote more expansion of and/or better optimized pathogenic Th17 cells that target the eye for disease. Of course, it is possible to consider that Card9 controls non-T cell responses as an additional mechanism through which EAU is induced. Card9 is expressed in myeloid cells such as macrophages, a population that we have observed to dominate the leukocyte infiltrate in the eye and was recently shown to be involved in microglial responses (34). Thus it is also possible that Card9 controls macrophage/microglia responses locally within the eye to further perpetuate ocular damage. Future studies to dissect the cellular mediators by which Card9 controls mechanisms of EAU induction will be informative in this regard.
Through the genetic approach used here, a key observation is that Mincle was identified as the foremost receptor in EAU induction via a mechanism that requires both Syk and Card9. Transcription of the prototypical CLRs that signal by way of Syk/Card9 (Clec7a, Clec4n, Clec4e—encoding Dectin-1, Dectin-2 and Mincle, respectively) was greatly increased within the retina. The in vivo genetic approach towards understanding their function in EAU demonstrated a significant role for Mincle, since Mincle KO mice exhibited significantly reduced uveitis, and a redundant or counter-regulatory role for Dectin-1 and Dectin-2, respectively. During preparation of this manuscript a pathogenic role for Dectin-1 in EAU was reported (35). Intriguingly, Dectin-1 did not play a role in other models of EAU employed by the authors and it was concluded that Dectin-1 participated in adjuvant-recognition functions. This conclusion contrasts that of our study and that of another publication reporting that Dectin-1 plays a non-essential role in the adjuvant effects of CFA vs that of Mincle (36). Such discrepancies are nonetheless interesting and could be related to differences in the purity of CFA or mycobacterium used, or methodological differences in the models of EAU induction such as the use of peptide alone versus our studies that used whole IRBP protein, which could elicit polyclonal T cell responses to multiple epitopes. Our studies here directly compared Dectin-1 to multiple other CLRs to demonstrate differential roles for Dectin-1 vs Dectin-2 vs Mincle in EAU. We also identified an entirely novel protective role for Dectin-2 in that Dectin-2 deficiency rendered mice more susceptible to EAU as evidenced by exacerbated vasculitis, more extensive retinal lesions and enhanced photoreceptor damage. This somewhat surprising observation would suggest counter-regulatory responses amongst the CLRs in the context of uveitis. The antagonistic relationship between Mincle and Dectin-1 in fungal infection has been previously reported (37), and our observations extend this antagonistic interaction across the CLR family to include Dectin-2.
Mincle expression has been shown to be important in susceptibility to both mycobacterial and fungal infections (19, 38, 39) and in sensing danger signals such as the endogenous protein SAP130 (40), thereby implicating multiple pathogenic mechanisms by which it could contribute to disease. Activation of Mincle by TDB sufficiently reproduced the EAU disease phenotype, a response that required Mincle and Card9 expression as well as Syk activity. Given the ability of TDB to elicit granulomas, the importance of Mincle for the eye would be consistent with the granulomatous-like pathology observed in both EAU and human uveitis (3). Indeed, almost all varieties of posterior uveitis seem to be mimicked by tuberculosis infection (41). A role for Card9 (and Mincle) in the development of granulomas and resistance to tuberculosis is thus not surprising, but in the context of uveitis provides invaluable insight into a core pathway of disease development. Fungal triggers are also important in activation of CLRs such as Dectin-1 and Dectin-2 and our recent preliminary observations indicate that EAU can be induced by the Dectin-1 agonists curdlan or zymosan via a Card9-dependent mechanism. Thus, such a Card9-specific response in EAU could potentially be driven by a variety of upstream CLRs that respond to either MtB or fungal components. While this pathway may be central in initiation of infectious uveitis, it could also be important in cases of non-infectious uveitis. It would be interesting to test the relevancy of this pathway in other models of uveitis that do not involve adjuvants, such as in a spontaneous model of uveitis recently reported (42, 43).
Our observations further connect the relationship amongst Mincle, Card9-signaling events and IL-1, which is a known early signal required for induction of EAU (11). Our data demonstrate induction of gene expression of molecules within the IL-1-inflammasome pathway (e.g. Nlpr3, Il-1, Asc, Caspase-1) that are regulated by Card9, thereby implicating Card9 as a signal upstream from IL-1. While Card9-mediated responses were found to be essential for development of autoimmune uveitis, a minor Card9-independent response involving mild retinal vasculitis was noted on clinical retinal exam. Intriguingly, gene expression levels of molecules of the IL-1 pathway were reduced in Card9 KO mice (Fig. 2) to a level that was significantly different from adjuvant controls, suggesting the existence of both Card9-dependent and independent IL-1 responses. Desel et al. (44) reported that TDB induced MyD88-dependent IL-1R signaling, thereby placing Mincle/Card9 upstream of IL-1. However, our studies demonstrated that EAU was not completely abrogated in Mincle KOs, suggesting an alternative way for IL-1 induction, a possibility supported by our gene expression studies showing that some IL-1 pathway genes were regulated to some extent independently of Card9.
An important outstanding question is: what mechanism is responsible for the residual uveitis that occurs in the absence of Mincle? Although relatively minor, we noted Mincle-independent ocular pathology that involved retinal vasculitis, leukocytic infiltration into the vitreous and occasional, focal retinal folding. During preparation of this manuscript, an additional CLR (Dectin-3, aka the CLR macrophage C-type lectin, MCL) was described as a co-receptor to TDB (45). It is possible that MCL compensates for the absence of Mincle to contribute to EAU induction through a Syk/Card9-dependent mechanism. Interestingly, an inductive role for MCL has been identified in another model of organ-specific autoimmunity, experimental autoimmune encephalomyelitis (EAE) (46). Collectively, these data suggest that there may be some level of redundancy in upstream receptor ligand interactions and even additional unknown ligand(s) which may activate Card9 and lead to disease.
Since the Syk-coupled Card9 pathway is highly homologous between mouse and man (47), our in vivo animal studies provide relevance for this conserved pathway as a possible target for treatment. In our hands, pharmacological inhibition of Syk dramatically abrogated EAU, thereby supporting this as a rational pathway to target. A caveat to consider though is that while piceatannol is known to reliably inhibit Syk kinases, it has been reported to have inhibitory activity against other kinases. Thus, we cannot rule out potential influence of such off-target effects on EAU. Current ongoing clinical trials for treatment of rheumatoid arthritis and other diseases with Syk inhibitors will prove informative. However, there are almost certainly caveats and limitations when therapeutics interfere with key host defense pathways. Another consideration brought forth from this study would be the possibility of strategies to prophylactically treat uveitis. Some forms of uveitis are characterized by recurrences that likely arise from continuous priming of new effectors that sustain the disease over time. Thus, although the mechanisms are not fully understood, it is conceivable that aberrant control of CARD9, such as when genetic alterations in an individual interact with certain environmental triggers/infection, could be reversed or altered by treatment to prevent the next uveitic episode.
In conclusion, the studies here contribute to a deeper understanding of innate immune receptors in autoimmune uveitis using the well-established T cell-dependent model of EAU. Starting with the hypothesis focused on the biological function of Card9 in EAU, we subsequently extended our findings to identify Mincle as a predominant activating CLR via a Syk/Card9-dependent signaling mechanism. Such studies reveal a previously unconsidered role for the innate immune family of CLRs as an important early initiating factor in uveitis, which we speculate could be relevant for therapeutic intervention.
Supplementary Material
Acknowledgements
We thank Dr. Gordon Brown (Institute of Medical Sciences, University of Aberdeen, Aberdeen, Scotland, UK) for provision of the Dectin-1 KO mice and his helpful discussions. We are thankful for the assistance of Dr. Justine Smith (Flinders University, Adelaide, Australia) and Dr. João Furtado (Federal University of São Paulo, São Paulo, Brazil) with establishing TEFI methodology. We thank Maya Lewinsohn, Rose Chuong, Riley Hazard, and Keith Wegmann at the VA Portland Health Care Systems for their technical support; and Dr. Michael Davey (OHSU, VA Medical Center, Portland, OR) for his helpful discussions and critical reading of the manuscript.
Grant Support: This work was supported by National Institutes of Health (RO1 EY025250) and Intramural funding from National Eye Institute (Project# EY00184) as well as the Department of Veterans Affairs Biomedical Laboratory Research & Development Service (Merit Review Award I01 BX002180).
Footnotes
Disclosures: The authors have no financial conflict of interest.
REFERENCES
- 1.Chang JH, Wakefield D. Uveitis: a global perspective. Ocul Immunol Inflamm. 2002;10:263–279. doi: 10.1076/ocii.10.4.263.15592. [DOI] [PubMed] [Google Scholar]
- 2.Martin TM, Rosenbaum JT. An update on the genetics of HLA B27-associated acute anterior uveitis. Ocul Immunol Inflamm. 2011;19:108–114. doi: 10.3109/09273948.2011.559302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forrester JV, Klaska IP, Yu T, Kuffova L. Uveitis in mouse and man. Int Rev Immunol. 2013;32:76–96. doi: 10.3109/08830185.2012.747524. [DOI] [PubMed] [Google Scholar]
- 4.Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120:3073–3083. doi: 10.1172/JCI42440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gery I, Egwuagu CE. Central tolerance mechanisms in control of susceptibility to autoimmune uveitic disease. Int Rev Immunol. 2002;21:89–100. doi: 10.1080/08830180212061. [DOI] [PubMed] [Google Scholar]
- 6.Peng Y, Han G, Shao H, Wang Y, Kaplan HJ, Sun D. Characterization of IL-17+ interphotoreceptor retinoid-binding protein-specific T cells in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2007;48:4153–4161. doi: 10.1167/iovs.07-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshimura T, Sonoda KH, Miyazaki Y, Iwakura Y, Ishibashi T, Yoshimura A, Yoshida H. Differential roles for IFN-gamma and IL-17 in experimental autoimmune uveoretinitis. Int Immunol. 2008;20:209–214. doi: 10.1093/intimm/dxm135. [DOI] [PubMed] [Google Scholar]
- 9.Medzhitov R. Approaching the asymptote: 20 years later. Immunity. 2009;30:766–775. doi: 10.1016/j.immuni.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Chang JH, McCluskey PJ, Wakefield D. Recent advances in Toll-like receptors (TLRs) and anterior uveitis. Clin Experiment Ophthalmol. 2012 doi: 10.1111/j.1442-9071.2012.02797.x. [DOI] [PubMed] [Google Scholar]
- 11.Su SB, Silver PB, Grajewski RS, Agarwal RK, Tang J, Chan CC, Caspi RR. Essential role of the MyD88 pathway, but nonessential roles of TLRs 2, 4, and 9, in the adjuvant effect promoting Th1-mediated autoimmunity. J Immunol. 2005;175:6303–6310. doi: 10.4049/jimmunol.175.10.6303. [DOI] [PubMed] [Google Scholar]
- 12.Fang J, Fang D, Silver PB, Wen F, Li B, Ren X, Lin Q, Caspi RR, Su SB. The role of TLR2, TRL3, TRL4, and TRL9 signaling in the pathogenesis of autoimmune disease in a retinal autoimmunity model. Invest Ophthalmol Vis Sci. 2010;51:3092–3099. doi: 10.1167/iovs.09-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drummond RA, Saijo S, Iwakura Y, Brown GD. The role of Syk/CARD9 coupled C-type lectins in antifungal immunity. Eur J Immunol. 2011;41:276–281. doi: 10.1002/eji.201041252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruland J. CARD9 signaling in the innate immune response. Ann N Y Acad Sci. 2008;1143:35–44. doi: 10.1196/annals.1443.024. [DOI] [PubMed] [Google Scholar]
- 15.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 16.Dorhoi A, Desel C, Yeremeev V, Pradl L, Brinkmann V, Mollenkopf HJ, Hanke K, Gross O, Ruland J, Kaufmann SH. The adaptor molecule CARD9 is essential for tuberculosis control. J Exp Med. 2010;207:777–792. doi: 10.1084/jem.20090067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross O, Gewies A, Finger K, Schafer M, Sparwasser T, Peschel C, Forster I, Ruland J. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature. 2006;442:651–656. doi: 10.1038/nature04926. [DOI] [PubMed] [Google Scholar]
- 18.Hsu YM, Zhang Y, You Y, Wang D, Li H, Duramad O, Qin XF, Dong C, Lin X. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol. 2007;8:198–205. doi: 10.1038/ni1426. [DOI] [PubMed] [Google Scholar]
- 19.Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, Beckhouse AG, Lo YL, Manzanero S, Cobbold C, et al. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol. 2008;180:7404–7413. doi: 10.4049/jimmunol.180.11.7404. [DOI] [PubMed] [Google Scholar]
- 20.Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caspi RR. Experimental autoimmune uveoretinitis in the rat and mouse. Curr Protoc Immunol. 2003 doi: 10.1002/0471142735.im1506s53. Chapter 15:Unit 15 16. [DOI] [PubMed] [Google Scholar]
- 23.Furtado JM, Davies MH, Choi D, Lauer AK, Appukuttan B, Bailey ST, Rahman HT, Payne JF, Stempel AJ, Mohs K, et al. Imaging Retinal Vascular Changes in the Mouse Model of Oxygen-Induced Retinopathy. Transl Vis Sci Technol. 2012;1:5. doi: 10.1167/tvst.1.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H, Koch P, Chen M, Lau A, Reid DM, Forrester JV. A clinical grading system for retinal inflammation in the chronic model of experimental autoimmune uveoretinitis using digital fundus images. Exp Eye Res. 2008;87:319–326. doi: 10.1016/j.exer.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Kezic JM, Glant TT, Rosenbaum JT, Rosenzweig HL. Neutralization of IL-17 ameliorates uveitis but damages photoreceptors in a murine model of spondyloarthritis. Arthritis Res Ther. 2012;14:R18. doi: 10.1186/ar3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wegmann KW, Wagner CR, Whitham RH, Hinrichs DJ. Synthetic Peptide dendrimers block the development and expression of experimental allergic encephalomyelitis. J Immunol. 2008;181:3301–3309. doi: 10.4049/jimmunol.181.5.3301. [DOI] [PubMed] [Google Scholar]
- 27.Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, Akira S, Yoshikai Y, Yamasaki S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206:2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strasser D, Neumann K, Bergmann H, Marakalala MJ, Guler R, Rojowska A, Hopfner KP, Brombacher F, Urlaub H, Baier G, et al. Syk kinase-coupled C-type lectin receptors engage protein kinase C-sigma to elicit Card9 adaptor-mediated innate immunity. Immunity. 2012;36:32–42. doi: 10.1016/j.immuni.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pointon JJ, Harvey D, Karaderi T, Appleton LH, Farrar C, Stone MA, Sturrock RD, Brown MA, Wordsworth BP. Elucidating the chromosome 9 association with AS; CARD9 is a candidate gene. Genes Immun. 2010;11:490–496. doi: 10.1038/gene.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivas MA, Beaudoin M, Gardet A, Stevens C, Sharma Y, Zhang CK, Boucher G, Ripke S, Ellinghaus D, Burtt N, et al. Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat Genet. 2011;43:1066–1073. doi: 10.1038/ng.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crane IJ, Xu H, Wallace C, Manivannan A, Mack M, Liversidge J, Marquez G, Sharp PF, Forrester JV. Involvement of CCR5 in the passage of Th1-type cells across the blood-retina barrier in experimental autoimmune uveitis. J Leukoc Biol. 2006;79:435–443. doi: 10.1189/jlb.0305130. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Bell BA, Yu M, Chan CC, Peachey NS, Fung J, Zhang X, Caspi RR, Lin F. Complement anaphylatoxin receptors C3aR and C5aR are required in the pathogenesis of experimental autoimmune uveitis. J Leukoc Biol. 2015 doi: 10.1189/jlb.3A0415-157R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 34.Baldwin KT, Carbajal KS, Segal BM, Giger RJ. Neuroinflammation triggered by beta-glucan/dectin-1 signaling enables CNS axon regeneration. Proc Natl Acad Sci U S A. 2015;112:2581–2586. doi: 10.1073/pnas.1423221112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoppelkamp S, Reid DM, Yeoh J, Taylor J, McKenzie EJ, Brown GD, Gordon S, Forrester JV, Wong SY. Murine pattern recognition receptor dectin-1 is essential in the development of experimental autoimmune uveoretinitis. Mol Immunol. 2015;67:398–406. doi: 10.1016/j.molimm.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Shenderov K, Barber DL, Mayer-Barber KD, Gurcha SS, Jankovic D, Feng CG, Oland S, Hieny S, Caspar P, Yamasaki S, et al. Cord factor and peptidoglycan recapitulate the Th17-promoting adjuvant activity of mycobacteria through mincle/CARD9 signaling and the inflammasome. J Immunol. 2013;190:5722–5730. doi: 10.4049/jimmunol.1203343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wevers BA, Kaptein TM, Zijlstra-Willems EM, Theelen B, Boekhout T, Geijtenbeek TB, Gringhuis SI. Fungal engagement of the C-type lectin mincle suppresses dectin-1-induced antifungal immunity. Cell Host Microbe. 2014;15:494–505. doi: 10.1016/j.chom.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Behler F, Maus R, Bohling J, Knippenberg S, Kirchhof G, Nagata M, Jonigk D, Izykowski N, Magel L, Welte T, et al. Macrophage-inducible C-type lectin Mincle-expressing dendritic cells contribute to control of splenic Mycobacterium bovis BCG infection in mice. Infect Immun. 2015;83:184–196. doi: 10.1128/IAI.02500-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Behler F, Steinwede K, Balboa L, Ueberberg B, Maus R, Kirchhof G, Yamasaki S, Welte T, Maus UA. Role of Mincle in alveolar macrophage-dependent innate immunity against mycobacterial infections in mice. J Immunol. 2012;189:3121–3129. doi: 10.4049/jimmunol.1201399. [DOI] [PubMed] [Google Scholar]
- 40.Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 41.Gupta V, Gupta A, Rao NA. Intraocular tuberculosis--an update. Surv Ophthalmol. 2007;52:561–587. doi: 10.1016/j.survophthal.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 42.Horai R, Chong WP, Zhou R, Chen J, Silver PB, Agarwal RK, Caspi RR. Spontaneous Ocular Autoimmunity in Mice Expressing a Transgenic T Cell Receptor Specific to Retina: A Tool to Dissect Mechanisms of Uveitis. Curr Mol Med. 2015;15:511–516. doi: 10.2174/1566524015666150731095201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horai R, Zarate-Blades CR, Dillenburg-Pilla P, Chen J, Kielczewski JL, Silver PB, Jittayasothorn Y, Chan CC, Yamane H, Honda K, et al. Microbiota-Dependent Activation of an Autoreactive T Cell Receptor Provokes Autoimmunity in an Immunologically Privileged Site. Immunity. 2015;43:343–353. doi: 10.1016/j.immuni.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desel C, Werninghaus K, Ritter M, Jozefowski K, Wenzel J, Russkamp N, Schleicher U, Christensen D, Wirtz S, Kirschning C, et al. The Mincle-activating adjuvant TDB induces MyD88-dependent Th1 and Th17 responses through IL-1R signaling. PLoS One. 2013;8:e53531. doi: 10.1371/journal.pone.0053531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao XQ, Zhu LL, Chang Q, Jiang C, You Y, Luo T, Jia XM, Lin X. C-type lectin receptor dectin-3 mediates trehalose 6,6'-dimycolate (TDM)-induced Mincle expression through CARD9/Bcl10/MALT1-dependent nuclear factor (NF)-kappaB activation. J Biol Chem. 2014;289:30052–30062. doi: 10.1074/jbc.M114.588574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyake Y, Toyonaga K, Mori D, Kakuta S, Hoshino Y, Oyamada A, Yamada H, Ono K, Suyama M, Iwakura Y, et al. C-type lectin MCL is an FcRgamma-coupled receptor that mediates the adjuvanticity of mycobacterial cord factor. Immunity. 2013;38:1050–1062. doi: 10.1016/j.immuni.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 47.Ostrop J, Jozefowski K, Zimmermann S, Hofmann K, Strasser E, Lepenies B, Lang R. Contribution of MINCLE-SYK Signaling to Activation of Primary Human APCs by Mycobacterial Cord Factor and the Novel Adjuvant TDB. J Immunol. 2015;195:2417–2428. doi: 10.4049/jimmunol.1500102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.