Abstract
Background
Elevated parathyroid hormone (PTH) levels have been associated with cardiovascular disease risk factors and events. We hypothesized that elevated PTH would also be associated with subclinical cerebrovascular disease. We examined the relationship of elevated PTH with white matter hyperintensities (WMH) and subclinical infarcts measured on brain MRI.
Methods
PTH was measured at baseline (1993–1994) among participants free of prior clinical stroke who underwent a brain MRI at baseline (n=1703) and a second brain MRI 10 years later (n=948). PTH levels ≥65 pg/ml were considered elevated (n=204). Participants who did not return for a follow-up MRI had, at baseline, higher PTH and a greater prevalence of cardiovascular risk factors (p<0.05 for all); therefore multiple imputation was used. The cross-sectional and prospective associations of PTH levels with WMH and MRI-defined infarcts (and their progression) were investigated using multivariable regression models.
Results
At baseline, participants were mean age of 62 years, 60% female and 49% black. Cross-sectionally, after adjusting for demographic and lifestyle factors, elevated PTH was associated with higher WMH score [β=0.19, 95%CI 0.04–0.35] and increased odds of prevalent infarcts [OR 1.56,1.02–2.36]. Results were attenuated after adjustment for potential mediators of this association (i.e. hypertension). No prospective associations were found between PTH and incident infarcts or change in estimated WMH volume, although estimates were imprecise.
Conclusions
Although associated cross-sectionally, we did not confirm any association of elevated PTH with progression of cerebrovascular changes on brain MRIs obtained 10 years apart. The relationship of PTH with subclinical brain disease warrants further study.
Keywords: Parathyroid hormone, cerebrovascular disease, Brain MRI
Introduction
Elevated parathyroid hormone (PTH) levels not only play a role in mineral metabolism pathology, but elevated levels also may increase the risk for brain injury and cardiovascular disease (CVD) outcomes. Higher serum PTH concentrations have been associated with subclinical vascular disease,1 impaired endothelial function, and increased aortic pulse pressure.2 Elevated PTH levels have also been associated with increased risk of incident hypertension,3 cardiovascular mortality,4 sudden cardiac death,5 heart failure,6,7 and all-cause mortality.8 A 2013 meta-analysis found elevated PTH conferred a 45% excess risk for CVD events,9 although a prior analysis from the Atherosclerosis Risk in Communities (ARIC) Study did not find PTH to be an independent risk factor for incident CVD.10 When the ARIC data were added to the previous meta-analysis, the association of PTH with incident CVD was weakened but remained significant.10
White matter hyperintensities (WMH) and subclinical infarcts are commonly seen on brain magnetic resonance imaging (MRI) studies of older adults.11 Because of their association with cardiac disease, prior stroke, and CVD risk factors, WMH are believed to be at least partially preventable through identification and treatment of modifiable risk factors. WMH, even in the absence of obvious neurologic deficits, are associated with reduced performance on cognitive testing,12,13 with more cognitive impairment noted in persons with more progression of WMH over time.14
We previously examined the association of 25-hydroxyvitamin D [25(OH)D] with WMH prevalence, WMH progression, and incident infarcts in the ARIC Brain MRI Ancillary Study.15 This study found no association of vitamin D with subclinical cerebrovascular disease, and results were unaltered after further adjustment for PTH. However, the primary association of PTH with brain changes has not been studied in our study nor in others. Furthermore, the risk that PTH may confer on CVD and cerebrovascular disease may be mediated through pathways independent of 25(OH)D.
Elevated PTH has been associated with cognitive decline,16 but only one prior study [the Prospective Investigation Of The Vasculature In Uppsala Seniors (PIVUS)] evaluated PTH with white matter disease in the brain.17 In the PIVUS study, the association of PTH with WMH remained significant even after adjustment for 25(OH)D. However, the PIVUS study was small (n=406), studied an older population (all age 70) that was only of white race, and had a brain MRI measurement at only one point in time, which warrants further exploration using the biethnic ARIC study. To our knowledge, no prior study has evaluated the prospective association of PTH with white matter changes in the brain on serial MRI studies.
The objective of our study was to determine the cross-sectional associations of PTH levels with WMH and subclinical infarcts and their progression. We hypothesized that elevated levels of PTH would be associated with increased subclinical cerebrovascular disease independent of demographic and socioeconomic factors, vascular risk factors including blood pressure and diabetes, and biomarkers of mineral metabolism such as calcium, phosphate, and 25(OH)D.
Methods
Participants
The ARIC study is an on-going prospective population-based cohort of 15,792 middle-aged, predominantly black and white, participants who were recruited from 4 U.S. communities between 1987–1989 (visit 1).18 The ARIC Brain MRI ancillary study is a subset of ARIC study participants age ≥55 years from the Forsyth County, NC and Jackson, MS sites who were invited for a cerebral MRI and cognitive testing during ARIC visit 3 in 1993–1994. Inclusion and exclusion criteria for the Brain MRI ancillary study have previously been published.13 A subsequent ancillary study newly measured PTH, 25(OH)D, calcium, and phosphate levels from visit 3 stored blood from the ARIC Brain MRI Study participants.15
Of the 1756 ARIC participants with both measured samples and interpretable brain MRIs at visit 3, we excluded individuals who suffered a stroke before visit 3 (n=3), who were not black or white (n=33), who were missing serum PTH values (n=8), who had an outlier PTH of either <10 pg/ml (n=1) or ≥200 pg/ml (n=4), or who were missing brain MRI results at ARIC visit 3 (n=4). A total of 1,703 participants were included for the cross-sectional analyses at ARIC visit 3. Of these, 948 participants returned for a second brain MRI ~10 years later.
The institutional review boards at all ARIC study sites and the ARIC Coordinating Center approved study protocols, and all participants provided written informed consent. Participants received modest financial compensation.
Laboratory Assays
Blood samples were collected during each full cohort visit under standardized conditions. Serum or plasma were separated at 4°C and promptly stored at −70°C. In 2012–2013, PTH, 25(OH)D, calcium, and phosphate were measured from stored serum obtained at ARIC study visit 3 (1993–1994). Serum PTH levels were measured using Elecsys 2010 (Roche Diagnostics, Indianapolis, Indiana). The interassay coefficient of variation (CV) was 7% for PTH. Calcium and phosphate were measured using a Roche Modular P-Chemistry Analyzer (Roche Diagnostics, Indianapolis, Indiana) using a colorimetric method. The laboratory inter-assay CVs for calcium and phosphate were 2.3% and 2.2%, respectively.
25(OH)D levels were measured using liquid chromatography-tandem mass spectrometry (Waters Alliance e2795, Milford, Massachusetts). The interassasy CVs for 25(OH)D2 were 6.2% and 5.3% at concentrations of 7.9 and 12.9 ng/mL, respectively. The interassay CVs for 25(OH)D3 were both 4.8% at concentrations of 30.1 and 55.8 ng/mL. Total 25(OH)D concentration was determined by summing 25(OH)D2 and 25(OH)D3. To account for seasonal variation, 25(OH)D levels were adjusted by month of lab draw separately by race as previously described.15 Vitamin D levels <20 ng/ml were considered deficient.
Brain MRI
The MRI scanning protocol for the ARIC Brain MRI ancillary study has been previously published.13 At ARIC visit 3, WMH severity was qualitatively scored from barely detectable white matter disease (Grade 1) to extensive confluent disease (Grade 8). The absence of WMH was scored as Grade 0, and those with WMH worse than Grade 8 were scored as Grade 9. We classify Grades 3 and greater as “high”. The rating scale (Grade 0–9) was previously developed and validated in the Cardiovascular Health Study.11
At the second ARIC Brain visit in 2004–2006, in addition to qualitatively scoring WMH into grades 0–9, volumes were measured quantitatively by a semiautomated procedure. Using a quadratic fit, these WMH volumes were found to correlate well with the qualitative scores (R2 =0.80). Since quantitative WMH were not available for visit 3 scans, WMH volumes were imputed for visit 3 (using the qualitative scores from visit 3, and the quadratic formula between the qualitative and quantitative volumes from the second MRI), as previously described.19 The 10-year WMH volume change is the difference in these quantitative volumes (i.e. the second Brain MRI WMH volume minus the first Brain MRI WMH volume).
At both ARIC brain visits, brain MRIs were also evaluated for subclinical infarcts by size and location. Lesions smaller than 3 mm were not reliably detected, therefore only lesions of 3 mm or more were considered.20 Incident infarcts were defined as those seen on the second brain MRI among individuals with no infarcts on their first brain MRI.
Covariates
Age, sex, race, education level, alcohol use, smoking status, history of stroke, and medication use was assessed with standard ARIC questionnaires. Physical examination at ARIC visit 3 included blood pressure (BP) and body mass index (BMI) per standard ARIC protocol.18 Prevalence of hypertension (blood pressure ≥140/90 mm Hg and/or anti-hypertensive medication use) and diabetes (self-reported physician diagnosis, medication use, fasting serum glucose ≥126 mg/dl, or nonfasting glucose ≥200 mg/dl) were determined at visit 3. The Baecke Physical Activity questionnaire qualitatively assessed leisure time intentional exercise through sports-related physical activity at visit 3, scored on a scale of 1 to 5 based on frequency and intensity.21 Estimated glomerular filtration rate (eGFR) was defined using the Chronic Kidney Disease Epidemiology Collaboration equation at visit 2 (1990–1992).22
Statistical Analyses
Of the 1703 participants available for cross-sectional analyses at ARIC visit 3, 755 did not return for a second brain MRI 10 years later. Participants with higher PTH levels were more likely to withdraw or die before the second brain MRI, potentially biasing the estimated relationship between PTH levels and cerebrovascular disease. For prospective analyses, we used multiple imputation by chained equations (MICE)23 to impute the brain MRI outcomes for the 755 missing participants based on their visit 3 clinical characteristics and visit 3 MRI findings. Covariates included age, sex, race, education, physical activity, diabetes, systolic and diastolic blood pressures, log-transformed PTH, cigarette smoking status, body mass index, self-reported poor health (assessed at visit 1), vitamin D deficiency status, eGFR, brain volume, death status at Brain MRI visit, HDL-C, triglycerides, WMH score, and prevalent infarcts at visit 3. Missing covariate patterns were explored and imputation models refined.
For each analysis, PTH levels were analyzed both as a continuous variable per 1 unit log(PTH) and also dichotomized at a clinical cutoff point for elevated PTH (≥65 pg/mL, n=204).
The cross-sectional association of PTH levels with WMH score (0–9) at visit 3 and the prospective association of PTH levels with WMH volume change were examined using multivariable-adjusted linear regression. The cross-sectional association of PTH levels with prevalent subclinical cerebral infarcts (≥3 mm) and the prospective association of PTH levels with incident infarcts (excluding those with prevalent infarcts at visit 3) were determined using multivariable-adjusted logistic regression.
We used progressively adjusted models for all analyses as follows: Model 1 (demographic variables) adjusted for age (years; continuous), sex (male; female), and race (whites; blacks); Model 2 (Model 1 + behavioral and socioeconomic variables) additionally adjusted for education (<High School; High School or Vocational School; College, Graduate, or Professional School), body mass index [BMI] (kg/m2; continuous), smoking status (never; former; current), alcohol drinking (non-current; current), and physical activity (based on the Baecke Physical Activity questionnaire; continuous); Model 3 (Model 2 + potential CVD risk mediators) further adjusted for systolic and diastolic blood pressure (mmHg; continuous), use of hypertension medication (yes; no), diabetes (yes; no), HDL cholesterol (mg/dl; continuous), total cholesterol (mg/dl; continuous), cholesterol lowering medications (yes; no) and estimated glomerular filtration rate (<60, 60–89, ≥90 ml/min/1.73 m2 at visit 2). Finally, Model 4: (Model 3 + mineral metabolites) further adjusted for 25(OH)D (mg/dl; continuous), calcium (mg/dl; continuous), and phosphorus (mg/dl; continuous).
To allow for a non-linear relationship between PTH levels with outcomes and for a more detailed dose-response analyses, we modeled PTH using restricted cubic splines with knots at the 5th, 35th, 65th, and 95th percentiles of their sample distributions using Model 2.
The significance of interaction by race was determined using Wald tests. Analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, North Carolina) and STATA/IC 12.1 (Stata Corp., College Station, Texas). All P values are two-sided and significance was set at p<0.05.
Results
ARIC visit 3 (the date of the first brain MRI and the PTH lab assays) was the baseline for this analysis. At visit 3, mean age of participants was 62 years, 60% were female, and 49% were black. The mean (SD) PTH level was 45.2 (18.8) pg/mL and 204 individuals (12%) had an elevated PTH level (≥65 pg/mL). Mean 25(OH)D level was 21.5 (SD 8.2) ng/ml with 47.1% of participants having vitamin D deficiency (<20 ng/ml).
Compared with participants with PTH <65 pg/ml, those with elevated PTH had a greater frequency of high WMH score ≥3 (18% vs 11%, p=0.007) and a greater prevalence of infarcts at baseline (19% vs 11%, p=0.001, Table 1). They were more likely to be black, female, diabetic, vitamin D deficient, have lower education, physical activity, eGFR, and to have higher BMI, and systolic and diastolic blood pressure.
Table 1.
Baseline Characteristics* by Elevated or Non-Elevated PTH levels at ARIC visit 3
| Overall (N=1,703) |
PTH<65 pg/ml (N=1,499) |
PTH ≥65 pg/ml (N=204) |
P-value | |
|---|---|---|---|---|
| PTH (pg/ml) | 45.2 ± 18.8 | 40.0 ± 11.3 | 83.0 ± 19.3 | <0.001 |
| 25(OH)D (ng/ml) | 21.5 ± 8.2 | 22.1 ± 8.1 | 16.9 ± 7.7 | <0.001 |
| Vitamin D deficiency <20 ng/ml (%) | 802 (47.1) | 653 (43.6) | 149 (73.0) | |
| Calcium (mg/dl) | 9.5 ± 0.4 | 9.5 ± 0.4 | 9.5 ± 0.6 | 0.91 |
| Phosphate (mg/dl) | 3.4 ± 0.5 | 3.5 ± 0.5 | 3.3 ± 0.5 | <0.001 |
| Age (years) | 62.4 ± 4.5 | 62.3 ± 4.5 | 62.9 ± 4.7 | 0.074 |
| Males (%) | 680 (39.9) | 627 (41.8) | 53 (26.0) | <0.001 |
| Race/center (%) | <0.001 | |||
| Forsyth County, NC Whites | 862 (50.6) | 803 (53.6) | 59 (28.9) | |
| Forsyth County, NC Blacks | 103 (6.0) | 79 (5.3) | 24 (11.8) | |
| Jackson, MS Blacks | 738 (43.3) | 617 (41.2) | 121 (59.3) | |
| Education† | 0.001 | |||
| <High School | 458 (26.9) | 381 (25.5) | 77 (37.7) | |
| High School or Vocational School | 582 (34.2) | 526 (35.1) | 56 (27.5) | |
| College, Graduate, or Professional School | 661 (38.9) | 590 (39.4) | 71 (34.8) | |
| BMI (kg/m2) | 27.9 ± 5.2 | 27.6 ± 4.9 | 30.7 ± 6.1 | <0.001 |
| Physical activity index | 2.5 ± 0.8 | 2.5 ± 0.8 | 2.4 ± 0.8 | 0.002 |
| Smoking Status | 0.018 | |||
| Never | 759 (44.7) | 649 (43.4) | 110 (53.9) | |
| Former | 632 (37.2) | 569 (38.1) | 63 (30.9) | |
| Current | 308 (18.1) | 277 (18.5) | 31 (15.2) | |
| Alcohol consumption | <0.001 | |||
| Non-current | 1,055 (62.1) | 900 (60.2) | 155 (76.0) | |
| Current | 645 (37.9) | 596 (39.8) | 49 (24.0) | |
| SBP (mm Hg) | 128.0 ± 20.7 | 126.7 ± 19.8 | 137.4 ± 24.3 | <0.001 |
| DBP (mm Hg) | 72.0 ± 10.9 | 71.6 ± 10.7 | 75.1 ± 12.0 | <0.001 |
| Use of hypertension meds (%) | 722 (42.4) | 606 (40.4) | 116 (56.9) | <0.001 |
| Total cholesterol (mg/dl) | 208.6 ± 38.1 | 208.9 ± 38.0 | 206.6 ± 38.5 | 0.42 |
| HDL-C (mg/dl) | 54.9 ± 19.9 | 55.0 ± 19.8 | 54.3 ± 20.8 | 0.60 |
| Triglycerides (mg/dl) | 134.7 ± 86.3 | 135.2 ± 88.3 | 130.8 ± 69.8 | 0.49 |
| Use of lipid lowering medications (%) | 137 (8.1) | 117 (7.8) | 20 (9.8) | 0.33 |
| Diabetes (%) | 298 (17.6) | 249 (16.7) | 49 (24.0) | 0.01 |
| eGFR < 60 ml/min/1.73 m2‡ | 30 (1.8) | 20 (1.3) | 10 (5.1) | <0.001 |
| WMH score | 1.00 (1.00 – 2.00) | 1.00 (1.00 – 2.00) | 1.00 (1.00 – 2.00) | 0.17 |
| High WMH score ≥3 (%) | 203 (11.9) | 167 (11.1) | 36 (17.6) | 0.007 |
| Prevalent infarct (%) | 197 (11.6) | 159 (10.6) | 38 (18.6) | 0.001 |
Data are mean ± SD or number (percentage)
Education information for visit 3 is derived from visit 1 (1987–1989)
eGFR for visit 3 is derived from visit 2 (1990–1992)
PTH: parathyroid hormone, 25(OH)D: 25-hydroxyvitamin D, BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, HDL-C: high density lipoprotein cholesterol, eGFR: estimated glomerular filtration rate, WMH: white matter hyperintensities
In cross-sectional analysis adjusted for demographic, behavioral and socioeconomic variables, elevated PTH levels were associated with higher WMH score and more prevalent infarcts (Table 2). The difference (95% CI) in cross-sectional WMH score was 0.19 (0.04, 0.35) and the odds ratio (95% CI) for prevalent infarcts was 1.56 (1.02, 2.36), comparing participants with PTH level ≥65 to <65 pg/mL (Table 2, Model 2). The associations were attenuated after adjusting for potential CVD risk mediators in the biologic pathway between PTH and cerebrovascular disease (such as hypertension).
Table 2.
Adjusted Associations of Parathyroid Hormone (PTH) With White Matter Hyperintensity (WMH) Score and Prevalent Infarcts at ARIC visit 3 (1993–1994)
| Per 1 unit increase in log(PTH) |
PTH≥65 vs <65 | |
|---|---|---|
| Over all No. | 1703 | 204 (12.0%) |
| WMH score (qualitative score, 0–9) | β coef (95% CI) | β coef (95% CI) |
| Model 1 | 0.11 (−0.03, 0.25) | 0.19 (0.03, 0.35) |
| Model 2 | 0.14 (−0.00, 0.27) | 0.19 (0.04, 0.35) |
| Model 3 | 0.09 (−0.05, 0.23) | 0.14 (−0.01, 0.30) |
| Model 4 | 0.12 (−0.03, 0.27) | 0.16 (−0.00, 0.32) |
| P-interaction for race (model 2) | P=0.04 | P=0.80 |
| Prevalent infarcts, ≥3 mm | OR (95% CI) | OR (95% CI) |
| Model 1 | 1.18 (0.79, 1.76) | 1.57 (1.05, 2.35) |
| Model 2 | 1.24 (0.82, 1.86) | 1.56 (1.02, 2.36) |
| Model 3 | 1.06 (0.70, 1.61) | 1.31 (0.84, 2.03) |
| Model 4 | 1.06 (0.67, 1.67) | 1.33 (0.84, 2.09) |
| P-interaction for race (model 2) | P=0.06 | P=0.17 |
Model 1 (demographic variables): age, sex, race
Model 2 (demographic + behavioral and socioeconomic variables): Model 1 + education, body mass index, smoking status, alcohol drinking, physical activity
Model 3 (demographic + behavioral and socioeconomic + comorbidities): Model 2 + systolic and diastolic blood pressure, use of hypertension medication, diabetes, HDL cholesterol, total cholesterol, cholesterol lowering medications, and estimated glomerular filtration rate
Model 4: (demographic + behavioral and socioeconomic + comorbidities + mediators): Model 3 + 25(OH)D, calcium, phosphorus
In spline regression analyses, we found a U-shaped association between continuous PTH levels with WMH score and risk of prevalent infarcts, with an increased burden of subclinical cerebrovascular disease at both extremes of the continuous distribution of PTH levels (P for non-linear spline terms = 0.25 for WMH score and 0.009 for prevalent infarcts; Figure 1 and Figure 2). However, confidence intervals overlapped the null at the low end of the PTH spectrum. To further understand this Ushaped relationship we calculated mean calcium and phosphate levels by PTH quartiles. We found those in the lowest PTH quartile had higher mean calcium and higher mean phosphate levels (p=0.02 and <0.001 for calcium and phosphate, respectively).
Figure 1.
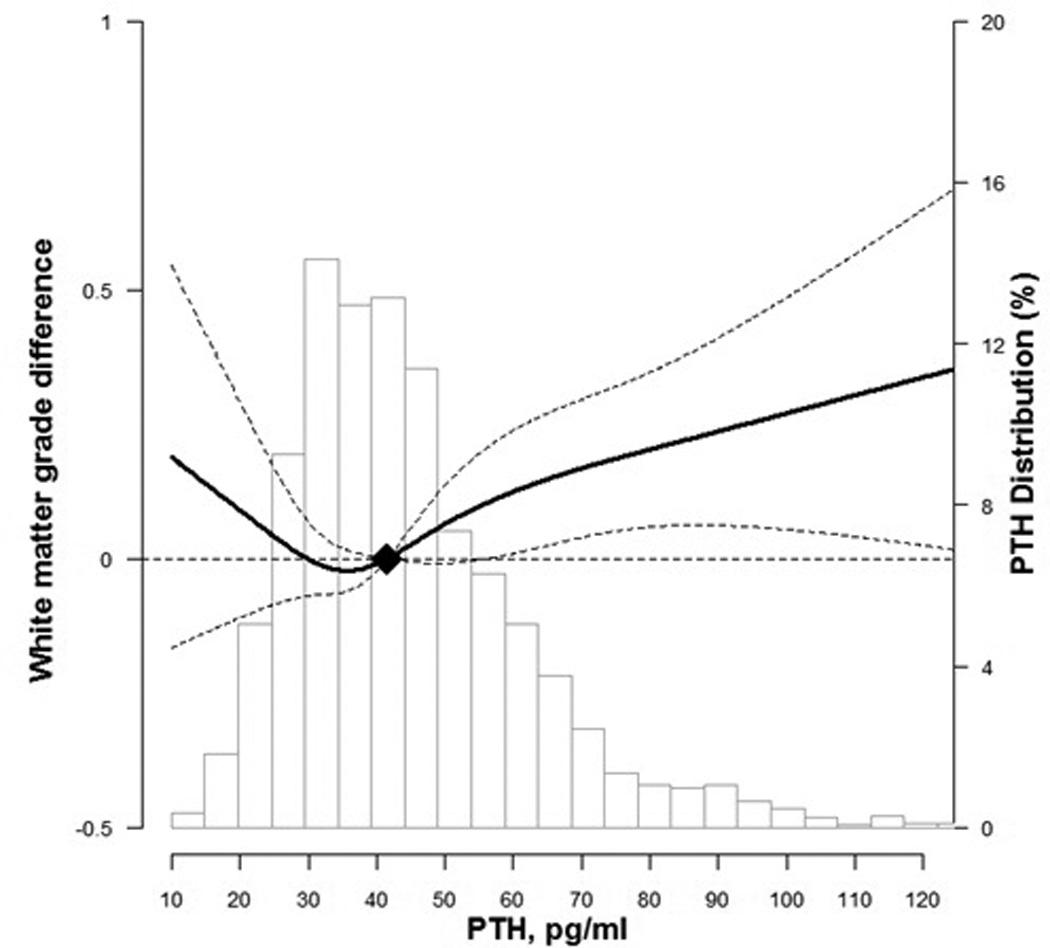
Difference in white matter grade by parathyroid hormone (PTH) levels at ARIC visit 3
Curves represent adjusted change (solid lines) and their 95% confidence intervals (dashed lines) based on restricted cubic splines for PTH and brain MRI outcomes with knots at the 5th, 35th, 65th and 95th percentiles of their sample distributions. The reference values (diamond dots) were set at the 50th percentile level (41.7 pg/ml). Models were adjusted for age, sex, race, education, body mass index, smoking status, alcohol drinking, and physical activity
Figure 2.
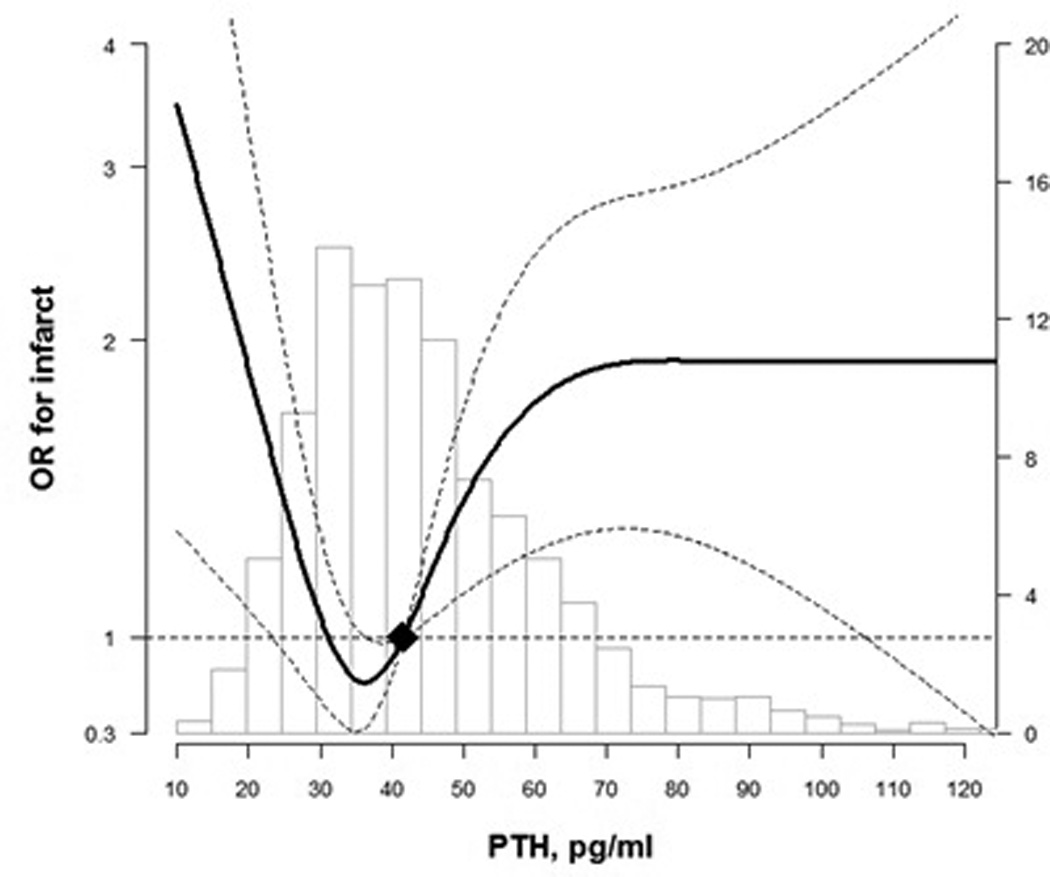
Odds Ratio for prevalent infarct by PTH levels at ARIC visit 3
Curves represent adjusted change (solid lines) and their 95% confidence intervals (dashed lines) based on restricted cubic splines for PTH and brain MRI outcomes with knots at the 5th, 35th, 65th and 95th percentiles of their sample distributions. The reference values (diamond dots) were set at the 50th percentile level (41.7 pg/ml). Models were adjusted for age, sex, race, education, body mass index, smoking status, alcohol drinking, and physical activity
Compared with those who remained in the study (n=948), participants who dropped out of the study (n=755) had higher PTH levels at ARIC visit 3 and differed by important clinical characteristics (Table 3). Participants who did not return for their second MRI were older, less physically active, had lower eGFR, education levels and HDL-C, and more likely to have higher BMI, systolic blood pressure, triglycerides, WMH score and subclinical infarcts. Given this problematic informative censoring, for the prospective analyses, we used imputation methods for those missing the follow-up brain MRI.
Table 3.
Characteristics* of participants at ARIC visit 3 (1993–1994) by attrition before Brain MRI visit (2004–2006)
| Remained in cohort (N=948) |
Dropped out (N=755) |
Overall (N=1,703) |
P value | |
|---|---|---|---|---|
| PTH (pg/ml) | 44.0 ± 16.6 | 46.6 ± 21.1 | 45.2 ± 18.8 | 0.005 |
| 25(OH)D (ng/ml) | 21.8 ± 8.2 | 21.0 ± 8.2 | 21.5 ± 8.2 | 0.05 |
| Vitamin D deficiency <20 ng/ml (%) | 430 (45.4) | 372 (49.3) | 802 (47.1) | 0.12 |
| Age (years) | 61.8 ± 4.4 | 63.1 ± 4.6 | 62.4 ± 4.5 | <0.001 |
| BMI (kg/m2) | 27.7 ± 4.7 | 28.3 ± 5.7 | 27.9 ± 5.2 | 0.011 |
| Waist circumference (cm) | 97.8 ± 12.4 | 100.5 ± 14.5 | 99.0 ± 13.5 | <0.001 |
| Physical activity index | 2.6 ± 0.8 | 2.5 ± 0.8 | 2.5 ± 0.8 | 0.006 |
| SBP (mm Hg) | 126.3 ± 19.5 | 130.0 ± 22.0 | 128.0 ± 20.7 | <0.001 |
| DBP (mm Hg) | 72.5 ± 10.5 | 71.5 ± 11.5 | 72.0 ± 10.9 | 0.084 |
| HDL-C (mg/dl) | 56.1 ± 19.1 | 53.5 ± 20.9 | 54.9 ± 19.9 | 0.007 |
| Total cholesterol (mg/dl) | 209.3 ± 37.9 | 207.7 ± 38.2 | 208.6 ± 38.1 | 0.37 |
| Triglycerides (mg/dl) | 129.7 ± 74.8 | 140.9 ± 98.5 | 134.7 ± 86.3 | 0.008 |
| eGFR < 60 ml/min/1.73 m2‡ | 10 (1.1) | 20 (2.7) | 30 (1.8) | 0.01 |
| Race | 0.46 | |||
| Whites | 472 (49.8) | 390 (51.7) | 862 (50.6) | |
| Blacks | 476 (50.2) | 365 (48.3) | 841 (49.4) | |
| Sex | 0.80 | |||
| Female | 572 (60.3) | 451 (59.7) | 1,023 (60.1) | |
| Male | 376 (39.7) | 304 (40.3) | 680 (39.9) | |
| Education† | <0.001 | |||
| <High School | 207 (21.9) | 251 (33.3) | 458 (26.9) | |
| High School or Vocational School | 330 (34.8) | 252 (33.4) | 582 (34.2) | |
| College, Graduate, or Professional School | 410 (43.3) | 251 (33.3) | 661 (38.9) | |
| Smoking Status | <0.001 | |||
| Never | 452 (47.8) | 307 (40.7) | 759 (44.7) | |
| Former | 365 (38.6) | 267 (35.4) | 632 (37.2) | |
| Current | 128 (13.5) | 180 (23.9) | 308 (18.1) | |
| Alcohol consumption | 0.009 | |||
| Non-current | 561 (59.3) | 494 (65.5) | 1,055 (62.1) | |
| Current | 385 (40.7) | 260 (34.5) | 645 (37.9) | |
| Diabetes | 137 (14.5) | 161 (21.4) | 298 (17.6) | <0.001 |
| Prevalent WMH score | 1.00 (1.00 – 2.00) | 1.00 (1.00 – 2.00) | 1.00 (1.00 – 2.00) | <0.001 |
| Prevalent infarct | 67 (7.1) | 130 (17.2) | 197 (11.6) | <0.001 |
| High WMH score ≥3 (%) | 77 (8.1) | 126 (16.7) | 203 (11.9) | <0.001 |
Data are mean ± SD, median (IQR) or number (percentage)
Education information for visit 3 is derived from visit 1 (1987–1989)
eGFR for visit 3 is derived from visit 2 (1990–1992)
PTH: parathyroid hormone, 25(OH)D: 25-hydroxyvitamin D, BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, HDL-C: high density lipoprotein cholesterol, eGFR: estimated glomerular filtration rate, WMH: white matter hyperintensities
In prospective analysis, elevated PTH levels were associated with an increased incidence of infarcts in demographic adjusted models. However, the associations between PTH levels and estimated WMH volume change from the first to second MRIs (~10 year interval) and with infarct incidence were statistically insignificant, albeit suggesting a positive trend, in multivariable models (Table 4). Comparing participants with PTH levels ≥65 to <65 pg/mL, there was 1.39 cm3 (−0.73, 3.51) more WMH progression and the odds ratio (95% CI) for incident infarcts was 1.42 (0.97, 2.06) (Table 4, Model 2). Spline regression models for the prospective analyses were consistent with the cross-sectional associations. Confidence intervals were wide and overlapped the null value for the low end of PTH distribution (P for non-linear spline terms = 0.12 for WMH volume change and 0.39 for incident infarcts; Figure 3 and Figure 4).
Table 4.
Adjusted Associations of PTH with Prospective WMH Volume Change and Risk of Incident Infarcts over follow-up from ARIC visit 3 (1993–1994) to ARIC Brain MRI visit (2004–2006).
| Per 1 unit increase in log(PTH) |
PTH≥65 vs <65 | |
|---|---|---|
| Over all No. | 1703 | 204 (12.0%) |
| WMH volume change, cm3 | β coef (95% CI) | β coef (95% CI) |
| Model 1 | 0.88 (−1.43, 3.19) | 1.48 (−0.56, 3.53) |
| Model 2 | 1.09 (−1.27, 3.44) | 1.39 (−0.73, 3.51) |
| Model 3 | 0.77 (−1.55, 3.09) | 0.92 (−1.21, 3.04) |
| Model 4 | 1.21 (−1.31, 3.73) | 1.17 (−0.90, 3.24) |
| P-interaction for race (Model 2) | P=0.32 | P=0.55 |
| Incident infarcts (≥3 mm) | OR (95% CI) | OR (95% CI) |
| Model 1 | 1.53 (1.01, 2.31) | 1.52 (1.05, 2.19) |
| Model 2 | 1.50 (0.97, 2.30) | 1.42 (0.97, 2.06) |
| Model 3 | 1.45 (0.90, 2.35) | 1.30 (0.86, 1.98) |
| Model 4 | 1.61 (0.95, 2.72) | 1.37 (0.88, 2.13) |
| P-interaction for race (Model 2) | P=0.06 | P=0.52 |
Model 1 (demographic variables): age, sex, race
Model 2 (demographic + behavioral and socioeconomic variables): Model 1 + education, body mass index, smoking status, alcohol drinking, physical activity
Model 3 (demographic + behavioral and socioeconomic + comorbidities): Model 2 + systolic and diastolic blood pressure, use of hypertension medication, diabetes, HDL cholesterol, total cholesterol, cholesterol lowering medications, and estimated glomerular filtration rate
Model 4: (demographic + behavioral and socioeconomic + comorbidities + mediators): Model 3 + 25(OH)D, calcium, phosphorus
Figure 3.
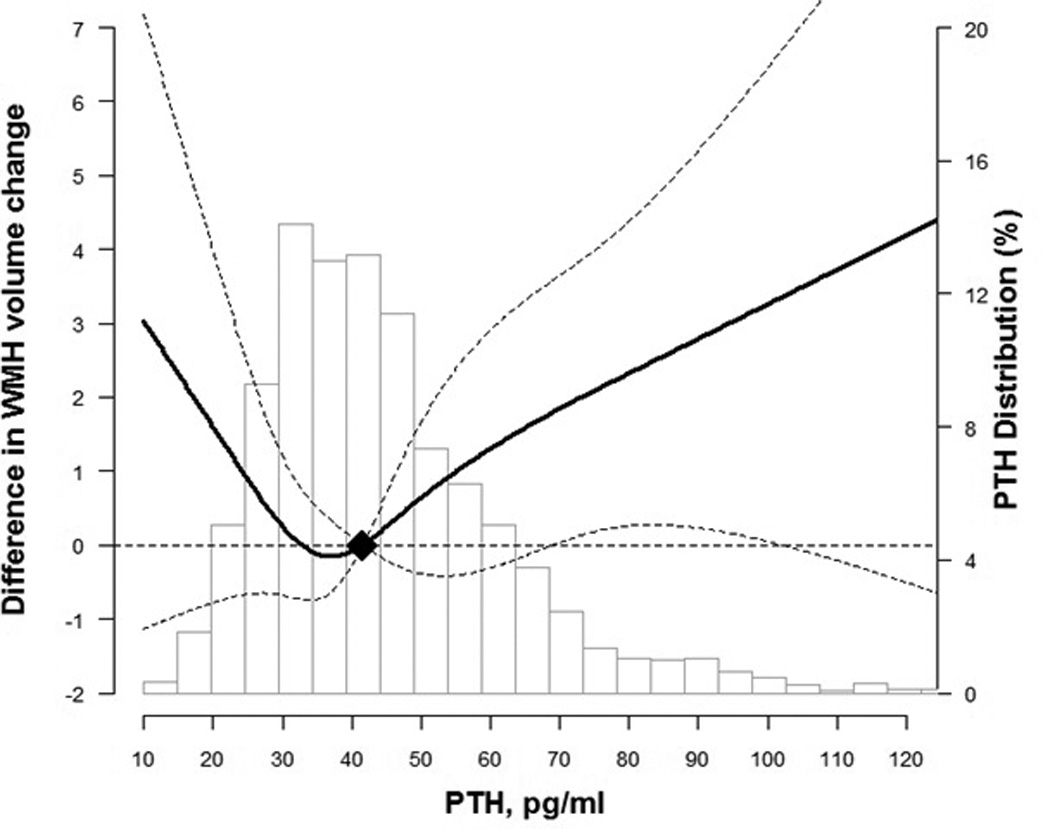
Change in white matter hyperintensity volume by parathyroid hormone (PTH) levels from visit 3 to Brain MRI visit ~10 years later
Curves represent adjusted change (solid lines) and their 95% confidence intervals (dashed lines) based on restricted cubic splines for PTH and brain MRI outcomes with knots at the 5th, 35th, 65th and 95th percentiles of their sample distributions. The reference values (diamond dots) were set at the 50th percentile level (41.7 pg/ml). Models were adjusted for age, sex, race, education, body mass index, smoking status, alcohol drinking, and physical activity
Figure 4.
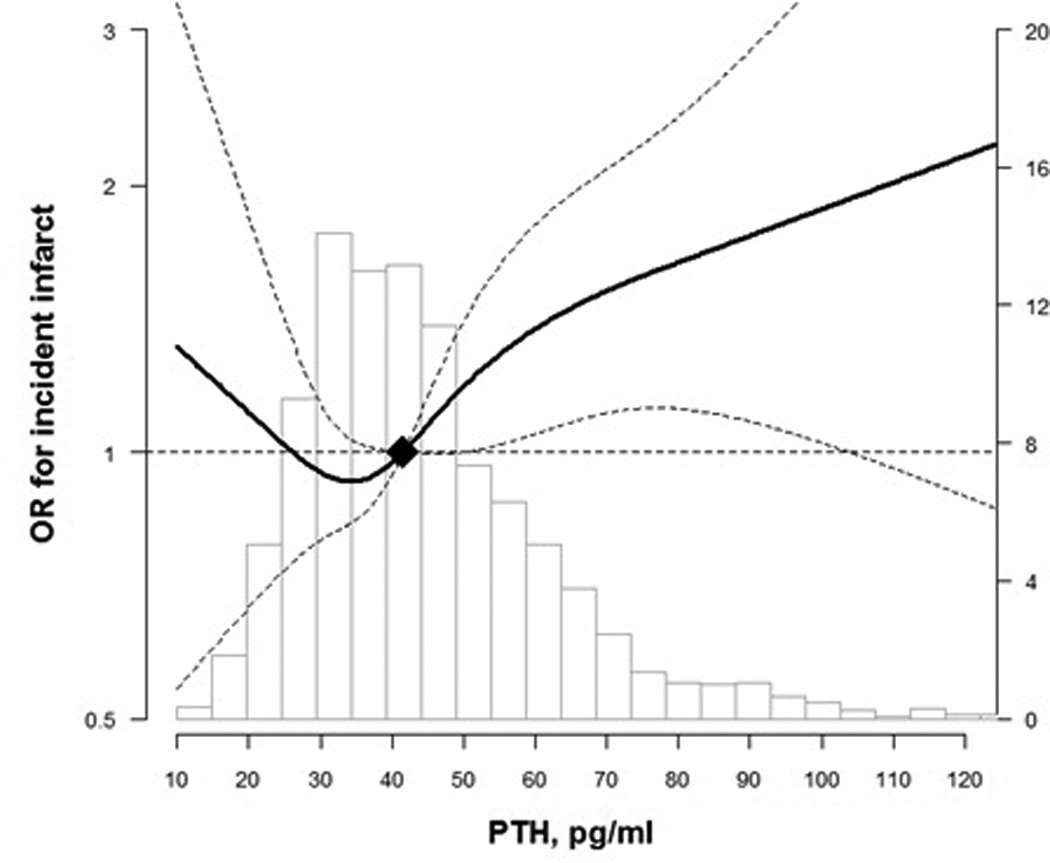
OR for incident infarct by PTH level from visit 3 to Brain MRI visit
Curves represent adjusted change (solid lines) and their 95% confidence intervals (dashed lines) based on restricted cubic splines for PTH and brain MRI outcomes with knots at the 5th, 35th, 65th and 95th percentiles of their sample distributions. The reference values (diamond dots) were set at the 50th percentile level (41.7 pg/ml). Models were adjusted for age, sex, race, education, body mass index, smoking status, alcohol drinking, and physical activity
No significant interactions by race were noted in the cross-sectional or prospective analyses for elevated PTH (≥65 pg/ml) compared to normal PTH levels.
Discussion
In a middle-aged population-based cohort of blacks and whites who were free of stroke and TIA, we found that elevated PTH levels were cross-sectionally associated with the presence of greater white matter disease and subclinical infarcts on brain MRI imaging. However, contrary to our hypothesis, we did not confirm any associations of elevated PTH with progression of cerebrovascular signs on brain MRIs obtained approximately 10 years apart. Elevated PTH was associated with incident infarcts in models adjusting for demographics only, but this was attenuated in models adjusting for lifestyle and CVD risk factors. Findings were unaltered after additional adjustment for markers of mineral metabolism such as 25(OH)D, calcium, and phosphate levels. However, a non-significant trend remained for the association of elevated PTH with incident infarcts. Only 204 individuals had an elevated PTH; thus given the relatively few numbers of individuals with elevated PTH, we had limited power for the prospective analyses. Therefore, there may be a true relationship with elevated PTH and incident subclinical cerebrovascular disease that we were unable to confirm.
We also evaluated the continuous distribution of PTH with outcomes in spline models. Indeed, these analyses suggested an increased risk for subclinical cerebrovascular disease progression at higher PTH levels. However, even with the removal of outliers (PTH <10 and ≥200 pg/ml), there also was a signal for a U-shaped distribution, although confidence intervals were wide and overlapped the null at the lower end of the PTH spectrum. While the association with high PTH and subclinical cerebrovascular disease was consistent with our hypotheses from the prior literature, it is unclear why low PTH would also confer increased risk. Certainly clinical hypoparathyroidism, which is rare and usually due to removal of parathyroid glands at the time of thyroid surgery, causes low calcium levels and metabolic derangements, but these PTH values were within the normal physiologic range. PTH levels are also appropriately suppressed when serum calcium levels are high, so it is possible that these individuals with PTH at the low-end of the normal spectrum had medical conditions or supplementation with calcium that led to higher serum levels of calcium and more vascular disease. Indeed the mean calcium and mean phosphate levels were significantly higher in the lowest PTH quartile. Our findings warrant further investigation into the relation of PTH and the effect it has on subclinical cerebrovascular disease.
Previous research on the association between PTH and CVD clinical events has been contradictory.10 There is a lack of quantity and quality of research regarding the association of elevated PTH and subclinical cerebrovascular disease. Only one prior study has involved neuroimaging data. This study by Hagström et al was conducted in the Uppsala Longitudinal Study of Adult Men (ULSAM) and the PIVUS cohorts, which are populations of white race in Sweden.17 The authors found that PTH was associated with both vascular dementia in ULSAM as well as increased WMH on brain MRI in PIVUS. These associations remained statistically significant even after adjustment for CVD risk factors and markers of mineral metabolism such as 25(OH)D levels. In PIVUS, there was only a one-time brain MRI performed that was 5 years after the PTH measurement, with no baseline brain MRI to examine white matter progression.17 Other limitations of that study are the inability to generalize to younger populations or other race/ethnicities. In our study, we had a baseline brain MRI and a second brain MRI 10 years later to observe for white matter progression. In addition, our study population was younger and included both blacks and white individuals.
In contrast to Hagström et al17, we did not observe any association of PTH with subclinical cerebrovascular disease after adjustment for CVD risk factors and 25(OH)D levels. Prior work in the ARIC Brain MRI study has found that cumulative systolic blood pressure was a strong predictor of WMH progression over follow-up,19 and since PTH is associated with incident hypertension,3 we had hypothesized that hypertension might be a mediator in the biologic pathway between elevated PTH levels and cerebrovascular disease. Indeed our results were attenuated when we adjusted for hypertension. Our lack of association between elevated PTH and subclinical cerebrovascular disease, however, was in accord with the findings of the larger ARIC PTH and CVD events study by Folsom et al.10 Neither study provides support that elevated PTH is a risk marker for cardiovascular and subclinical cerebrovascular events. PTH also was not associated with carotid atherosclerosis in prior studies.24,25
Limitations and Strengths
The study is limited by the high rate of attrition of participants with elevated PTH levels, which could bias results towards the null, so we used multiple imputation analysis to attempt to account for this. Although our study sample was much larger than the one in the previously published paper by Hagström et al17 (n=1703 in our sample vs n=406 in their study), our study may still be underpowered for prospective analyses as mentioned above. Because the sample was relatively young at the time of both brain MRIs, most participants had relatively minimal WMH, with relatively low rates of infarcts at both MRIs. In addition, our definition of incident infarcts does not allow for progression of number of infarcts, which could be an indication of progression of cerebrovascular disease; we can only define new infarcts as being present in people who did not previously have any infarcts on imaging, based on the way scans were analyzed.
A strength of our study is the ARIC cohort of generally middle-aged to older community-dwelling black and white adults with no history of stroke at baseline, a cohort which has been well characterized with comprehensive measurement of confounding and mediating variables including 25(OH)D, calcium, and blood pressure.
Conclusion
In summary, we did not find that elevated PTH was independently associated brain infarcts and WMH disease or their progression after accounting for other CVD risk factors and markers of mineral metabolism. However, these results suggest that further study of the relationship between PTH with subclinical cerebrovascular disease is warranted, perhaps in an older cohort with more cerebrovascular disease. Future studies are needed to determine if treatment of elevated PTH, such as correcting vitamin D deficiency, can prevent these subclinical cerebrovascular disease changes and improve brain health.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
FUNDING SOURCES
This work was supported by NIH/NINDS grant R01NS072243. The ARIC Brain MRI study was supported by grant R01-HL70825. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
REFERENCES
- 1.Hagström E, Ahlström T, Ärnlöv J, Larsson A, Melhus H, Hellman P, Lind L. Parathyroid hormone and calcium are independently associated with subclinical vascular disease in a community-based cohort. Atherosclerosis. 2015;238(2):420–426. doi: 10.1016/j.atherosclerosis.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Bosworth C, Sachs MC, Duprez D, Hoofnagle AN, Ix JH, Jacobs DR, Jr, Peralta CA, Siscovick DS, Kestenbaum B, de Boer IH. Parathyroid hormone and arterial dysfunction in the multi-ethnic study of atherosclerosis. Clin Endocrinol (Oxf) 2013;79(3):429–436. doi: 10.1111/cen.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Ballegooijen AJ, Kestenbaum B, Sachs MC, de Boer IH, Siscovick DS, Hoofnagle AN, Ix JH, Visser M, Brouwer IA. Association of 25-hydroxyvitamin D and parathyroid hormone with incident hypertension: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2014;63(12):1214–1222. doi: 10.1016/j.jacc.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagström E, Hellman P, Larsson TE, Ingelsson E, Berglund L, Sundström J, Melhus H, Held C, Lind L, Michaëlsson K, Arnlöv J. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009;119:2765–2771. doi: 10.1161/CIRCULATIONAHA.108.808733. [DOI] [PubMed] [Google Scholar]
- 5.Deo R, Katz R, Shlipak MG, Sotoodehnia N, Psaty BM, Sarnak MJ, Fried LF, Chonchol M, de Boer IH, Enquobahrie D, Siscovick D, Kestenbaum B. Vitamin D, parathyroid hormone, and sudden cardiac death: results from the Cardiovascular Health Study. Hypertension. 2011;58(6):1021–1028. doi: 10.1161/HYPERTENSIONAHA.111.179135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bansal N, Zelnick L, Robinson-Cohen C, Hoofnagle AN, Ix JH, Lima JA, Shoben AB, Peralta CA, Siscovick DS, Kestenbaum B, de Boer IH. Serum parathyroid hormone and 25-hydroxyvitamin D concentrations and risk of incident heart failure: the multiethnic study of atherosclerosis. J Am Heart Assoc. 2014;3(6):e001278. doi: 10.1161/JAHA.114.001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kestenbaum B, Katz R, de Boer I, Hoofnagle A, Sarnak MJ, Shlipak MG, Jenny NS, Siscovick DS. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol. 2011;58(14):1433–1441. doi: 10.1016/j.jacc.2011.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Ballegooijen AJ, Reinders I, Visser M, Dekker JM, Nijpels G, Stehouwer CD, Pilz S, Brouwer IA. Serum parathyroid hormone in relation to all-cause and cardiovascular mortality: the Hoorn study. J Clin Endocrinol Metab. 2013;98(4):E638–E645. doi: 10.1210/jc.2012-4007. [DOI] [PubMed] [Google Scholar]
- 9.van Ballegooijen AJ, Reinders I, Visser M, Brouwer IA. Parathyroid hormone and cardiovascular disease events: A systematic review and meta-analysis of prospective studies. Am Heart J. 2013;165(5):655–664. 664.e1–664.e5. doi: 10.1016/j.ahj.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Folsom AR, Alonso A, Misialek JR, Michos ED, Selvin E, Eckfeldt JH, Coresh J, Pankow JS, Lutsey PL. Parathyroid hormone concentration and risk of cardiovascular diseases: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2014;168(3):296–302. doi: 10.1016/j.ahj.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manolio TA, Kronmal RA, Burke GL, Poirier V, O'Leary DH, Gardin JM, Fried LP, Steinberg EP, Bryan RN. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994;25:318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- 12.Breteler MM, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JHW, van Harskamp F, Tanghe HLJ, de Jong PTVM, van Gijn J, Hofman A. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam study. Neurology. 1994;44:1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 13.Mosley TH, Knopman DS, Catellier DJ, Bryan N, Hutchinson RG, Grothues CA, Folsom AR, Cooper LS, Burke GL, Liao D, Szklo M. Cerebral MRI findings and cognitive functioning: The Atherosclerosis Risk in Communities Study. Neurology. 2005;64:2056–2062. doi: 10.1212/01.WNL.0000165985.97397.88. [DOI] [PubMed] [Google Scholar]
- 14.Silbert LC, Howieson DB, Dodge H, Kaye JA. Cognitive impairment risk: white matter hyperintensity progression matters. Neurology. 2009;73(2):120–125. doi: 10.1212/WNL.0b013e3181ad53fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michos ED, Carson KA, Schneider ALC, Lutsey PL, Xing L, Sharrett AR, Alonso A, Coker LH, Gross M, Post W, Mosley TH, Gottesman RF. Vitamin D and Subclinical Cerebrovascular Disease: The Atherosclerosis Risk in Communities Brain Magnetic Resonance Imaging Study. JAMA Neurol. 2014;71(7):863–871. doi: 10.1001/jamaneurol.2014.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Björkman MP, Sorva AJ, Tilvis RS. Does elevated parathyroid hormone concentration predict cognitive decline in older people? Aging Clin Exp Res. 2010;22(2):164–169. doi: 10.1007/BF03324791. [DOI] [PubMed] [Google Scholar]
- 17.Hagström E, Kilander L, Nylander R, Larsson EM, Michaëlsson K, Melhus H, Ahlström H, Johansson L, Lind L, Arnlöv J. Plasma parathyroid hormone is associated with vascular dementia and cerebral hyperintensities in two community-based cohorts. J Clin Endocrinol Metab. 2014;99(11):4181–4189. doi: 10.1210/jc.2014-1736. [DOI] [PubMed] [Google Scholar]
- 18.The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 19.Gottesman RF, Coresh J, Catellier DJ, Sharrett AR, Rose KM, Coker LH, Shibata DK, Knopman DS, Jack CR, Mosley TH. Blood pressure and white matter disease progression in a biethnic cohort: Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2010;41:3–8. doi: 10.1161/STROKEAHA.109.566992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longstreth WT, Jr, Dulberg C, Manolio TA, Lewis MR, Beauchamp NJ, Jr, O'Leary D, Carr J, Furberg CD. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2002;33(10):2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 21.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 22.Pugliese G, Solini A, Bonora E, Orsi E, Zerbini G, Giorgino F, Cavalot F, Pontiroli AE, Baroni MG, Morano S, Nicolucci A, Penno G. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation provides a better definition of cardiovascular burden associated with CKD than the Modification of Diet in Renal Disease (MDRD) study formula in subjects with type 2 diabetes. Atherosclerosis. 2011;218(1):194–199. doi: 10.1016/j.atherosclerosis.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 23.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 24.Blondon M, Sachs M, Hoofnagle AN, Ix JH, Michos ED, Korcarz C, Gepner AD, Siscovick DS, Kaufman JD, Stein JH, Kestenbaum B, de Boer IH. 25-Hydroxyvitamin D and parathyroid hormone are not associated with carotid intima-media thickness or plaque in the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33(11):2639–2645. doi: 10.1161/ATVBAHA.113.301781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reis JP, von Mühlen D, Michos ED, Miller ER, 3rd, Appel LJ, Araneta MR, Barrett-Connor E. Serum vitamin D, parathyroid hormone levels, and carotid atherosclerosis. Atherosclerosis. 2009;207(2):585–590. doi: 10.1016/j.atherosclerosis.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]


