Abstract
In the past few decades, nucleoside analog drugs have been used to treat a large variety of cancers. These antimetabolite drugs mimic nucleosides and interfere with chain lengthening upon incorporation into the DNA or RNA of actively replicating cells. However, efficient delivery of these drugs is limited due to their pharmacokinetic properties, and tumors often develop drug resistance. In addition, nucleoside analogs are generally hydrophilic, resulting in poor bioavailability and impaired blood-brain barrier penetration. Conjugating these drugs to lipids modifies their pharmacokinetic properties and may improve in vivo efficacy. This review will cover recent advances in the field of conjugation of phospholipids to nucleoside analogs. This includes conjugation of myristic acid, 12-thioethyldodecanoic acid, 5-elaidic acid esters, phosphoramidate, and self-emulsifying formulations. Relevant in vitro and in vivo data will be discussed for each drug, as well as any available data from clinical trials.
Keywords: Nucleoside analog, lipid conjugate, drug development, liposomes
1. Introduction
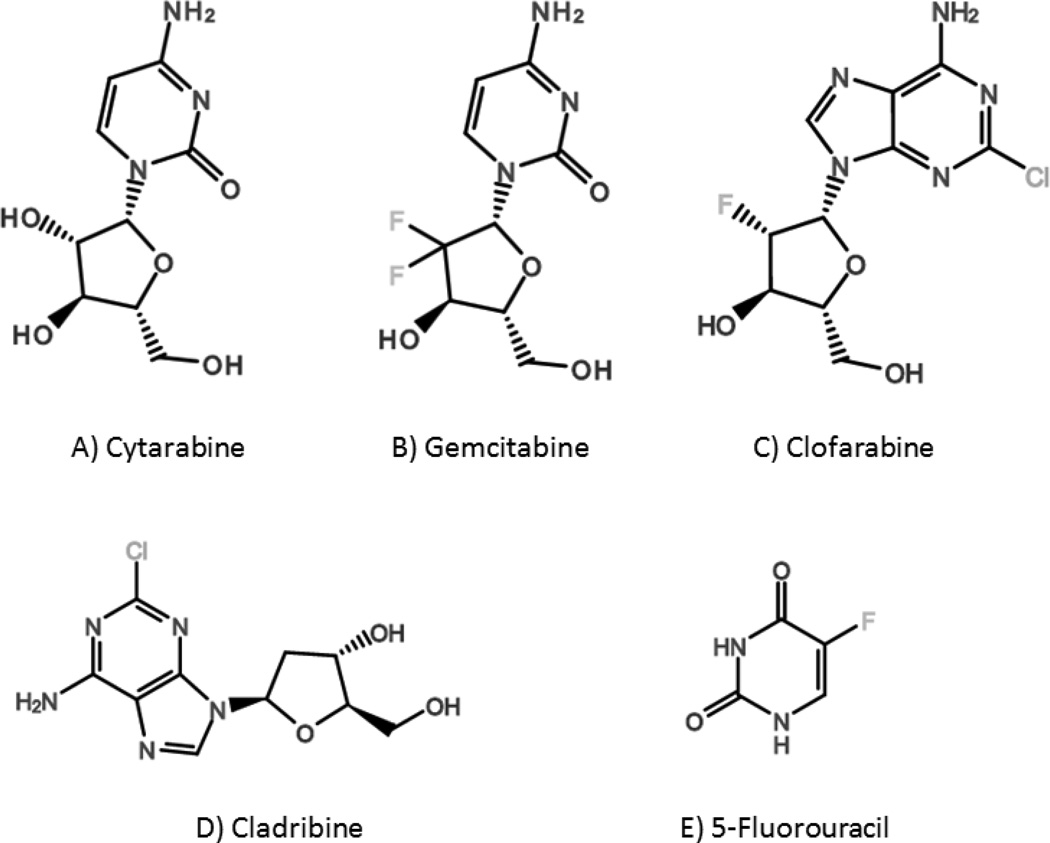
Nucleoside analogs are a class of synthetic cytotoxic antimetabolite drugs commonly used as primary treatment for a variety of cancers, particularly hematologic malignancies (Figure 1). These drugs closely resemble endogenous purine and pyrimidine nucleosides, and their primary mechanism of action is chain termination upon incorporation into nucleic acid strands via inhibition of DNA or RNA polymerases.
Figure 1.
Chemical structures of conventional nucleoside analogs: A) Cytarabine, B) Gemcitabine, C) Clofarabine, D) Cladribine, and E) 5-Fluorouracil.
Nucleoside analogs require transport by the human equilibrative nucleoside transporter 1 (hENT1) to enter cells [1]. Once inside the cell, phosphorylation to the monophosphate form of the drug is mediated by cellular kinases, which is often the rate-limiting step of activation [2]. Two further phosphorylation steps are required to produce the active triphosphate form, which can then be incorporated into growing nucleic acid chains. Once incorporated, these drugs cause DNA chain termination and DNA strand breaks, leading to apoptosis [3]. The nucleoside analogs, gemcitabine and clofarabine, also can inhibit ribonucleotide reductase [4, 5], and two others, decitabine and azacytidine, can inhibit DNA methyltransferases [6]. The activity of these drugs depends on their incorporation into replicating DNA during S phase of the cell cycle and is not specific to cancer cells; rapidly dividing normal cells (including those in the bone marrow, gastrointestinal tract, and hair follicles) are often damaged as well. This results in the unwanted side effects that limit clinical administration of nucleoside analogs including myelosuppression, mucositis, and hair loss.
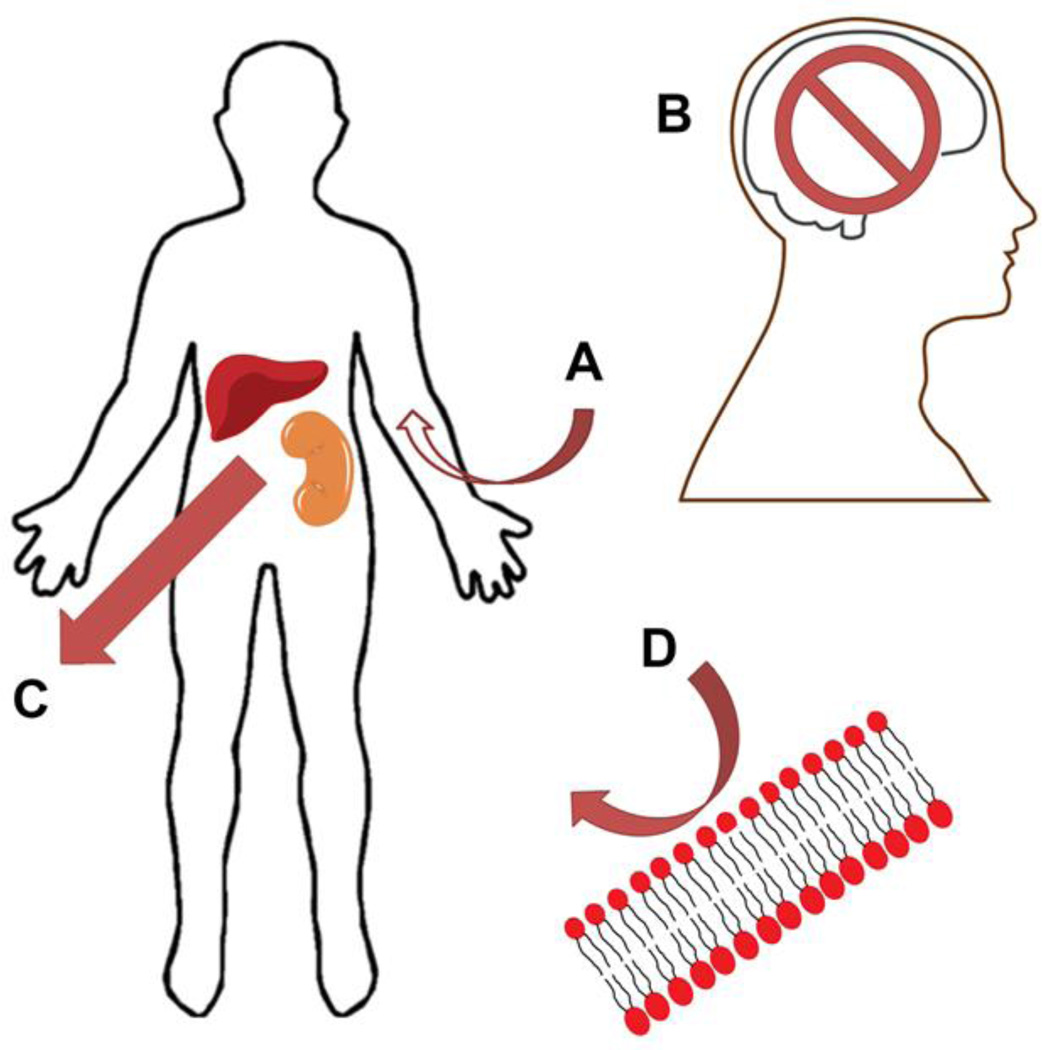
Many patients develop resistance to nucleoside analog agents, ultimately reducing their clinical benefit. Resistance mechanisms include reduced drug uptake due to decreased expression of transport proteins such as hENT1, increased activity of the P-glycoprotein drug efflux pump, lower rates of drug activation due to loss of deoxycytidine kinase (dCK) expression, and inactivation due to deamination by cytidine deaminase for Ara-C and gemcitabine or ribonucleotide reductase and nuclear exonucleases for fludarabine [7]. Conventional nucleoside analogs exhibit poor passive diffusion across the gastrointestinal tract and require active transport by either concentrative or equilibrative transporters [8]. All these factors limit oral bioavailability of these drugs, so they must be given intravenously [1]. The short comings of current nucleoside analogs are summarized in figure 2. Researchers began modifying nucleoside analogs shortly after they were first approved for clinical use. The development of novel pro-drugs or conjugates by attaching various lipid moieties to the parental drugs has improved their pharmacokinetic properties, including uptake, plasma half-life, and activity in vivo. This review will focus on recent advances in nucleoside analogs conjugated to phospholipid groups, fatty acids or packaged in liposomes.
Figure 2.
Graphical depiction of the common short comings of nucleoside analogs. A) Inconvenient administration, B) Minimal blood brain barrier penetration at standard doses, C) Rapid elimination and short half-life D) Do not penetrate the tumor cell membrane.
2. Lipid-Drug Conjugate Chemistry
Most lipid-drug conjugate chemistry utilizes liposomal incorporation, salt formation with a fatty acid, or covalent linkage of esters, ethers, glycerides, or phospholipids to create novel pro-drugs [9–11]. Fatty acid conjugates generally attach a drug with an amino or alcohol function to the carboxylate group of the fatty acid or attach the drug to the ω-position of a modified fatty acid. In phospholipid conjugates, the drug is linked via the phosphate group or glycerol backbone of the phospholipid. Alternatively, one or two fatty acids of the phospholipid can be replaced by the drug. Glycerides can form drug conjugates via an ester bond with carboxylate-containing drugs [11]. Unilamellar liposomes are primarily composed of cholesterol and phospholipids (either phosphoglycerides or sphingolipids). Liposomal drugs are well-studied and have altered tissue distribution compared to their parental drugs, as the conjugated drugs tend to take on the pharmacokinetic properties of the liposomal carrier [12]. Liposomeen-capsulated drugs exhibit reduced elimination by the body [12] and generally have milder toxicity profiles [13, 14], as well as increased solubility, stability, and circulation [15–17] and can improve blood-brain barrier (BBB) penetration of the active drug [18]. These effects can vary depending on liposomal size, charge, rigidity, and dose [12].
2.1 Lipid-conjugated Drugs and the Blood-Brain Barrier
The BBB protects the brain and central nervous system (CNS) from circulating substances and is composed of several types of barriers, including the vascular BBB and blood-cerebrospinal fluid barrier (primarily the choroid plexus) [19]. The various barriers include the brain and choroid plexus endothelium (endothelial and capillary cells), astrocytes, pericytes, and microglia. Endothelial cells form tight and adherens junctions strengthened by astrocytes and pericytes and also highly express various transporter and efflux proteins. The BBB utilizes the ATP-binding cassette p-glycoprotein efflux pumps, including the multidrug resistant protein (MRP) family members, to transport biologically active molecules away from the brain [20]. In particular, hydrophilic drugs have difficulty crossing the BBB due to the lack of active transport and drug efflux pumps. These features act as a physical and transport barrier that effectively block most drugs (98% of all small-molecule drugs) from entering the brain and CNS [21].
Drugs can cross the BBB passively by transmembrane diffusion, especially if they are lipid soluble and have a molecular weight under 600 Da. To a lesser degree, other factors that influence drug solubility across the BBB include tertiary structure, degree of protein binding, and charge [19]. Small lipid and amphipathic molecules including phosphatidylcholine and phosphatidylethanolamine are known to be effectively effluxed by p-glycoprotein pumps in an ATP-dependent fashion [22, 23]. An estimated 50% of clinically used anti-cancer therapeutic agents are effluxed by this system spanning a variety of drug types including taxanes, vinca alkaloids, campothecin, mitomycins, and anthracyclines [24]. Despite the fact that nucleoside analogs are generally not considered to be substrates of p-glycoprotein for efflux [25], the activity of these transporters must be taken into consideration when administering lipid-conjugated nucleoside analogs. Studies have found increased cellular concentrations of drug-lipid conjugates containing amphiphilic glycerides, polysorbates, and polyethoxylated castor oil. The increase in accumulation is believed to be due to inhibition of the activity of P-glycoprotein efflux pumps by the anionic amphiphilic lipid groups, resulting in reduced drug efflux [26]. Changes in uptake mechanisms and inhibition of P-glycoproteins are the most likely explanations for the increase in BBB penetration of lipid-conjugated drugs. The size and degree of lipid solubility are factors that must be taken into careful consideration for lipid-containing drugs to successfully deliver their payload with the brain and CNS.
2.2 Clinically Approved Cytarabine Conjugates
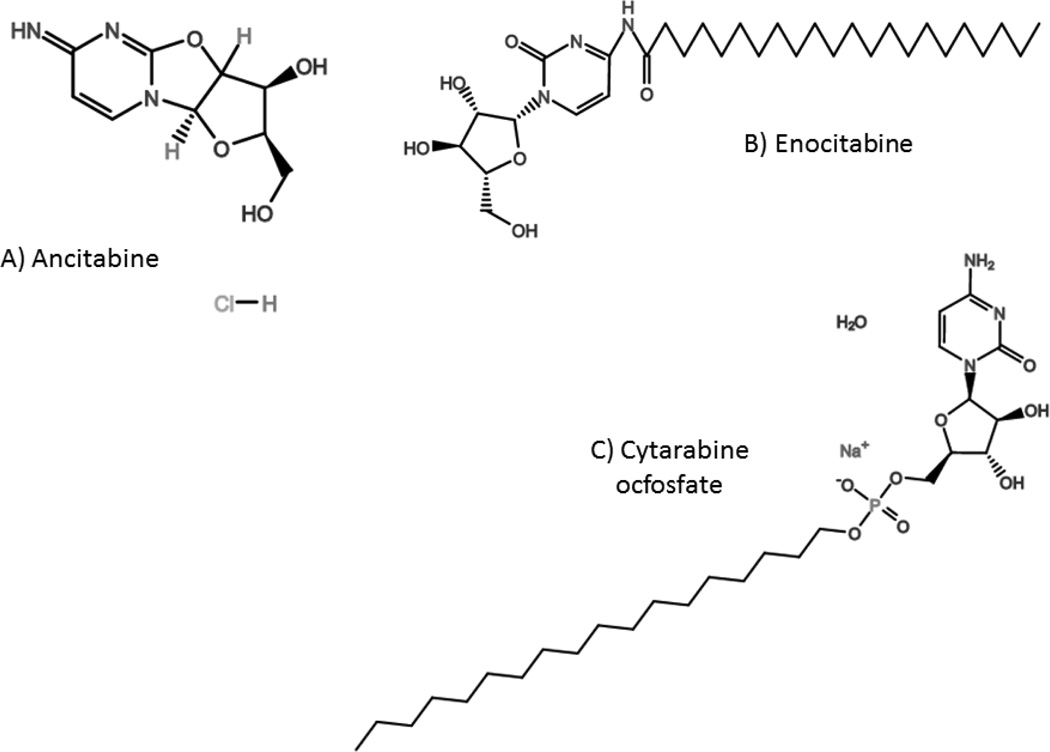
Based on advances in drug delivery, several lipid conjugates of cytarabine have been used clinically in Japan for the treatment of acute leukemias, including enocitabine, ancitabine and cytarabine ocfosfate (Figure 3). Enocitabine and ancitabine require dCK for activation and must be infused intravenously.
Figure 3.
Chemical structures of clinically approved cytarabine conjugates: A) Ancitabine, B) Enocitabine, and C) Cytarabine ocfosfate.
After demonstrating efficacy as a single agent or synergy in combination with cyclophosphamide, daunorubicin or vinblastine in a mouse model of leukemia [27], ancitabine was tested as a single agent in patients with melanoma [28] or combined with daunorubicin [29] or amsacrine [30] in children with refractory acute nonlymphocytic leukemia. This drug had virtually no effect in patients with melanoma and did not demonstrate superiority when combined with daunorubicin over patients treated with Ara-C and daunorubicin. Both studies also found significant toxicity, including thrombocytopenia and cardiotoxicity. Combination with amsacrine produced complete remission in almost half of evaluated patients, but about 10% of patients did not survive therapy.
Enocitabine was shown to significantly increase survival compared to Ara-C in a mouse model of leukemia [31, 32] and demonstrated prolonged release when administered i.v. in patients with acute leukemia [33, 34]. Despite the drug’s lipophilicity, poor CSF penetration was observed, while a significant increase in bone marrow fluid availability was seen compared to plasma [34]. In a Phase II study in patients with naïve AML, 36% of patients reached complete remission and 24% achieved partial remission, and increased response was correlated with higher doses [35]. Toxicity was found to be mild and acceptable. Enocitabine combined with mitoxantrone, 6-mercaptopurine and prednisolone was shown to be an effective treatment for naïve patients with acute leukemia [36], and combination with aclarubicin and prednisolone was an effective salvage therapy in patients with AML [37]. However, a more recent study found reduced remission and survival rates in patients with naïve AML treated with enocitabine compared to cytarabine [38]. These studies suggest that enocitabine has limited efficacy as a single agent, particularly in the relapsed setting, but can be highly effective when administered in combination with other therapeutics.
Cytarabine ocfosfate, a prodrug of cytarabine 5′-monophosphate conjugated to a long-chain fatty alcohol group, can be delivered orally and is resistant to deamination. It first demonstrated efficacy in a mouse model of leukemia [39, 40] and colorectal adenocarcinoma [41], and lipophilicity was found to be improved by conjugation of the long-chain fatty acyl group. Phase II trials testing subcutaneous or oral administration of cytarabine ocfosfate with interferon-α2b and found some success in improving survival and response rate in patients with hematological malignancies. Patients were unable to continue treatment due to side effects, and a modified dosing regimen might hold promise in the future [42–44]. Oral administration of cytarabine ocfosfate in AML patients with relapsed disease induced complete remission in ~10% of patients and partial remission and stable disease in some of the other patients studied. Pharmacokinetic analysis suggested intestinal and hepatic absorption of the drug, resulting in prolonged release mimicking continuous i.v. infusion. Off-target toxicities limited dose-escalation in these studies as well [45, 46]. A small combination study of orally administered cytarabine ocfosfate and etoposide showed higher induction of complete remission in treating patients with hematological malignancies compared to previous single agent or combination trials [47], and two recent follow-up studies supported these findings [48, 49].
Ancitabine, enocitabine and cytarabine ocfosfate have not been approved for clinical use in the United States. Their clinical efficacy appears to be ultimately limited by dCK dependence for activation and various toxic side effects [50]. They may have potential to treat hematological malignancies when used in combination with other drugs, but there appear to be few clinical trials still interested in testing these drugs.
DepoCyt (DTC 101) is an extended release formulation of cytarabine (8,9,11,17), produced by Sigma-Tau [51]. Cytarabine is encapsulated inside vesicles within a lipid foam composed mainly of cholesterol and dioleoylphosphatidylcholine [52]. This drug was developed to treat neoplastic and lymphomatous meningitis, fairly common symptoms of CNS infiltration by solid and hematologic malignancies [53, 54] that can quickly become fatal if left untreated [55]. Previous treatment options involved combinations of intrathecal chemotherapy using Ara-C, methotrexate, and steroids [56], high-dose systemic chemotherapy using methotrexate (5–8 g/m2) or Ara-C (1–7.5 g/m2), and radiation therapy [57–59]. These therapeutic options come with undesirable side effects, including increased toxicity, cognitive deficiency, infection, chemical meningitis, and inaccurate lumbar puncture [58]. Depocyt was shown to be more effective than intrathecally administered methotrexate [60, 61] or cytarabine [56, 62] in multiple tumor types. However, Depocyt still requires intrathecal administration and caused neurotoxicity in approximately 15% of patients tested [56]. The T1/2 of Depocyt was 141 hours in the brain and 277 hours in the lumbar space, compared to 3.4 and 2 hours for cytarabine, respectively [63–65]. This large difference in half-life is the most likely explanation for the increase in toxicity. Ideally, CNS involved cancer would be treated by a drug that does not require intrathecal administration and has reduced neurotoxic side effects.
2.3 Drug Duplex Conjugates
One strategy of modifying the pharmacokinetic properties of nucleoside analogs is to conjugate two drug molecules together. The lipophilicity of these conjugates can be increased by using a lipid backbone or by using a drug with lipid moieties attached. Utilizing drug duplexes is expected to improve cellular uptake and alter distribution profiles in vivo due to the conjugated drug’s lipophilic and amphiphilic properties. Previous studies suggest that the conjugated drugs act similarly to free drugs upon uptake and cleavage inside the cell. Delivering nucleoside analogs in this fashion may protect the drugs from inactivation or degradation due to lack of substrate recognition, resulting in improved intracellular delivery [66].
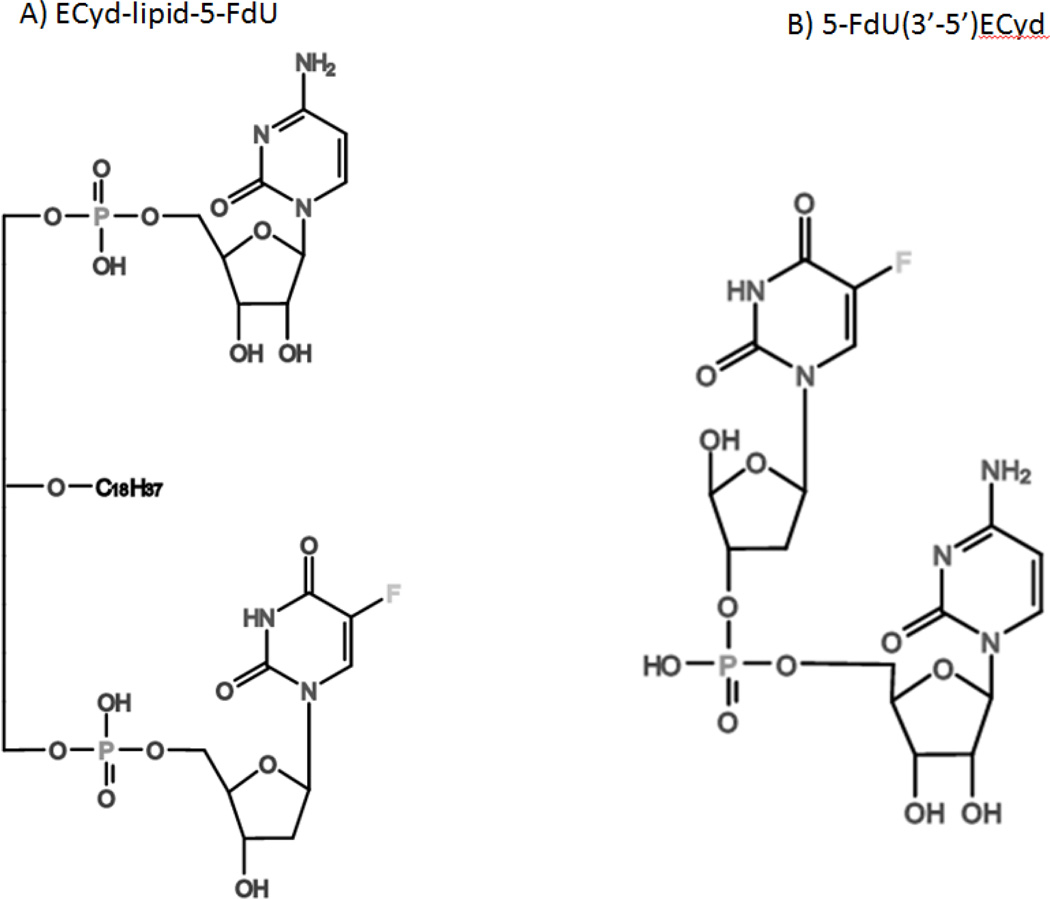
Teams led by Drs. Herbert Schott and Reto Schwendener created two novel drug duplexes by conjugating the 5-Fluorouracil (5-FU) derivative 2’-deoxy-5-fluorouridine (5-FdU) and a 3-ethynyl nucleoside (ECyd). These nucleoside analogs were either coupled directly via a phosphodiester bond – producing 2’-deoxy-5-fluorouridylyl-(3’–5’)-3’-C-ethynylcytidine (5-FdU(3’–5’)ECyd) – or indirectly via a lipophilic octadecylglycerophospholipid backbone, producing 3’-C-ethynylcytidinylyl-(5’→1-O)-2-O-octadecyl-sn-glycerylyl-(3’-O→5’)-2’-deoxy-5-luorouridine (ECyd-lipid-5-FdU) (Figure 4). Combining these two drugs should inhibit both DNA and RNA synthesis and may reduce chemoresistance. Both complexes are believed to require phosphodiesterase cleavage for activation. Cleavage of ECyd-lipid-5-FdU results in the monophosphate form of both drugs, while cleavage of 5-FdU(3’–5’)ECyd produces one drug in the monophosphate form and one that requires phosphorylation, depending on which side of the phosphate group is cleaved.
Figure 4.
Chemical structures of duplexes created by Drs. Herbert Schott and Reto Schwendener: A) ECyd-lipid-5-FdU and B) 5-FdU(3’–5’)ECyd. Both complexes contain the 5-FU derivative 2’-deoxy-5-fluorouridine and the novel 3’-ethynyl nucleoside 1-(3-C-ethynyl-beta-D-ribopentofuranosyl)cytosine.
5-FdU(3’–5’)ECyd and ECyd-lipid-5-FdU were screened against the NCI-60 cancer cell line panel. 5-FdU(3’–5’)ECyd acted additively, synergistically, or antagonistically in different cell lines compared to individual drug treatment of 5-FdU or ECyd [67]. These differences in cytotoxicity are hypothesized to be due to the chemical properties of the duplexes, including cytostatic potential and the direction of the cleaved phosphodiester bond [67]. These results highlight the importance of comparing the toxicity of multidrug complexes to simultaneous administration of their component drugs.
5-FdU(3’–5’)ECyd and ECyd-lipid-5-FdU were tested in various pre-clinical cancer models. Both drugs did not significantly affect survival in DBA/2J mice bearing L1210 murine leukemia cells. ECyd-lipid-5-FdU did have greater anti-leukemic activity compared to 5-FdU(3’–5’)ECyd treatment, most likely due to a combination of increased lipophilicity and intracellular release of two monophosphate nucleoside analogs. The duplexes had more off-target toxicity than 5-FdU alone but less than ECyd [68]. 5-FdU(3’–5’)ECyd was effective against in vivo melanoma models, but efficacy depended on dose and schedule [69]. 5-FdU(3’–5’)ECyd was found to be cleaved extracellularly and required nucleoside transporters for transport, unlike ECyd-lipid-5-FdU. Treatment with 5-FdU(3’–5’)ECyd, caused rapid nucleotide accumulation in the tumor site followed by the liver, whereas ECyd-lipid-5-FdU resulted in gradual nucleotide accumulation in the tumor and more rapid nucleoside uptake by the liver. However, these pharmacokinetic differences did not affect the anti-tumor response [70].
In vitro studies of these duplex drugs may underestimate their clinical benefit due to the benefit of their unique pharmacokinetic properties in vivo. Association with the lipid membrane by duplex drugs may delay their cytoplasmic release and active metabolism, resulting in prolonged intracellular exposure compared to unmodified parental drugs. Delivering these modified drugs in liposomal formulations may increase drug delivery by increasing solubility and stability, decreasing toxicity, and enhancing receptor-free tumor accumulation compared to the parental drugs [69]. More in vivo studies are required to determine if 5-FdU(3’–5’)ECyd and ECyd-lipid-5-FdU can be successfully delivered orally in other tumor models and to determine optimal dosing schedules. Rational combinations of drugs linked by lipid complexes may be a promising avenue for increasing anti-tumor efficacy of treatments and preventing chemoresistance.
2.4 Myristic Acid and 12-Thioethyldodecanoic Acid Conjugated Analogs
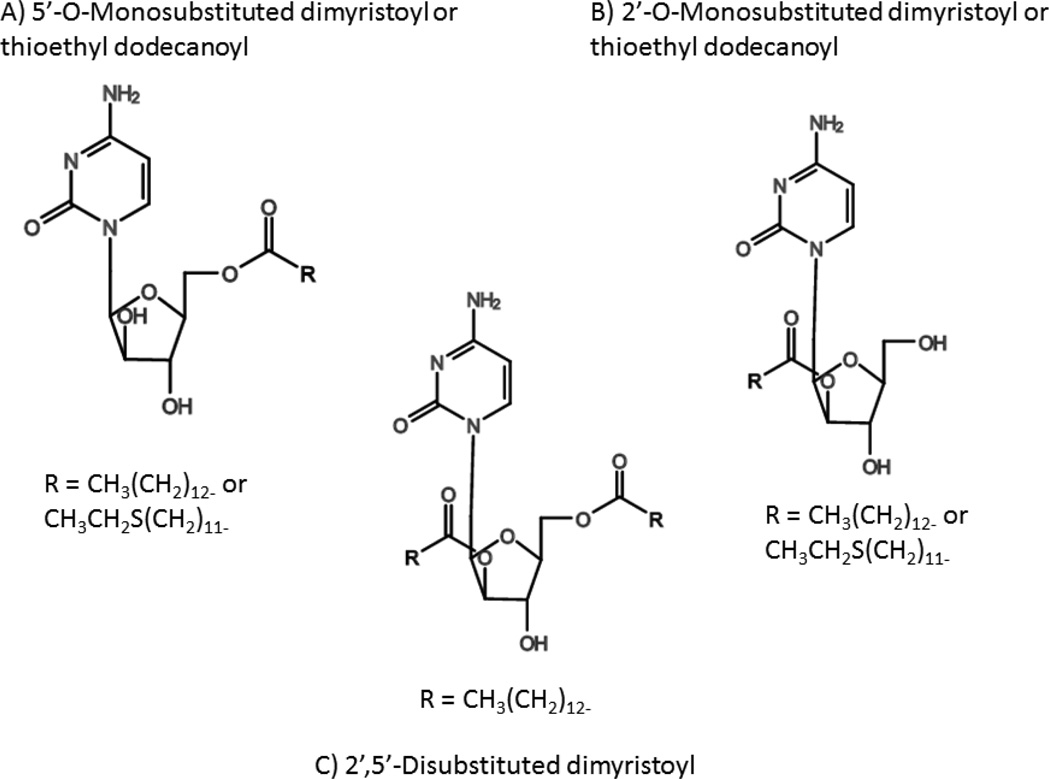
Previous work by the Parang group has shown that conjugation of fatty acid derivatives to antiviral agents changes their biological properties compared to the parent drugs. They recently applied this approach against cancer cells in vitro using several cytarabine conjugates, with the expectation of sustained release and improved cellular uptake of the drugs based on previous studies [71–73]. Chhikara et al. created cytarabine prodrugs by synthesizing three classes of lipophilic fatty acyl conjugates – 5’-O-substituted, 2’-O-substituted, and 2’,5’-disubstituted using myristic acid or 12-thioethyldodecanoic acid (Figure 5). These conjugates displayed varying antitumor effects in vitro against a human T cell leukemia line. The 5’-O-substituted compounds did not inhibit growth, suggesting that the location of substitution may prevent the release of cytarabine. The 2’,5’-dimyristoyl derivative was as effective as cytarabine in a time-dependent fashion, while 2’-fatty acyl derivatives of cytarabine demonstrated lower degrees of inhibition. These results show a clear dependence on chemical conformation of cytarabine derivatives for biological activity, and time-dependent cytotoxicity suggests a gradual intracellular release of the active drug. Further in vivo testing of these drugs is required to determine if the lipophilic formulation also increases bioavailability and cellular uptake, and the potential benefit of conjugating the monophosphate form of cytarabine to avoid dependency on dCK activation [74].
Figure 5.
Chemical structures of cytarabine prodrugs synthesized by Dr. Keykavous Parang: A) 5’-O-Monosubstituted dimyristoyl/thioethyl dodecanoyl, B) 2’-O-Monosubstituted dimyristoyl/thioethyl dodecanoyl, and C) 2’,5’-Disubstituted dimyristoyl.
2.5 Phosphoramidate Conjugated Analogs
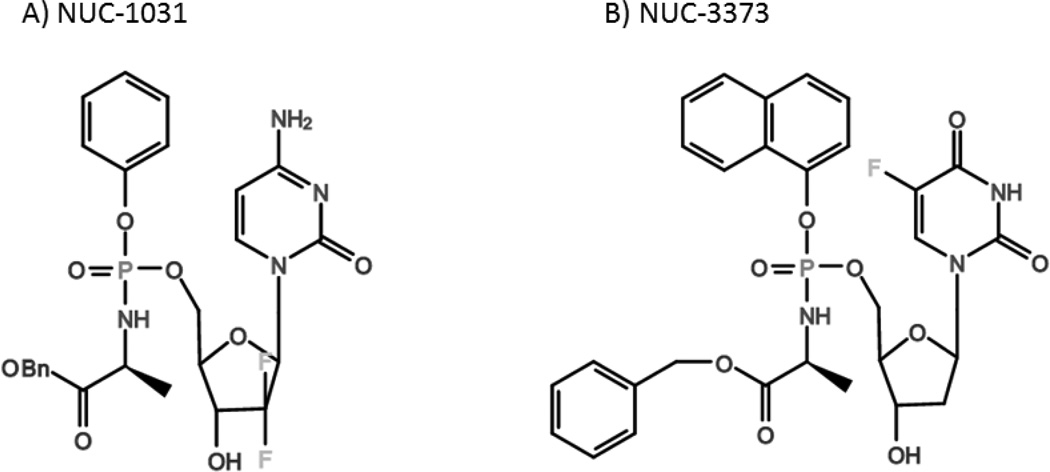
NuCana has developed several nucleoside analog pro-drugs by adding a phosphoramidate moiety (ProTide) to the monophosphate form of the drug (Figure 6) [75]. This moiety has been shown to protect the phosphate group, reducing metabolic degradation [76], as well as potentially increasing intracellular delivery of the active drug. NUC-1031 (Acelarin) is a gemcitabine monophosphate analog designed to bypass hENT1 uptake, dCK activation, and deactivation by cytidine deaminase [77, 78] and was found to be active against gemcitabine-resistant human pancreatic cancer cell lines in a xenograft model [79]. NUC-1031 is currently being tested in an ongoing Phase I/II trial in patients with various refractory solid cancers. Patients received multiple four-week cycles of NUC-1031 with two different schedules. The drug could be safely administered at four times the maximum tolerated dose (MTD) of gemcitabine, and over half the patients treated had stable disease. Five out of 36 patients were even able to achieve tumor shrinkage of over 30%.
Figure 6.
Chemical structures of NuCana’s phosphoramidate-conjugated prodrugs: A) NUC-1031 (Gemcitabine monophosphate) and B) NUC-3373 (5-FU).
Pharmacokinetic data revealed a 13-fold increase in the intracellular active form of gemcitabine (dFdCTP) compared to the parental form of gemcitabine, suggesting greater activity independent of increased dose [80]. NuCana plans on beginning Phase III trials in 2015 for patients with pancreatic, ovarian, non-small cell lung, and biliary cancers. The safety and efficacy of NUC-1031 in combination with carboplatin is currently being tested in a Phase IB trial for patients with recurrent ovarian cancer and will also be tested in patients with biliary cancers mid 2015.
NuCana is also modifying the pyrimidine analogs 5-FU and 5-fluoro-2'-deoxyuridine (FUdR) by attaching a phosphoramidate moiety to the active form of these drugs, fluorodeoxyuridine monophosphate (FdUMP). These modified drugs, NUC-3373 and NUC-3641, respectively, prevent degradation by phosphorolytic enzymes, bypass thymidine kinase-mediated activation, and are taken up passively. A screen of 39 FUDR conjugates against several human tumor cell lines found these two drugs to be much more cytotoxic in vitro than 5-FU, independent of thymidine kinase and hENT1 [81]. NuCana plans to begin testing NUC-3373 in clinical trials for patients with colorectal and non-small cell lung cancers mid 2015. The company is also in the early stages of applying the same lipid conjugate technology to fludarabine (NUC-4545), clofarabine (NUC-5435), and cladribine.
2.6 Phospholipid Gemcitabine Conjugate
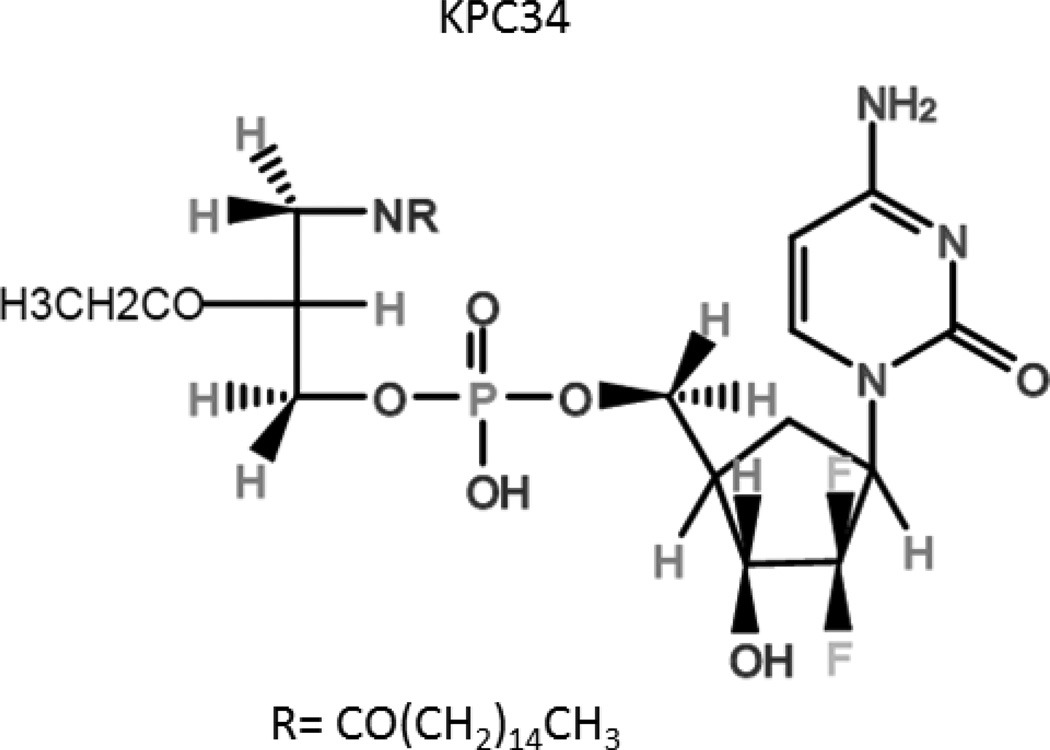
Kucera et al. has conjugated gemcitabine monophosphate to an amido-containing phospholipid moiety [82], producing the drug KPC34 (Figure 7). It was rationally designed to overcome multiple forms of chemoresistance, including dCK-independent activation and hENT1-free uptake, improve pharmacokinetics, increase BBB penetration, and inhibit PKC. KPC34 has been shown to be as effective or superior compared to gemcitabine treating several types of cancer in vitro and in vivo. In the human promyelocytic cell line HL60, pharmacological inhibition of hENT1 by dipyridamole decreased the IC50 of gemcitabine 35-fold, compared to only 4-fold for KPC34. In the same study, KPC34’s cytotoxicity was tested in the human breast cancer cell line MCF-7 and a clone over-expressing the multidrug resistance protein 1 (MDR1) efflux pump, BC-19. KPC34 maintained much of its cytotoxicity when compared to doxorubicin, a known substrate of MDR1. [82] Based on drugs previously synthesized with similar chemistry [83], KPC34’s phospholipid group is predicted to act as a diacyglycerol mimetic upon cleavage by a phospholipase C-like enzyme. Diacylglycerol is necessary for the activation of classical members of the PKC family by recruiting them to the plasma membrane. Increased PKC signaling is associated with chemoresistance in acute leukemia [84]. KPC34’s cleaved moiety may antagonize PKC signaling by sequestering PKC away from the plasma membrane and reducing its activation. Treatment of acute lymphoblastic leukemic cells in vitro with KPC34 inhibited PKC signaling by over 50% as assessed by immunoblotting for p-PKC α and βII (Alexander et al., manuscript in preparation).
Figure 7.
Chemical structure of KPC34, the gemcitabine phospholipid conjugate developed by Kucera, et al.
In the Lewis lung mouse tumor model 50 mg/kg of KPC34 prolonged survival as effectively as 120 mg/kg of gemcitabine. KPC34 could be administered orally with similar survival benefit, was well-tolerated, and had a greatly improved plasma half-life compared to gemcitabine [82]. In a syngeneic immunocompetent model of murine acute lymphoblastic leukemia, KPC34 was also well-tolerated and oral dosing provided a greater survival benefit compared to intraperitoneal administration of cytarabine, gemcitabine, or KPC34 (Alexander et al., manuscript in preparation). KPC34 overcame induced cytarabine chemoresistance in vivo and was more effective than gemcitabine. Cytarabine and gemcitabine share similar mechanisms of resistance. The efficacy of KPC34 in this model of chemoresistance suggests the value of phospholipid conjugation for improving outcomes.
The amphipathic nature of KPC34 may result in the formation of water-soluble lipid aggregates. In one report, KPC34 formed spherical particles in aqueous media with an average size of 115 nm [82]. The hENT-1 independent uptake suggests that KPC34 may enter the cell by endocytosis or passive diffusion across the cell membrane. Previous studies have shown that water-soluble small lipid molecules (400–600 Da) can cross the BBB via diffusion through the endothelial plasma membranes [85]. Improved survival seen with KPC34 versus gemcitabine in a naïve model of acute lymphoid leukemia may be due to increased clearance of CNS-involved disease, as hind limb paralysis occurred later in the KPC34 treated animals (studies ongoing). Further, KPC34 successfully restored motor function in mice that developed hind limb paralysis after treatment with cytarabine and doxorubicin, indirect evidence of its ability to cross the BBB (Alexander et al., unpublished data). KPC34 appears to be a promising new option for patients with relapsed acute lymphoblastic leukemia, and this drug is scheduled to enter early phase clinical trials in the next few years.
2.7 5-Elaidic Acid Ester Conjugated Analogs
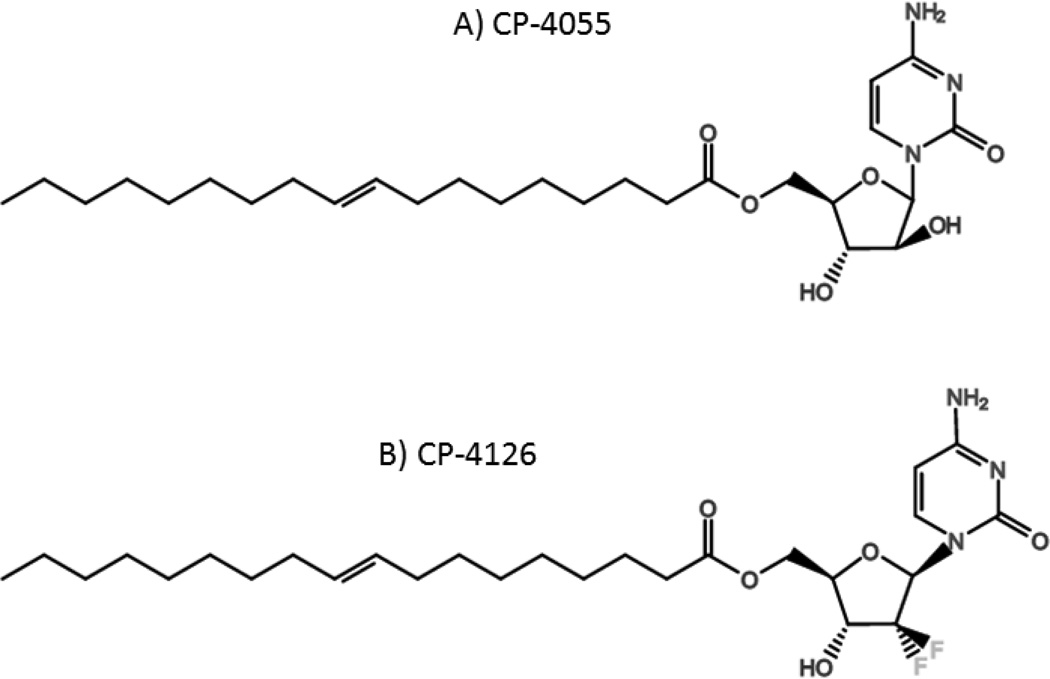
Clavis Pharmaceuticals developed and tested two novel lipid-conjugated nucleoside analogs, CP-4055 and CP-4126, in an attempt to improve the pharmacokinetics of cytarabine and gemcitabine respectively. CP-4055 (elacytarabine) is cytarabine covalently conjugated to elaidic acid ester at the 5’-OH of the ribose sugar, and CP-4126 is gemcitabine similarly attached to the same hydrophobic lipid moiety (Figure 8). The rationale for this conjugation is that linkage of fatty acids to the nucleoside analogs is predicted to increase receptor-independent drug uptake by passive diffusion across the plasma membrane, resulting in increased levels of active intracellular drug. The lipid formulation is also believed to increase plasma half-life and prevent intracellular deamination. These compounds are hydrolytically cleaved into their respective parent nucleoside analog, possibly by carboxylesterases, and require phosphorylation by dCK to reach the active triphosphate form [86]. In vitro studies demonstrated that the lipid formulation increased uptake of the prodrugs in a hENT-1-independent fashion compared to the parental forms of the drugs [87, 88]. Both novel drugs demonstrated gradual increased nuclear accumulation of the active drug compared to the parental drugs, even after drug removal. The gradual intracellular release of drug suggests that the lipid formulation may trap conjugates in an intracellular compartment after uptake [87]. Both CP-4055 and CP-4126 overcame resistance to cytarabine and gemcitabine in a human T-cell leukemia cell line lacking hENT1 [89, 90]. In mouse models using a variety of human cancer xenografts, CP-4126 was as effective as gemcitabine when administered intraperitoneally. When administered orally, CP-4126 was well tolerated and as effective as intraperitoneal delivery [88]. A significant increase in survival after CP-4055 treatment was seen compared to cytarabine in a variety of human xenograft models in mice [91].
Figure 8.
Chemical structures of lipid-conjugated nucleoside analogs developed by Clavis Pharma: A) CP-4055 (cytarabine) and B) CP-4126 (gemcitabine).
These promising results led to clinical trials of CP-4055 and CP-4126 as orphan drugs in Europe and America, with fast-track designation by the FDA. Both drugs have undergone various Phase I and II clinical trials for solid and hematologic malignancies. A Phase I study of CP-4126 administered orally to patients with solid tumors once a week found few adverse side effects, with a maximum tolerated dose of 3,000 mg/day. However, the best anti-tumor outcome was stable disease, and no patients achieved partial or complete response. Low plasma levels of active gemcitabine were attributed to high intestinal absorption and deamination by the liver [86]. These preliminary results led Clavis to abandon testing oral delivery of CP-4126 for intravenous delivery. CP-4126 was tested in a Phase II study with pancreatic cancer patients who expressed low levels of hENT1. No difference was found in overall survival compared to gemcitabine treatment [92]. As a result, Clavis suspended all further developmental work on CP-4126.
A Phase I dose escalation study of CP-4055 in patients with solid tumors was well-tolerated and produced few significant adverse effects. The major dose-limiting toxicity (DLT) was myelosuppression [93]. CP-4055 was then tested in a Phase I study of 77 patients with refractory leukemia starting at 2000 mg/m2 on days 1–5 with various infusion times. Patient deaths were not attributed to CP-4055 or affected by dose, and 11% of patients displayed a complete remission. The DLT was hyperbilirubinemia, which lasted up to 13 days and was reversible once treatment was discontinued. Optimal scheduling of CP-4055 appeared to be 2,000 mg/m2 per day for 5 days by continuous intravenous administration (CIV), as minimal toxicities were seen at this dose [94].
CP-4055 was tested as a single agent therapy in a Phase II trial for patients with refractory acute myeloid leukemia (AML) in whom two other therapies were not effective. When CP-4055 was given (2,000 mg/m2/d for 5 d by CIV) a remission rate of 18% and an overall survival rate of 5.3 months were observed. The adverse events profile was similar to that of cytarabine [95]. A randomized Phase III clinical trial in AML patients found no significant difference in overall survival or adverse events between patients given CP-4055 (same dose as the Phase II study) and investigator’s choice of treatment [96]. However, a lower dose of CP-4055 (1,000 mg/m2/d CIV on days 1–5) combined with idarubicin (12 mg/m2/d IV on days 1–3) produced a 40% complete remission rate [97].
Despite discouraging results as single-agent therapy, CP-4055 may be useful in combination with other drugs, or as an option for patients with refractory disease. In vitro studies found synergy between CP-4055 and gemcitabine, irinotecan, and topotecan in a promyelocytic cell line, while co-treatment with cloretazine or idarubicin were additive. Further consideration must be given to the scheduling of combination therapies, as 24-hour pre-treatment with topoisomerase inhibitors was antagonistic when combined with CP-4055 in vitro [98].
2.8 Self-Emulsifying Compounds
InnoPharmax has developed an orally available self-microemulsifying formulation of gemcitabine, known as D07001-F4 using proprietary OralPAS® technology. Self- microemulsifying formulation of poorly absorbed lipophilic drugs has been shown to improve oral delivery of these poorly water-soluble molecules by incorporation of oils, solvents, surfactants, and co-solvents/surfactants [99]. D07001-F4 was found to be more bioavailable, 3-fold less susceptible to deamination, and more effective at inhibiting growth in a panel of tumor cell lines compared to gemcitabine. In mouse models of pancreatic and colon cancer, oral delivery of D07001-F4 was well-tolerated and more effective than intravenous gemcitabine [100]. InnoPharmax has started a Phase I dose-escalation trial in patients with advanced solid tumors or lymphoma. So far, no dose-limiting toxicity has been observed and the drug has shown some signs of anti-tumor activity against solid tumors. Further clinical testing is planned upon completion of this Phase I trial.
2.9 Liopsome Encapsulation
Celator Pharmaceuticals has developed CPX-351, a liposomal formulation of cytarabine combined with daunorubicin in a fixed molar ratio of 5:1. The rationale for this is that the precise ratio of cytarabine to daunorubicin has been shown to effect anti-leukemic efficacy in preclinical studies [101]. Liposomal encapsulation of the two drugs allows for improved half-life and circumvents the issue of constantly changing ratios between simultaneously administered therapies with different pharmacokinetics [102]. This compound has shown promise in treating secondary AML patients when compared to the current standard of care, continuous infused cytarabine and bolus daunorubicin (7+3) [103]. These findings have led to a phase III randomized study in older patients with high risk AML that has recently completed accrual. The results of this trial are eagerly awaited.
3. Discussion
The past few years have produced great advances in the development of lipid-conjugated nucleoside analogs. The various chemical modifications described above have been shown to increase drug circulation and stability in the body, circumvented hENT1 uptake and dCK activation, and overcome chemoresistance in vitro and in vivo. The observed change in uptake from carrier-mediated to passive diffusion may be efficacious for the treatment of relapsed disease, where reduced active drug uptake is a common mechanism of resistance. Continued incremental modifications can help elucidate the effects of choice and position of phospholipid group conjugation on biological activity of nucleoside analogs, as not every modification studied has proven to be successful pre-clinically or in clinical trials. Further research is needed to better understand the precise effects of such modifications on drug pharmacokinetics, uptake, and intracellular activation. Still, conjugation of fatty acids or the use of liposomes shows great promise as methods to deliver drugs to the lymphatic system or to avoid the first-pass effect by the liver when delivered orally.
The failure of CP-4055 highlights the need for better pre-clinical models of chemoresistance in testing novel drug conjugates. Given that many drugs will be first tested clinically in patients resistant to traditional therapy, pre-clinical testing should have a strong focusing on relapsed disease. Most studies have tested overcoming common mechanisms of drug resistance in vitro, which while still useful is limited as pharmacokinetic properties cannot be tested in a dish. Further, most in vivo efficacy testing is performed against previously untreated tumors, often using immunocompromised mouse models. A more relevant animal model would include chemoresistant disease in an immunocompetent model, ideally arising within the same animal to better mimic clinical conditions. There are benefits and drawbacks of testing both human and murine cancer models, but ideally drugs would be tested in both types to fully account for the effects of the immune system and differences in species.
Despite the poor outcomes of clinical trials testing the efficacy CP-4055 and CP-4126, many other phospholipid nucleoside analog conjugates are currently undergoing or are poised to enter clinical trials, and many more are entering the early stages of pre-clinical development. As is currently ongoing with CP-4055, switching treatment to a different type of cancer may help salvage drugs that initially fail Phase III trials. Potential mechanisms to improve the efficacy of phospholipid drug conjugates involve increasing cytotoxicity and reducing unwanted side effects. This may be accomplished by incorporating active tumor targeting mechanisms via conjugation of antibodies or tumor-specific substrates, by creating multi-drug complexes, or by incorporating moieties that have additional anti-carcinogenic effects. Once the efficacy, safety profiles, and mechanisms of action of these novel drugs are better understood, rational combinations with other clinically approved anti-neoplastic agents can be further explored in clinical trials. Such combinations could test anthracyclines, other nucleoside analogs, anti-tumor antibodies, and kinase inhibitors with nucleoside analog conjugates. Continued rational modification of lipid-conjugated nucleoside analogs, combinations with other anti-neoplastic agents, and application to other types of cancer will hopefully lead to improved outcomes for patients suffering from advanced cancer.
Acknowledgments
This work was supported by the Frances P. Tutwiler Fund, the Doug Coley Foundation for Leukemia Research, The McKay Cancer Research Foundation, Wake Innovations and the National Institute of Health (PA is supported by Wake Innovations, TSP is supported by NCI 1K08CA169809-03, GC is supported by NCI Cancer Center Support Grant (CCSG) P30CA012197). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Biography
Timothy S. Pardee MD, PhD
Dr Pardee received his PhD in biochemistry and MD degrees from the University of Buffalo. He did his residency training in internal medicine at Massachusetts General Hospital and his post-doctoral training at Cold Spring Harbor in the laboratory of Dr Scott Lowe. He is currently an associate professor in the Department of Internal Medicine, Section on Hematology and Oncology with a cross appointment in the Department of Cancer Biology. He serves as the Director of Leukemia Translational Research at the Comprehensive Cancer Center of Wake Forest University. He is a practicing hematologist and medical oncologist with a clinical focus on leukemia and MDS. In addition to his clinical duties he heads a research program focused on the role of cellular metabolism in leukemia cell survival and resistance to therapy as well as novel therapeutics. He also has an interest in early phase clinical trials and is PI for several completed and ongoing phase I and phase II studies. He is an active member of the leukemia correlative science committee for the Alliance for Clinical Trials in Oncology. He has served as the Chair of the Leukemia/Lymphoma Maintenance of Certification (MOC) Module Working Group for the American Society of Clinical Oncology, is currently a member of the ASCO University question writing faculty, a member of the Data Safety Monitoring Board for Cincinnati Children’s Hospital and a member in good standing in the American Society of Hematology, the American Society of Clinical Oncology and the American Association for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
GK holds a patent on KPC34. The authors report no other conflicts of interest relevant to the manuscript.
References
- 1.Galmarini CM, Mackey JR, Dumontet C. Nucleoside analogues and nucleobases in cancer treatment. Lancet Oncol. 2002;3(7):415–424. doi: 10.1016/s1470-2045(02)00788-x. [DOI] [PubMed] [Google Scholar]
- 2.Staub M, Eriksson S. The Role of Deoxycytidine Kinase in DNA Synthesis and Nucleoside Analog Activation. In: Peters G, editor. Deoxynucleoside Analogs In Cancer Therapy. Humana Press; 2007. pp. 29–52. [Google Scholar]
- 3.Genini D, et al. Deoxyadenosine analogs induce programmed cell death in chronic lymphocytic leukemia cells by damaging the DNA and by directly affecting the mitochondria. Blood. 2000;96(10):3537–3343. [PubMed] [Google Scholar]
- 4.Baker CH, et al. 2'-Deoxy-2'-methylenecytidine and 2'-deoxy-2',2'-difluorocytidine 5'-diphosphates: potent mechanism-based inhibitors of ribonucleotide reductase. J Med Chem. 1991;34(6):1879–1884. doi: 10.1021/jm00110a019. [DOI] [PubMed] [Google Scholar]
- 5.Aye Y, et al. Clofarabine targets the large subunit (alpha) of human ribonucleotide reductase in live cells by assembly into persistent hexamers. Chem Biol. 2012;19(7):799–805. doi: 10.1016/j.chembiol.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beumer JH, et al. Concentrations of the DNA methyltransferase inhibitor 5-fluoro-2'-deoxycytidine (FdCyd) and its cytotoxic metabolites in plasma of patients treated with FdCyd and tetrahydrouridine (THU) Cancer Chemother Pharmacol. 2008;62(2):363–368. doi: 10.1007/s00280-007-0603-8. [DOI] [PubMed] [Google Scholar]
- 7.Peters GJ, Schornagel JH, Milano GA. Clinical pharmacokinetics of anti-metabolites. Cancer Surv. 1993;17:123–156. [PubMed] [Google Scholar]
- 8.Balimane PV, Sinko PJ. Involvement of multiple transporters in the oral absorption of nucleoside analogues. Adv Drug Deliv Rev. 1999;39(1–3):183–209. doi: 10.1016/s0169-409x(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 9.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303(5665):1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 10.Yatvin MB, et al. Covalent lipid-drug conjugates for drug targeting. Google Patents; 1992. [Google Scholar]
- 11.Lambert DM. Rationale and applications of lipids as prodrug carriers. European Journal of Pharmaceutical Sciences. 2000;11:S15–S27. doi: 10.1016/s0928-0987(00)00161-5. [DOI] [PubMed] [Google Scholar]
- 12.Drummond DC, et al. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev. 1999;51(4):691–743. [PubMed] [Google Scholar]
- 13.Gregoriadis G. Overview of liposomes. J Antimicrob Chemother. 1991;28(Suppl B):39–48. doi: 10.1093/jac/28.suppl_b.39. [DOI] [PubMed] [Google Scholar]
- 14.Gregoriadis G, Florence AT. Liposomes and cancer therapy. Cancer Cells. 1991;3(4):144–146. [PubMed] [Google Scholar]
- 15.Mori A, et al. Influence of the steric barrier activity of amphipathic poly(ethyleneglycol) and ganglioside GM1 on the circulation time of liposomes and on the target binding of immunoliposomes in vivo. FEBS Lett. 1991;284(2):263–266. doi: 10.1016/0014-5793(91)80699-4. [DOI] [PubMed] [Google Scholar]
- 16.Klibanov AL, et al. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268(1):235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 17.Blume G, Cevc G. Molecular mechanism of the lipid vesicle longevity in vivo. Biochim Biophys Acta. 1993;1146(2):157–168. doi: 10.1016/0005-2736(93)90351-y. [DOI] [PubMed] [Google Scholar]
- 18.Lai F, Fadda AM, Sinico C. Liposomes for brain delivery. Expert Opin Drug Deliv. 2013;10(7):1003–1022. doi: 10.1517/17425247.2013.766714. [DOI] [PubMed] [Google Scholar]
- 19.Banks WA. Characteristics of compounds that cross the blood-brain barrier. Bmc Neurology. 2009;9 doi: 10.1186/1471-2377-9-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Liu L. Modern methods for delivery of drugs across the blood–brain barrier. Advanced Drug Delivery Reviews. 2012;64(7):640–665. doi: 10.1016/j.addr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.vanHelvoort A, et al. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell. 1996;87(3):507–517. doi: 10.1016/s0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- 23.Bosch I, et al. Phosphatidylcholine and phosphatidylethanolamine behave as substrates of the human MDR1 P-glycoprotein. Biochemistry. 1997;36(19):5685–5694. doi: 10.1021/bi962728r. [DOI] [PubMed] [Google Scholar]
- 24.Constantinides PP, Wasan KM. Lipid formulation strategies for enhancing intestinal transport and absorption of P-glycoprotein (P-gp) substrate drugs: in vitro/in vivo case studies. J Pharm Sci. 2007;96(2):235–248. doi: 10.1002/jps.20780. [DOI] [PubMed] [Google Scholar]
- 25.Litman T, et al. From MDR to MXR: new understanding of multidrug resistance systems, their properties and clinical significance. Cell Mol Life Sci. 2001;58(7):931–959. doi: 10.1007/PL00000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chime SA, Onyishi IV. Lipid-based drug delivery systems (LDDS): Recent advances and applications of lipids in drug delivery. Afr. J. Pharm. Pharmacol. 2013;7(8):3034–3059. [Google Scholar]
- 27.Hoshi A, et al. Effect and toxicity of combination treatment including cyclocytidine or cytosine arabinoside in L-1210 and sarcoma-180 systems. Gan. 1975;66(5):539–546. [PubMed] [Google Scholar]
- 28.McKelvey EM, et al. Cyclocytidine chemotherapy for malignant melanoma. Cancer Treat Rep. 1978;62(3):469–471. [PubMed] [Google Scholar]
- 29.Movassaghi N, et al. Evaluation of cyclocytidine in reinduction and maintenance therapy of children with acute nonlymphocytic leukemia previously treated with cytosine arabinoside: a report from Children's Cancer Study Group. Med Pediatr Oncol. 1984;12(5):352–356. doi: 10.1002/mpo.2950120512. [DOI] [PubMed] [Google Scholar]
- 30.Miller LP, et al. Successful reinduction therapy with amsacrine and cyclocytidine in acute nonlymphoblastic leukemia in children. A report from the Childrens Cancer Study Group. Cancer. 1991;67(9):2235–2240. doi: 10.1002/1097-0142(19910501)67:9<2235::aid-cncr2820670904>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 31.Aoshima M, et al. Antitumor activities of newly synthesized N4-acyl-1-beta-D-arabinofuranosylcytosine. Cancer Res. 1976;36(8):2726–2732. [PubMed] [Google Scholar]
- 32.Aoshima M, et al. N4-Behenoyl-1-Beta-D-Arabinofuranosylcytosine as a Potential New Antitumor Agent. Cancer Research. 1977;37(8):2481–2486. [PubMed] [Google Scholar]
- 33.Yoshida T, et al. Plasma and leukemic cell pharmacokinetics of high-dose N4-behenoyl-1-beta-D-arabinofuranosylcytosine in acute leukemia patients. J Clin Pharmacol. 1994;34(1):52–59. doi: 10.1002/j.1552-4604.1994.tb03966.x. [DOI] [PubMed] [Google Scholar]
- 34.Ueda T, et al. Pharmacokinetics of N4-behenoyl-1-beta-D-arabinofuranosylcytosine in patients with acute leukemia. Cancer Res. 1983;43(7):3412–3416. [PubMed] [Google Scholar]
- 35.Kimura K, et al. Treatment of acute myelogenous leukemia in adults with N4-behenoyl-1-beta-D-arabinofuranosylcytosine. Cancer. 1985;56(8):1913–1917. doi: 10.1002/1097-0142(19851015)56:8<1913::aid-cncr2820560803>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 36.Masaoka T, et al. [A phase III study of BHAC-MMP (behenoyl-ara-C, mitoxantrone, 6-mercaptopurine prednisolone) in acute leukemia. Hanshin Cooperative Study Group of Hematological Disorders] Gan To Kagaku Ryoho. 1986;13(9):2829–2834. [PubMed] [Google Scholar]
- 37.Dobashi N, et al. Aclarubicin plus behenoyl cytarabine and prednisolone for previously treated acute myeloid leukemia patients. Leuk Lymphoma. 2006;47(10):2203–2207. doi: 10.1080/10428190600756490. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi T, et al. Randomized trials between behenoyl cytarabine and cytarabine in combination induction and consolidation therapy, and with or without ubenimex after maintenance intensification therapy in adult acute myeloid leukemia. Journal of Clinical Oncology. 1996;14(1):204–213. doi: 10.1200/JCO.1996.14.1.204. [DOI] [PubMed] [Google Scholar]
- 39.Saneyoshi M, et al. Synthetic nucleosides and nucleotides. XVI. Synthesis and biological evaluations of a series of 1-beta-D-arabinofuranosylcytosine 5'-alkyl or arylphosphates. Chem Pharm Bull (Tokyo) 1980;28(10):2915–2923. doi: 10.1248/cpb.28.2915. [DOI] [PubMed] [Google Scholar]
- 40.Kodama K, et al. Antitumor activity and pharmacology of 1-beta-D-arabinofuranosylcytosine-5'-stearylphosphate: an orally active derivative of 1-beta-D-arabinofuranosylcytosine. Jpn J Cancer Res. 1989;80(7):679–685. doi: 10.1111/j.1349-7006.1989.tb01696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koga K, et al. Characteristic antitumor activity of cytarabine ocfosfate against human colorectal adenocarcinoma xenografts in nude mice. Cancer Chemother Pharmacol. 1995;36(6):459–462. doi: 10.1007/BF00685794. [DOI] [PubMed] [Google Scholar]
- 42.Maloisel F, et al. Results of a phase II trial of a combination of oral cytarabine ocfosfate (YNK01) and interferon alpha-2b for the treatment of chronic myelogenous leukemia patients in chronic phase. Leukemia. 2002;16(4):573–580. doi: 10.1038/sj.leu.2402433. [DOI] [PubMed] [Google Scholar]
- 43.Mollee P, et al. Interferon-alpha-2b and oral cytarabine ocfosfate for newly diagnosed chronic myeloid leukaemia. Ann Oncol. 2004;15(12):1810–1815. doi: 10.1093/annonc/mdh468. [DOI] [PubMed] [Google Scholar]
- 44.Rosti G, et al. A phase II study of alpha-interferon and oral arabinosyl cytosine (YNK01) in chronic myeloid leukemia. Leukemia. 2003;17(3):554–559. doi: 10.1038/sj.leu.2402850. [DOI] [PubMed] [Google Scholar]
- 45.Braess J, et al. Oral cytarabine ocfosfate in acute myeloid leukemia and non-Hodgkin's lymphoma--phase I/II studies and pharmacokinetics. Leukemia. 1998;12(10):1618–1626. doi: 10.1038/sj.leu.2401152. [DOI] [PubMed] [Google Scholar]
- 46.Tatsumi N, et al. [Phase II study of YNK01 (1-beta-D-arabinofuranosylcytosine-5'-stearylphosphate) on hematological malignancies] Gan To Kagaku Ryoho. 1990;17(12):2387–2395. [PubMed] [Google Scholar]
- 47.Kiyama Y, et al. [Treatment of refractory hematologic malignancies by combination of cytarabine ocfosfate and etoposide] Gan To Kagaku Ryoho. 1996;23(12):1717–1720. [PubMed] [Google Scholar]
- 48.Horikoshi A, et al. Efficacy of Oral Cytarabine Ocfosfate and Etoposide in the Treatment of Elderly Patients with Higher-Risk Myelodysplastic Syndromes Compared to That in Elderly Acute Myeloid Leukemia Patients. Chemotherapy. 2013;59(2):152–158. doi: 10.1159/000351114. [DOI] [PubMed] [Google Scholar]
- 49.Horikoshi A, et al. The value of oral cytarabine ocfosfate and etoposide in the treatment of refractory and elderly AML patients. International Journal of Hematology. 2008;87(2):118–125. doi: 10.1007/s12185-007-0019-6. [DOI] [PubMed] [Google Scholar]
- 50.Hamada A, Kawaguchi T, Nakano M. Clinical pharmacokinetics of cytarabine formulations. Clin Pharmacokinet. 2002;41(10):705–718. doi: 10.2165/00003088-200241100-00002. [DOI] [PubMed] [Google Scholar]
- 51.Murry DJ, Blaney SM. Clinical pharmacology of encapsulated sustained-release cytarabine. Ann Pharmacother. 2000;34(10):1173–1178. doi: 10.1345/aph.19347. [DOI] [PubMed] [Google Scholar]
- 52.Karami L, Jalili S. Effects of cholesterol concentration on the interaction of cytarabine with lipid membranes: a molecular dynamics simulation study. J Biomol Struct Dyn. 2015;33(6):1254–1268. doi: 10.1080/07391102.2014.941936. [DOI] [PubMed] [Google Scholar]
- 53.Gonzalez-Vitale JC, Garcia-Bunuel R. Meningeal carcinomatosis. Cancer. 1976;37(6):2906–2911. doi: 10.1002/1097-0142(197606)37:6<2906::aid-cncr2820370648>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 54.Bleyer WA, Byrne TN. Leptomeningeal cancer in leukemia and solid tumors. Curr Probl Cancer. 1988;12(4):181–238. doi: 10.1016/s0147-0272(88)80001-1. [DOI] [PubMed] [Google Scholar]
- 55.Chamberlain MC. Neoplastic meningitis. Neurologist. 2006;12(4):179–187. doi: 10.1097/01.nrl.0000219732.33321.cc. [DOI] [PubMed] [Google Scholar]
- 56.Jabbour E, et al. Neurologic complications associated with intrathecal liposomal cytarabine given prophylactically in combination with high-dose methotrexate and cytarabine to patients with acute lymphocytic leukemia. Blood. 2007;109(8):3214–3218. doi: 10.1182/blood-2006-08-043646. [DOI] [PubMed] [Google Scholar]
- 57.Marks DI. Treating the "older" adult with acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2010;2010:13–20. doi: 10.1182/asheducation-2010.1.13. [DOI] [PubMed] [Google Scholar]
- 58.Jabbour E, et al. Central nervous system prophylaxis in adults with acute lymphoblastic leukemia: current and emerging therapies. Cancer. 2010;116(10):2290–2300. doi: 10.1002/cncr.25008. [DOI] [PubMed] [Google Scholar]
- 59.Blaney SM, Poplack DG. Neoplastic meningitis: diagnosis and treatment considerations. Med Oncol. 2000;17(3):151–162. doi: 10.1007/BF02780522. [DOI] [PubMed] [Google Scholar]
- 60.Glantz MJ, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999;5(11):3394–3402. [PubMed] [Google Scholar]
- 61.Cole BF, et al. Quality-of-life-adjusted survival comparison of sustained-release cytosine arabinoside versus intrathecal methotrexate for treatment of solid tumor neoplastic meningitis. Cancer. 2003;97(12):3053–3060. doi: 10.1002/cncr.11449. [DOI] [PubMed] [Google Scholar]
- 62.Glantz MJ, et al. Randomized trial of a slow-release versus a standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis. J Clin Oncol. 1999;17(10):3110–3116. doi: 10.1200/JCO.1999.17.10.3110. [DOI] [PubMed] [Google Scholar]
- 63.Kim S, et al. Extended CSF cytarabine exposure following intrathecal administration of DTC 101. J Clin Oncol. 1993;11(11):2186–2193. doi: 10.1200/JCO.1993.11.11.2186. [DOI] [PubMed] [Google Scholar]
- 64.Chamberlain MC, et al. Pharmacokinetics of intralumbar DTC-101 for the treatment of leptomeningeal metastases. Arch Neurol. 1995;52(9):912–917. doi: 10.1001/archneur.1995.00540330094020. [DOI] [PubMed] [Google Scholar]
- 65.Chabner BA, Longo DL. Cancer Chemotherapy and Biotherapy: Principles and Practice. Wolters Kluwer Health; 2011. [Google Scholar]
- 66.Cattaneo-Pangrazzi RM, Schott H, Schwendener RA. The novel heterodinucleoside dimer 5-FdU-NOAC is a potent cytotoxic drug and a p53-independent inducer of apoptosis in the androgen-independent human prostate cancer cell lines PC-3 and DU-145. Prostate. 2000;45(1):8–18. doi: 10.1002/1097-0045(20000915)45:1<8::aid-pros2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 67.Schott H, Schott S, Schwendener RA. Synthesis and in vitro activities of new anticancer duplex drugs linking 2'-deoxy-5-fluorouridine (5-FdU) with 3'-C-ethynylcytidine (ECyd) via a phosphodiester bonding. Bioorg Med Chem. 2009;17(19):6824–6831. doi: 10.1016/j.bmc.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 68.Novotny L, Rauko P, Schott H. Cytotoxicity and antileukaemic activity of new duplexes linking 3-C-ethynylcytidine and 5-fluorodeoxyuridine. Anticancer Res. 2010;30(12):4891–4898. [PubMed] [Google Scholar]
- 69.Schott S, et al. Cytotoxicity of new duplex drugs linking 3'-C-ethynylcytidine and 5-fluor-2'-deoxyuridine against human melanoma cells. Int J Cancer. 2012;131(9):2165–2174. doi: 10.1002/ijc.27476. [DOI] [PubMed] [Google Scholar]
- 70.Bijnsdorp IV, et al. Cellular pharmacology of multi- and duplex drugs consisting of ethynylcytidine and 5-fluoro-2'-deoxyuridine. Invest New Drugs. 2011;29(2):248–257. doi: 10.1007/s10637-009-9353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parang K, Wiebe LI, Knaus EE. Synthesis, in vitro anti-human immunodeficiency virus structure-activity relationships and biological stability of 5'-O-myristoyl analogue derivatives of 3'-azido-2',3'-dideoxythymidine (AZT) as potential prodrugs. Antivir Chem Chemother. 1998;9(4):311–323. [PubMed] [Google Scholar]
- 72.Parang K, Wiebe LI, Knaus EE. Pharmacokinetics and tissue distribution of (+/−)-3'-azido-2',3'-dideoxy-5'-O-(2-bromomyristoyl)thymidine, a prodrug of 3'-azido-2',3'-dideoxythymidine (AZT) in mice. J Pharm Pharmacol. 1998;50(9):989–996. doi: 10.1111/j.2042-7158.1998.tb06913.x. [DOI] [PubMed] [Google Scholar]
- 73.Parang K, et al. In vitro anti-hepatitis B virus activities of 5"-O-myristoyl analogue derivatives of 3"-fluoro-2",3"-dideoxythymidine (FLT) and 3"-azido-2",3"-dideoxythymidine (AZT) J Pharm Pharm Sci. 1998;1(3):108–114. [PubMed] [Google Scholar]
- 74.Chhikara BS, Mandal D, Parang K. Synthesis and evaluation of fatty acyl ester derivatives of cytarabine as anti-leukemia agents. Eur J Med Chem. 2010;45(10):4601–4608. doi: 10.1016/j.ejmech.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 75.Cahard D, McGuigan C, Balzarini J. Aryloxy phosphoramidate triesters as pro-tides. Mini Rev Med Chem. 2004;4(4):371–381. doi: 10.2174/1389557043403936. [DOI] [PubMed] [Google Scholar]
- 76.McGuigan C, et al. Synthesis and evaluation of some novel phosphate and phosphinate derivatives of araA. Studies on the mechanism of action of phosphate triesters. Nucleic Acids Res. 1989;17(24):10171–10177. doi: 10.1093/nar/17.24.10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saif M, Lee Y, Kim R. Harnessing gemcitabine metabolism: a step towards personalized medicine for pancreatic cancer. Ther Adv Med Oncol. 2012;4(6):341–346. doi: 10.1177/1758834012453755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Slusarczyk M, et al. Application of ProTide technology to gemcitabine: a successful approach to overcome the key cancer resistance mechanisms leads to a new agent (NUC-1031) in clinical development. J Med Chem. 2014;57(4):1531–1542. doi: 10.1021/jm401853a. [DOI] [PubMed] [Google Scholar]
- 79.McGuigan C, et al. A phosphoramidate ProTide (NUC-1031) and acquired and intrinsic resistance to gemcitabine. ASCO Meeting Abstracts. 2011;29(15_suppl):e13540. [Google Scholar]
- 80.Ghazaly EA, et al. ProGem1: Phase I first-in-human study of the novel nucleotide NUC-1031 in adult patients with advanced solid tumors. ASCO Meeting Abstracts. 2013;31(15_suppl):2576. [Google Scholar]
- 81.McGuigan C, et al. Phosphoramidate ProTides of the anticancer agent FUDR successfully deliver the preformed bioactive monophosphate in cells and confer advantage over the parent nucleoside. J Med Chem. 2011;54(20):7247–7258. doi: 10.1021/jm200815w. [DOI] [PubMed] [Google Scholar]
- 82.Pickin KA, et al. Phospholipid/deoxycytidine analogue prodrugs for the treatment of cancer. Journal of Drug Delivery Science and Technology. 2009;19(1):31–36. [Google Scholar]
- 83.Alexander RL, et al. A novel phospholipid gemcitabine conjugate is able to bypass three drugresistance mechanisms. Cancer Chemother Pharmacol. 2005;56(1):15–21. doi: 10.1007/s00280-004-0949-0. [DOI] [PubMed] [Google Scholar]
- 84.Volm M, Sauerbrey A, Zintl F. Prognostic-significance of protein-kinase-C in newly-diagnosed childhood acute lymphoblastic-leukemia. Int J Oncol. 1994;4(2):363–368. doi: 10.3892/ijo.4.2.363. [DOI] [PubMed] [Google Scholar]
- 85.Pardridge WM. CNS drug design based on principles of blood-brain barrier transport. J Neurochem. 1998;70(5):1781–1792. doi: 10.1046/j.1471-4159.1998.70051781.x. [DOI] [PubMed] [Google Scholar]
- 86.Stuurman FE, et al. Phase I study of oral CP-4126, a gemcitabine derivative, in patients with advanced solid tumors. Invest New Drugs. 2013;31(4):959–966. doi: 10.1007/s10637-013-9925-z. [DOI] [PubMed] [Google Scholar]
- 87.Adema AD, et al. Metabolism and accumulation of the lipophilic deoxynucleoside analogs elacytarabine and CP-4126. Invest New Drugs. 2012;30(5):1908–1916. doi: 10.1007/s10637-011-9756-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bergman AM, et al. Antiproliferative activity, mechanism of action and oral antitumor activity of CP-4126, a fatty acid derivative of gemcitabine, in in vitro and in vivo tumor models. Invest New Drugs. 2011;29(3):456–466. doi: 10.1007/s10637-009-9377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Galmarini CM, Myhren F, Sandvold ML. CP-4055 and CP-4126 are active in ara-C and gemcitabine-resistant lymphoma cell lines. Br J Haematol. 2009;144(2):273–275. doi: 10.1111/j.1365-2141.2008.07467.x. [DOI] [PubMed] [Google Scholar]
- 90.Sandvold ML, et al. The activity of the lipophilic nucleoside derivatives elacytarabine and CP-4126 in a panel of tumor cell lines resistant to nucleoside analogues. Nucleosides Nucleotides Nucleic Acids. 2010;29(4–6):386–393. doi: 10.1080/15257771003729625. [DOI] [PubMed] [Google Scholar]
- 91.Breistol K, et al. Antitumor activity of P-4055 (elaidic acid-cytarabine) compared to cytarabine in metastatic human tumor xenograft models. Cancer Res sc. 1999;59(12):2944–2949. [PubMed] [Google Scholar]
- 92.Poplin E, et al. Randomized multicenter, phase II study of CO-101 versus gemcitabine in patients with metastatic pancreatic ductal adenocarcinoma (mPDAC) and a prospective evaluation of the of the association between tumor hENT1 expression and clinical outcome with gemcitabine treatment. ASCO Meeting Abstracts. 2013;31(15_suppl):4007. doi: 10.1200/JCO.2013.51.0826. [DOI] [PubMed] [Google Scholar]
- 93.Dueland S, et al. Intravenous administration of CP-4055 (ELACYT) in patients with solid tumours. A Phase I study. Acta Oncol. 2009;48(1):137–145. doi: 10.1080/02841860802183620. [DOI] [PubMed] [Google Scholar]
- 94.Giles FJ, et al. Phase I and pharmacokinetic study of elacytarabine, a novel 5'-elaidic acid derivative of cytarabine, in adults with refractory hematological malignancies. Leukemia. 2012;26(7):1686–1689. doi: 10.1038/leu.2012.1. [DOI] [PubMed] [Google Scholar]
- 95.O'Brien S, et al. Elacytarabine has single-agent activity in patients with advanced acute myeloid leukaemia. Br J Haematol. 2012;158(5):581–588. doi: 10.1111/j.1365-2141.2012.09186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roboz GJ, et al. International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol. 2014;32(18):1919–1926. doi: 10.1200/JCO.2013.52.8562. [DOI] [PubMed] [Google Scholar]
- 97.Giles F, et al. Elacytarabine, a novel 5'-elaidic acid derivative of cytarabine, and idarubicin combination is active in refractory acute myeloid leukemia. Leuk Res. 2012;36(4):e71–e73. doi: 10.1016/j.leukres.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Adams DJ, et al. Anti proliferative activity of ELACY (CP-4055) in combination with cloretazine (VNP40101M), idarubicin, gemcitabine, irinotecan and topotecan in human leukemia and lymphoma cells. Leuk Lymphoma. 2008;49(4):786–797. doi: 10.1080/10428190801935752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58(3):173–182. doi: 10.1016/j.biopha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 100.Hao WH, et al. In vitro and in vivo studies of pharmacokinetics and antitumor efficacy of D07001-F4, an oral gemcitabine formulation. Cancer Chemother Pharmacol. 2013;71(2):379–388. doi: 10.1007/s00280-012-2017-5. [DOI] [PubMed] [Google Scholar]
- 101.Tardi P, et al. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33(1):129–139. doi: 10.1016/j.leukres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 102.Feldman EJ, et al. First-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J Clin Oncol. 2011;29(8):979–985. doi: 10.1200/JCO.2010.30.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lancet JE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014;123(21):3239–3246. doi: 10.1182/blood-2013-12-540971. [DOI] [PMC free article] [PubMed] [Google Scholar]