Abstract
Obesity has been identified as a risk factor for postoperative atrial fibrillation (POAF) following coronary artery bypass grafting (CABG). However, no studies have addressed the influence of race on this association. A total of 13,594 patients undergoing first-time, isolated CABG without preoperative atrial fibrillation between 1992 and 2011 were included in our study. The association between body mass index (BMI) and POAF was compared by race. Relative risk and 95% confidence intervals were computed using maximum likelihood log-binomial regression. Increasing levels of BMI were associated with higher POAF risk following CABG among black but not white patients (Pinteraction=0.0009).
Keywords: postoperative atrial fibrillation, coronary artery bypass grafting, obesity, race
Introduction
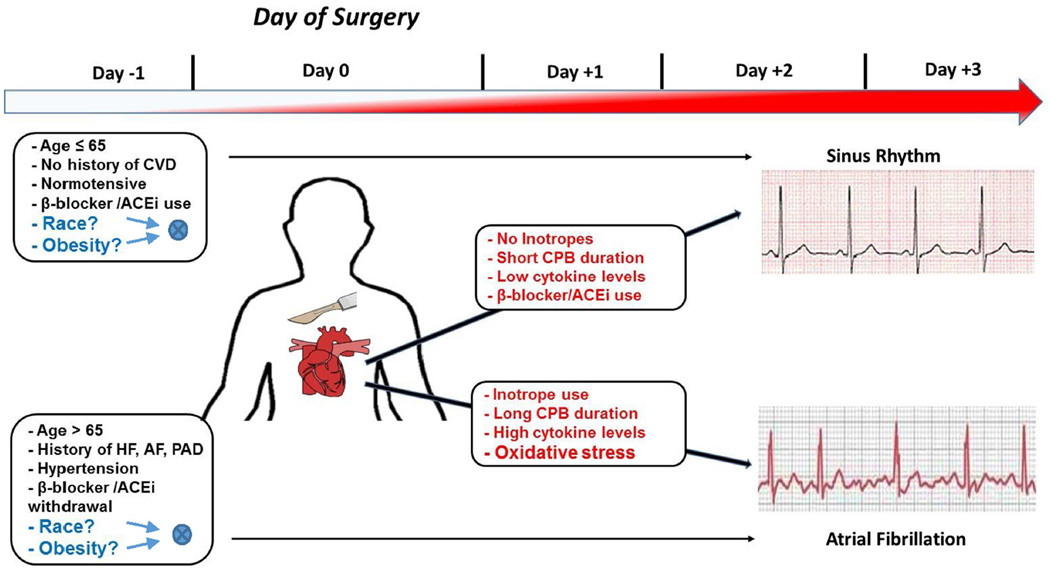
Postoperative atrial fibrillation (POAF) is a common complication following coronary artery bypass grafting (CABG), and obesity is a well-established risk factor for developing POAF.(1) However, no studies have addressed the differential influence of race on this association (Figure 1). Recently, increased body mass index (BMI) has been shown to be a stronger predictor for incident atrial fibrillation (AF) in blacks compared with whites in the general population.(2) To our knowledge, a similar finding has not been examined in patients undergoing CABG. This is important as black patients who develop POAF are at increased risk for mortality following CABG.(3) Identifying high risk groups for POAF will allow for the development of targeted interventions to reduce mortality, hospital readmissions, and total hospital costs among CABG patients.(4) Similar to the general nonsurgical obese population, we hypothesized that obese black patients undergoing CABG are at a higher risk for developing POAF.
Figure 1. Summary of the preoperative (Day −1) and intra/postoperative risk factors known to be associated with POAF.
The schematic shown above highlights the major question addressed by the present study, namely the unknown interacting role of obesity and race (shown at left in blue text) as preoperative risk factors for POAF. The time-scale at top illustrates the increasing prevalence of POAF (red shading of arrow) that peaks within postoperative day +2 and +3 following surgery. In the boxes in red text are the intra-and postoperative variables that we and others have shown to play a role in determining whether a patient is likely to have sinus rhythm or POAF following heart surgery. ACEi=angiotensin converting enzyme inhibitors; AF=atrial fibrillation; CPB=cardiopulmonary bypass; CVD=cardiovascular disease; HF=heart failure; PAD=peripheral artery disease.
Methods
This was a retrospective cohort study of patients undergoing first-time, isolated CABG at the East Carolina Heart Institute (ECHI) between 1992 and 2011. Patient demographics, prior medical history/comorbidities, preoperative medications and operative data were collected at the time of surgery. Patients with POAF were compared with those without POAF. Only black and white patients were included to minimize the potential for residual confounding (~1% other races). Racial identity was self-reported. The study and a waiver of participant consent for the period from 1992 to 2011 was approved by the Institutional Review Board at the Brody School of Medicine, East Carolina University (UMCIRB 12-002107).
Clinical variables were defined according to standard Society of Thoracic Surgeons (STS) protocol and documented by hospital notes, medication reports, outpatient medical records, radiology readings, and physician documentation.(5)
POAF denoted a first-time episode of AF (chaotic/irregular atrial rhythm with a variable rate and irregular ventricular rhythm) lasting longer than 1 hour following surgery and requiring treatment. Patients with a history of preoperative paroxysmal, persistent, or permanent AF/atrial flutter were excluded.
The World Health Organization (WHO) classification of BMI was used to group obesity into 3 classes.(6) Non-obese patients were designated as the reference group for statistical comparisons. Underweight patients have a known risk profile for adverse cardiac outcomes and mortality and were excluded from our analysis to minimize confounding bias (n=102).(7)
Data for this study were obtained from the STS Adult Cardiac Surgery Database and the electronic medical record (EMR) at the Brody School of Medicine. Cardiovascular surgery information at our facility was first reported to the STS in 1989 with routine data submission starting in 1992. An EMR was introduced at the Brody School of Medicine in 1997. Patient information and clinical data from 1989 to 1997 were retrospectively scanned into the EMR. The STS database is linked to the EMR through a unique patient medical record number. The National Death Index, using social security numbers, was used to validate operative mortality information in our EMR. However, in accordance with §205(r) of the Social Security Act, the use of social security numbers was proscribed within our university system at the end of 2011.
Categorical variables were expressed as frequency and percentage, whereas continuous variables were reported as median and interquartile range. Statistical significance (P<0.05) for categorical variables was computed using the Fisher’s exact test and the Deuchler-Wilcoxon procedure for continuous variables. Trend across categories of obesity was computed using an exact Cochran-Armitage trend test.(8) An iterative expectation-maximization algorithm was used to account for missing BMI values (<0.01%; imputation efficiency >99.9%).(9)
Log-binomial regression was used to directly estimate relative risk (RR) and 95% confidence intervals (CI) for POAF.(10) Goodness-of-fit was assessed by examining Akaike’s Information Criteria and leverage/casewise diagnostic statistics, generalized to log-binomial regression.(11) P-values for the interaction between race and obesity level was computed using a likelihood ratio test by entering the appropriate cross-term into our regression models. All models achieved convergence and satisfied admissibility criteria (i.e., linear predictor constrained to be negative).(10)
The initial multivariable models included variables in our dataset that have been previously associated with POAF in the literature, regardless of their statistical significance in the current analysis (e.g., patient age, diabetes mellitus, heart failure, hypertension, obesity, peripheral artery disease, sex, and three-vessel coronary disease). Post-hoc inclusion of other prior medical history/comorbidities and preoperative medications into the model was performed in a pairwise fashion. P-values for point estimates were computed assuming asymptotic normality. Rounding was performed using the method of Holly and Whittemore.(12) SAS Version 9.4 (Cary, NC) was used for all analyses.
Results
A total of 13,594 patients were included in the study (48% age >65 years, 70% males, 17% blacks) (Table 1). A greater percentage of black patients were diabetic, hypertensive, and used angiotensin converting enzyme inhibitors/angiotensin receptor blockers (ACEi/ARBs), beta-blockers, diuretics, and lipid lowering agents as preoperative medications (P<0.0001). In contrast, a greater percentage of white patients were male and preoperatively used antiplatelet agents (P<0.0001). A decreasing trend of digitalis use and an increasing trend of ACEi/ARB and beta-blocker use with increasing obesity severity was observed for white (Ptrend<0.0001) but not black patients (Ptrend>0.05). Adjusting for age and sex in our total sample, POAF was significantly associated with obesity (P=0.0019) and white race (P<0.0001), which is consistent with previous research (not shown in tables).(1)
Table 1.
Patient characteristics (N=13,594)
| Patient Characteristics | Black | White | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-Obese | Obesity Class₴ | Ptrend§ | Non-Obese | Obesity Class₴ | Ptrend§ | |||||
| I | II | III | I | II | III | |||||
| Overall | 1165 (50%) | 734 (32%) | 277 (12%) | 153 (7%) | --- | 6846 (61%) | 3073 (27%) | 939 (8%) | 407 (4%) | --- |
| Demographics | ||||||||||
| Age (>65) | 532 (46%) | 293 (40%) | 90 (32%) | 48 (31%)‡ | <0.0001 | 3734 (55%) | 1382 (45%) | 333 (35%) | 134 (33%)‡ | <0.0001 |
| Male | 758 (65%) | 406 (55%) | 140 (51%) | 48 (31%)‡ | <0.0001 | 5063 (74%) | 2284 (74%) | 622 (66%) | 233 (57%)‡ | <0.0001 |
| Prior Medical History/Comorbidities | ||||||||||
| Diabetes | 434 (37%) | 436 (58%) | 160 (58%) | 100 (65%)‡ | <0.0001 | 1779 (26%) | 1133 (37%) | 454 (48%) | 227 (56%)‡ | <0.0001 |
| Dialysis | 80 (7%) | 35 (5%) | 14 (5%) | 4 (3%)† | 0.016 | 60 (1%) | 16 (1%) | 7 (1%) | 1 (<1%) | 0.073 |
| Elective status | 437 (3%) | 289 (39%) | 123 (44%) | 67 (44%) | 0.023 | 2644 (39%) | 1206 (39%) | 448 (48%) | 172 (42%)‡ | <0.0001 |
| Hypertension | 958 (82%) | 657 (90%) | 247 (89%) | 138 (90%)‡ | <0.0001 | 4439 (65%) | 2276 (74%) | 740 (79%) | 336 (83%)‡ | <0.0001 |
| LMCA disease | 245 (21%) | 152 (21%) | 47 (17%) | 30 (20%) | 0.27 | 1446 (21%) | 592 (19%) | 165 (18%) | 79 (19%)‡ | 0.0072 |
| LVEF <35% | 253 (22%) | 156 (21%) | 48 (17%) | 22 (14%) | 0.020 | 1296 (19%) | 516 (17%) | 137 (15%) | 54 (13%)‡ | <0.0001 |
| PAD | 193 (17%) | 83 (11%) | 29 (10%) | 15 (10%)‡ | 0.0003 | 838 (12%) | 278 (9%) | 80 (9%) | 36 (9%)‡ | <0.0001 |
| Prior MI | 512 (44%) | 315 (43%) | 129 (47%) | 66 (43%) | 0.85 | 2679 (39%) | 1198 (39%) | 342 (36%) | 155 (38%) | 0.23 |
| Recent smoker | 331 (28%) | 167 (23%) | 73 (26%) | 19 (12%)‡ | 0.0001 | 1746 (26%) | 692 (23%) | 212 (23%) | 77 (19%)‡ | <0.0001 |
| Three-vessel disease | 805 (69%) | 503 (69%) | 174 (63%) | 94 (61%) | 0.017 | 4501 (66%) | 2049 (67%) | 604 (64%) | 254 (62%) | 0.32 |
| Unstable heart failure | 230 (20%) | 141 (19%) | 63 (23%) | 38 (25%) | 0.13 | 810 (12%) | 369 (12%) | 147 (16%) | 63 (15%)† | 0.0011 |
| Preoperative Medications | ||||||||||
| ACEi/ARBs | 437 (38%) | 304 (31%) | 119 (43%) | 62 (41%)† | 0.092 | 1786 (26%) | 939 (31%) | 328 (35%) | 149 (37%)‡ | <0.0001 |
| Aspirin | 783 (67%) | 510 (69%) | 205 (74%) | 112 (73%)† | 0.017 | 4792 (70%) | 2148 (70%) | 687 (73%) | 276 (68%) | 0.60 |
| Anticoagulants | 404 (35%) | 232 (32%) | 90 (32%) | 41 (27%) | 0.051 | 2279 (33%) | 1004 (33%) | 288 (31%) | 118 (29%) | 0.028 |
| Antiplatelets | 549 (47%) | 360 (49%) | 130 (47%) | 63 (41%) | 0.42 | 3736 (55%) | 1627 (53%) | 480 (51%) | 188 (46%)‡ | 0.0003 |
| Beta blockers | 705 (61%) | 446 (61%) | 187 (68%) | 98 (64%) | 0.086 | 3619 (53%) | 1736 (56%) | 549 (58%) | 251 (62%)‡ | <0.0001 |
| CCB | 361 (31%) | 259 (35%) | 101 (36%) | 49 (32%)† | 0.14 | 2051 (30%) | 964 (31%) | 292 (31%) | 124 (30%) | 0.30 |
| Digitalis | 68 (6%) | 39 (5%) | 14 (5%) | 10 (7%) | 0.92 | 478 (9%) | 145 (5%) | 50 (5%) | 16 (4%)‡ | <0.0001 |
| Diuretics | 287 (25%) | 229 (31%) | 103 (37%) | 59 (39%)‡ | <0.0001 | 1149 (17%) | 645 (21%) | 251 (27%) | 138 (34%)‡ | <0.0001 |
| Inotropes | 18 (2%) | 10 (1%) | 5 (2%) | 2 (1%) | 1.0 | 84 (1%) | 27 (1%) | 7 (1%) | 1 (<1%)† | 0.015 |
| Hypolipidemics | 490 (42%) | 329 (45%) | 141 (51%) | 75 (49%)‡ | 0.0061 | 2516 (37%) | 1228 (40%) | 426 (45%) | 197 (48%)‡ | <0.0001 |
| Nitrates | 170 (15%) | 110 (15%) | 48 (17%) | 17 (11%) | 0.92 | 1201 (18%) | 529 (17%) | 141 (15%) | 58 (14%) | 0.024 |
Isolated primary coronary artery bypass grafting procedures between 1992–2011.
Exact Cochran-Armitage trend test.
P<0.05,
P<0.01 for Fisher’s exact test comparing obese with non-obese individuals.
Missing values imputed using expectation-maximization (EM) algorithm (n=10 simulations; imputation efficiency>99.5%).
ACEi=angiotensin converting enzyme inhibitor; ARB=angiotensin receptor blocker; BMI=body mass index (kg/m2); Non-Obese: 18.5≤BMI<30, Obesity Class I: 30≤BMI<35, Obesity Class II: 35≤BMI<40, Obesity Class III: BMI≥ 40; CCB=calcium channel blocker; LMCA=left main coronary artery; LVEF=left ventricular ejection fraction; MI=myocardial infarction; PAD=peripheral arterial disease
Approximately 91% of patients underwent cardiopulmonary bypass (Table 2). The median length of hospital stay following surgery was 5 days (IQR=3.0). Increasing severity of obesity was associated with an increasing percentage of patients with renal failure (Ptrend=0.0022) and POAF (Ptrend=0.0021) among black but not white patients (Ptrend≥0.5).
Table 2.
Perioperative characteristics (N=13,594)
| Black | White | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-Obese | Obesity Class₴ | Ptrend § | Non-Obese | Obesity Class₴ | Ptrend § | |||||
| I | II | III | I | II | III | |||||
| Median [IQR] | Median [IQR] | |||||||||
| Overall | 1165 (50%) | 734 (32%) | 277 (12%) | 153 (7%) | --- | 6846 (61%) | 3073 (27%) | 939 (8%) | 407 (4%) | --- |
| Characteristics | ||||||||||
| CPB | 1045 (90%) | 660 (90%) | 242 (87%) | 135 (88%) | 0.35 | 6240 (91%) | 2837 (92%) | 863 (92%) | 368 (90%) | 0.42 |
| Cross-clamp time (min) | 57 [27] | 58 [29] | 57 [26] | 54 [24] | 0.15 | 59 [28] | 60 [30] | 61 [29] | 59 [33]‡ | 0.10 |
| Perfusion time (min) | 92 [41] | 91 [43] | 90 [42] | 87 [38] | 0.27 | 95 [42] | 96 [43] | 96 [46] | 96 [46] | 0.30 |
| Total ICU time (hours) | 26 [26] | 24 [15] | 27 [25] | 29 [40]† | 0.65 | 24 [20] | 24 [19] | 24 [23] | 25 [17] | 0.77 |
| Hospital LOS (days) | 5 [3] | 5 [3] | 5 [3] | 6 [3] | 0.34 | 5 [3] | 5 [2] | 5 [3] | 5 [3] | 0.36 |
| Superficial SI¥ | 3 (<1%) | 3 (<1%) | 3 (1%) | 1 (1%) | 0.16 | 15 (<1%) | 10 (<1%) | 2 (<1%) | 4 (1%) | 0.042 |
| Deep SI¥ | 6 (1%) | 5 (1%) | 0 (0%) | 2 (1%) | 0.76 | 18 (<1%) | 17 (1%) | 11 (1%) | 5 (1%) | <0.0001 |
| Sepsis¥ | 11 (1%) | 4 (1%) | 3 (1%) | 1 (1%) | 0.80 | 53 (1%) | 16 (1%) | 6 (1%) | 4 (1%) | 0.67 |
| Myocardial infarction¥ | 3 (<1%) | 1 (<1%) | 1 (<1%) | 0 (0%) | 0.82 | 41 (1%) | 13 (<1%) | 3 (<1%) | 0 (0%) | 0.044 |
| Stroke¥ | 31 (3%) | 17 (2%) | 8 (3%) | 2 (1%) | 0.51 | 81 (1%) | 24 (1%) | 8 (1%) | 4 (1%) | 0.16 |
| Pneumonia¥ | 25 (2%) | 13 (2%) | 8 (3%) | 3 (2%) | 0.87 | 117 (2%) | 39 (1%) | 17 (2%) | 7 (2%) | 0.67 |
| ARDS¥ | 11 (1%) | 3 (<1%) | 1 (<1%) | 3 (2%) | 1.0 | 63 (1%) | 24 (1%) | 7 (1%) | 6 (1%) | 0.90 |
| Renal failure¥ | 21 (2%) | 14 (2%) | 12 (4%) | 8 (5%) | 0.0022 | 80 (1%) | 39 (1%) | 10 (1%) | 7 (2%) | 0.59 |
| POAF¥ | 174 (15%) | 108 (15%) | 58 (21%) | 36 (34%) | 0.0021 | 1627 (24%) | 685 (22%) | 215 (23%) | 100 (25%) | 0.50 |
| Operative mortality¥ | 36 (3%) | 16 (2%) | 5 (2%) | 10 (7%) | 0.45 | 210 (3%) | 51 (2%) | 22 (2%) | 12 (3%) | 0.023 |
Isolated primary coronary artery bypass grafting procedures between 1992 and 2011.
Exact Cochran-Armitage trend test.
P<0.05,
P<0.01 for comparing obese with non-obese individuals (Fisher’s exact test for categorical characteristics; Deuchler-Wilcoxon for continuous characteristics).
Missing values imputed using expectation-maximization (EM) algorithm (n=10 simulations; imputation efficiency>99.5%).
Includes events within 30 days of CABG in or out of our hospital and after 30 days during the same hospitalization following surgery.
ARDS=acute respiratory distress syndrome; BMI=body mass index (kg/m2), Non-Obese: 18.5≤BMI<30, Obesity Class I: 30≤BMI<35, Obesity Class II: 35≤BMI<40, Obesity Class III: BMI≥ 40; CABG=coronary artery bypass graft surgery; CPB=cardiopulmonary bypass; ICU=intensive care unit; IQR=interquartile range; LOS=length of stay; POAF=postoperative atrial fibrillation; SI=sternal infection.
Similarly, the relative risk for POAF, adjusted for demographics and prior medical history/comorbidities, increased with obesity severity among black (Ptrend<0.0001) but not white (Ptrend=0.33) patients (Pinteraction=0.0009) (Table 3). The adjusted interaction effect remained statistically significant when the analysis was further stratified by age group, a known risk factor for POAF (≤65 years: Pinteraction=0.022; >65 years, Pinteraction=0.013) (not shown in tables).
Table 3.
Univariable and multivariable relative risk for postoperative atrial fibrillation (POAF) (N=13,594)ⱴ
| Characteristics₰ | Univariable§ | Multivariable§ | ||
|---|---|---|---|---|
| Black RR (95% CI)Ϟ |
White RR (95% CI)Ϟ |
Black RR (95% CI)Ϟ |
White RR (95% CI)Ϟ |
|
| Obesity₴ | ||||
| Non-obese (18.5≤BMI<30) | 1.0 Referent | 1.0 Referent | 1.0 Referent | 1.0 Referent |
| Class 1 (30≤BMI<35) | 0.99 (0.79–1.2) | 0.94 (0.87–1.01) | 1.0 (0.83–1.3) | 0.97 (0.90–1.1) |
| Class 2 (35≤BMI<40) | 1.4 (1.1–1.8) | 0.96 (0.85–1.1) | 1.6 (1.2–2.1) | 1.0 (0.92–1.2) |
| Class 3 (BMI≥ 40) | 1.6 (1.1–2.2) | 1.0 (0.87–1.2) | 1.8 (1.3–2.5) | 1.2 (0.97–1.4) |
| Ptrend¥=0.0021 | Ptrend¥=0.49 | Ptrend¥<0.0001 | Ptrend¥=0.33 | |
| Demographics | ||||
| Age >65 | 2.0 (1.7–2.4) | 1.9 (1.8–2.0) | 2.1 (1.7–2.5) | 1.9 (1.7–2.0) |
| Male | 1.1 (0.87–1.3) | 1.1 (1.008–1.2) | 1.3 (1.04–1.5) | 1.2 (1.1–1.3) |
| Prior Medical History/Comorbidities | ||||
| Diabetes | 1.1 (0.92–1.3) | 1.1 (1.009–1.1) | 0.97 (0.80–1.2) | 0.99 (0.92–1.1) |
| Unstable heart failure | 1.5 (1.2–1.8) | 1.5 (1.4–1.6) | 1.4 (1.1–1.7) | 1.4 (1.3–1.5) |
| Hypertension | 1.5 (1.1–2.1) | 1.4 (1.3–1.5) | 1.4 (1.02–1.9) | 1.3 (1.2–1.4) |
| Peripheral artery disease | 1.2 (0.97–1.6) | 1.4 (1.2–1.5) | 1.1 (0.88–1.4) | 1.2 (1.1–1.3) |
| Three-vessel disease | 1.1 (0.93–1.4) | 1.3 (1.2–1.4) | 1.1 (0.88–1.3) | 1.1 (1.07–1.2) |
Isolated primary coronary artery bypass grafting procedures between 1992 and 2011.
Comparison group for binary variables was the absence of the characteristic.
RR and 95% CI computed using maximum likelihood log-binomial regression.
Missing values imputed using expectation-maximization (EM) algorithm (n=10 simulations; imputation efficiency>99.5%).
Likelihood ratio test for interaction of Race × Obesity (univariable, P=0.0020; multivariable, P=0.0009).
Likelihood ratio test for linear trend.
BMI=body mass index; CI=confidence interval; POAF=postoperative atrial fibrillation; RR=relative risk.
The pairwise addition of other variables listed in Table 1 did not substantively change our results. Adjusting for year period of surgery (<2000 vs. ≥2000) also had little effect.
Discussion
In both the general population and following cardiac surgery, blacks have a lower incidence of AF than whites, despite having more cardiovascular risk factors and increased incidence of stroke.(2, 13) However, in a recent population-based study of 5,685 community-dwelling adults ≥65 years, increasing BMI was linearly associated with chronic/persistent AF risk among blacks but not whites (Pinteraction=0.01), prompting the current stratified analysis by race.(2) While the association of obesity and POAF is well established in the CABG literature, our study, to our knowledge, is the first to examine and demonstrate a differential influence of race on this association.(1)
Obese patients, owing to their generally increased blood pressure levels, are believed to tolerate higher doses of certain medications known to be cardioprotective against AF with a side effect of hypotension (e.g., beta-blockers, renin-angiotensin-aldosterone-system inhibitors, and aldosterone antagonists).(14) However, their use in black patients is frequently questioned, especially among hypertensive and heart failure patients.(15) Thiazide diuretics or calcium channel blockers, for example, are the preferred first-line therapy for hypertension (HTN) among black patients compared with thiazide diuretics or beta-blockers among white patients.(16) Accordingly, POAF risk may be higher among black CABG patients because of their lower use of prophylactic AF medications.
Chronic, low-grade inflammation has been reported to play a role in the pathogenesis of obesity and its relationship to cardiovascular disease.(17, 18) Acute phase proteins (e.g., C-reactive protein (CRP)) and proinflammatory cytokines (e.g., IL-1, IL-6, TNF-α) are commonly increased in obesity.(17, 18) For example, obese individuals have greater plasma concentrations and more than ten times the amount of IL-6 in adipose tissue than their leaner counterparts.(18) The enlarged adipose tissue is a major site of inflammatory mediators and releases an abundance of proinflammatory cytokines and CRP into circulation.(17) Increased postoperative levels of IL-2, IL-6, IL-8 and CRP have been observed to be associated with AF among CABG patients.(19–21) In theory, obese blacks, who tend to manifest higher levels of serum IL-6 and CRP than obese whites, may be more susceptible to POAF following CABG.(22) Future research efforts will benefit from the collection of inflammatory biomarkers associated with obesity and POAF.
Our results may be explained by factors not completely controlled for in our analysis. For example, the left ventricular septum is more hypertrophic in the black population, which is believed to represent cardiac remodeling related to HTN.(23) To minimize the potential confounding effect of this risk factor for POAF, we excluded patients with preoperative AF and adjusted our multivariable models for HTN. While this strategy was effective, some residual confounding may still exist given that preoperative HTN was recorded as a dichotomous variable.
In contrast, increased left atrial diameter, a known risk factor for AF, is more prevalent among the white population and also is associated with obesity.(23) Again, by excluding pre-operative AF, we likely reduced the predominance of patients with increased left atrial diameters. However, because left atrial diameter measurements were not routinely documented in the health records during the study period, we cannot rule out that some of these patients remained in our analysis. Nonetheless, such bias probably would have been towards the null given that increased left atrial diameter is less commonly associated with being black. Additionally, increased levels of b-type natriuretic peptide (BNP), which has been shown to confer a significant risk for POAF, is associated with diastolic dysfunction and a subsequent enlarged left atrium, independent of signs or symptoms of cardiac dysfunction.(24, 25) We were unable to adjust our results for preoperative levels of BNP, which was only collected if a patient manifested fluid overload. However, our multivariable analysis was adjusted for unstable heart failure.
The increased risk of POAF observed among obese black patients could be attributed to an overall lower use of certain cardioprotective medications. In our data, there was a linearly increasing trend of ACEi/ARB and beta-blocker use with respect to obesity, but only among white patients (Table 1). Nonetheless, the pairwise post-hoc inclusion of preoperative medications into our multivariable model did not substantively change our results.
BMI has been criticized as being a less than ideal surrogate marker for body fat in coronary heart disease patients and lacking broad construct validity.(26) Specifically, this measure fails to take into account fat mass/fat-free mass ratio, cardiorespiratory fitness, and body fat distribution.(27) Categorization by BMI also may have contributed to internal validity bias.(28) However, BMI was grouped according to the widely implemented WHO classification scheme.(6) Furthermore, BMI is generally recommended as a practical approach for assessing body fat in the clinical setting and has been shown to be a better gauge of total body fat than body weight alone.(29)
Newly identified genetic polymorphisms, such as rs10504554 (LY96) and rs2200733 (PITX2), have been associated with increased POAF risk. While such markers may be important to our findings, DNA was not collected in our study.(30)
Acknowledgments
The authors would like to thank the East Carolina Heart Institute for providing resources to conduct this study. Portions of this study were funded by the National Institutes of Health grant R01-HL122863 to Ethan J. Anderson, Jimmy T. Efird and Alan P. Kypson.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors of this manuscript have no conflict of interest to disclose.
References
- 1.Filardo G, Hamilton C, Hamman B, Hebeler RF, Jr, Grayburn PA. Relation of obesity to atrial fibrillation after isolated coronary artery bypass grafting. Am J Cardiol. 2009;103:663–666. doi: 10.1016/j.amjcard.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 2.Jensen PN, Thacker EL, Dublin S, Psaty BM, Heckbert SR. Racial differences in the incidence of and risk factors for atrial fibrillation in older adults: the cardiovascular health study. J Am Geriatr Soc. 2013;61:276–280. doi: 10.1111/jgs.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Neal WT, Efird JT, Davies SW, O'Neal JB, Anderson CA, Ferguson TB, et al. Impact of race and postoperative atrial fibrillation on long-term survival after coronary artery bypass grafting. J Card Surg. 2013;28:484–491. doi: 10.1111/jocs.12178. [DOI] [PubMed] [Google Scholar]
- 4.LaPar DJ, Speir AM, Crosby IK, Fonner E, Jr, Brown M, Rich JB, et al. Postoperative atrial fibrillation significantly increases mortality, hospital readmission, and hospital costs. Ann Thorac Surg. 2014;98:527–533. doi: 10.1016/j.athoracsur.2014.03.039. discussion 33. [DOI] [PubMed] [Google Scholar]
- 5.Adult cardiac surgery database v2.81 training manual [Internet] The Society of Thoracic Surgeons. 2015 Available from: http://www.sts.org/sites/default/files/documents/MayTrainingManual.pdf. [Google Scholar]
- 6.Organization WH. BMI Classification 2006. [updated 10/13/2015]; Available from: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
- 7.De Schutter A, Lavie CJ, Kachur S, Patel DA, Milani RV. Body composition and mortality in a large cohort with preserved ejection fraction: untangling the obesity paradox. Mayo Clin Proc. 2014;89:1072–1079. doi: 10.1016/j.mayocp.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Corcoran C, Mehta C. Exact level and power of permutation, bootstrap, and asymptotic tests of trend. J Mod Appl Stat Meth. 2002;1:42–51. [Google Scholar]
- 9.Little RJ, D'Agostino R, Cohen ML, Dickersin K, Emerson SS, Farrar JT, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367:1355–1360. doi: 10.1056/NEJMsr1203730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blizzard L, Hosmer DW. Parameter estimation and goodness-of-fit in log binomial regression. Biom J. 2006;48:5–22. doi: 10.1002/bimj.200410165. [DOI] [PubMed] [Google Scholar]
- 11.Bozdogan H. Model selection and Akaike's Information Criterion (AIC): The general theory and its analytical extensions. Psychometrika. 1987;52:345–370. [Google Scholar]
- 12.Holly EA, Whittemore AS, Aston DA, Ahn DK, Nickoloff BJ, Kristiansen JJ. Anal cancer incidence: genital warts, anal fissure or fistula, hemorrhoids, and smoking. J Natl Cancer Inst. 1989;81:1726–1731. doi: 10.1093/jnci/81.22.1726. [DOI] [PubMed] [Google Scholar]
- 13.Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35:426–431. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 14.Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102. doi: 10.1016/j.jchf.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Johnson JA. Ethnic differences in cardiovascular drug response: potential contribution of pharmacogenetics. Circ. 2008;118:1383–1393. doi: 10.1161/CIRCULATIONAHA.107.704023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shulman NB. Treatment of hypertension in black patients with angiotensin-converting enzyme inhibitors. J Natl Med Assoc. 1988;80:265–272. [PMC free article] [PubMed] [Google Scholar]
- 17.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 19.Li T, Sun ZL, Xie QY. Meta-analysis identifies serum C-reactive protein as an indicator of atrial fibrillation risk after coronary artery bypass graft. Am J Ther. 2015 doi: 10.1097/MJT.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 20.Wu ZK, Laurikka J, Vikman S, Nieminen R, Moilanen E, Tarkka MR. High postoperative interleukin-8 levels related to atrial fibrillation in patients undergoing coronary artery bypass surgery. World J Surg. 2008;32:2643–2649. doi: 10.1007/s00268-008-9758-7. [DOI] [PubMed] [Google Scholar]
- 21.Hak L, Mysliwska J, Wieckiewicz J, Szyndler K, Siebert J, Rogowski J. Interleukin-2 as a predictor of early postoperative atrial fibrillation after cardiopulmonary bypass graft (CABG) J Interferon Cytokine Res. 2009;29:327–332. doi: 10.1089/jir.2008.0082.2906. [DOI] [PubMed] [Google Scholar]
- 22.Morimoto Y, Conroy SM, Ollberding NJ, Kim Y, Lim U, Cooney RV, et al. Ethnic differences in serum adipokine and C-reactive protein levels: the multiethnic cohort. Int J Obes (Lond) 2014;38:1416–1422. doi: 10.1038/ijo.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcus GM, Olgin JE, Whooley M, Vittinghoff E, Stone KL, Mehra R, et al. Racial differences in atrial fibrillation prevalence and left atrial size. Am J Med. 2010;123:375, e1–e7. doi: 10.1016/j.amjmed.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amar D, Zhang H, Shi W, Downey RJ, Bains MS, Park BJ, et al. Brain natriuretic peptide and risk of atrial fibrillation after thoracic surgery. J Thorac Cardiovasc Surg. 2012;144:1249–1253. doi: 10.1016/j.jtcvs.2012.06.051. [DOI] [PubMed] [Google Scholar]
- 25.Raman T, Roistacher N, Liu J, Zhang H, Shi W, Thaler HT, et al. Preoperative left atrial dysfunction and risk of postoperative atrial fibrillation complicating thoracic surgery. J Thorac Cardiovasc Surg. 2012;143:482–487. doi: 10.1016/j.jtcvs.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond) 2008;32:959–966. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heymsfield SB, Cefalu WT. Does body mass index adequately convey a patient's mortality risk? JAMA. 2013;309:87–88. doi: 10.1001/jama.2012.185445. [DOI] [PubMed] [Google Scholar]
- 28.Filardo G, Hamilton C, Hamman B, Ng HK, Grayburn P. Categorizing BMI may lead to biased results in studies investigating in-hospital mortality after isolated CABG. J Clin Epidemiol. 2007;60:1132–1139. doi: 10.1016/j.jclinepi.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults--The evidence report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 30.Kertai MD, Li YJ, Ji Y, Qi W, Lombard FW, Shah SH, et al. Genome-wide association study of new-onset atrial fibrillation after coronary artery bypass grafting surgery. Am Heart J. 2015;170:580–590. e28. doi: 10.1016/j.ahj.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]