Abstract
Objective
To evaluate differences in meniscal T1ρ and T2 quantification in patients with acute anterior cruciate ligament (ACL) injuries and to determine correlations of these differences with MR morphological grading and patient-reported outcomes.
Design
Bilateral knees of 52 patients with acute ACL injury and 20 healthy controls were scanned using 3T MRI T1ρ and T2 mapping in this prospective study. Quantitative analysis of the meniscus was performed in anterior and posterior horns of the lateral and medial menisci. Morphological meniscal damage was assessed using modified whole-organ MRI scores (WORMS). Measurements were compared between injured, uninjured contralateral, and control knees using a mixed-effects regression model. Correlations between meniscal T1ρ/T2, WORMS and Knee Injury and Osteoarthritis Outcome Scores (KOOS) were examined using partial correlation analysis.
Results
Mean meniscal T1ρ and T2 values were significantly higher in ACL-injured knees compared to control and contralateral knees. Menisci of ACL-injured knees without tears, including those limited to modified meniscal WORMS grade 0, also had significantly higher T1ρ and T2 values compared to menisci of uninjured knees. Within ACL-injured knees, T1ρ and T2 values showed significant positive associations with meniscal WORMS and significant negative associations with KOOS.
Conclusion
Acute ACL injuries are associated with significantly increased meniscal T1ρ and T2 values in both patients with and without meniscal lesions or tears, suggesting quantitative MRI provides more sensitive measures of meniscal differences compared to traditional morphological MRI sequences. Correlation between meniscal T1ρ/T2 and KOOS suggest that quantitative MRI is reflective of the extent of patients’ clinical symptoms.
Keywords: Meniscus, Magnetic resonance imaging, T1ρ, T2, Anterior cruciate ligament, Osteoarthritis
Introduction
Acute anterior cruciate ligament (ACL) injury is a high-risk factor for the development of post-traumatic osteoarthritis1-3. Previous studies have shown that even with ACL reconstruction surgery, 50 to 70 percent of ACL-injured patients have radiological signs of osteoarthritis (OA) within 10-15 years post-injury4, 5. ACL injury often occurs along with damage to other internal structures of the knee, including the meniscus, articular cartilage, subchondral bone, and other ligaments6. Specifically, studies have found that the most frequent injury associated with an ACL tear is the lateral meniscus tear in the posterior horn6-9. These concomitant meniscal injuries are associated with increased incidence of OA and worse outcomes in ACL-injured patients10, 11.
The meniscal fibrocartilage structure is comprised primarily of type I collagen (98%), proteoglycans (<1%), and water (1%)12. As shown in previous studies, meniscal damage is linked to biochemical changes in the meniscus as defined by damage to the collagen-proteoglycan matrix, which is strongly associated with osteoarthritic cartilage loss13-15.
Quantitative magnetic resonance imaging (MRI) techniques have the ability to assess these differences in the changing biochemical composition of the meniscus13. Previous studies used MR T1ρ and T2 to evaluate the differences between the menisci of healthy controls and patients with mild or severe OA and found that meniscal MR quantification can be used to differentiate the three groups13, 16. Additionally, Bolbos et al. demonstrated the utility of T1ρ and T2 quantification in indicating significant biochemical changes in the meniscus of patients with early OA15.
Quantitative MRI thus provides the opportunity for early detection of compositional differences within a damaged meniscus. This non-invasive method is advantageous in its utility in detecting biochemical differences in the collagen-proteoglycan matrix prior to the prospective occurrence of morphological changes during tissue degeneration17, 18. Therefore, quantitative MRI is potentially more sensitive than standard MRI in identifying early signs of meniscus deterioration, which thereby allows for earlier evaluation of the risk of OA development13. Although cartilage matrix changes in ACL-injured knees have been studied previously6, 9, 15, 19-24, prior assessments of quantitative MR evaluation of meniscus after acute ACL injuries have not investigated T2 quantification and have also been limited to cohorts of less than 20 patients6, 9. Furthermore, no previous studies have looked specifically at menisci without lesions or tears to assess whether quantitative MRI can detect meniscus differences not reflected by morphological grading. A previous study of meniscal T1ρ and T2 in osteoarthritic patients revealed correlations between T1ρ and T2 measurements and morphological grading scores13, but as of yet, there has been no literature regarding such relationships in the context of acute ACL injuries. Additionally, to the best of our knowledge, there have been no studies evaluating the relationship between meniscal T1ρ and T2 quantification and patient-reported outcomes.
Therefore, the goals of our study were (i) to evaluate acute posttraumatic differences in meniscal T1ρ and T2 in patients with acute ACL injuries, including those limited to no meniscal tears or lesions, and (ii) to correlate these differences with MRI morphological grading and patient-reported outcomes in comparison with a healthy control cohort.
Materials and methods
Subjects
Two groups of subjects were recruited for this study: 20 controls – six females, age ranging between 19-40 years (average = 30.4 ± 5.4 years) and body mass index (BMI) of 24.4 ± 2.7 kg/m2; and 52 patients with acute ACL injuries – 22 females, age ranging between 15-50 years (average = 29.8 ± 8.5 years) and BMI = 24.2 ± 3.1 kg/m2. The controls were without clinical symptoms of osteoarthritis or other knee injuries and were recruited to match age and BMI of the ACL-injured patients. Only patients scanned within 6 months of ACL injury were included in this study. All subjects gave informed consent, and the study was approved by and carried out in accordance with the rules and regulations of the Committee for Human Research at our institution.
Questionnaires
On the day of MR scan, subjects completed the Knee Injury and Osteoarthritis Outcome Score (KOOS) and Marx activity scale surveys25, 26. The KOOS is a validated self-assessed questionnaire with five categories: pain, other symptoms, function in sport and recreation, function in daily living (ADL), and knee-related quality of life (QOL). The KOOS scoring scale ranges from 0-100, with 0 being the worst and 100 being the best. The Marx activity scale is a validated self-administered questionnaire that surveys subjects regarding their level of physical activity, specifically inquiring about the frequency of various physical actions (running, changing directions while running, decelerating, and pivoting) during the subject's healthiest and most active state in the past year. The Marx scoring system was defined as follows: 0 = less than one time in a month, 1 = one time in a month, 2 = one time in a week, 3 = two or three times in a week, and 4 = four or more times in a week.
Magnetic Resonance Imaging Protocol
MR images of all subject knees were acquired using a 3 Tesla GE MR Scanner (General Electric, Milwaukee, WI, USA) with an 8-channel phased array knee coil (Invivo, Gainesville, FL, USA). Injured patients were scanned within 6 months of injury (average of 1.8 months with a standard deviation of 1.2 months) and prior to surgical ACL reconstruction. In injured patients, the injured knee was scanned prior to the uninjured contralateral knee. Knees had been unloaded for 20-30 minutes prior to scanning by T1ρ and T2 sequences.
Imaging protocol included sagittal intermediate-weighted, fluid sensitive, fat-saturated three-dimensional (3D) fast spin-echo (CUBE) images [repetition time (TR)/echo time (TE) = 1500/25 ms, field of view (FOV) = 16 cm, matrix = 384 × 384, slice thickness = 1 mm, echo train length = 50, bandwidth (BW) = 50 kHz, number of excitations (NEX) = 0.5]. The CUBE images were used for both meniscus segmentation and clinical assessment of the morphological abnormalities related to the ACL injury.
The sagittal multi-slice T1ρ- and T2-weighted sequences were obtained using a previously developed 3D sequence based on combined T1ρ and T2 acquisition techniques23. The acquisition parameters were: TR/TE = 9 ms/min full, FOV = 14 cm, matrix = 256 × 128, slice thickness = 4 mm, views per segment (VPS) = 64, time of recovery = 1.2 s, spin-lock frequency = 500 Hz, ARC phase AF = 2, time of spin lock (TSL) = 0/10/40/80 ms for T1ρ, and preparation TE = 0/13.7/27.3/54.7 ms for T2.
MR Imaging Analysis
Morphological Analysis
Semi-quantitative gradings of the meniscus were performed using the 3D fast spin-echo (CUBE) images by two experienced radiologists with 11 (MK) and 8 (LN) years of experience. The radiologists were blinded to subject information and meniscal T1ρ and T2 values. Meniscal abnormalities were evaluated using the meniscus grading scale from a modified whole-organ magnetic resonance imaging scoring (WORMS) method27. The anterior and posterior horns of the lateral and medial menisci were graded from 0 to 4 with each score defined as follows: 0 = no lesion, 1 = intrasubstance degeneration, 2 = non-displaced tear, 3 = displaced or complex tear without deformity, and 4 = complete maceration of the meniscus. Meniscal scores 0 and 1 were combined into one category to represent subjects without a meniscal tear, whereas meniscal scores 2 through 4 were combined into another category to represent subjects with a meniscal tear.
Quantitative Assessment
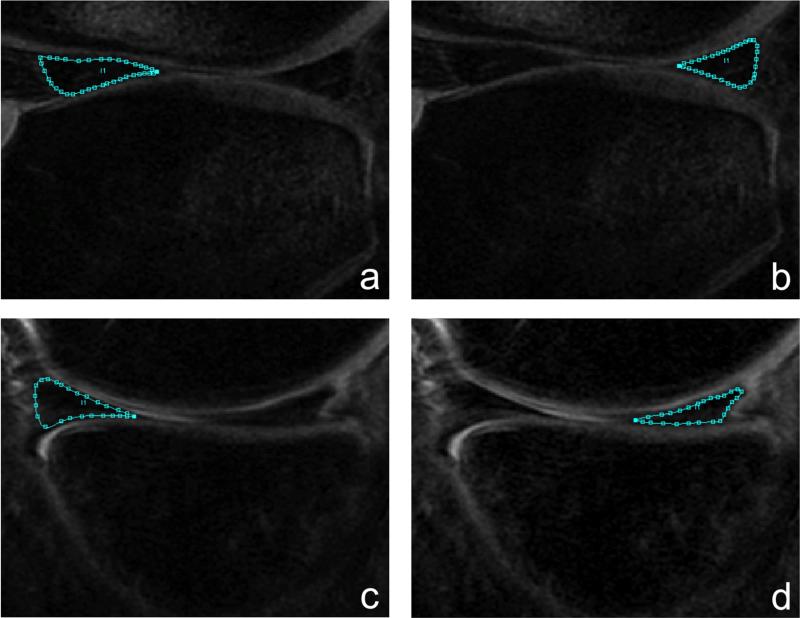
CUBE images were rigidly registered and downsampled in the slice thickness direction to match the first TSL image of the T1ρ-weighted sequence. Registration was performed using the VTK CISG Registration Toolkit28. Menisci were segmented semi-automatically on the registered CUBE images into four subcompartments: anterior horn of the lateral/medial meniscus (AHLAT/AHMED) and posterior horn of the lateral/medial meniscus (PHLAT/PHMED) (Figure 1). Each subcompartment was segmented on three consecutive slices. Segmentations were completed using an in-house developed program with MATLAB (Mathworks, Natick, MA, USA) based on edge detection and Bezier splines, which demonstrated excellent scan/rescan reproducibility of meniscal T1ρ measurements (coefficient of variation < 5%) in Bolbos et al6, 29.
Figure 1.
Segmentation of the four meniscal subcompartments: (a) lateral anterior horn (AHLAT), (b) lateral posterior horn (PHLAT), (c) medial anterior horn (AHMED), and (d) medial posterior horn (PHMED).
These regions of interest (ROI) were transferred onto T1ρ and T2 maps, and mean T1ρ and T2 values were calculated for each ROI. T1ρ and T2 maps were reconstructed by fitting T1ρ-weighted and T2-weighted images pixel-by-pixel to the following respective equations below using an in-house developed Levenberg-Marquardt mono-exponential fitting algorithm:
Prior to fitting, VTK CISG registration was applied on the second and third TSL/TE images to align them onto the first image. The T1ρ and T2 sequences were originally optimized to look at cartilage, in which average T1ρ relaxation time is 35-40 ms, and average T2 relaxation time is 25-30 ms6, 13, 22. In meniscus, average T1ρ relaxation time is 16-20 ms, and average T2 relaxation time is 11-13 ms6, 13. Therefore, only the first three echo images (TSL = 0, 10, 40 ms; TE = 0, 13.7, 27.3 ms) were used for calculated meniscal T1ρ and T2 values, because the last image had a very low signal-to-noise ratio in the meniscus.
Statistical Analysis
Mean and standard deviation of T1ρ and T2 values were calculated from the three segmented slices of each meniscus subcompartment in all control, injured, and contralateral knees. These values were initially adjusted for age, gender, BMI, level of physical activity, and time to injury for each subject, using a mixed-effects restricted maximum likelihood (REML) regression, which accounts for any correlations in the outcome data (T1ρ and T2 values). Since time to injury was not significantly associated with meniscal T1ρ and T2 values, we removed it from the final model. The regression analysis was generated separately by subcompartment, and pairwise comparisons were made within T1ρ, T2, and WORMS data sets. Pairwise comparisons included ACL-injured vs. control, ACL-injured vs. contralateral, ACL-injured without meniscal tears vs. control, ACL-injured without meniscal tears vs. contralateral, ACL-injured with modified WORMS = 0 vs. control with modified WORMS = 0, and ACL-injured with modified WORMS = 0 vs. contralateral with modified WORMS = 0. To reduce the effect of small number bias, all anterior subcompartments with meniscal tears (control: n = 2; contralateral: n = 0, ACL-injured: n = 1) and posterior subcompartments of control and contralateral knees with meniscal tears (control: n = 1; contralateral: n = 8) were not included. Pearson partial correlation coefficients were calculated between meniscal T1ρ/T2 values and WORMS/KOOS, after adjustment for the variables mentioned above. To avoid false positives, the Bonferroni correction was applied as a multiple comparison adjustment for the four subcompartments, which may not be independent of one another within the same subject. Dividing the standard 0.05 by the four subcompartments (0.05/4=0.0125), an alpha of less than 0.0125 was considered significant.
Results
Clinical Findings
Table 1 illustrates the distribution of modified meniscal WORMS grades by subject group and subcompartment. Among 52 ACL-injured knees, 29 (56%) had at least one lateral meniscal lesion (modified meniscal WORMS ≥ 1), 25 (48%) had at least one medial meniscal lesion, 17 (32%) had both lateral and medial meniscal lesions, and 37 (71%) had at least one meniscal lesion of either type. Twenty (38%) ACL-injured knees had at least one lateral meniscal tear (modified meniscal WORMS ≥ 2), 20 (38%) had at least one medial meniscal tear, 10 (19%) had both lateral and medial meniscal tears, and 30 (58%) had at least one meniscal tear of either type. The most common types of meniscal lesions and tears in ACL-injured knees included contusion (morphous intrameniscal signal abutting an articular surface but without a linear component to suggest a tear, n = 13), horizontal tear (horizontally oriented line of increased intrameniscal signal that extends to the superior or inferior surface of the meniscus near the free edge, n = 13), and complex tear (extends in more than one plane creating separate flaps of meniscus and extensive distortion, n = 13)30. Of the 52 contralateral knees, there were 12 (23%) with lesions and 6 (12%) with tears, whereas of the 40 control knees, there were 7 (18%) with lesions and 2 (5%) with tears. Both modified WORMS grades and frequency of lesions and tears were significantly higher in ACL-injured knees compared to control and contralateral knees (p < 0.0001) (Figure 2).
Table 1.
Modified Meniscal WORMS by Group and Subcompartment
| ACL-Injured Knees (n=52) | |||||
|---|---|---|---|---|---|
| Grade = 0 | Grade = 1 | Grade = 2 | Grade = 3 | Grade = 4 | |
| AHLAT | 48 | 3 | 1 | 0 | 0 |
| PHLAT | 25 | 8 | 15 | 4 | 0 |
| AHMED | 52 | 0 | 0 | 0 | 0 |
| PHMED | 27 | 5 | 13 | 6 | 1 |
| Contralateral Knees (n=52) | |||||
|---|---|---|---|---|---|
| Grade = 0 | Grade = 1 | Grade = 2 | Grade = 3 | Grade = 4 | |
| AHLAT | 52 | 0 | 0 | 0 | 0 |
| PHLAT | 45 | 5 | 2 | 0 | 0 |
| AHMED | 51 | 1 | 0 | 0 | 0 |
| PHMED | 43 | 3 | 4 | 2 | 0 |
| Control Knees (n=40) | |||||
|---|---|---|---|---|---|
| Grade = 0 | Grade = 1 | Grade = 2 | Grade = 3 | Grade = 4 | |
| AHLAT | 38 | 0 | 2 | 0 | 0 |
| PHLAT | 37 | 2 | 1 | 0 | 0 |
| AHMED | 40 | 0 | 0 | 0 | 0 |
| PHMED | 35 | 5 | 0 | 0 | 0 |
Legend:
AHLAT = anterior horn of lateral meniscus; PHLAT = posterior horn of lateral meniscus; AHMED = anterior horn of medial meniscus; PHMED = posterior horn of medial meniscus
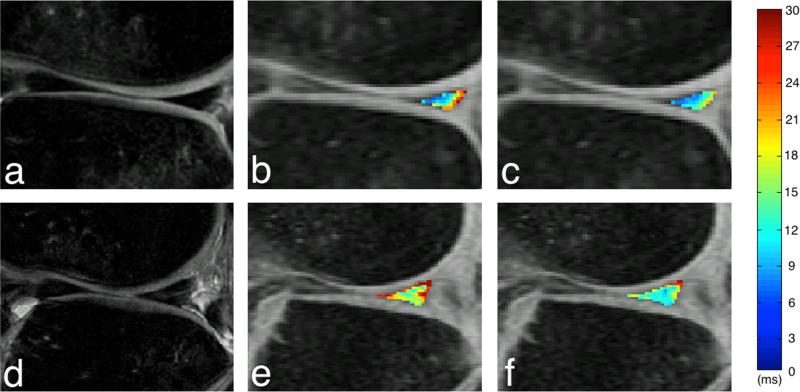
Figure 2.
MR images showing the posterior horn of the lateral meniscus in an ACL-injured patient with modified meniscal WORMS grade of 0 (A, B, C) and an ACL-injured patient with modified meniscal WORMS grade of 1 (D, E, F). The CUBE (A and D), T1ρ (B and E), and T2 (C and F) images illustrate the discrepancies between subjects with modified meniscal WORMS grades of 0 and 1. The color bar indicates the relaxation measure gradient.
Survey Results
Table 2 summarizes the KOOS and Marx survey data reported by both the control and ACL-injured groups. Compared to control subjects, ACL-injured patients had significantly lower KOOS scores in all five categories. In response to the Marx questionnaire, ACL-injured patients reported significantly higher levels of physical activity involving cutting, decelerating, and pivoting compared to the control group, whereas differences in running were not significant (p = 0.075).
Table 2.
KOOS and MARX Survey Results
| KOOS Questionnaire | |||
|---|---|---|---|
| Control (n = 18*) | ACL-Injured (n = 50*) | t-test | |
| Symptoms | 97.62 ± 3.46 | 69.14 ± 19.33 | < 0.0001 |
| Pain | 99.69 ± 0.90 | 75.50 ± 17.17 | < 0.0001 |
| ADL | 100 ± 0 | 83.09 ± 17.19 | < 0.0001 |
| Sports | 99.44 ± 2.36 | 55.00 ± 27.72 | < 0.0001 |
| QOL | 96.18 ± 8.34 | 44.13 ± 24.37 | < 0.0001 |
| Marx Activity Scale | |||
|---|---|---|---|
| Control (n = 18*) | ACL-Injured (n = 50*) | t-test | |
| Running | 2.56 ± 1.50 | 3.10 ± 0.91 | 0.075 |
| Cutting | 1.39 ± 1.50 | 2.72 ± 1.18 | 0.00030 |
| Decelerating | 1.56 ± 1.50 | 2.82 ± 1.16 | 0.00049 |
| Pivoting | 0.67 ± 0.97 | 2.74 ± 1.26 | < 0.0001 |
Legend:
KOOS = Knee Injury and Osteoarthritis Outcome Score; ADL = function in daily living; QOL = quality of life
*Note: Survey data was available for only 18 of 20 control subjects and 50 of 52 ACL-injured patients.
There was a significant negative correlation between age and KOOS symptoms (p = 0.011). No relationships were found between KOOS and gender, BMI, or time to injury (Table 3).
Table 3.
Correlation between KOOS and Patient Background
| SYMPTOMS | PAIN | ADL | SPORTS | QOL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p-value | r | p-value | r | p-value | r | p-value | r | p-value | |
| Gender | −0.002 | 0.99 | −0.17 | 0.24 | 0.026 | 0.86 | −0.035 | 0.81 | −0.10 | 0.50 |
| Age | −0.36 | 0.011 | −0.13 | 0.37 | −0.18 | 0.21 | −0.083 | 0.57 | −0.33 | 0.019 |
| BMI | −0.19 | 0.18 | −0.15 | 0.29 | −0.19 | 0.20 | 0.013 | 0.93 | −0.14 | 0.34 |
| Time to Injury | −0.007 | 0.96 | 0.052 | 0.72 | 0.12 | 0.40 | −0.023 | 0.88 | −0.025 | 0.87 |
Legend: KOOS = Knee Injury and Osteoarthritis Outcome Score; ADL = function in daily living; QOL = quality of life, r = Pearson's correlation coefficient
MR T1ρ and T2 Values
Table 4 summarizes the adjusted mean T1ρ and T2 values for each group and subcompartment.
Table 4.
Adjusted T1ρ and T2 Values
| Knee Group | Subgroup | Subcompartment | Sample Size | T1ρ (ms) | T2 (ms) |
|---|---|---|---|---|---|
| ACL-Injured | All | AHLAT | n = 52 | 19.2 ± 0.4 | 13.2 ± 0.3 |
| PHLAT | n = 52 | 19.0 ± 0.5 | 13.4 ± 0.4 | ||
| AHMED | n = 52 | 17.7 ± 0.3 | 12.4 ± 0.2 | ||
| PHMED | n = 52 | 18.3 ± 0.5 | 12.9 ± 0.3 | ||
| Without meniscal tears | AHLAT | n = 51 | 19.0 ± 0.4 | 13.1 ± 0.3 | |
| PHLAT | n = 33 | 18.6 ± 0.4 | 13.0 ± 0.3 | ||
| AHMED | n = 52 | 17.7 ± 0.3 | 12.4 ± 0.2 | ||
| PHMED | n = 32 | 16.8 ± 0.4 | 12.1 ± 0.3 | ||
| WORMS = 0 | AHLAT | n = 48 | 19.0 ± 0.4 | 13.0 ± 0.3 | |
| PHLAT | n = 25 | 18.6 ± 0.4 | 13.0 ± 0.3 | ||
| AHMED | n = 52 | 17.7 ± 0.3 | 12.3 ± 0.2 | ||
| PHMED | n = 27 | 16.7 ± 0.4 | 11.9 ± 0.2 | ||
| Contralateral | Without meniscal tears | AHLAT | n = 52 | 18.7 ± 0.4 | 12.3 ± 0.3 |
| PHLAT | n = 50 | 16.9 ± 0.3 | 12.3 ± 0.3 | ||
| AHMED | n = 52 | 17.7 ± 0.3 | 12.0 ± 0.2 | ||
| PHMED | n = 46 | 17.4 ± 0.4 | 11.6 ± 0.2 | ||
| WORMS = 0 | AHLAT | n = 52 | 18.7 ± 0.4 | 12.3 ± 0.3 | |
| PHLAT | n = 45 | 16.7 ± 0.3 | 12.2 ± 0.3 | ||
| AHMED | n = 51 | 17.7 ± 0.3 | 12.0 ± 0.2 | ||
| PHMED | n = 43 | 17.4 ± 0.3 | 11.4 ± 0.2 | ||
| Control | Without meniscal tears | AHLAT | n = 38 | 19.3 ± 0.6 | 12.4 ± 0.4 |
| PHLAT | n = 39 | 17.0 ± 0.4 | 11.5 ± 0.3 | ||
| AHMED | n = 40 | 19.4 ± 0.5 | 12.0 ± 0.3 | ||
| PHMED | n = 40 | 18.0 ± 0.5 | 11.4 ± 0.3 | ||
| WORMS = 0 | AHLAT | n = 38 | 19.4 ± 0.5 | 12.4 ± 0.4 | |
| PHLAT | n = 37 | 17.0 ± 0.4 | 11.5 ± 0.3 | ||
| AHMED | n = 40 | 19.4 ± 0.5 | 12.0 ± 0.3 | ||
| PHMED | n = 35 | 17.9 ± 0.4 | 11.3 ± 0.2 |
Legend:
AHLAT = anterior horn of lateral meniscus; PHLAT = posterior horn of lateral meniscus; AHMED = anterior horn of medial meniscus; PHMED = posterior horn of medial meniscus; WORMS = whole-organ magnetic resonance imaging score
Table 5 provides a summary of the T1ρ and T2 pairwise comparison results generated by the regression analysis. Mean T1ρ and T2 values were significantly higher in ACL-injured knees compared to both control and contralateral knees in the PHLAT (p < 0.0005) and PHMED (p < 0.0005) (Table 5).
Table 5.
p-Values of T1ρ and T2 Comparisons
| ACL-Injured vs. Uninjured Knees | ||||
|---|---|---|---|---|
| T1ρ | T2 | |||
| Injured vs. control | Injured vs. contralateral | Injured vs. control | Injured vs. contralateral | |
| AHLAT | 0.035 | 0.016 | 0.048 | 0.044 |
| PHLAT | < 0.0005 | < 0.0005 | < 0.0005 | < 0.0005 |
| AHMED | 0.006* | 0.92 | 0.23 | 0.058 |
| PHMED | < 0.0005 | < 0.0005 | < 0.0005 | < 0.0005 |
| ACL-Injured Knees without Meniscal Tears vs. Uninjured Knees | ||||
|---|---|---|---|---|
| T1ρ | T2 | |||
| Injured (no tear) vs. control | Injured (no tear) vs. contralateral | Injured (no tear) vs. control | Injured (no tear) vs. contralateral | |
| AHLAT | 0.73 | 0.33 | 0.13 | 0.019 |
| PHLAT | 0.006 | 0.002 | 0.001 | 0.053 |
| AHMED | 0.006* | 0.92 | 0.23 | 0.058 |
| PHMED | 0.064 | 0.26 | 0.057 | 0.070 |
| ACL-Injured Knees with WORMS = 0 vs. Uninjured Knees with WORMS = 0 | ||||
|---|---|---|---|---|
| T1ρ | T2 | |||
| [WORMS = 0] Injured vs. control | [WORMS = 0] Injured vs. contralateral | [WORMS = 0] Injured vs. control | [WORMS = 0] Injured vs. contralateral | |
| AHLAT | 0.59 | 0.31 | 0.19 | 0.028 |
| PHLAT | 0.006 | 0.001 | 0.002 | 0.056 |
| AHMED | 0.001* | 0.85 | 0.38 | 0.038 |
| PHMED | 0.026 | 0.15 | 0.048 | 0.030 |
Legend:
AHLAT = anterior horn of lateral meniscus; PHLAT = posterior horn of lateral meniscus; AHMED = anterior horn of medial meniscus; PHMED = posterior horn of medial meniscus; WORMS = whole-organ magnetic resonance imaging score
Bold =ACL-injured values significantly higher than control/contralateral values.
*ACL-injured values significantly lower than control values.
ACL-injured knees without meniscal tears (modified meniscal WORMS = 0 or 1) had significantly higher T1ρ values in the PHLAT compared to those of control knees (p = 0.006) and contralateral knees (p = 0.002) (Table 5). Mean T2 values were significantly higher in ACL-injured knees without meniscal tears compared to control knees in the PHLAT (p = 0.001).
Among ACL-injured, control, and contralateral knees with modified meniscal WORMS = 0, ACL-injured knees had significantly higher T1ρ values in the PHLAT compared to control knees (p = 0.006) and contralateral knees (p = 0.001) (Table 5). Mean T2 values were also significantly higher in ACL-injured knees with modified meniscal WORMS = 0 compared to control knees in the PHLAT (p = 0.002).
Compared to control knees, mean T1ρ values in the AHMED were significantly lower in ACL-injured knees without meniscal tears (p = 0.006) and ACL-injured knees with modified meniscal WORMS = 0 (p = 0.001) (Table 5).
There were significant positive correlations between modified meniscal WORMS and mean T1ρ values of ACL-injured knees in the PHLAT (p < 0.0001) and PHMED (p < 0.0001) as well as between modified meniscal WORMS and mean T2 values of ACL-injured knees in the PHLAT (p = 0.0079) and PHMED (p < 0.0001) (Table 6). No significant relationships with modified meniscal WORMS were observed in the AHLAT or AHMED.
Table 6.
Correlations of T1ρ and T2 with Modified WORMS and KOOS
| T1ρ | T2 | ||||
|---|---|---|---|---|---|
| Pearson's r | p-value | Pearson's r | p-value | ||
| Modified WORMS | AHLAT | 0.182 | 0.041 | 0.147 | 0.10 |
| PHLAT | 0.352 | < 0.0001 | 0.235 | 0.0079 | |
| AHMED | −0.012 | 0.89 | 0.112 | 0.21 | |
| PHMED | 0.459 | < 0.0001 | 0.558 | < 0.0001 | |
| KOOS Categories | |||||
| Symptoms | AHLAT | −0.225 | 0.011 | −0.342 | < 0.0001 |
| PHLAT | −0.088 | 0.33 | −0.222 | 0.012 | |
| Pain | AHLAT | −0.139 | 0.12 | −0.236 | 0.0079 |
| PHLAT | −0.124 | 0.17 | −0.218 | 0.014 | |
| ADL | AHLAT | −0.249 | 0.0049 | −0.319 | 0.0003 |
| PHLAT | −0.115 | 0.20 | −0.183 | 0.040 | |
| Sports | AHLAT | −0.219 | 0.014 | −0.300 | 0.0006 |
| PHLAT | −0.127 | 0.16 | −0.189 | 0.034 | |
| QOL | AHLAT | −0.226 | 0.011 | −0.229 | 0.0099 |
| PHLAT | −0.242 | 0.0063 | −0.205 | 0.021 | |
Legend:
AHLAT = anterior horn of lateral meniscus; PHLAT = posterior horn of lateral meniscus; PHMED = posterior horn of medial meniscus; WORMS = Whole-Organ Resonance Magnetic Imaging Score; ADL = function in daily living; QOL = quality of life
Significant negative associations were found between MR quantification values and KOOS in the AHLAT between T1ρ and symptoms (p = 0.011), ADL (p = 0.0049), and QOL (p = 0.011) and between T2 and all five categories (symptoms: p < 0.0001; pain: p = 0.0079; ADL: p = 0.0003; sports: p = 0.0006; QOL: p = 0.0099) (Table 6). A significant negative association was also found in the PHLAT between T1ρ and QOL (p = 0.0063). However, no significant relationships with KOOS were evident in the AHMED or PHMED.
Discussion
In this cross-sectional study, quantitative MRI was used to investigate the effects of acute ACL injury on T1ρ and T2 measurements in the meniscus and their relationship with morphological grading methods and patient-reported outcomes. To our best knowledge, this is the first study to document not only an overall trend of higher meniscal T1ρ and T2 values in ACL-injured knees, but also, more notably, the significant elevation in meniscal T1ρ and T2 values in ACL-injured knees without meniscal tears and with modified meniscal WORMS grade of 0. Correlation data demonstrate positive association between quantification values and modified WORMS grading and negative association between quantification values and KOOS scores.
Clinical Findings
In regards to meniscal tears in the anterior and posterior horns, our observation of a 58% incidence among ACL-injured knees, 12% incidence among contralateral knees, and 5% incidence among control knees is comparable to previous reports of meniscal tears found in ACL-reconstructed, ACL-deficient, as well as osteoarthritic knees13, 31, 32. The slightly lower incidence of meniscal tears within our control group compared to other studies is likely due to our focus on a younger subject population (average age of control group = 30 vs. 39 years), as incidence of meniscal tears increases with age33, 34. The marginally higher incidence of meniscal tears among contralateral knees compared to control knees can possibly be attributed to the ACL-injured group's greater levels of physical activity as defined by the Marx activity scale, since evidence has demonstrated the significance of sporting activities involving knee torsion in acute meniscal tears35.
Consistent with previous findings, we observed a higher occurrence of meniscal abnormalities in the posterior horn of both the lateral and medial menisci compared to the anterior horn13, 33, 34, 36. Additionally, as supported by previous studies, the number of meniscal lesions and tears associated with acute ACL injury was higher in the PHLAT compared to the PHMED37, 38. This is in contrast with OA and chronic ACL-injured patients, who experience a higher frequency of lesions and tears in the medial meniscus13, 36. It is likely that this discrepancy is dependent on time since injury; whereas the lateral compartment suffers the most direct damage at the time of acute ACL injury7, the medial compartment experiences the highest weight-bearing pressure and thus becomes increasingly susceptible to gradual damage with the progression of time13. Given that this study is based on knee condition at 1.8 ± 1.2 months after injury and prior to ACL reconstruction, it is probable that degeneration in the medial meniscus had not yet manifested to a degree comparable to that of OA knees13. This question requires further investigation in our ongoing longitudinal study.
T1ρ and T2 Quantification
The results of this study demonstrate that acute ACL-injured knees display increased meniscal T1ρ and T2 values compared to uninjured knees, which is in alignment with previous findings on meniscal T1ρ and T2 quantification6, 9, 13. It should be noted that increases in T2 values were more consistent throughout all four subcompartments, also in agreement with previous reports16, 39, suggesting that T2 quantification is potentially more effective at detecting biochemical differences in the meniscus. Histological studies have shown that the meniscal degeneration process constitutes deterioration of the collagen network and decline in proteoglycan content40, 41. Given that T1ρ is more sensitive to proteoglycan whereas T2 is more sensitive to collagen, such a decline in proteoglycan content, in conjunction with the naturally low concentration of proteoglycan in the meniscus (< 1%) relative to that in hyaline cartilage (3-10%), can potentially explain why T2 is more reliable than T1ρ in detecting differences in the meniscal matrix12, 15, 42. Interestingly, a previous study using delayed gadolinium-enhanced MRI of the meniscus (dGEMRIM) showed a trend towards lower T1GD, indicative of proteoglycan loss, in degenerated menisci43. However, since T1ρ quantification nevertheless demonstrates considerable and comparable levels of significance, this trend must be further evaluated in our longitudinal studies and confirmed by large-scale studies.
We observed a particularly significant association between meniscal damage and elevated T1ρ and T2 measurements in the PHLAT, which further suggests that acute ACL injuries affect the PHLAT of the meniscus to a greater extent than the other subcompartments. This quantitative data is consistent with our qualitative observation that the PHLAT has the highest frequency of meniscal lesions and tears, and is also supported by previous studies presenting the significance of acute ACL injury on the PHLAT in particular7, 44. Conversely, we observed significantly lower T1ρ measurements in the AHMED of ACL-injured knees in comparison to control knees. Based on modified meniscal WORMS grading, no lesions were found in this subcompartment among all ACL-injured knees, suggesting that the AHMED sustains minimal injury during ACL rupture, which could explain our observation. A larger cohort is necessary to evaluate this trend.
Interestingly, significantly elevated T1ρ and T2 values were found in the PHLAT of ACL-injured knees without meniscal tears, and, furthermore, in the PHLAT of ACL-injured knees with modified meniscal WORMS grade 0, indicating that quantitative MR imaging is more sensitive than morphological imaging in detecting compositional damage in the meniscus. A recent study also witnessed elevated MR quantification values in clinically intact meniscus of ACL-injured patients, albeit using T2* measurements8. This particular longitudinal study found that elevations in meniscal T2* measurements observed prior to ACL reconstruction surgery returned to lower values similar to those found in uninjured controls, suggesting that healing had occurred8. Temporarily elevated T1ρ and T2 values can be explained in the context of posttraumatic meniscal contusions rather than degeneration, with possible causes including edemas and micro-ruptures of the collagen network45. Since contusions are often reversible, the predictive value of T1ρ and T2 measurements for the onset of a structural lesion remains unclear45. Whether such trends exist in meniscal T1ρ and T2 values must be investigated in the next steps of our longitudinal study. However, regardless of whether the increased T1ρ and T2 values we observed are short- or long-term, its implications regarding MR quantification's ability to detect biochemical differences within the meniscus earlier than currently used morphological grading methods serve as one of the most significant and novel findings in our study.
Relationships to Modified WORMS and KOOS
To our knowledge, this is the first study to evaluate the correlation between WORMS grading and meniscal T1ρ and T2 measurements in ACL-injured patients. Consistent with previous studies involving patients who had already developed OA, we report a significant positive correlation between WORMS grading and meniscal T1ρ and T2 values in posterior horns of the lateral and medial menisci13, 16. Significant associations were not observed in anterior horns, likely due to their low prevalence of morphological abnormalities as reflected by modified meniscal WORMS grades of 0 in 138 of 144 AHLAT and 143 of 144 AHMED subcompartments reviewed.
Using unadjusted data, KOOS was tested for correlations with gender, age, BMI, and time to injury. The only association found was between age and KOOS symptoms, characterized by decreasing KOOS scores at increasing ages. This finding is consistent with previous studies demonstrating negative associations between KOOS and age46, 47. Contrary to our findings, a large-scale clinical study (n = 10164) with a comparable age profile (27.0 ± 9.8 years) observed worse KOOS scores in women both before and after ACL reconstruction48. It is possible that this discrepancy between genders is not observed in our study due to relatively small sample size (n = 52).
In the context of acute ACL injuries, there has been little to no investigation of the correlation between KOOS and meniscal T1ρ and T2 measurements11, 49. Our finding of a negative association between both meniscal T1ρ and T2 values and KOOS suggests a weak but notable relationship between meniscal damage and patient outcomes after acute injuries. According to previous longitudinal studies, concomitant meniscal damage during ACL-injury is a predictor of lower KOOS scores two to six years after ACL reconstruction11, 49. This finding signifies that MR quantification is not only capable of detecting biochemical differences within the meniscus but also reflective of the extent of physical symptoms experienced by patients.
Limitations
Due to this being a cross-sectional study, one of the limitations is that we were unable to distinguish between meniscal contusions and meniscal degeneration, making it unclear whether elevated T1ρ and T2 values were due to contusions or early degeneration. We are currently following up on longitudinal data that may help determine whether contusions or degeneration had a greater role in the elevated baseline T1ρ and T2 values. Another limitation is the relatively small sample size; longitudinal studies with a larger cohort are necessary to confirm these findings.
Conclusion
Quantitative MR imaging can be valuable in ongoing evaluation of meniscal condition and possible early detection of meniscal degeneration. Most significantly, because T1ρ and especially T2 measurements are more sensitive to compositional differences within the meniscus, this data may be used to diagnose and track the early stages of meniscal change and potential degeneration prior to the possibility of diagnosis by a morphological grading system.
Acknowledgements
The authors would like to thank Samuel Wu and Michael Hoppe for their help with data collection and processing. This study was supported by NIH/NIAMS P50 AR060752, and statistical analysis was supported by CTSA grant # UL1 RR024131.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Conception and design: Xiaojuan Li, C Benjamin Ma
- Collection and assembly of data: Amy Wang, Favian Su, Elijah Abramson
- Analysis and interpretation of the data: Xiaojuan Li, Amy Wang, Valentina Pedoia, Favian Su, Elijah Abramson
- Drafting of the article: Amy Wang, Xiaojuan Li, Valentina Pedoia, Favian Su, Martin Kretzschmar
- Critical revision of the article for important intellectual content: All authors
- Final approval of the article: All authors
- Provision of study materials or patients: Xiaojuan Li, C Benjamin Ma
- Statistical Expertise: Charles M. McCulloch, Chengshi Jin
- Obtaining of funding: Xiaojuan Li, C Benjamin Ma
Amy Wang (ca.amywang@gmail.com) declares responsibility for the integrity of the work.
Competing Interests
The authors have no competing interests to disclose.
References
- 1.Friel NA, Chu CR. The role of ACL injury in the development of posttraumatic knee osteoarthritis. Clin Sports Med. 2013;32:1–12. doi: 10.1016/j.csm.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–69. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 3.Wong JM, Khan T, Jayadev CS, Khan W, Johnstone D. Anterior cruciate ligament rupture and osteoarthritis progression. Open Orthop J. 2012;6:295–300. doi: 10.2174/1874325001206010295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145–52. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 5.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63:269–73. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolbos RI, Link TM, Ma CB, Majumdar S, Li X. T1rho relaxation time of the meniscus and its relationship with T1rho of adjacent cartilage in knees with acute ACL injuries at 3 T. Osteoarthritis Cartilage. 2009;17:12–8. doi: 10.1016/j.joca.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikolic DK. Lateral meniscal tears and their evolution in acute injuries of the anterior cruciate ligament of the knee. Arthroscopic analysis. Knee Surg Sports Traumatol Arthrosc. 1998;6:26–30. doi: 10.1007/s001670050068. [DOI] [PubMed] [Google Scholar]
- 8.Chu CR, Williams AA, West RV, Qian Y, Fu FH, Do BH, et al. Quantitative Magnetic Resonance Imaging UTE-T2* Mapping of Cartilage and Meniscus Healing After Anatomic Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2014;42:1847–1856. doi: 10.1177/0363546514532227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Chang G, Bencardino J, Babb JS, Rokito A, Jazrawi L, et al. T1rho MRI at 3T of menisci in patients with acute anterior cruciate ligament (ACL) injury. J Magn Reson Imaging. 2015;41:544–549. doi: 10.1002/jmri.24594. [DOI] [PubMed] [Google Scholar]
- 10.Oiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009;37:1434–43. doi: 10.1177/0363546509338827. [DOI] [PubMed] [Google Scholar]
- 11.Cox CL, Huston LJ, Dunn WR, Reinke EK, Nwosu SK, Parker RD, et al. Are articular cartilage lesions and meniscus tears predictive of IKDC, KOOS, and Marx activity level outcomes after anterior cruciate ligament reconstruction? A 6-year multicenter cohort study. Am J Sports Med. 2014;42:1058–67. doi: 10.1177/0363546514525910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y, Mauerhan DR, Kneisl JS, Norton HJ, Zinchenko N, Ingram J, et al. Histological examination of collagen and proteoglycan changes in osteoarthritic menisci. Open Rheumatol J. 2012;6:24–32. doi: 10.2174/1874312901206010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarins ZA, Bolbos RI, Pialat JB, Link TM, Li X, Souza RB, et al. Cartilage and meniscus assessment using T1rho and T2 measurements in healthy subjects and patients with osteoarthritis. Osteoarthritis Cartilage. 2010;18:1408–16. doi: 10.1016/j.joca.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54:795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 15.Bolbos RI, Ma CB, Link TM, Majumdar S, Li X. In vivo T1rho quantitative assessment of knee cartilage after anterior cruciate ligament injury using 3 Tesla magnetic resonance imaging. Invest Radiol. 2008;43:782–788. doi: 10.1097/RLI.0b013e318184a451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauscher I, Stahl R, Cheng J, Li X, Huber MB, Luke A, et al. Meniscal measurements of T1rho and T2 at MR imaging in healthy subjects and patients with osteoarthritis. Radiology. 2008;249:591–600. doi: 10.1148/radiol.2492071870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedersen DR, Klocke NF, Thedens DR, Martin JA, Williams GN, Amendola A. Integrating carthage-specific T1rho MRI into knee clinic diagnostic imaging. Iowa Orthop J. 2011;31:99–109. [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, et al. Quantitative MRI using T1rho and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging. 2011;29:324–34. doi: 10.1016/j.mri.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lozano J, Li X, Link TM, Safran M, Majumdar S, Ma CB. Detection of posttraumatic cartilage injury using quantitative T1rho magnetic resonance imaging. A report of two cases with arthroscopic findings. J Bone Joint Surg Am. 2006;88:1349–1352. doi: 10.2106/JBJS.E.01051. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Ma CB, Bolbos RI, Stahl R, Lozano J, Zuo J, et al. Quantitative assessment of bone marrow edema-like lesion and overlying cartilage in knees with osteoarthritis and anterior cruciate ligament tear using MR imaging and spectroscopic imaging at 3 Tesla. J Magn Reson Imaging. 2008;28:453–461. doi: 10.1002/jmri.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theologis AA, Kuo D, Cheng J, Bolbos RI, Carballido-Gamio J, Ma CB, et al. Evaluation of bone bruises and associated cartilage in ACL injured and reconstructed knees using quantitative T1ρ MRI: One-year cohort study. Arthroscopy. 2011;27:65–76. doi: 10.1016/j.arthro.2010.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Kuo D, Theologis A, Carballido-Gamio J, Stehling C, Link TM, et al. Cartilage in Anterior Cruciate Ligament–Reconstructed Knees: MR Imaging T1ρ and T2—Initial Experience with 1-year Follow-up. Radiology. 2011;258:505–514. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Wyatt C, Rivoire J, Han E, Chen W, Schooler J, et al. Simultaneous acquisition of T1ρ and T2 quantification in knee cartilage – reproducibility and diurnal variation. J Magn Reson Imaging. 2014;39:1287–1293. doi: 10.1002/jmri.24253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su F, Hilton JF, Nardo L, Wu S, Liang F, Link TM, et al. Cartilage Morphology and T1ρ and T2 Quantification in ACL-reconstructed Knees: A 2-year Follow-up. Osteoarthritis Cartilage. 2013;21:1058–1067. doi: 10.1016/j.joca.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)--development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28:88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 26.Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29:213–8. doi: 10.1177/03635465010290021601. [DOI] [PubMed] [Google Scholar]
- 27.Laberge MA, Baum T, Virayavanich W, Nardo L, Nevitt MC, Lynch J, et al. Obesity increases the prevalence and severity of focal knee abnormalities diagnosed using 3T MRI in middle-aged subjects—data from the Osteoarthritis Initiative. Skeletal Radiol. 2012;41:633–641. doi: 10.1007/s00256-011-1259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–21. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- 29.Carballido-Gamio J, Bauer J, Lee KY, Krause S, Majumdar S. Combined image processing techniques for characterization of MRI cartilage of the knee. Conf Proc IEEE Eng Med Biol Soc. 2005;3:3043–6. doi: 10.1109/IEMBS.2005.1617116. [DOI] [PubMed] [Google Scholar]
- 30.Smet AAD. How I diagnose meniscal tears on knee MRI. AJR Am J Roentgenol. 2012;199:481–499. doi: 10.2214/AJR.12.8663. [DOI] [PubMed] [Google Scholar]
- 31.Thompson WO, Fu FH. The meniscus in the cruciate-deficient knee. Clin Sports Med. 1993;12:771–96. [PubMed] [Google Scholar]
- 32.Lee JJ, Choi YJ, Shin KY, Choi CH. Medial meniscal tears in anterior cruciate ligament-deficient knees: effects of posterior tibial slope on medial meniscal tear. Knee Surg Relat Res. 2011;23:227–30. doi: 10.5792/ksrr.2011.23.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kornick J, Trefelner E, McCarthy S, Lange R, Lynch K, Jokl P. Meniscal abnormalities in the asymptomatic population at MR imaging. Radiology. 1990;177:463–5. doi: 10.1148/radiology.177.2.2217786. [DOI] [PubMed] [Google Scholar]
- 34.Fukuta S, Masaki K, Korai F. Prevalence of abnormal findings in magnetic resonance images of asymptomatic knees. J Orthop Sci. 2002;7:287–91. doi: 10.1007/s007760200049. [DOI] [PubMed] [Google Scholar]
- 35.Baker P, Coggon D, Reading I, Barrett D, McLaren M, Cooper C. Sports injury, occupational physical activity, joint laxity, and meniscal damage. J Rheumatol. 2002;29:557–63. [PubMed] [Google Scholar]
- 36.Smith JP, 3rd, Barrett GR. Medial and lateral meniscal tear patterns in anterior cruciate ligament-deficient knees. A prospective analysis of 575 tears. Am J Sports Med. 2001;29:415–9. doi: 10.1177/03635465010290040501. [DOI] [PubMed] [Google Scholar]
- 37.Brindle T, Nyland J, Johnson DL. The meniscus: review of basic principles with application to surgery and rehabilitation. J Athl Train. 2001;36:160–169. [PMC free article] [PubMed] [Google Scholar]
- 38.Cipolla M, Scala A, Gianni E, Puddu G. Different patterns of meniscal tears in acute anterior cruciate ligament (ACL) ruptures and in chronic ACL-deficient knees. Classification, staging and timing of treatment. Knee Surg Sports Traumatol Arthrosc. 1995;3:130–134. doi: 10.1007/BF01565470. [DOI] [PubMed] [Google Scholar]
- 39.Fox MG. MR imaging of the meniscus: review, current trends, and clinical implications. Magn Reson Imaging Clin N Am. 2007;15:103–23. doi: 10.1016/j.mric.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Reicher MA, Hartzman S, Duckwiler GR, Bassett LW, Anderson LJ, Gold RH. Meniscal injuries: detection using MR imaging. Radiology. 1986;159:753–7. doi: 10.1148/radiology.159.3.3754645. [DOI] [PubMed] [Google Scholar]
- 41.Hajek PC, Gylys-Morin VM, Baker LL, Sartoris DJ, Haghighi P, Resnick D. The high signal intensity meniscus of the knee. Magnetic resonance evaluation and in vivo correlation. Invest Radiol. 1987;22:883–90. doi: 10.1097/00004424-198711000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Choi JA, Gold G. MR Imaging of Articular Cartilage Physiology. Magn Reson Imaging Clin N Am. 2011;19:249–282. doi: 10.1016/j.mric.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Tiel J KG, Reijman M, Bos PK, Bron EE, Klein S, Verhaar JA, Krestin GP, Weinans H, Oei EH. Delayed gadolinium-enhanced MRI of the meniscus (dGEMRIM) in patients with knee osteoarthritis: relation with meniscal degeneration on conventional MRI, reproducibility, and correlation with dGEMRIC. Eur Radiol. 2014;24:2261–2270. doi: 10.1007/s00330-014-3204-z. [DOI] [PubMed] [Google Scholar]
- 44.Li G, Zhang S, Wang X. [Biomechanical effect of anterior cruciate ligament rupture on posterior horn of lateral meniscus]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010;24:14–6. [PubMed] [Google Scholar]
- 45.Cothran RL, Major NM, Helms CA, Higgins LD. MR imaging of meniscal contusion in the knee. AJR Am J Roentgenol. 2001;177:1189–1192. doi: 10.2214/ajr.177.5.1771189. [DOI] [PubMed] [Google Scholar]
- 46.Paradowski PT, Bergman S, Sunden-Lundius A, Lohmander LS, Roos EM. Knee complaints vary with age and gender in the adult population. Population-based reference data for the Knee injury and Osteoarthritis Outcome Score (KOOS). BMC Musculoskelet Disord. 2006;7:38. doi: 10.1186/1471-2474-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dunn WR, Spindler KP, Amendola A, Andrish JT, Brophy RH, Flanigan DC, et al. Which preoperative factors, including bone bruise, are associated with knee pain/symptoms at index anterior cruciate ligament reconstruction (ACLR)? A Multicenter Orthopaedic Outcomes Network (MOON) ACLR Cohort Study. Am J Sports Med. 2010;38:1778–1787. doi: 10.1177/0363546510370279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ageberg E, Forssblad M, Herbertsson P, Roos EM. Sex differences in patient-reported outcomes after anterior cruciate ligament reconstruction: data from the Swedish knee ligament register. Am J Sports Med. 2010;38:1334–42. doi: 10.1177/0363546510361218. [DOI] [PubMed] [Google Scholar]
- 49.Rotterud JH, Sivertsen EA, Forssblad M, Engebretsen L, Aroen A. Effect of meniscal and focal cartilage lesions on patient-reported outcome after anterior cruciate ligament reconstruction: a nationwide cohort study from Norway and Sweden of 8476 patients with 2-year follow-up. Am J Sports Med. 2013;41:535–43. doi: 10.1177/0363546512473571. [DOI] [PubMed] [Google Scholar]