Abstract
Background & Aims
It has been a challenge to confirm the association between laryngeal symptoms and physiologic reflux disease. We examined the ability of oropharyngeal pH tests (with the Restech Dx-pH system) and salivary pepsin tests (with Peptest) to discriminate between asymptomatic volunteers (controls) and subjects with a combination of laryngeal and reflux symptoms (laryngeal±reflux).
Methods
We performed a physician-blinded prospective cohort study of 59 subjects at a single academic institution. Adult volunteers were recruited and separated into 3 groups based on GerdQ and reflux symptom index scores: controls (n=20), laryngeal symptoms (n=20), or laryngeal+reflux symptoms (n=19). Subjects underwent laryngoscopy and oropharyngeal pH tests and submitted saliva samples for analysis of pepsin concentration. Primary outcomes included abnormal acid exposure and composite (RYAN) score for oropharyngeal pH tests and abnormal mean salivary pepsin concentration based on normative data.
Results
Complete oropharyngeal pH data were available from 53 subjects and complete salivary pepsin data from 35 subjects. We did not observe any significant differences between groups in percent time spent below pH 4.0, 5.0, 5.5, 6.0 or RYAN scores; or percent of subjects with positive results from tests for salivary pepsin (53% vs 40% vs 75%; P=.50, respectively). The laryngeal+reflux group had a significantly higher estimated mean concentration of salivary pepsin (117.9±147.4ng/mL) than the control group (32.4±41.9ng/mL) or laryngeal symptom group (7.5±11.2ng/mL) (P=.01 and P=.04, respectively).
Conclusions
Using current normative thresholds, oropharyngeal pH testing and salivary pepsin analysis are not able to distinguish between healthy volunteers and subjects with a combination of laryngeal and reflux symptoms.
Keywords: Extra-esophageal reflux, Gastroesophageal reflux, Oropharyngeal pH testing, Salivary pepsin analysis
Background
Establishing an association between pathologic acidic gastroesophageal reflux disease (GERD) and symptoms of laryngeal irritation, commonly referred to as laryngopharyngeal reflux (LPR), is challenging.1, 2 Current tests are limited in their ability to reliably diagnose LPR and reliance on these tools can lead to over-diagnosis. For instance, the reflux finding score (RFS), a tool developed to document the physical findings and severity of LPR on laryngoscopy 3 is fraught with poor interrater reliability and poor specificity, as up to 90% of asymptomatic subjects may have signs of posterior laryngeal irritation.4 On the other hand, mucosal abnormalities such as esophagitis on upper gastrointestinal (GI) endoscopy are found in only 5 to 30% of patients with suspected LPR.5 Although ambulatory 24-hour pH monitoring was once considered to be the gold standard, recent studies demonstrate its poor sensitivity and specificity and limited ability to predict treatment response.1, 6 Similarly, initial studies examining impedance testing report a poor correlation and the authors caution against over-reliance on impedance data.7
In the face of unreliable diagnostic tests, patients with laryngeal complaints are commonly treated with an empiric trial of proton pump inhibitor (PPI) therapy. A meta-analysis of eight studies found less than a 40 to 50% improvement in symptoms with PPI therapy, and concluded that this represented a clinically insignificant reduction of symptoms when compared to placebo.8 With limited therapeutic options, anti-reflux surgery is at times considered and performed for patients with persistent laryngeal symptoms, although studies demonstrating a sustained post-surgical improvement in laryngeal symptoms are lacking.9 Not surprisingly, the expense of managing patients with suspected extra-esophageal reflux is burdensome, estimated to cost over five times that of patients with typical GERD.10 Thus, minimally invasive, cost effective and reliable diagnostic tools to accurately identify LPR are needed.
Two tools for evaluating LPR have been recently developed. The Restech Dx-pH (Respiratory Technology Corp, San Diego, CA) probe is a minimally invasive device for detection of posterior oropharyngeal acid. It uses a nasopharyngeal catheter to measure pH in liquid or aerosolized droplets. Normative data and a composite score have been developed for this device though its clinical application for detecting LPR is unclear. 11 Peptest™ is an office-based noninvasive technique to measure salivary pepsin concentration. Pepsin is secreted as pepsinogen from chief cells in the gastric fundus, activated in the acidic environment and may cause laryngeal damage in up to a pH of 6.0; consequently, pepsin has been implicated in the pathogenesis of LPR.12
The aims of this study were to examine the ability of 1) oropharyngeal pH testing with the Restech Dx-pH system and 2) salivary pepsin testing with Peptest™ to discriminate between asymptomatic volunteers and subjects with laryngeal symptoms with or without traditional reflux symptoms.
Methods
The Northwestern University institutional review board approved the study protocol.
We conducted a physician blinded prospective cohort pilot study at a single academic medical center. Adult subjects were recruited from January 2014 to February 2015. Subjects were excluded if pregnant, allergic to anesthetic spray used during laryngoscopy, unable to discontinue PPI for five days prior to initial testing, or due to the presence of significant comorbidities compromising the subject's health and safety. Informed consent was obtained from each participant. Based on responses to two validated symptom questionnaires, the Reflux Symptom Index (RSI) and GerdQ, subjects were separated into three cohorts: Control: RSI ≤ 13 and GerdQ < 8; Laryngeal: RSI > 13 and GerdQ < 8; and Laryngeal+reflux: RSI > 13 and GerdQ ≥ 8. The control population consisted of healthy volunteers, while subjects in the laryngeal or laryngeal+reflux cohorts were recruited from gastroenterology or otolaryngology clinics.
Subjects on PPI therapy were advised to hold their PPI for at least five days prior to the study. On day one subjects underwent laryngoscopy with subsequent oropharyngeal probe placement. The probe was removed 16 to 24 hours after being placed. Midway through the study, the option to collect sputum samples for pepsin analysis was made available, and thus a subgroup of patients also submitted two sputum samples for pepsin analysis, one on day of probe placement and one on the day of removal.
Laryngoscopy
First, patients were anesthetized using 1% tetracaine nasal spray, after which an office-based flexible fiber optic video laryngoscopy was performed by an otolaryngologist. The laryngoscopy studies were video recorded, de-identified and reviewed in a blinded fashion by an otolaryngologist who reported the laryngoscopic findings and RFS for each subject.
Oropharyngeal pH Testing
The Restech pH probe is a 1.5-mm diameter oropharyngeal catheter with a tear-drop tip and a colored light-emitting diode (LED) which aids with insertion and assurance that the sensor is properly positioned. The probe measures pH in both liquid and aerosolized droplets. The probes were calibrated in pH 7 and pH 4 buffer solutions and were placed transnasally until the flashing LED was seen 1 cm below the uvula, as recommended by the manufacturer. The subjects carried a wireless receiver and were advised to carry out their regular routine while the probe was in place. Subjects indicated their supine periods, oral intake of solids/liquids and symptoms (cough, throat clearing, heartburn) by pushing the respective buttons on the transponder and via hand-written paper diary. On the second day of the study, subjects returned to have the probe removed, and the data from the digital recorder were downloaded to a password-protected computer and analyzed with DataView software (AEMC Instruments, Foxborough, MA). The software generated a graphical tracing of all events. The respondent electronic button indications were adjusted according to paper diary recordings. Mealtimes with 5-minute pre- and post-prandial intervals were excluded. Both the study team and the manufacturer reviewed the data recordings.
Salivary Pepsin
Expectorated saliva samples were collected from subjects and placed into 15-mL sterile plastic tubes containing 0.5mL of 0.01 mol/L citric acid at pH 2.5. The samples were transferred to the refrigerator at 4°C. Pepsin was detected using the Peptest™ lateral flow device (LFD) (RD Biomed Ltd). Within seven days of collection, samples were vortexed for one minute and then centrifuged for 5 minutes at 4,000 rpm in a bench top centrifuge, and the supernatants were collected. Eighty μL of the supernatants layer was then mixed with 240 μL of migration buffer solution, and 80 μL of the mixture was added to the well of the LFD. Within a few minutes a line appeared under the control indicator on the LFD. If pepsin was present in the saliva sample, a second line appeared under the letter T (test) between 5 to 15 minutes after sample application. Photographs of the LFD results were taken at 15 minutes. This test has the ability to detect pepsin concentrations of 16 ng/mL or greater. Semi-quantitative assessment of pepsin in the samples was carried out with the scale 0, +1, +2, +3, +4 corresponding to approximately 0, 25, 100, 250, 500 ng/mL of pepsin, respectively. This semi-quantitative measurement was based on the intensity of the test sample line compared to the control line, as recommended by the manufacturer [Figure 1]. The research team assessed the samples and the manufacturer assessed the sample images.
Figure 1.
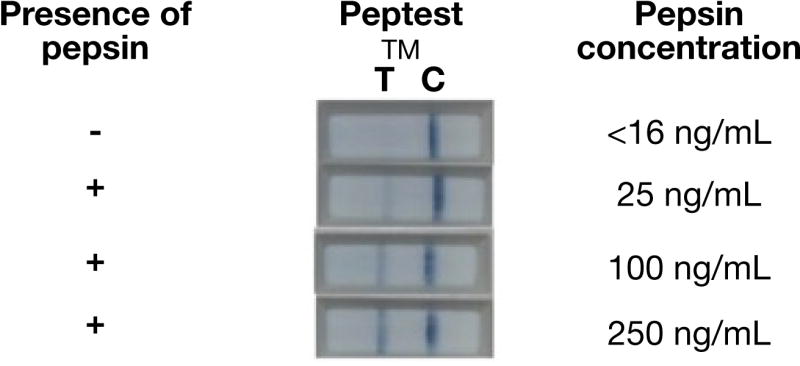
Semi-quantitative visual estimation of salivary pepsin concentration.
Outcomes
Oropharyngeal pH recordings
The primary outcomes for oropharyngeal pH tracings were 1) abnormal acid exposure defined by existing reported normative data as recommended by the manufacturer, with abnormal being > 0.02% for pH below 4.0, > 2.33% for pH below 5.0, > 16.6% for pH below 5.5, > 21.41% for pH below 6.0, and 2) abnormal composite score, referred to as the RYAN score, with an abnormal upright RYAN score defined as greater than 9.4 and abnormal supine RYAN score defined as greater than 6.89. 11, 13 The RYAN score was developed by Ayazi et al. in 2009 and consists of three components: number of reflux episodes, duration of longest reflux episode and percent time spent below the defined pH thresholds of 5.5 in the upright and 5.0 in the supine position.13 The secondary outcome was mean acid exposure below pH of 4.0, 5.0, 5.5, and 6.0 and the median pH.
Salivary pepsin
The primary outcome was abnormal salivary pepsin concentration, defined by the manufacturer as one or more samples with detectable pepsin (>16ng/mL).12 A secondary outcome was estimated mean pepsin concentration.
The physicians and manufacturers were blinded to cohort assignments when interpreting laryngoscopy, oropharyngeal pH testing and salivary pepsin results.
Statistical Analysis
One-way ANOVA and Kruskal-Wallis test were conducted as appropriate to analyze for variance between the three cohorts. Chi-squared tests were conducted to assess bivariate associations between cohorts. Wilcoxon Rank-Sum test was performed to assess for differences between controls and all subjects with laryngeal symptoms.
Results
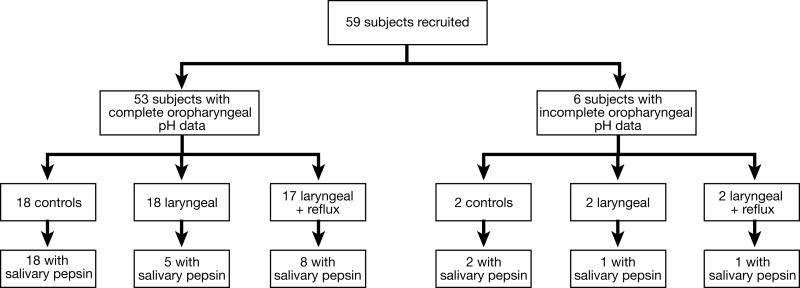
Fifty-nine subjects were recruited: 20 were healthy volunteers, 20 met criteria for the laryngeal cohort and 19 met criteria for the laryngeal+reflux cohort. Two subjects from each cohort, a total of six subjects, were excluded due to oropharyngeal pH data recording error. Thus, 53 subjects were included in the oropharyngeal pH analysis: 18 controls, 18 laryngeal, and 17 laryngeal+reflux. Saliva samples were collected from 35 total subjects: 20 controls, 6 laryngeal, and 9 laryngeal+reflux subjects. Seventeen laryngoscopies were not interpretable either due to poor quality of images or technical difficulties with the software. Figure 2 depicts the breakdown of included subjects.
Figure 2.
Breakdown of subjects.
Fifty-nine subjects were recruited: 20 controls, 20 laryngeal and 19 laryngeal+reflux. Six (2, 2, 2, respectively) were excluded from oropharyngeal pH testing results due to data recording error. Thirty-five subjects overall submitted saliva for pepsin analysis, 31 also had interpretable oropharyngeal pH data.
Baseline Characteristics
Baseline characteristics of the subjects are presented in Table 1. Seventy percent of subjects were female and the overall mean body mass index (BMI) was 24.5 ± 4.8 kg/m2. The overall mean age was 35.8 ± 11.9 years; the control group was significantly younger compared to the laryngeal and the laryngeal+reflux group (p<0.05); however, age did not correlate with mean acid exposure (Spearman's rho R=0.23, p=0.1). GerdQ and RSI scores differed significantly across the three cohorts as intended. Predominant laryngeal complaints amongst the laryngeal and laryngeal+reflux cohorts included throat clearing (60%), chronic cough (54%), post nasal drip (54%), globus (51%), dysphagia (37%), sore throat (31%), and hoarseness (40%). On laryngoscopy, the cohorts had significantly different RFS, with the lowest RFS in the control group.
Table 1. Baseline clinical variables for subjects by cohort.
| Clinical Variables | Control (n=18) | Laryngeal (n=18) | Laryngeal + GERD (n=17) | P-value |
|---|---|---|---|---|
| Female gender, n (%) | 10 (56%) | 13 (72%) | 14 (82%) | 0.21 |
| BMI (kg/m2 ), Mean ± SD | 23.3 ± 3.5 | 24.8 ± 3.5 | 25.5 ± 6.8 | 0.39 |
| Age (years), Mean ± SD | 27.8 ± 4.6 | 38.1 ± 13.8 | 42.3 ± 10.6 | < 0.05 |
| GerdQ Score, Mean ± SD | 5.6 ± 1.6 | 3.8 ± 2.4 | 11.0 ± 2.8 | < 0.001 |
| RSI Score, Mean ± SD | 3.1 ± 4.0 | 22.5 ± 7.2 | 28.0 ± 6.6 | < 0.001 |
| Reflux Finding Score*, Mean ± SD | 3.4 ± 0.9 | 7.2 ± 2.9 | 6.4 ± 3.0 | < 0.001 |
Reflux Finding Score available for 15 controls, 15 laryngeal and 12 laryngeal+reflux subjects
Oropharyngeal pH Results
Table 2 and supplemental table 1 depict the proportion of abnormal oropharyngeal pH studies and the mean acid exposure across various pH thresholds for each cohort. The median pH amongst all three cohorts did not differ (control: 6.51 laryngeal: 6.57, laryngeal+reflux: 6.58; p=0.99). Overall, there were no significant differences detected across the three cohorts using the RYAN score or percentage time spent below various pH thresholds. One-third of control subjects had an abnormal upright RYAN score and 16.7% of controls had an abnormal exposure time below pH of 5.5. A non-significant trend towards differences amongst the cohorts was seen for mean acid exposure, particularly for total acid exposure below pH 5.0, with the lowest acid exposure in the control group and the highest acid exposure in the laryngeal+reflux group.
Table 2. Oropharyngeal pH Results by Cohort.
| Control (18) | Laryngeal (18) | Laryngeal + Reflux (17) | P-value | |
|---|---|---|---|---|
| Abnormal % Time pH < 5.0, n (%) | 0 (0) | 2 (11.1) | 2 (11.8) | 0.3 |
| % Time pH < 5.0, Total, Mean (SD) | 0.1 (0.2) | 1.1 (2.7) | 1.5 (3.9) | 0.3 |
| % Time pH < 5.0, Upright, Mean (SD) | 0.1 (0.2) | 1.0 (2.7) | 0.4 (1.2) | 0.3 |
| % Time pH < 5.0, Supine, Mean (SD) | 0.3 (0.6) | 1.1 (2.8) | 4.0 (10.4) | 0.2 |
| Abnormal % Time pH < 5.5, n (%) | 3 (16.7) | 1 (5.6) | 3 (17.6) | 0.5 |
| % Time pH < 5.5, Total, Mean (SD) | 4.4 (8.9) | 5.5 (8.7) | 6.3 (8.6) | 0.8 |
| % Time pH < 5.5, Upright, Mean (SD) | 0.4 (0.6) | 1.5 (2.7) | 1.1 (2.0) | 0.2 |
| % Time pH < 5.5, Supine, Mean (SD) | 10.2 (22.3) | 10.8 (15.4) | 1.1 (2.0) | 0.9 |
| Abnormal Upright RYAN Score, n (%) | 6 (33.3) | 11 (61.1) | 8 (47.1) | 0.3 |
| Abnormal Supine RYAN Score, n (%) | 0 (0) | 4 (22.2) | 3 (17.6) | 0.1 |
| Abnormal Upright RYAN Score Adjusted, n (%) | 4 (22.2) | 10 (55.6) | 8 (47.1) | 0.1 |
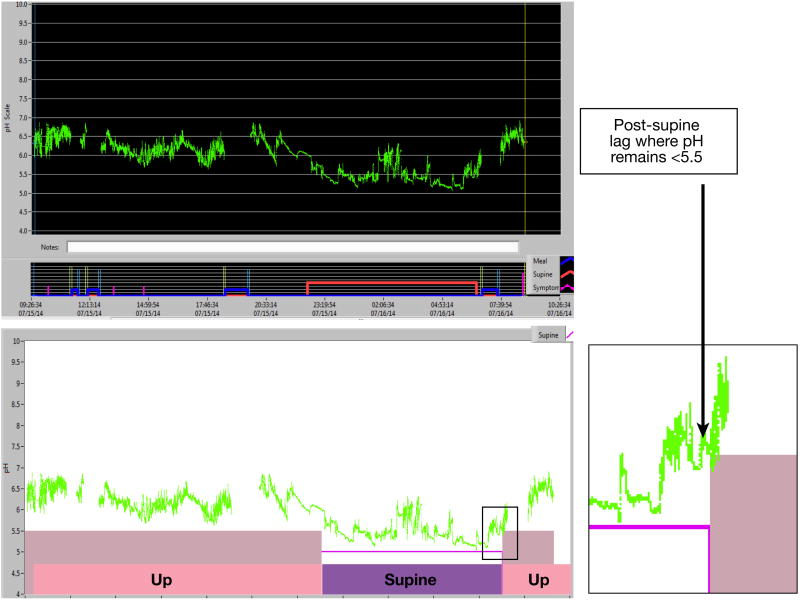
In further analysis, a 7 to 10 minute delay in pH equilibration when transitioning from supine to upright position was detected in 11 subjects (21%), resulting in an elevated upright RYAN score. During this delay the oropharyngeal pH remained below 5.5 despite a clear upward trend in pH when transitioning from the supine to upright position [Figure 3]. In three cases the upright RYAN score normalized when accounting for this post-supine lag period. Using this adjusted upright RYAN score resulted in a greater trend towards differences between the three cohorts; however, this analysis did not reach statistical significance and 22% of controls still had an abnormal upright RYAN score.
Figure 3.
(Top) Baseline oropharyngeal pH tracing with mealtimes excluded for an asymptomatic healthy volunteer. (Bottom) At baseline, the upright RYAN score is abnormal (11.58) due to a 9-minute post-supine lag period where the oropharyngeal pH remains below 5.5 in the upright position. When this 9-minute post-supine lag period is accounted for, the upright RYAN score normalizes to 2.12.
We observed poor symptom association with oropharyngeal pH drops below 5.5, with median symptom association in both laryngeal and laryngeal+reflux groups being 0% (range 0-50%).
Salivary Pepsin Results
Based on current normative thresholds, there were no significant differences denoted in the proportion of positive salivary pepsin concentration results among the three cohorts; results were positive in 53% controls, 40% of laryngeal subjects, and 75% of laryngeal+reflux subjects (p=0.5). Variation in the estimated mean pepsin concentration was found between cohorts, with a significantly higher pepsin concentration in the laryngeal+reflux group (117.9 ± 147.4) when compared to either control (32.4 ± 41.9) or laryngeal group (7.5 ± 11.2), p=0.01 and 0.04, respectively [Table 3; Supplementary Figure 1].
Table 3. Salivary Pepsin Results by Cohort.
| Control (18) | Laryngeal (6) | Laryngeal + Reflux (9) | P-value | |
|---|---|---|---|---|
| Abnormal Peptest™ Result | 9 (53%) | 2 (40%) | 6 (75%) | 0.5 |
| Mean Salivary Pepsin (ng/mL) | 32.4 ± 41.9 | 7.5 ± 11.2 | 117.9 ± 147.4 | 0.1 |
Correlation Between Studies
Overall twenty-two subjects successfully underwent all three tests: oropharyngeal pH testing, laryngoscopy and salivary sputum collection. There was no statistically significant correlation between oropharyngeal pH testing (using mean total acid exposure below pH 5.0) and mean pepsin concentration (Spearman's Rho R=0.13, p=0.49) or between mean acid exposure and RFS (R=0.21, p=0.18). There was however a moderately significant correlation between RSI and RFS (R=0.58, p<0.01).
No association between endoscopic findings or reflux testing was seen with oropharyngeal pH testing or salivary pepsin analysis. Eleven of 35 laryngeal and laryngeal+reflux subjects had an upper endoscopy; 6 (55%) had normal findings while only 3 (27%) demonstrated distal esophageal reactive changes. Three of these subjects, two with a normal endoscopic findings and one with reactive esophageal changes, had an abnormal oropharyngeal pH study. One subject underwent 96-hour wireless pH monitoring off of medication, which demonstrated abnormal acid exposure on days 1, 3, & 4 (DeMeester score 23.5, 32.5, 59.0, respectively); however, had a normal oropharyngeal pH study and undetectable levels of salivary pepsin.
Conclusions
Minimally invasive diagnostic tools to identify patients with LPR are needed; thus we examined the ability of two novel diagnostic modalities, oropharyngeal pH testing and salivary pepsin analysis, to reliably discriminate between asymptomatic and symptomatic patients. Based on current normative thresholds both oropharyngeal pH testing with the Restech Dx-pH system and salivary pepsin analysis with Peptest™ could not distinguish between healthy volunteers and symptomatic subjects. Trends towards differences in mean acid exposure between the cohorts did exist, and subjects with laryngeal and reflux symptoms exhibited the highest oropharyngeal acid exposure below pH of 5.0. Importantly, subjects with both laryngeal and reflux symptoms had significantly higher estimated pepsin concentrations compared to healthy volunteers or subjects with only laryngeal symptoms.
Oropharyngeal pH Testing
A multitude of factors may explain the inability to distinguish between control subjects and those with symptoms when utilizing the oropharyngeal pH test and its normative data. Three different studies have reported normative data for the oropharyngeal pH catheter. On closer examination it is recognized that each of these studies investigated normative thresholds in a unique subset of asymptomatic subjects. For instance, when Chheda, et al. examined 20 healthy volunteers, the authors admittedly used stringent exclusion cutoffs for recruitment (RSI of 10 or greater and RFS of 6 or greater).14 On the other hand, Sun, et al. did not use validated tools to assess for laryngeal symptoms and also excluded subjects if they had a known motility disorder.11 Similarly, Ayazi, et al. excluded subjects with motility disorders as well as those with hiatal hernia greater than 2 cm and DeMeester score > 14.7 on esophageal pH monitoring.13 The variation in the definition of “normal” in these studies is highlighted by the heterogeneity in the reported mean acid exposures for each study's asymptomatic population [Supplementary Table 2]. The lack of a standard definition of an “asymptomatic” patient for the existing normative data poses a problem in clinical practice, diminishing the generalizability and applicability of this device's ability to perform as a sole diagnostic test.
More than 5% of our subjects had false positive upright RYAN scores due to a delay in pH equilibration when changing positions from supine to upright. This artifact has not previously been reported and is not accounted for in the automated software analysis; our findings suggest that a 7 to 10 minute post-supine period should be accounted for and potentially eliminated in data analysis. Drops in oropharyngeal pH without a corresponding drop in esophageal pH in the supine positions have been demonstrated in previous studies, and the precise physiology for this decrease in pH is not understood.14 Even after accounting for this post-supine lag period, we found a high false positive rate of abnormal upright RYAN scores in our control population, again suggesting caution when applying existing normative data in clinical practice as discriminatory diagnostic cut-offs.
While the Restech Dx pH system is sensitive11, the clinical role of this diagnostic tool is currently undefined. In our study, based on its current thresholds it cannot serve as a stand-alone diagnostic tool to reliably identify symptomatic patients with LPR. Smaller single center studies have suggested that oropharyngeal pH testing may have a role in predicting response to anti-reflux therapy15, 16 and larger multicenter studies to evaluate the oropharyngeal pH probe's role in predicting treatment outcomes are needed.
Salivary Pepsin Analysis
While there were no appreciable differences in salivary pepsin positivity between cohorts, detectable differences in mean salivary pepsin concentration exists. This suggests that Peptest™ may be able to reliably distinguish between asymptomatic and symptomatic subjects. If similar variations are found in larger prospective studies, it is possible that this low cost, non-invasive and rapid office based test will find a role in the clinical diagnostic approach to this challenging group of patients. Nonetheless, the precise normative thresholds and relationship of salivary pepsin to time of day and oral intake need to be further examined and delineated. We quantified salivary pepsin based on visual semi-quantitative assessment, and future studies would benefit from use of a quantitative reader. Irrespective of this, there was 100% inter-rater reliability for determining pepsin concentration positivity and 85% inter-rater agreement for estimated pepsin quantification.
Interestingly, in our analyses, salivary pepsin concentrations did not correlate with mean acid exposure below pH 5.0, questioning the cause and effect theory between laryngeal symptoms and acidic reflux.
This study is limited by a small sample size; it is possible that with larger cohorts, differences in mean acid exposure and pepsin concentration will become increasingly apparent. This was designed as a pilot exploratory study, and thus sample size calculations were not performed prior to the study. Our intention was to recruit 20 subjects in four cohorts, and while we met our goal for the control, laryngeal, and laryngeal+reflux cohorts, we were unable to recruit an adequate cohort of subjects with reflux symptoms alone. Examining the performance of these diagnostic tools in subjects with reflux symptoms without the presence of laryngeal symptoms would provide an additional important perspective to this analysis. As is the case with pH monitoring, unreliable and inconsistent patient reporting complicates data interpretation. Using the RSI as a metric of separation is a limitation as its correlation to clinical outcomes and other diagnostic tools is unknown. Given that the RFS is even more limited in terms of poor interrater reliability and poor specificity, RFS was not used to distinguish groups. Despite these known limitations, we found a significant correlation between our subjects' RSI and RFS, providing subjective and objective evidence of laryngeal irritation in these groups. While comparison to 24 hour dual pH-metry may have provided further insight into the directionality of reflux and association between esophageal acid burden and oropharyngeal acid exposure, our study was not designed as such and rather aimed to simply explore the ability of these diagnostic tools to discriminate between healthy volunteers and those with laryngeal symptoms. The introduction of salivary pepsin analysis midway during the study is a limitation as all recruited subjects were not able to submit saliva, and thus the correlation between salivary pepsin analysis and oropharyngeal pH testing for each subject remains unclear. Additionally, we did not standardize the time of day and relationship to meals for saliva collection. The optimal procedure to collect salivary samples is not known and needs to be considered further; prior studies have demonstrated overall elevated levels of pepsin following meals.12
In conclusion, our findings suggest that based on current normative data, neither oropharyngeal pH testing nor salivary pepsin analysis can be used as stand-alone diagnostic tools in clinical practice. However, the increased levels of salivary pepsin in subjects with both laryngeal and reflux symptoms is of interest, and further work to explore this signal is needed. In addressing the aims of this study, our data raises several concerns and questions. For one, owing to the complexity and wide spectrum of LPR presentations, it may be that using a single device to diagnose patients with LPR is not feasible. The disagreement between test positivity between these two devices additionally questions the true cause and effect mechanism behind acidic gastroesophageal reflux and laryngeal symptoms and raises the question of whether it is the burden of refluxate rather than acidic burden which drives laryngeal complaints. Finally, while reliable minimally invasive diagnostic tests for LPR are undoubtedly needed, further work is required to first clinically define a gold standard for LPR.
Supplementary Material
Supplementary Figure 1. Mean pepsin concentration by cohort.
Supplementary Table 1. Oropharyngeal Results by Cohort
Supplementary Table 2. Mean oropharyngeal acid exposure for asymptomatic subjects reported across studies.
Footnotes
Disclosures/Conflicts of Interest: The authors do not have any disclosures or potential conflicts of interest, with the exception of: RY: Supported by T32 DK101363-02 grant; JEP: Consults for Covidien, Sandhill Scientific, and Given.
Author Contributions: Rena Yadlapati: Study oversight, study concept & design; acquisition of data; analysis and interpretation of data; drafting of manuscript; critical revision of the manuscript for important intellectual content
Christopher Adkins: Study concept & design; acquisition of data; analysis and interpretation of data; drafting of manuscript; critical revision of the manuscript for important intellectual content
Diana-Marie Jaiyeola: Study concept & design; acquisition of data; analysis and interpretation of data; drafting of manuscript; critical revision of the manuscript for important intellectual content
Andrew Gawron: Study concept & design; analysis and interpretation of data; drafting of manuscript; critical revision of the manuscript for important intellectual content
Bruce K. Tan: Study concept & design; acquisition of data; drafting of manuscript; critical revision of the manuscript for important intellectual content
Nadine Shabeeb: Study concept & design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Alcina K. Lidder: Study concept & design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Caroline PE Price: Study concept & design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Neelima Agrawal: Study concept & design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Michael Ellenbogen: Study concept & design; acquisition of data; critical revision of the manuscript for important intellectual content
Stephanie S. Smith: Acquisition of data; critical revision of the manuscript for important intellectual content
Michiel Bove: Acquisition of data; critical revision of the manuscript for important intellectual content
John E. Pandolfino: Study oversight, study concept & design; analysis and interpretation of data; drafting of manuscript; critical revision of the manuscript for important intellectual content;
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vaezi MF, Hicks DM, Abelson TI, et al. Laryngeal signs and symptoms and gastroesophageal reflux disease (GERD): a critical assessment of cause and effect association. Clin Gastroenterol Hepatol. 2003;1(5):333–44. doi: 10.1053/s1542-3565(03)00177-0. [DOI] [PubMed] [Google Scholar]
- 2.Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101(8):1900–20. doi: 10.1111/j.1572-0241.2006.00630.x. quiz 43. [DOI] [PubMed] [Google Scholar]
- 3.Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS) Laryngoscope. 2001;111(8):1313–7. doi: 10.1097/00005537-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Milstein CF, Charbel S, Hicks DM, et al. Prevalence of laryngeal irritation signs associated with reflux in asymptomatic volunteers: impact of endoscopic technique (rigid vs. flexible laryngoscope) Laryngoscope. 2005;115(12):2256–61. doi: 10.1097/01.mlg.0000184325.44968.b1. [DOI] [PubMed] [Google Scholar]
- 5.Qua CS, Wong CH, Gopala K, et al. Gastro-oesophageal reflux disease in chronic laryngitis: prevalence and response to acid-suppressive therapy. Aliment Pharmacol Ther. 2007;25(3):287–95. doi: 10.1111/j.1365-2036.2006.03185.x. [DOI] [PubMed] [Google Scholar]
- 6.Joniau S, Bradshaw A, Esterman A, et al. Reflux and laryngitis: a systematic review. Otolaryngol Head Neck Surg. 2007;136(5):686–92. doi: 10.1016/j.otohns.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Kavitt RT, Yuksel ES, Slaughter JC, et al. The role of impedance monitoring in patients with extraesophageal symptoms. Laryngoscope. 2013;123(10):2463–8. doi: 10.1002/lary.23734. [DOI] [PubMed] [Google Scholar]
- 8.Qadeer MA, Phillips CO, Lopez AR, et al. Proton pump inhibitor therapy for suspected GERD-related chronic laryngitis: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2006;101(11):2646–54. doi: 10.1111/j.1572-0241.2006.00844.x. [DOI] [PubMed] [Google Scholar]
- 9.So JB, Zeitels SM, Rattner DW. Outcomes of atypical symptoms attributed to gastroesophageal reflux treated by laparoscopic fundoplication. Surgery. 1998;124(1):28–32. [PubMed] [Google Scholar]
- 10.Francis DO, Rymer JA, Slaughter JC, et al. High economic burden of caring for patients with suspected extraesophageal reflux. Am J Gastroenterol. 2013;108(6):905–11. doi: 10.1038/ajg.2013.69. [DOI] [PubMed] [Google Scholar]
- 11.Sun G, Muddana S, Slaughter JC, et al. A new pH catheter for laryngopharyngeal reflux: Normal values. Laryngoscope. 2009;119(8):1639–43. doi: 10.1002/lary.20282. [DOI] [PubMed] [Google Scholar]
- 12.Saritas Yuksel E, Hong SK, Strugala V, et al. Rapid salivary pepsin test: blinded assessment of test performance in gastroesophageal reflux disease. Laryngoscope. 2012;122(6):1312–6. doi: 10.1002/lary.23252. [DOI] [PubMed] [Google Scholar]
- 13.Ayazi S, Lipham JC, Hagen JA, et al. A new technique for measurement of pharyngeal pH: normal values and discriminating pH threshold. J Gastrointest Surg. 2009;13(8):1422–9. doi: 10.1007/s11605-009-0915-6. [DOI] [PubMed] [Google Scholar]
- 14.Chheda NN, Seybt MW, Schade RR, et al. Normal values for pharyngeal pH monitoring. Ann Otol Rhinol Laryngol. 2009;118(3):166–71. doi: 10.1177/000348940911800302. [DOI] [PubMed] [Google Scholar]
- 15.Worrell SG, DeMeester SR, Greene CL, et al. Pharyngeal pH monitoring better predicts a successful outcome for extraesophageal reflux symptoms after antireflux surgery. Surg Endosc. 2013;27(11):4113–8. doi: 10.1007/s00464-013-3076-3. [DOI] [PubMed] [Google Scholar]
- 16.Friedman M, Maley A, Kelley K, et al. Impact of pH monitoring on laryngopharyngeal reflux treatment: improved compliance and symptom resolution. Otolaryngol Head Neck Surg. 2011;144(4):558–62. doi: 10.1177/0194599811399240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Mean pepsin concentration by cohort.
Supplementary Table 1. Oropharyngeal Results by Cohort
Supplementary Table 2. Mean oropharyngeal acid exposure for asymptomatic subjects reported across studies.