Abstract
The management of metastatic colorectal cancer substantially improved over the last 10 years and median overall survival of patients might exceed 30 months. The selection of an effective first-line treatment represents a crucial point in order to achieve good outcome results. In the last years, the intensive FOLFOXIRI regimen in association with bevacizumab became a new standard option in this setting. In the present review we summarized the main steps of FOLFOXIRI regimen development from the first pilot study to the recent findings with biological agents, with a specific focus on practical aspects, such as patient’s selection, adverse event management, treatment schedules and post-progression strategies. Possible predictive markers, open issues and ongoing clinical trials have been also deeply described.
Keywords: metastatic colorectal cancer, first-line, FOLFOXIRI, bevacizumab, anti-EGFR
Introduction
Colorectal cancer (CRC) ranks as the third most common cancer worldwide [1]. More than 20% of patients have metastatic disease when diagnosed, and more than one-third of patients eventually develop metastases [2]. It has been shown that more effective chemotherapy together with improved surgical techniques might allow more secondary resection of metastases with improved survival and potentially cure [3,4]. However, the majority of patients with metastatic colorectal cancer (mCRC) cannot be cured and receive palliative chemotherapy.
There has been great progress in the first-line treatment of metastatic colorectal cancer (mCRC) patients in the past ten years and up today median overall survival (OS) might exceed 30 months [5,6]. Such results derive from the adoption of targeted therapies, the anti-epidermal growth factor receptor (EGFR)- monoclonal antibodies, cetuximab and panitumumab, and the anti-vascular endothelial growth factor (VEGF) antibody, bevacizumab; from the identification of predictive and/or prognostic molecular factors (such as RAS and BRAF) and from the development of new intensive or tailored medical treatments [7–10]. In particular the intensive combination of oxaliplatin, irinotecan, and 5-fluorouracil (5-FU) (FOLFOXIRI) in association with bevacizumab demonstrated in a phase III trial to improve outcome and became a new standard regimen for initial therapy of mCRC patients [10].
In the present review we summarize the FOLFOXIRI regimen development from the first pilot study to the recent findings with biological agents. We focused on practical aspects, such as patient’s selection, adverse event, treatment schedules, and possible predictive markers, in order to refine the first-line clinical management of mCRC patients.
1) The beginning of the story: the FOLFOXIRI regimen’s development
For over 30 years 5-FU has been the only treatment for patients with mCRC, subsequently the two-drug combinations, FOLFIRI or FOLFOX, have demonstrated to improve the outcome compared to 5-FU alone and became the standard treatment in mCRC patients [11,12]. Interestingly, the phase III GERCOR study demonstrated that the use of a sequential strategy (FOLFOX followed by FOLFIRI or the opposite combination) led to prolonged survival and similar efficacy results [11]. Moreover, a pooled analysis of seven phase III trials in mCRC has demonstrated that the exposition to all the three active drugs (5-FU, irinotecan, and oxaliplatin) in the course of the disease might maximize the survival benefit of patients [13]. Such data are also supported by preclinical studies, where a clear synergism or additivity between 5-FU and irinotecan; 5-FU and oxaliplatin or irinotecan and oxaliplatin was observed [14,15].
Based on these observations a pilot study was conducted and showed that the FOLFOXIRI was feasible and had acceptable and manageable toxicities as well as no apparent relevant pharmacokinetics interactions in patients with mCRC [16]. To confirm such preliminary results the Gruppo Oncologico Nord Ovest (GONO) conducted a phase III trial comparing first-line FOLFIRI versus FOLFOXIRI treatment. Among 244 unresectable mCRC patients enrolled, the experimental treatment led to an increase of progression-free survival (PFS) (9.5 months versus 6.6 months, HR 0.59, p<0.001) and OS (23.4 months versus 16.7 months, HR 0.74, p=0.026) with an acceptable toxicity profile. Moreover, a higher response rate (RR) was also observed (60% versus 34%, p<0.0001), thus resulting in the improvement of secondary resection rate (15% versus 6%, p=0.033) [17,18]. Another randomized phase III trial, conducted by the Hellenic Oncology Research Group (HORG), compared the triplet chemotherapy to FOLFIRI in 285 patients. The trial failed to demonstrate any superiority for the FOLFOXIRI arm. However, it should be noted that the study populations were selected according to different eligibility criteria and different schedules of the three drugs [19] (Table 1).
Table 1.
Main results of trials evaluating triplet chemotherapy alone, in combination with bevacizumab or with anti-EGFRs
| Author | Phase | Primary endpoint |
Regimen | N | Response rate (%) |
Odds Ratio P-value |
Progression-free survival (95%CI) (months) |
HR P-value |
Overall survival (95%CI) (months) |
HR P-value |
R0 resection rate (%) |
R0 resection rate in pts with LLD (%) |
Relapse-free survival (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Falcone A, et al. 2007 | III | RR | FOLFOXIRI FOLFIRI |
122 122 |
66 41 |
P=0.0002 | 9.8 6.9 |
0.63 P=0.0006 |
22.6 16.7 |
0.70 P=0.032 |
15 6 |
36 12 |
|
| Souglakos J, et al. 2006 | III | OS | FOLFOXIRI FOLFIRI |
137 146 |
43 33.6 |
P=0.17 | 8.4 (7.0–10.2)* 6.9 (6.0–7.7)* |
P=0.17 | 21.5 19.5 |
P=0.34 | |||
| Bevacizumab | |||||||||||||
| Loupakis F, et al. 2014 | III | PFS | FOLFOXIRI+bev FOLFIRI+bev |
252 256 |
65.1 53.1 |
1.64 P=0.006 |
12.1 (10.9–13.2) 9.7 (9.3–10.9) |
0.75 P=0.003 |
31.0 (26.9–35.1) 25.8 (22.7–30.8) |
0.79 P=0.054 |
15 12 |
||
| Masi G, et al. 2010 | II | PFS | FOLFOXIRI+bev | 57 | 77 | 13.1 (10.9–15.2) | 30.9 (24.9–35.2) | 26 | 40 | ||||
| Gruenberger T, et al. 2015 | II | Resection rate |
FOLFOXIRI+bev | 41 Liver limited |
81 | 18.6 (12.9–22.3) | NA | 49 | 17.1** | ||||
| Bruera G, et al. 2010 | II | RR | Fir-bev/FOx | 50 | 82 | 12 (range; 3–46) | 28 (range; 3–47) | 22 | |||||
| Stein A, et al. 2015 | II | PFS | FOLFOXIRI+bev | 90 | 64 | 11.1 (9.4–12.0) | 32.2 (22.6–36.9) | 18 | |||||
| Cetuximab | |||||||||||||
| Saridaki Z, et al. 2012 | II | RR | FOLFOXIRI+cet | 30 KRAS wt |
70 | 10.2 (7.1–13.4)* | 30.3 (18.8–41.9) | 37 | 62 | 10.2 | |||
| Garufi C,et al. 2010 | II | Resection rate |
chrono-IFLO+cet | 43 Liver limited |
79.1 | 14 (11–17) | 37 (21–53) | 60 | 11** | ||||
| Assenat E, et al. 2011 | II | Complete RR |
FOLFIRINOX+cet | 24 KRAS wt |
83.3 | 9.9 (6.2–12.5) | NR | ||||||
| Folprecht G, et al. 2014 | I | MTD | FOLFOXIRI+cet | 20 | 75 | 16.0 (12.6–19.4) | 33.0 (26.2–39.8) | ||||||
| Panitumumab | |||||||||||||
| Fornaro L, et al. 2013 | II | RR | FOLFOXIRI+pani | 37 RAS/BRAF wt |
89 | 11.3 (9.7–12.9) | NR | 35 | |||||
TTP; time to tumor progression
patients underwent R0/R1 resection or all patients resected
Abbreviation: RR, response rate; PFS, progression-free survival; OS, overall survival; MTD, maximum tolerable dosage; LLD, liver-limited disease; NA, not applicable; NR, not reached; FIr-bev/FOx, 5-FU plus alternating irinotecan/bevacizumab or oxaliplatin
With regard to secondary resection of metastases, the pooled analysis of 196 unresectable mCRC patients treated with the GONO-FOLFOXIRI regimen in two phase II and phase III trials showed a 19% radical resection rate, with 5- and 8-year survivals of 42% and 33%, respectively [20,21]. In addition, the perioperative complication rate was 27% including liver injury and all complications resolved without sequelae. Recently, after the development of the capecitabine-based doublet regimens [22–25], a dose-finding trial and a multicenter phase II trial evaluated the association of such oral fluoropyrimidine in combination with oxaliplatin and irinotecan (XELOXIRI). This regimen demonstrated to be feasible and active but a high grade of diarrhea (30%), neutropenia (30%), and febrile neutropenia (11%) were observed [26]. Another phase I/II trial of XELOXIRI plus bevacizumab showed similar toxicity [27]. Therefore, standard triplet regimen should be based on continuous administration of 5-FU, and both bolus 5-FU and capecitabine are not considered as valid alternative options.
2) FOLFOXIRI plus bevacizumab, a new standard option in mCRC patients
In the last 10 years, the introduction of biologics marked a turning point in the first-line treatment of mCRC patients. The combination of chemotherapy plus a monoclonal antibody is deemed a standard treatment in the initial therapy [7–9,28]. More recently, several studies have evaluated the combination of the triplet with bevacizumab. In a phase II FOIB trial, FOLFOXIRI plus bevacizumab (FOLFOXIRI-bev) demonstrated high rate of responses (77%), disease-control (100%), and secondary radical resection (26%) among 57 unresectable mCRC patients [29]. Such results were validated in the randomized phase III TRIBE study, which enrolled 508 patients and showed that FOLFOXIRI-bev significantly improved PFS (12.1 months versus 9.7 months, HR 0.75, p=0.003) and RR (65% versus 53%, p=0.006) compared to FOLFIRI plus bevacizumab, thus leading to longer OS (29.8 months versus 25.8 months, HR 0.80, p=0.030) (Table 1). In the TRIBE trial, among patients assigned to FOLFIRI plus bevacizumab arm, 78% of patients received oxaliplatin in the subsequent treatments [10]. The intensified treatment of FOLFOXIRI-bev was associated with a higher rate of grade 3 or 4 neurotoxicity, stomatitis, diarrhea, and neutropenia, but no increase of serious adverse events or deaths due to treatment-related toxic effects were observed. Moreover, the subgroup analysis of BRAF and RAS status has demonstrated that the benefit from FOLFOXIRI-bev was consistent across all molecular subgroups [30]. The phase II OPAL study identified that FOLFOXIRI-bev had a high efficacy in terms of RR, OS, and secondary resection rate in molecularly unselected mCRC patients [31]. Thus, FOLFOXIRI-bev can markedly improve the efficacy of first-line therapy for mCRC patients, with an acceptable toxicity profile. The randomized phase II STEAM trial of sequential or concurrent FOLFOXIRI-bev versus FOLFOX plus bevacizumab for the first-line treatment is under way to evaluate bevacizumab with FOLFOXIRI in the United States (NCT01765582)[32].
Whether the initial treatment with triplet plus bevacizumab is effective in patients with initially unresectable colorectal liver metastases (CLM) was specifically evaluated in the phase II OLIVIA trial. Among 80 randomized patients, overall resection rate, the primary endpoint of the study, was significantly increased in the FOLFOXIRI-bev arm compared to the mFOLFOX6 plus bevacizumab arm (61% versus 49%). Promising results were also observed in terms of RR (81% versus 62%) and PFS (18.6 months versus 11.5 months, HR 0.43) [33]. Such results demonstrate that an intensified treatment with FOLFOXIRI-bev may represent a new option also for patients with CLM, according to the hypothesis that CLM patients may become cured after downsizing of metastases by the active induction chemotherapy. Initial FOLFOXIRI-bev treatment as perioperative therapy in patients with resectable CLM is currently under evaluation in the randomized phase II trial PERIMAX [34]. In addition, the CAIRO5 trial is under way, which investigates the optimal induction chemotherapy for patients with initially unresectable CLM. Doublet chemotherapy (FOLFOX or FOLFIRI) plus bevacizumab and FOLFOXIRI-bev will be compared for median PFS in the trial [35] (Table 1).
3) The promising results of the combination of FOLFOXIRI plus anti-EGFRs
More recently the efficacy of an anti-EGFR antibody, cetuximab or panitumumab, in combination with the triplet has been evaluated. The first trial was performed in molecular-unselected patients [36]. With the identification of RAS as a predictive biomarker for EGFR inhibitor efficacy, subsequent studies tested such combination in a selected-population of KRAS or RAS wild-type patients (Table 1). In a phase II trial evaluating FOLFOXIRI plus cetuximab, RR and resection rate were 70% and 37%, respectively, although high rate of grade 3 or 4 neutropenia (23%) and diarrhea (53%) occurred. In particular, in patients with unresectable CLM only, the resection rate was 62% [37]. In the phase II TRIP trial, FOLFOXIRI plus panitumumab in a highly molecularly selected-population of RAS wild-type patients demonstrated a particularly high RR of 89% and R0 resection rate of 35%, with increased incidence and severity of diarrhea (grade 3–4 35%) [38]. In conclusion, the combination of FOLFOXIRI plus an anti-EGFR has showed to be promising in terms of efficacy with high RR; however some concerns about safety such as severe non-hematological toxicity have been raised up (Table 2) [36–39].
Table 2.
Dosage and toxicities of trials evaluating triplet chemotherapy alone, in combination with bevacizumab or with anti-EGFRs
| Author | phase | regimen | N | irinotecan | Oxaliplatin | 5-FU | Median cycle |
Gr3-4 toxicity (%) |
Gr3-4 neutropenia (FN) (%) |
Gr3-4 diarrhea (%) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/m2) |
rDI | Dose (mg/m2) |
rDI | Dose mg/m2) |
rDI | ||||||||
| Triplet regimen | |||||||||||||
| Falcone A, et al. 2007 | III | FOLFOXIRI | 122 | 165 | 82 | 85 | 83 | 3200 | 82 | 11 | 50 (5) |
20 | |
| Masi G, et al. 2004 | II | FOLFOXIRI | 32 | 165 | 86 | 85 | 89 | 3200 | 90 | 12 | 59 (12) |
16 | |
| Souglakos J, et al. 2006 | III | FOLFOXIRI | 137 | 150 | 85 | 65 | 84 | Bolus 800 CI 1200 |
88 | 10 | 35 (9) |
27.7 | |
| Souglakos J, et al. 2002 | II | FOLFOXIRI | 31 | 150 | 85 | 65 | 84 | Bolus 800 CI 1200 |
88 | 12 | 45 (6) |
32 | |
| Ychou, et al. 20 | II | FOLFIRINOX | 34 Liver limited |
180 | 87 | 85 | 88 | Bolus 400 CI 2400 |
91 | 9.5 | 76.5 | 64.8 (3) |
29.4 |
| Sunakawa Y, et al. 2012 | I | FOLFOXIRI | 6 3 |
150 165 |
79 88 |
85 85 |
79 88 |
2400 2400 |
79 88 |
8.5 | 27 (0) |
0 | |
|
Combination with bevacizumab |
|||||||||||||
| Loupakis F, et al. 2014 | III | FOLFOXIRI+bev | 252 | 165 | 74 | 85 | 75 | 3200 | 73 | 11 | 50.0 (8.8***) |
18.8 | |
| Masi G, et al. 2010 | II | FOLFOXIRI+bev | 57 | 165 | 85 | 85 | 85 | 3200 | 84 | 12 | 49 (2) |
14 | |
| Gruenberger T, et al. 2015 | II | FOLFOXIRI+bev | 41 Liver limited |
165 | 85 | 3200 | 6 | 95 | 50 (13) |
30 | |||
| Satake, et al. 2015 | I | FOLFOXIRI+bev | 6 | 165 | 87 | 85 | 87 | 3200 | 90 | 67 (0) |
0 | ||
|
Combination with anti-EGFR antivbody |
|||||||||||||
| Saridaki Z, et al. 2012 | II | FOLFOXIRI+cet | 30 KRAS wt |
150 | 85.3 | 65 | 93.2 | Bolus 800 CI 1200 |
83.2 | 23.3 (6.7) |
53.3 | ||
| Assenat E, et al. 2011 | II | FOLFIRINOX+cet | 42 | 180 | 86 | 85 | 85 | Bolus 400 CI 2400 |
92 | 9 | 88.1 | 38.0 (4.8) |
52.4 |
| Folprecht G, et al. 2014 | I | FOLFOXIRI+cet | 6 | 125 | 85 | 3200 | 9.7 | 28.6 | 28.6 | ||||
| Fornaro L, et al. 2013 | II | FOLFOXIRI+pani | 37 RAS/BRAF wt |
150 | 74 | 85 | 75 | 2400 | 76 | 11 | 48 (5) |
35 | |
TTP
patients underwent R0/R1 resection or all patients resected
Gr3-4
Abbreviation: NA, not applicable; NR, not reached; FIr-bev/FOx, 5-FU plus alternating irinotecan/bev or oxaliplatin; FN, febrile neutropenia; rDI, relative dose intensity; CI, continuous infusion
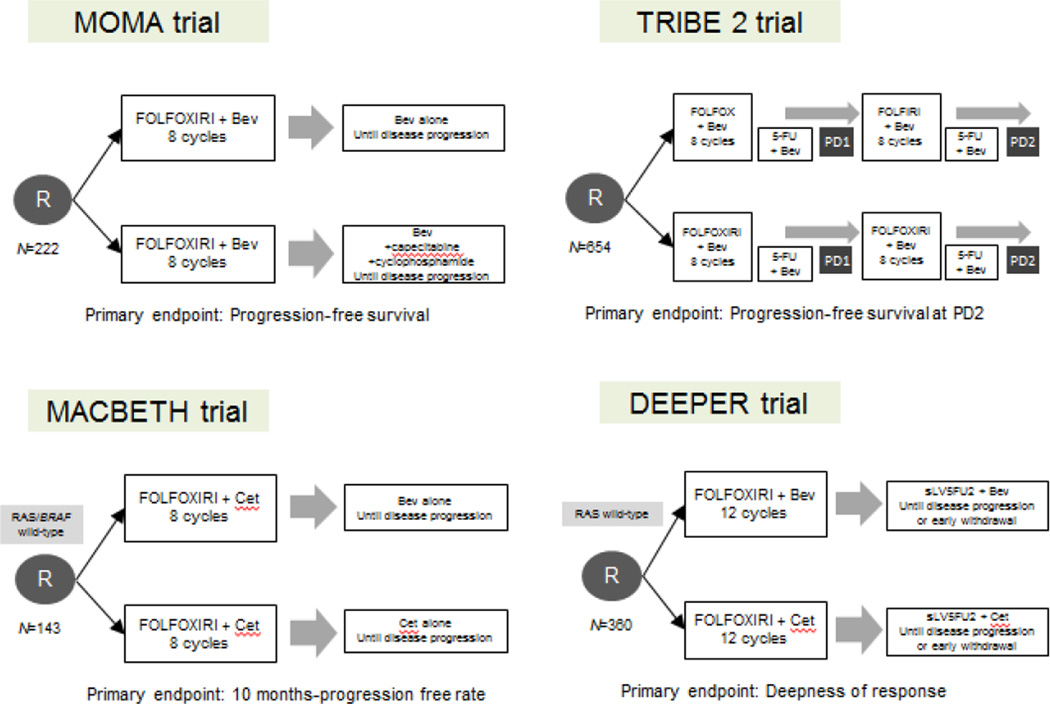
To date, the use of such a combination is not recommended outside clinical trials, but due to the promising efficacy results the combination regimen requires further consideration and some clinical trials are currently ongoing. In particular, a German phase II trial is randomizing patients to FOLFOXIRI plus or minus panitumumab in order to compare RR and secondary resection rate in RAS wild-type mCRC (NCT01328171); the randomized phase II MACBETH trial is evaluating the activity of initial FOLFOXIRI plus cetuximab followed by a subsequent maintenance with cetuximab or bevacizumab (NCT02295930). Preliminary results on the first 72 enrolled patients, were presented and showed encouraging activity (71% RR) and a safe toxicity profile: 35% grade 3 or 4 neutropenia, 21% diarrhea, 7% stomatitis, 15% skin rash, and 3% febrile neutropenia [40]. It should be noted that the GONO-FOLFOXIRI schedule was modified when combined with anti-EGFRs due to the high rate of toxicities. Finally, the randomized phase II DEEPER (JACCRO CC-13) trial is currently recruiting patients and is evaluating the addition of bevacizumab or cetuximab to FOLFOXIRI as a first-line therapy in Japanese mCRC patients with RAS wild-type tumors (UMIN000018217) (Figure 1).
Figure 1.
Main ongoing trials evaluating the triplet chemotherapy plus biologic agents in patients with mCRC
4) Practical issues: Toxicities and dosage
FOLFOXIRI-based regimens have been investigated in European and Japanese clinical trials (Table 2). In the TRIBE study, FOLFOXIRI consisted of 165 mg/m2 irinotecan, 85 mg/m2 oxaliplatin, and 3200 mg/m2 continuous 5-FU in association with bevacizumab (5 mg/kg). In the first pilot study the GONO identified 175 mg/m2 irinotecan, 100 mg/m2 oxaliplatin, and 3800 mg/m2 48-h chronomodulated continuous infusion 5-FU as a recommended dose of FOLFOXIRI [16]. However, subsequently the schedule was modified to the current version due to the frequent occurrence of sever hematological toxicity observed in a phase II trial [41]. Souglakos et al have developed a different FOLFOXIRI regimen including 150 mg/m2 irinotecan, 85 mg/m2 oxaliplatin and 400 mg/m2 bolus administration of 5-FU followed by 600 mg/m2 22-h continuous infusion on days 2 and 3. The failure by the HORG to demonstrate a survival benefit of the FOLFOXIRI might be partially explained by the different FOLFOXIRI schedule [19]. In Japan, the recommended dose is still controversial. In the initial report, the dosage of GONO-FOLFOXIRI appeared to be not optimal for Japanese patients [42], while another study indicated that it may be tolerable although high percentage of neutropenia was observed: 67% grade 3 or 4 neutropenia [43]. It should be noted that in the TRIBE study FOLFOXIRI-bev was active and feasible but it was adopted in a clinically selected-population: patients younger than 70 years or if aged 70 to 75 with ECOG performance status (PS) <1. Moreover, although grade 3–4 neutropenia, diarrhea, and stomatitis were slightly increased in patients treated with the triplet plus bevacizumab, no differences were reported in terms of serious and/or fatal adverse events, nor in terms of treatment related deaths.
From a practical point of view, the use of G-CSF is not recommended as primary prophylaxis of neutropenia. A dose reduction of irinotecan and oxaliplatin is suggested after the occurrence of a long lasting grade 4 neutropenia (>5 days), febrile neutropenia, or grade 4 thrombocytopenia. The management of diarrhea and stomatitis should be careful and sudden. A dose reduction of irinotecan and 5-FU is recommended after the occurrence of grade 3–4 diarrhea, and a reduction of 5-FU is suggested in case of grade 3–4 stomatitis. The STEAM trial based in the United States is ongoing to verify whether sequential FOLFOXIRI (alternating treatment with FOLFOX and FOLFIRI) may improve the tolerability of the regimen without impacting efficacy (NCT01765582) [32].
More controversial is the issue of a combination regimen with FOLFOXIRI and an anti-EGFR antibody due to their toxicity profile. Higher rates of severe diarrhea were observed in the clinical trials [37,38], thus resulting in dose reduction of irinotecan and 5-FU when combined with an anti-EGFR monoclonal antibody [44].
In conclusion, the GONO-FOLFOXIRI might be proposed as the standard FOLFOXIRI (to be used in association or not with bevacizumab) [10,29]. Personalized regimens adopted in specific-population require further validation. At the moment the combination of FOLFOXIRI plus anti-EGFR is not yet recommended outside clinical trials.
5) How should we treat patients after FOLFOXIRI-based therapy? Maintenance and second-line options
It remains unclear, whether and when a maintenance treatment should be adopted after an induction treatment with FOLFOXIRI plus a biologics. Several studies have indicated the efficacy of bevacizumab as a maintenance treatment, mainly in combination with 5-FU [45,46]; while data on maintenance with anti-EGFRs are lacking.
In the TRIBE trial after 12 cycles of induction treatment patients were treated with maintenance of 5-FU plus bevacizumab until progression or unacceptable toxicity. Thus, maintenance treatment with 5-FU plus bevacizumab represents a current standard treatment after FOLFOXIRI-bev. However, several trials are ongoing in order to investigate the best maintenance treatment after induction of FOLFOXIRI-based chemotherapy. The MOMA study is a phase II randomized trial investigating the role of a maintenance treatment with bevacizumab versus bevacizumab plus a metronomic chemotherapy after initial FOLFOXIRI-bev (NCT02271464). The already mentioned MACBETH trial is evaluating the role of maintenance with cetuximab versus bevacizumab after an induction of FOLFOXIRI plus cetuximab (NCT02295930) (Figure 1).
A major concern regarding FOLFOXIRI-based therapy is that the up-front use of all three cytotoxic agents may limit disease control with second-line treatments. Retrospective analyses of two phase II trials showed that initial FOLFOXIRI treatment did not impair the possibility to obtain objective responses and delay tumor progression with second-line treatments containing the same agents used in first-line. In particular, patients receiving a rechallenge of FOLFOXIRI reported RR of 38%, median PFS of 8.2 months, and median OS of 19.3 months [47,48].
Certainly, the time elapsed between the end of first-line treatment and the start of second-line may be a crucial point. If disease progression occurs during treatment with FOLFOXIRI plus or minus bevacizumab other therapeutic options such as anti-EGFRs based treatment in RAS wild-type patients or regorafenib or TAS-102 should be considered. In patients with a progression-free interval from the discontinuation of first-line FOLFOXIRI of at least 3 months, a second-line treatment containing a doublet or a triplet might be considered as a suitable therapy [47]. In the TRIBE study, 78% of patients in the FOLFOXIRI-bev arm received components of the primary regimen as their second-line treatment, while 82% of patients in FOLFIRI plus bevacizumab arm received second-line treatment [10]. In particular among patients in the FOLFOXIRI-arm 8%, 37%, and 15% of patients were treated with FOLFOXIRI, irinotecan-, and oxaliplatin-based regimen, respectively. In patients experiencing neurotoxicity, irinotecan-containing regimen should be more reasonable. On the other hand, oxaliplatin-based treatments could be preferred in patients experiencing higher rates of mucositis and diarrhea during the first-line treatment. The rechallenge with FOLFOXIRI plus or minus bevacizumab may also represent a valid option.
In order to prospectively validate the option of FOLFOXIRI-bev rechallenge strategy, the GONO is conducting the phase III TRIBE-2 trial. In this study patients are randomized to receive first-line FOLFOXIRI-bev followed by reintroduction of FOLFOXIRI-bev at progression versus a standard strategy of FOLFOX plus bevacizumab followed by FOLFIRI plus bevacizumab at progression in first- and second-line treatment (NCT02339116) (Figure 1).
6) Novel appropriate/surrogate end-points for FOLFOXIRI-based treatment?
Basically, OS as an endpoint is clinically meaningful, objectively assessed, and used for regulatory approval. Prolongation of OS is generally the most relevant measure of clinical benefit in a randomized clinical trial of an experimental treatment. The PFS incorporates not only with survival but also with the reduction in cancer burden and the delay in cancer progression, leading to the use as an endpoint to avoid the requirement of large patient number and long-term follow-up. The results from several pooled analyses have suggested that while PFS might be a surrogate endpoint for OS [49–51], tumor shrinkage and RR have a moderate correlation with OS and are not deemed an optimal new endpoints for phase III trials in mCRC [50,52]. An analysis of published mCRC trials of chemotherapy plus or minus targeted-drug, which evaluated the surrogacy of PFS and RR for OS, revealed the correlation coefficient between PFS and OS and weak correlation between RR and OS. In this analysis, improvements in PFS are strongly correlated with improvements in OS, suggesting that PFS remains a valid surrogate endpoint for OS with current treatment regimens in mCRC [53]. Another analysis of published trials containing bevacizumab further suggested PFS to be a suitable surrogate endpoint for chemotherapy plus bevacizumab, while data concerning cetuximab- or panitumumab-based chemotherapy needs further validation [54]. Therefore, it still remains controversial if PFS is a suitable surrogate marker for OS when targeted therapies are used.
There was no correlation between PFS and OS in a phase III FIRE-3 trial evaluating the efficacy of FOLFIRI plus cetuximab compared to FOLFIRI plus bevacizumab in the first-line treatment of KRAS exon2 wild-type mCRC patients [5]. This trial showed a statistically significant difference in favor of FOLFIRI plus cetuximab in terms of OS, but not in terms of PFS. A possible explanation of this phenomenon could be extrapolated taking into account data on early tumor shrinkage (ETS) defined as a reduction ≥20% of target lesions’ diameters measured after 6–8 weeks from treatment start, depth of response (DpR), defined as the percentage of maximal tumor shrinkage observed compared with baseline and survival post-progression (SPP) [55]. Indeed, in the FIRE-3 trial, ETS and DpR were increased in the cetuximab-arm and were significantly correlated with longer PFS and OS in both arms. It was hypothesized that patients showing a deeper response could have earlier disease progression, but the lower tumor load might explain the longer OS. Similarly, a retrospective analysis from CRYSTAL and OPUS trials have demonstrated that the addiction of cetuximab to chemotherapy was able to increase the ETS, which was significantly associated with PFS and OS [56]. Cetuximab was able to increase the DpR, which was significantly associated with SPP and OS [57]. In a subgroup analysis of PEAK study, although RR appeared to be similar or only numerically improved in the panitumumab arm, the responses appeared to occur earlier, last longer and be deeper [58]. In a subgroup analysis of the TRIBE study, patients treated with FOLFOXIRI-bev achieved earlier response and a better extent of DpR compared to those treated with FOLFIRI plus bevacizumab [59]. Moreover, both ETS and DpR could predict PFS, PPS, and OS in patients treated with chemotherapy plus bevacizumab, thus confirming the importance of achieving an early response in order to improve long-term prognosis. A retrospective analysis of subgroup populations with RAS/BRAF wild-type patients treated in 3 first-line trials by the GONO suggested that the triplet plus anti-EGFR may be associated with a higher extent of ETS and a better DpR than bevacizumab [60]. Recently, a systematic literature has demonstrated that ETS is an early indicator of the potentially achievable response, while DpR may serve as a predictor of antiproliferative or anti-EGFR directed therapy [61]. Consequently, both parameters may be considered as determinants of long-term outcome and potential endpoints for clinical trials, in particular when adopting FOLFOXIRI-based regimen or anti-EGFR-containing regimen. Four key trials approaching the investigation of new endpoint are summarized in Table 3.
Table 3.
Outcome parameters according to early tumor shirnkage and deepness of response of FOLFOXIRI plus biologics, FIRE-3 and PEAK trials
| Study | TRIBE | GONO retrospective analysis RAS/BRAF wild-type |
FIRE3 (N=330) RAS wild-type |
PEAK (N=169) RAS wild-type |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regimen | FOLFOXIRI +bev (N=225) |
FOLFIRI +bev (N=216) |
P-value | FOLFOXIRI +bev (N=62) |
FOLFOXIRI +anti-EGFR (N=101) |
P-value | FOLFIRI +bev (N=173) |
FOLFIRI +cet (N=157) |
P-value | FOLFOX +bev (N=216) |
FOLFOX +pani (N=) |
P-value | ||
| Response rate ETS | 71.6% | 62.0% | 0.043* | 71% | 82% | 0.12* | 56.1% | 72.0% | 0.003* | 61.7% | 64.8% | |||
| Median | 31.2% | 23.5% | <0.001** | 26.4% | 40.8% | 0.003*** | ||||||||
| ETS>20% | 62.7% | 51.9% | 62% | 74% | 0.15* | 49.1% | 68.2% | 0.0005* | 62% | 75% | 0.12 | |||
| ETS<20% | 30.2% | 39.8% | 38% | 26% | 38% | 25% | ||||||||
| Not evaluated | 7.1% | 8.3% | ||||||||||||
| DpR | ||||||||||||||
| Median | 43.4% | 37.8% | 0.003** | 32.3% | 48.9% | <0.0001 | 46% | 65% | 0.0007 | |||||
| Median within 4 months | 37.8 | 48.6 | 0.0047*** | |||||||||||
| Evaluable population (n=407) |
||||||||||||||
| ETS>20% (n=253) |
ETS<20% (n=154) |
ETS (n=85) |
No ETS (n=88) |
ETS (n=107) |
No ETS (n=50) |
ETS>20% | ETS>20% | |||||||
| Median PFS (months) | 8.8† |
7.2† | 11.7 | 8.3 | 9.7 | 5.8 | 11.1 | 13.2 | ||||||
| HR 0.78, P=0.027 | HR 0.71, P=0.03 | HR 0.59, P=0.0037 | HR 0.69, P=0.13 | |||||||||||
| Medina PPS (months) | 17.1† | 11.5† | ||||||||||||
| HR 0.65, P=0.030 | ||||||||||||||
| Median OS (months) | 31.9† | 21.9† | 31.9 | 21.2 | 38.3 | 20.5 | ||||||||
| HR 0.63, P=0.001 | HR 0.48, P=0.0001 | HR 0.52, P=0.0023 | ||||||||||||
ETS was defined as the relative change in the sum of longest diameters of RECIST target lesions at week 8 in TRIBE and GONO studies, week 6 in FIRE3 study, compared to baseline.
DpR was defined as the relative change in the sum of longest diameters of RECIST target lesions at the nadir, in the absence of new lesions or progression of non-target lesions, as compared to baseline.
Fisher’s exact test;
Kruskal Wallis test;
Mann-Whitney test
Not including the first 4 months after randomization
Abbreviation: bev, bevacizumab; cet, cetuximab; pani, panitumumab; NA, not applicable; NR, not reached; FIr-bev/FOx, 5-FU plus alternating irinotecan/bev or oxaliplatin; FN, febrile neutropenia; rDI, relative dose intensity; CI, continuous infusion; PPS, post progression survival
7) Promising predictive biomarker
According to accumulating data on the molecular pathways underlying CRC, few valid prognostic markers for CRC-specific survival have been recognized. BRAF V600E mutations in mCRC are the only notable exceptions [62]. Similarly, only few data are available concerning predictive markers of response/resistance to treatments and the only validated factor is RAS mutational status that is a well-known predictive factor of resistance to anti-EGFRs [9,63].
At the moment no validated biomarkers but only clinical considerations are available in order to select patients candidate to receive an intensive treatment such as the FOLFOXIRI. Among clinical characteristics, age and PS should be taken into account, according previously specified TRIBE selection criteria. Moreover, patients who have received adjuvant oxaliplatin-based chemotherapy may be candidates for an intensified chemotherapy, according to the results of a subgroup analysis of the TRIBE study that revealed an interaction between previous exposure to adjuvant chemotherapy which contained oxaliplatin in 64% of cases and treatment effect.
Several studies have addressed the identification of possible predictors of efficacy of FOLFOXIRI-based treatment. Loupakis F, et al. evaluated plasma levels of VEGF, PIGF, sVEGFR-2, and TSP-1 in a subgroup of patients treated with FOLFOXIRI-bev. Treatment with FOLFOXIRI-bev determined a prolonged and significant reduction of plasma VEGF levels. Interestingly, VEGF concentrations remained lower than at baseline as well as at the time of tumor progression. Conversely, PlGF levels increased during treatment if compared with baseline, suggesting a possible role in tumor resistance; moreover, sVEGFR-2 and TSP-1 levels increased at the time of tumor progression [64]. A genome-wide-associated-study using an exome array identified the association of VEGFR2 rs10008360 with OS in patients treated with FOLFOXIRI-bev in the TRIBE study (HR 9.81, 95%CI 3.59–26.81, p= 8.52×10−6) [65]. Finally, the analysis of mRNA levels among patients treated in the TRIBE study, showed that patients with high intratumoral expression of ERCC1 or VEGFC but not low expression had longer PFS in the FOLFOXIRI-bev arm compared to the FOLFIRI plus bevacizumab arm (12.0 months versus 9.7 months), suggesting a predictive role of ERCC1 and VEGFC mRNA levels [66]. Such results, although very appealing, are based on retrospective findings and require further validation.
Conclusions
Initial combination chemotherapy with FOLFOXIRI-bev is considered as one of the standard treatments for mCRC patients, which may be the preferred option in patients requiring a meaningful tumor shrinkage or rapid symptom control, with good PS and regardless of molecular status of RAS and BRAF. The association of an anti-EGFR antibody in combination with FOLFOXIRI would be a promising treatment in RAS wild-type mCRC patients, but deserve further validation in phase II and III trials.
Many clinical trials are ongoing to answer important questions regarding the role of maintenance treatment, subsequent treatment strategies, and the possible adoption of new endpoint such as ETS, DpR, or SPP in the evaluation of triplet plus anti-EGFRs or anti-VEGF activity and efficacy. The identification of predictive biomarkers for response to FOLFOXIRI-bev is also urgently needed in order to refine patient selection.
Highligths.
FOLFOXIRI plus bevacizumab represents a new standard option in mCRC patients
Adequate schedules and early adverse event management are crucial for such treatment
FOLFOXIRI plus anti-EGFRs showed promising results in clinical trials
Novel surrogate end-points for FOLFOXIRI-based treatment are under investigation
Predictive markers of efficacy are urgently needed to refine patients selection
Acknowledgments
We thank the Wunderglo Project and the National Institutes of Health [grant number P30CA14089-27S1] that partly funded this study.
Funding
This study was partly supported by award number P30CA014089 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Biographies
Yu Sunakawa; Medical oncologist, assistant professor of Showa University in Japan, who performed the first trial of FOLFOXIRI regimen in Japan and has worked as a postdoctoral research fellow of the University of Southern California/Norris Comprehensive Cancer Center between February 2013 and June 2015.
Marta Schirripa; Medical oncologist who is working as a researcher of the GONO group in Italy and has been working as a guest researcher at the University of Southern California/Norris Comprehensive Cancer Center.
Heinz-Josef Lenz; Medical oncologist, professor of Medicine and Preventive Medicine, University of Southern California, who serve as a director of GI Oncology Program at the University of Southern California/Norris Comprehensive Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have declared no conflicts of interest.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Nordlinger B, Van Cutsem E, Gruenberger T, Glimelius B, Poston G, Rougier P, et al. Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann Oncol. 2009;20:985–992. doi: 10.1093/annonc/mdn735. [DOI] [PubMed] [Google Scholar]
- 4.Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 5.Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–75. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 6.Venook AP, Niedzwiecki D, Lenz H-J, Innocenti F, Mahoney MR, O'Neil BH, et al. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC) ASCO Meeting Abstracts. 2014;32:LBA3. [Google Scholar]
- 7.Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 8.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 9.Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 10.Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609–1618. doi: 10.1056/NEJMoa1403108. [DOI] [PubMed] [Google Scholar]
- 11.Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 12.Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol. 2005;23:4866–4875. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 13.Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 14.Mans DR, Grivicich I, Peters GJ, Schwartsmann G. Sequence-dependent growth inhibition and DNA damage formation by the irinotecan-5-fluorouracil combination in human colon carcinoma cell lines. Eur J Cancer. 1999;35:1851–1861. doi: 10.1016/s0959-8049(99)00222-1. [DOI] [PubMed] [Google Scholar]
- 15.Raymond E, Buquet-Fagot C, Djelloul S, Mester J, Cvitkovic E, Allain P, et al. Antitumor activity of oxaliplatin in combination with 5-fluorouracil and the thymidylate synthase inhibitor AG337 in human colon, breast and ovarian cancers. Anticancer Drugs. 1997;8:876–885. doi: 10.1097/00001813-199710000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Falcone A, Masi G, Allegrini G, Danesi R, Pfanner E, Brunetti IM, et al. Biweekly chemotherapy with oxaliplatin, irinotecan, infusional Fluorouracil, and leucovorin: a pilot study in patients with metastatic colorectal cancer. J Clin Oncol. 2002;20:4006–4014. doi: 10.1200/JCO.2002.12.075. [DOI] [PubMed] [Google Scholar]
- 17.Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 18.Masi G, Vasile E, Loupakis F, Cupini S, Fornaro L, Baldi G, et al. Randomized trial of two induction chemotherapy regimens in metastatic colorectal cancer: an updated analysis. J Natl Cancer Inst. 2011;103:21–30. doi: 10.1093/jnci/djq456. [DOI] [PubMed] [Google Scholar]
- 19.Souglakos J, Androulakis N, Syrigos K, Polyzos A, Ziras N, Athanasiadis A, et al. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG) Br J Cancer. 2006;94:798–805. doi: 10.1038/sj.bjc.6603011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masi G, Cupini S, Marcucci L, Cerri E, Loupakis F, Allegrini G, et al. Treatment with 5-fluorouracil/folinic acid, oxaliplatin, and irinotecan enables surgical resection of metastases in patients with initially unresectable metastatic colorectal cancer. Ann Surg Oncol. 2006;13:58–65. doi: 10.1245/ASO.2006.03.094. [DOI] [PubMed] [Google Scholar]
- 21.Masi G, Loupakis F, Pollina L, Vasile E, Cupini S, Ricci S, et al. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann Surg. 2009;249:420–425. doi: 10.1097/SLA.0b013e31819a0486. [DOI] [PubMed] [Google Scholar]
- 22.Cassidy J, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. XELOX vs FOLFOX-4 as first-line therapy for metastatic colorectal cancer: NO16966 updated results. Br J Cancer. 2011;105:58–64. doi: 10.1038/bjc.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ducreux M, Bennouna J, Hebbar M, Ychou M, Lledo G, Conroy T, et al. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/leucovorin plus oxaliplatin (FOLFOX-6) as first-line treatment for metastatic colorectal cancer. Int J Cancer. 2011;128:682–690. doi: 10.1002/ijc.25369. [DOI] [PubMed] [Google Scholar]
- 24.Cassidy J, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006–2012. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- 25.Skof E, Rebersek M, Hlebanja Z, Ocvirk J. Capecitabine plus Irinotecan (XELIRI regimen) compared to 5-FU/LV plus Irinotecan (FOLFIRI regimen) as neoadjuvant treatment for patients with unresectable liver-only metastases of metastatic colorectal cancer: a randomised prospective phase II trial. BMC Cancer. 2009;9:120. doi: 10.1186/1471-2407-9-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bajetta E, Celio L, Ferrario E, Di Bartolomeo M, Denaro A, Dotti K, et al. Capecitabine plus oxaliplatin and irinotecan regimen every other week: a phase I/II study in first-line treatment of metastatic colorectal cancer. Ann Oncol. 2007;18:1810–1816. doi: 10.1093/annonc/mdm347. [DOI] [PubMed] [Google Scholar]
- 27.Bazarbashi S, Aljubran A, Alzahrani A, Mohieldin A, Soudy H, Shoukri M. Phase I/II trial of capecitabine, oxaliplatin, and irinotecan in combination with bevacizumab in first line treatment of metastatic colorectal cancer. Cancer Med. 2015 doi: 10.1002/cam4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 29.Masi G, Loupakis F, Salvatore L, Fornaro L, Cremolini C, Cupini S, et al. Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:845–852. doi: 10.1016/S1470-2045(10)70175-3. [DOI] [PubMed] [Google Scholar]
- 30.Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015 doi: 10.1016/S1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 31.Stein A, Atanackovic D, Hildebrandt B, Stubs P, Brugger W, Hapke G, et al. Upfront FOLFOXIRI+bevacizumab followed by fluoropyrimidin and bevacizumab maintenance in patients with molecularly unselected metastatic colorectal cancer. Br J Cancer. 2015;113:872–877. doi: 10.1038/bjc.2015.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bendell JC, Tan BR, Reeves JA, Xiong HQ, Laeufle R, Byrtek M, et al. STEAM: A randomized, open-label, phase 2 trial of sequential and concurrent FOLFOXIRI-bevacizumab (BEV) versus FOLFOX-BEV for the first-line (1L) treatment (tx) of patients (pts) with metastatic colorectal cancer (mCRC) ASCO Meeting Abstracts. 2014;32 TPS3652. [Google Scholar]
- 33.Gruenberger T, Bridgewater J, Chau I, Garcia Alfonso P, Rivoire M, Mudan S, et al. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26:702–708. doi: 10.1093/annonc/mdu580. [DOI] [PubMed] [Google Scholar]
- 34.Stein A, Glockzin G, Wienke A, Arnold D, Edelmann T, Hildebrandt B, et al. Treatment with bevacizumab and FOLFOXIRI in patients with advanced colorectal cancer: presentation of two novel trials (CHARTA and PERIMAX) and review of the literature. BMC Cancer. 2012;12:356. doi: 10.1186/1471-2407-12-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huiskens J, van Gulik TM, van Lienden KP, Engelbrecht MR, Meijer GA, van Grieken NC, et al. Treatment strategies in colorectal cancer patients with initially unresectable liver-only metastases, a study protocol of the randomised phase 3 CAIRO5 study of the Dutch Colorectal Cancer Group (DCCG) BMC Cancer. 2015;15:365. doi: 10.1186/s12885-015-1323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garufi C, Torsello A, Tumolo S, Ettorre GM, Zeuli M, Campanella C, et al. Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. Br J Cancer. 2010;103:1542–1547. doi: 10.1038/sj.bjc.6605940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saridaki Z, Androulakis N, Vardakis N, Vamvakas L, Kabouraki E, Kalbakis K, et al. A triplet combination with irinotecan (CPT-11), oxaliplatin (LOHP), continuous infusion 5-fluorouracil and leucovorin (FOLFOXIRI) plus cetuximab as first-line treatment in KRAS wt, metastatic colorectal cancer: a pilot phase II trial. Br J Cancer. 2012;107:1932–1937. doi: 10.1038/bjc.2012.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fornaro L, Lonardi S, Masi G, Loupakis F, Bergamo F, Salvatore L, et al. FOLFOXIRI in combination with panitumumab as first-line treatment in quadruple wild-type (KRAS, NRAS, HRAS, BRAF) metastatic colorectal cancer patients: a phase II trial by the Gruppo Oncologico Nord Ovest (GONO) Ann Oncol. 2013;24:2062–2067. doi: 10.1093/annonc/mdt165. [DOI] [PubMed] [Google Scholar]
- 39.Assenat E, Desseigne F, Thezenas S, Viret F, Mineur L, Kramar A, et al. Cetuximab plus FOLFIRINOX (ERBIRINOX) as first-line treatment for unresectable metastatic colorectal cancer: a phase II trial. Oncologist. 2011;16:1557–1564. doi: 10.1634/theoncologist.2011-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cremolini C, Loupakis F, Salvatore L, Lonardi S, Battaglin F, Gamucci T, et al. Modified FOLFOXIRI plus cetuximab (cet) as induction treatment in unresectable metastatic colorectal cancer (mCRC) patients (pts): Preliminary results of the phase II randomized Macbeth trial by GONO group. ASCO Meeting Abstracts. 2014;32:3596. [Google Scholar]
- 41.Masi G, Allegrini G, Cupini S, Marcucci L, Cerri E, Brunetti I, et al. First-line treatment of metastatic colorectal cancer with irinotecan, oxaliplatin and 5-fluorouracil/leucovorin (FOLFOXIRI): results of a phase II study with a simplified biweekly schedule. Ann Oncol. 2004;15:1766–1772. doi: 10.1093/annonc/mdh470. [DOI] [PubMed] [Google Scholar]
- 42.Sunakawa Y, Fujita K, Ichikawa W, Ishida H, Yamashita K, Araki K, et al. A phase I study of infusional 5-fluorouracil, leucovorin, oxaliplatin and irinotecan in Japanese patients with advanced colorectal cancer who harbor UGT1A1*1/*1,*1/*6 or *1/*28. Oncology. 2012;82:242–248. doi: 10.1159/000337225. [DOI] [PubMed] [Google Scholar]
- 43.Satake H, Tsuji A, Kotake T, Okita Y, Hatachi Y. First report of a Japanese phase I study of triplet plus bevacizumab for chemotherapy-naive metastatic colorectal cncer (J1-TRIBE study) Cancer Treat Commun. 2015;4:75–80. [Google Scholar]
- 44.Folprecht G, Hamann S, Schutte K, Trarbach T, Stoehlmacher-Williams J, Ehninger G. Dose escalating study of cetuximab and 5-FU/folinic acid (FA)/oxaliplatin/irinotecan (FOLFOXIRI) in first line therapy of patients with metastatic colorectal cancer. BMC Cancer. 2014;14:521. doi: 10.1186/1471-2407-14-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simkens LH, van Tinteren H, May A, ten Tije AJ, Creemers GJ, Loosveld OJ, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015;385:1843–1852. doi: 10.1016/S0140-6736(14)62004-3. [DOI] [PubMed] [Google Scholar]
- 46.Koeberle D, Betticher DC, von Moos R, Dietrich D, Brauchli P, Baertschi D, et al. Bevacizumab continuation versus no continuation after first-line chemotherapy plus bevacizumab in patients with metastatic colorectal cancer: a randomized phase III non-inferiority trial (SAKK 41/06) Ann Oncol. 2015;26:709–714. doi: 10.1093/annonc/mdv011. [DOI] [PubMed] [Google Scholar]
- 47.Masi G, Marcucci L, Loupakis F, Cerri E, Barbara C, Bursi S, et al. First-line 5-fluorouracil/folinic acid, oxaliplatin and irinotecan (FOLFOXIRI) does not impair the feasibility and the activity of second line treatments in metastatic colorectal cancer. Ann Oncol. 2006;17:1249–1254. doi: 10.1093/annonc/mdl119. [DOI] [PubMed] [Google Scholar]
- 48.Fornaro L, Vasile E, Masi G, Loupakis F, Baldi GG, Allegrini G, et al. Outcome of second-line treatment after first-line chemotherapy with the GONO FOLFOXIRI regimen. Clin Colorectal Cancer. 2012;11:71–76. doi: 10.1016/j.clcc.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 49.Buyse M, Burzykowski T, Carroll K, Michiels S, Sargent DJ, Miller LL, et al. Progression-free survival is a surrogate for survival in advanced colorectal cancer. J Clin Oncol. 2007;25:5218–5224. doi: 10.1200/JCO.2007.11.8836. [DOI] [PubMed] [Google Scholar]
- 50.Tang PA, Bentzen SM, Chen EX, Siu LL. Surrogate end points for median overall survival in metastatic colorectal cancer: literature-based analysis from 39 randomized controlled trials of first-line chemotherapy. J Clin Oncol. 2007;25:4562–4568. doi: 10.1200/JCO.2006.08.1935. [DOI] [PubMed] [Google Scholar]
- 51.Saad ED, Katz A, Hoff PM, Buyse M. Progression-free survival as surrogate and as true end point: insights from the breast and colorectal cancer literature. Ann Oncol. 2010;21:7–12. doi: 10.1093/annonc/mdp523. [DOI] [PubMed] [Google Scholar]
- 52.Louvet C, de Gramont A, Tournigand C, Artru P, Maindrault-Goebel F, Krulik M. Correlation between progression free survival and response rate in patients with metastatic colorectal carcinoma. Cancer. 2001;91:2033–2038. [PubMed] [Google Scholar]
- 53.Sidhu R, Rong A, Dahlberg S. Evaluation of progression-free survival as a surrogate endpoint for survival in chemotherapy and targeted agent metastatic colorectal cancer trials. Clin Cancer Res. 2013;19:969–976. doi: 10.1158/1078-0432.CCR-12-2502. [DOI] [PubMed] [Google Scholar]
- 54.Giessen C, Laubender RP, Ankerst DP, Stintzing S, Modest DP, Mansmann U, et al. Progression-free survival as a surrogate endpoint for median overall survival in metastatic colorectal cancer: literature-based analysis from 50 randomized first-line trials. Clin Cancer Res. 2013;19:225–235. doi: 10.1158/1078-0432.CCR-12-1515. [DOI] [PubMed] [Google Scholar]
- 55.Morita S, Sakamaki K, Yin G. Detecting overall survival benefit derived from survival postprogression rather than progression-free survival. J Natl Cancer Inst. 2015 doi: 10.1093/jnci/djv133. 10710.1093/jnci/djv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piessevaux H, Buyse M, Schlichting M, Van Cutsem E, Bokemeyer C, Heeger S, et al. Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol. 2013;31:3764–3775. doi: 10.1200/JCO.2012.42.8532. [DOI] [PubMed] [Google Scholar]
- 57.Mansmann UR, Sartorius U, Laubender RP, Giessen CA, Esser R, Heinemann V. Quantitative analysis of the impact of deepness of response on post-progression survival time following first-line treatment in patients with mCRC. ASCO Meeting Abstracts. 2013;31:3630. [Google Scholar]
- 58.Rivera F, Karthaus M, Hecht JR, Fasola G, Canon J-L, Koukakis R, et al. Tumor response outcomes in first-line treatment of wild-type (WT) RAS metastatic colorectal carcinoma (mCRC) following modified FOLFOX6 (mFOLFOX6) + either panitumumab (pmab) or bevacizumab (bev) ASCO Meeting Abstracts. 2015;33:3535. [Google Scholar]
- 59.Cremolini C, Loupakis F, Antoniotti C, Lonardi S, Masi G, Salvatore L, et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol. 2015 doi: 10.1093/annonc/mdv112. [DOI] [PubMed] [Google Scholar]
- 60.Salvatore L, Cremolini C, Loupakis F, Masi G, Schirripa M, Marmorino F, et al. 515PFOLFOXIRI PLUS BEVACIZUMAB (BV) OR PLUS ANTI-EGFR ANTIBODIES IN RAS AND BRAF WILD-TYPE (WT) METASTATIC COLORECTAL CANCER (MCRC) PATIENTS (PTS): ANALYSIS OF TUMOR RESPONSE. Annals of Oncology. 2014;25:iv174. [Google Scholar]
- 61.Heinemann V, Stintzing S, Modest DP, Giessen-Jung C, Michl M, Mansmann UR. Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC) Eur J Cancer. 2015 doi: 10.1016/j.ejca.2015.06.116. [DOI] [PubMed] [Google Scholar]
- 62.Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623–4632. doi: 10.1002/cncr.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lenz H, Niedzwiecki D, Innocenti F, Blanke C, Mahony MR, O'Neil BH, et al. 501OCALGB/SWOG 80405: PHASE III TRIAL OF IRINOTECAN/5-FU/LEUCOVORIN (FOLFIRI) OR OXALIPLATIN/5-FU/LEUCOVORIN (MFOLFOX6) WITH BEVACIZUMAB (BV) OR CETUXIMAB (CET) FOR PATIENTS (PTS) WITH EXPANDED RAS ANALYSES UNTREATED METASTATIC ADENOCARCINOMA OF THE COLON OR RECTUM (MCRC) Annals of Oncology. 2014 2510.1093/annonc/mdu438.13. [Google Scholar]
- 64.Loupakis F, Cremolini C, Fioravanti A, Orlandi P, Salvatore L, Masi G, et al. Pharmacodynamic and pharmacogenetic angiogenesis-related markers of first-line FOLFOXIRI plus bevacizumab schedule in metastatic colorectal cancer. Br J Cancer. 2011;104:1262–1269. doi: 10.1038/bjc.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lenz H-J, Schumacher F, Loupakis F, Cremolini C, Ning Y, Stremitzer S, et al. Effect of genetic variation on overall survival in a clinical trial of metastatic colorectal cancer (mCRC) ASCO Meeting Abstracts. 2015;33:3562. [Google Scholar]
- 66.Yang D, Loupakis F, Cremolini C, Antoniotti C, Schirripa M, Salvatore L, et al. mRNA expression levels of candidate genes and clinical outcome in mCRC patients treated with FOLFOXIRI plus bevacizumab (bev) or FOLFIRI plus bev in the TRIBE study. ASCO Meeting Abstracts. 2014;32:3640. [Google Scholar]