Abstract
Objective
To establish a standardized protocol for histopathological assessment of murine menisci that can be applied to evaluate transgenic, knock-out/in, and surgically induced OA models.
Methods
Knee joints from C57BL/6J mice (6 to 36 months) as well as from mice with surgically-induced OA were processed, and cut into sagittal sections. All sections included the anterior and posterior horns of the menisci and were graded for (1) surface integrity, (2) cellularity, (3) Safranin-O staining distribution and intensity. Articular cartilage in the knee joints was also scored.
Results
The new histopathological grading system showed good inter- and intra-class correlation coefficients. The major age-related changes in murine menisci in the absence of OA included decreased Safranin O staining intensity, abnormal cell distribution and the appearance of acellular areas. Menisci from mice with surgically-induced OA showed severe fibrillations, partial/total loss of tissue, and calcifications. Abnormal cell arrangements included both regional hypercellularity and hypocellularity along with hypertrophy and cell clusters. In general, the posterior horns were less affected by age and OA.
Conclusion
A new standardized protocol and histopathological grading system has been developed and validated to allow for a comprehensive, systematic evaluation of changes in aging and OA-affected murine menisci. This system was developed to serve as a standardized technique and tool for further studies in murine meniscal pathophysiology models.
Keywords: meniscus, aging, osteoarthritis, histopathology
INTRODUCTION
Osteoarthritis (OA) is a whole-joint disorder that invariably affects articular cartilage, subchondral bone, menisci, synovium, ligaments, and muscles[1, 2]. Among these joint tissues, articular cartilage is the most susceptible to developing OA and shows the most profound age-related changes. However, the relationship between articular cartilage and meniscus is known to be critical in the dynamics of knee joint loading and transmission [3-9]. This relationship is emphasized by the fact that meniscal lesions, partial/total meniscectomy, and meniscal degeneration contribute to the development or progression of OA [10-16]. Despite its pivotal role in knee joint loading and OA development, the mechanisms of meniscal pathophysiology in aging and OA are not well understood. Better understanding these mechanistic relationships will improve therapeutic strategies for repairing meniscal injuries as well as preventing and treating OA.
Recent progress in understanding the mechanisms related to aging pathology of cartilaginous tissues for the study of human diseases includes the development and use of animal models (i.e., dogs, rabbits, guinea pigs, rats, and mice) [17-23]. Murine models are the most versatile due to ease of genetic manipulation, leading to a plethora of transgenic and knockout and knockin strains for various biological studies [24]. Furthermore, the availability of murine models allows for extensive studies that answer key questions concerning the spatial and temporal relationships of changes in menisci as well as in correlation with other joint tissues in aging and disease development. The C57BL/6J inbred strain stain of mice has been widely used to create mutants to study developmental biology and OA pathogenesis [25-30]. C57BL/6J mice spontaneously develop OA as a function of increasing age [31, 32]. OA can also be surgically induced in mice models with destabilization of the medial meniscus (DMM) being the most widely used model [33].
Several histopathological assessment systems exist to evaluate OA-related changes in articular cartilage. The Osteoarthritis Society International (OARSI) histopathology initiative developed a grading system that focuses on specific pathological features in articular cartilage as well as subchondral bone and synovium [17-23]. Using this grading system, several studies showed that murine OA models exhibited similar histopathological characteristics as humans, including loss of Safranin O staining, fibrillation, and erosion in articular cartilage [19]. However, similar systems for the assessment of aging, degeneration and OA development in mouse menisci are not available. We reported on a grading system for human meniscal pathology [34]. Due to obvious differences between human and murine menisci, a comprehensive system to evaluate the histopathological status of the tissue (i.e., surface, cell morphology, and tissue composition) at the microscale is required.
The objectives of this study were to (1) establish a standardized protocol for histopathological studies of meniscal physiology in murine menisci for aging and OA, (2) develop and validate a new histopathological grading system for murine menisci to evaluate and assess major changes in aging and OA, and (3) identify major aging-related changes and OA in murine menisci. In this study, we have used C57BL/6J mice to represent normal aging and OA development to develop and validate the histological grading system.
MATERIALS AND METHODS
Mouse knee joints
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee at The Scripps Research Institute (TSRI). Pathogen-free C57BL/6J mice were purchased from TSRI breeding facility. GFP-LC3 transgenic mice with C57BL/6J genetic background [35] were obtained from the RIKEN BioResource Center. The mice were housed in a temperature-controlled environment with 12-hour light/dark cycles and allowed feed and water ad libitum. The mice were sacrificed at various ages and knee joints were collected for analysis. Both male and female mice were included in this study. A total of 62 mice from six different ages were assessed: 6 (n = 13), 18 (n = 16), 24 (n = 12), 27 (n=8), 30 (n = 8), and 36 (n = 5) month-old mice.
OA was experimentally induced in 2-month-old mice by surgical DMM. The meniscotibial ligament, which anchors the medial meniscus to the tibial plateau, was transected by microsurgical technique, which induced mechanical instability in the knee joint [33]. These mice were sacrificed at 2-, 4-, 6-, 8, and 12-weeks post-destabilization (10 mice per time point) and the knee joints were collected for analysis.
Tissue processing and staining
Entire knee joints were collected by a detailed protocol for tissue collection as previously described [36]. Tissue specimens were harvested and fixed using zinc-buffered formalin (Z-Fix; ANATECH LTD, Battle Creek, MI). Knee joints were decalcified in TBD-2 (Shandon Inc., Pittsburgh, PA) for 12 hours on a shaker and then washed.
After fixation, samples were embedded in paraffin. Knee joint sagittal sections (4 μm thick) were cut from the medial compartment at the junction between the middle region of the menisci and the anterior/posterior horns. These sections were cut until the anterior and posterior horns appeared as triangles between the femoral condyle and tibial plateau. The sections were then stained with Safranin O/Fast Green. For all sections, Safranin O staining was used to evaluate proteoglycan content and pathological changes.
Development of the histological grading system
The histological grading system reported in this study was developed by carefully reviewing age-related changes in meniscal sagittal sections (including anterior and posterior locations) of 62 mice sacrificed at various ages (Fig. 1). The DMM mice (n = 60) were also reviewed 2-, 4-, 6-, 8, and 12-weeks post-destabilization (Fig. 2). Three criteria were selected for histological assessment of menisci: (1) tissue surface structures, (2) cellularity, and (3) Safranin O staining distribution and intensity.
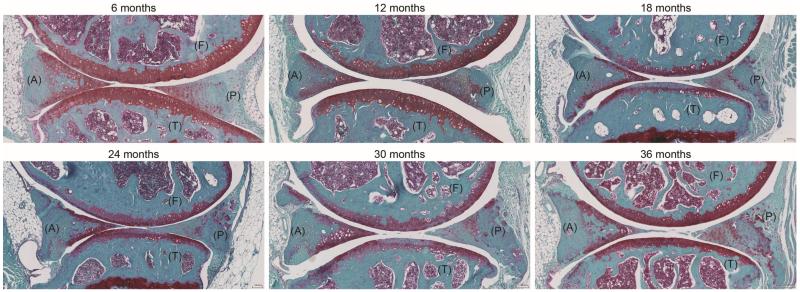
Figure 1.
Representative images of C57BL/6J mice knee joints (6 to 36 months old) showing the femur (F), tibia (T) as well as anterior (A) and posterior (P) location of the menisci (Safranin O staining).
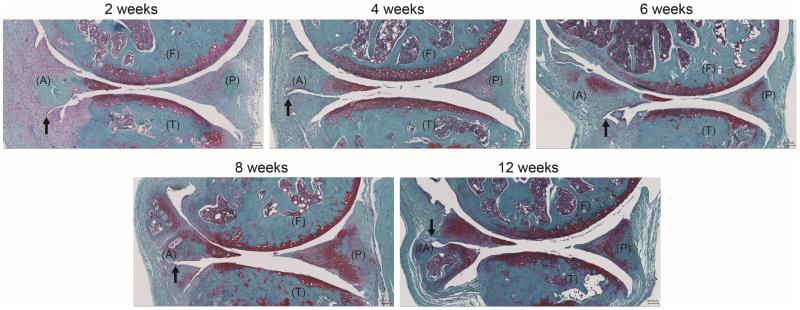
Figure 2.
Representative images of the surgical/injury DMM model of C57BL/6J mice knee joints (2-, 4-, 6-, 8- and 12-weeks post-destabilization) showing the femur (F), tibia (T) as well as anterior (A) and posterior (P) location of the menisci (Safranin O staining). The transection of the meniscotibial ligament (black arrow) intersects the medial menisci, leading to joint stability, meniscal degeneration, and development of OA in articular cartilage
Validation of the histological grading system
The total sum of the scores of all criteria (structure, cellularity, and matrix staining) can range from 0 – 24. This score is subdivided into 5 grades representing meniscal degeneration state: score 0–5 = Grade 0, score 6–10 = grade 1, score 11–15 = Grade 2, score 16–20 = Grade 3, score 21–25 = Grade 4.
Three individuals familiar with histopathological grading of joint tissues were given a set of 62 sections to grade using the new histological grading system for menisci. These sections were selected to cover the spectrum from healthy normal to severely degenerated menisci and were randomized for the graders who were blinded with respect to age or disease state. The three sets of scores were used to assess the interclass reliability of the histological scoring system. In addition, one grader scored the same collection of sections twice (3 weeks apart) to assess the intraclass reproducibility of the grading system.
Statistical analysis
All data were presented as mean ± standard error of the mean. Change in score with age was assessed with linear regression to determine whether there was a positive increase in score with age using Excel (Microsoft, Seattle, WA). The statistical significance of differences between age groups were assessed by the Kruskal-Wallis test followed by a Wilcoxon test with a Benjamin and Hochberg correction for multiple comparisons using R Project 3.1.0. The Kruskal-Wallis test assumes that the sample population is not normally distributed. P-values less than 0.05 were considered significant. The reliability and reproducibility of the histological grading system were assessed by comparing the scores from all graders for all the histological specimens using interclass/intraclass correlation coefficients (ICCs) according to Shrout and Fleiss’ schema using a custom written script in MATLAB (MathWorks, Natick, MA) [37]. The ICC is measured for consistency when systematic differences between graders are irrelevant. The 95% confidence intervals were computed using bootstrapping in MATLAB.
RESULTS
Development of the histological grading system
Aging-related changes in the menisci were observed in 6 to 36 month old mice (Fig. 1). With increasing age, the femoral (F) and tibial (T) surfaces of the menisci can undergo fibrillation and undulation that increase in severity with aging resulting in partial or total loss of meniscal structure. Cellularity also changes with aging as reflected by the emergence of areas with hyper- and hypocellularity. Age-related changes in the matrix involve a focal reduction of Safranin O staining, which is normally stained homogeneously at the surface. More severe age-related changes were observed in the anterior horn of the menisci. Based on these observations, a histological grading system of menisci was developed in the context of age-related changes.
For the histological evaluation of meniscus, criteria were selected based on the major age-related changes [27, 34, 38] independently observed in the anterior and posterior regions as well as different regions. These criteria included: (1) surface integrity (femoral and tibial aspects, and inner rim); (2) cellularity (vascular and avascular regions, and superficial zone); and (3) Safranin O staining distribution and intensity (vascular and avascular regions, and superficial zone) (Table 1). Each criterion describes four observations, scored 0–3, in varying regions (Fig. 3). Following evaluation of each criterion, a total score was calculated representing the pathological state of the meniscus. The presence or absence of blood vessel formation, calcification (ossification), and inflammation were recorded separately.
Table 1.
Criteria, scores, and observations for histological assessment of anterior and posterior menisci. The range of possible total score is 0–25.
| Criteria | Score | Anterior | Posterior |
|---|---|---|---|
| Tissue Surface Structure | |||
|
Femoral and tibial
side, inner rim |
0 | Smooth | Smooth |
| 1 | Slight fibrillation or undulating | Slight fibrillation or undulating | |
| 2 | Moderate fibrillation or undulating | Moderate fibrillation or undulating | |
| 3 | Severe fibrillation or undulating, - Disruption or total loss of tissue |
Severe fibrillation or undulating, - Disruption or total loss of tissue |
|
| Cellularity | |||
| Outer region | 0 | Normal distribution of fusiform cells | Normal distribution of fusiform cells |
| 1 | Hypercellularity | Hypercellularity | |
| 2 | Diffused hypocellularity - Few empty lacuna |
Diffused hypocellularity - Few empty lacuna |
|
| 3 | Hypocellularity - Empty lacuna, cyst, matrix separation |
Hypocellularity - Empty lacuna, cyst, matrix separation |
|
| Inner region | 0 | Normal distribution of round cells | Normal distribution of round cells |
| 1 | Hypercellularity | Hypercellularity | |
| 2 | Diffused hypo/acellular zones | Diffused hypo/acellular zones | |
| 3 | Hypocellularity - Empty lacuna, cyst |
Hypocellularity | |
| Superficial zone | 0 | Normal distribution of round cells | --- |
| 1 | Hypercellularity - Cell clustering |
--- | |
| 2 | Diffused hypo/acellularity - Cell shrinkage |
--- | |
| 3 | Hypocellularity | --- | |
| Matrix Staining | |||
| Outer region | 0 | Normal - slight staining of PCM | Normal - slight staining of PCM |
| 1 | Slightly disrupted | Slightly disrupted | |
| 2 | Moderately disrupted | Moderately disrupted | |
| 3 | Severely disrupted | Severely disrupted | |
| Inner region | 0 | --- | Normal - slight staining of ECM |
| 1 | --- | Slightly disrupted | |
| 2 | --- | Moderately disrupted | |
| 3 | --- | Severely disrupted | |
| Superficial zone | 0 | Normal - homogenous staining of ECM | --- |
| 1 | Slightly disrupted | --- | |
| 2 | Moderately disrupted | --- | |
| 3 | Severely disrupted | --- | |
Bone formation: present +, ++, +++
Inflammation: present +, ++, +++
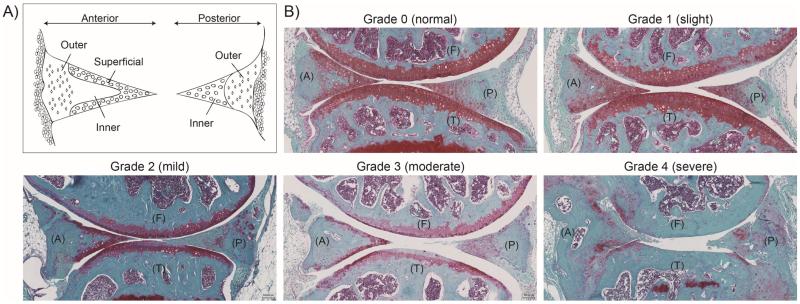
Figure 3.
(A) Diagram demonstrating the different regions of the anterior and posterior portions to be scored by histological assessment. (B) Representative images of histopathological grade 0 through 4, showing the femur (F), tibia (T) as well as anterior (A) and posterior (P) location of the menisci (Safranin O staining). Grades were converted from the total scores from the histological assessment: Grade 0 = 0 – 4, Grade 1 = 5 – 9, Grade 2 = 10 – 14; Grade 3 = 15 – 19; Grade 4 = 20 – 24.
Validation of the histological grading system
A total of 62 mouse menisci (normal aging and surgical OA models) were graded independently by three graders using the new histological system. The total scores for all criteria (structure, cellularity, and matrix staining) were summed together which can range from 0 to 24. This range was subdivided into grades 0 through 4 to present progressive stages of meniscal degeneration (Score 0–5 = Grade 0; Score 6–10 = Grade 1; Score 11–15 = Grade 2; Score 16–20 = Grade 3; Score 21–25 = Grade 4). Figure 3 shows representative histopathologic changes of each grade.
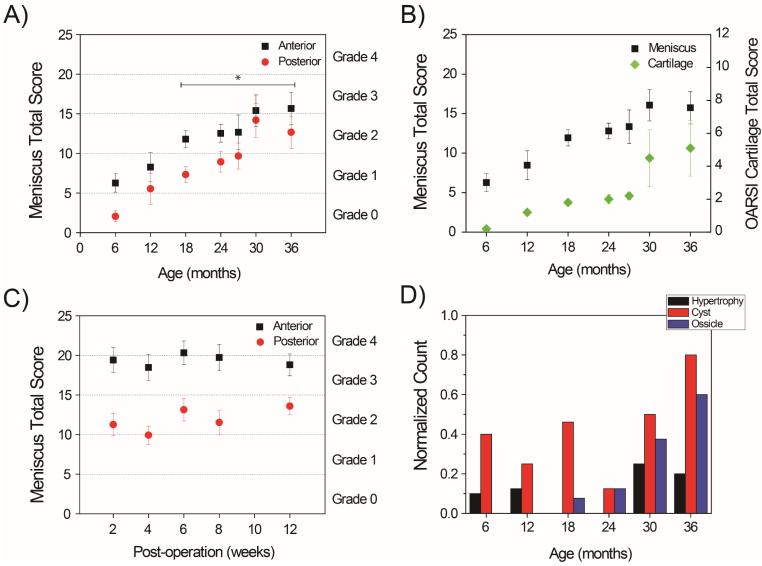
Overall there was an age-dependent statistically significant increase in scores (R2 = 0.952) in the C57BL/6J mice as assessed with the new grading system (Fig. 4A). Statistically significant differences in scores were detected starting at 18-months-old mice compared to 6-months (P < 0.05). The articular cartilage was also scored by the OARSI grading system and an age-dependent increase in scores was present as well (Fig. 4B).
Figure 4.
Meniscus total scores and grades for menisci (anterior and posterior) were obtained using the new grading system in this study for A) C57BL/6J normal aging mice (asterisk = P < 0.05 versus 6-month-old mice) and B) surgically-induced OA mice (DMM model). C) Meniscus total scores were compared with articular cartilage scoring by OARSI method. D) Frequency counts of hypertrophy, cyst and ossicle formation in anterior meniscus showing increased incidences with aging.
Scores for menisci from mice with DMM surgery were significantly higher at 2 weeks compared to normal aging mice without surgery (P < 0.001). However, scores for the surgical OA group (DMM model) did not increase further (Fig. 4C) although cartilage scores increased with post-operation time.
Inter-observer variability between three graders was low with an ICC of 0.7423 and 0.6914 for the anterior and posterior regions, respectively (See Supplementary Table 1). The ICC between the readers for the surface structural parameter ranged from 0.708 to 0.856 and 0.702 to 0.853 for the anterior and posterior regions, respectively, while the other two parameters (e.g., cellularity and Safranin O staining intensity) revealed lower ICCs. The ICC for the inter-observer variability between the readers was highest for the structural parameter, followed by ICC for overall grade. In addition, one grader repeated the scoring after a minimum period of 3 weeks to assess intra-class grading variations. The absolute agreement between replicate scores was within 0.702 to 0.853. The 95% confidence intervals computed using bootstrapping was 0.7292 and 0.8311 for the anterior region, and 0.6139 and 0.8168 for the posterior region.
Analysis of menisci in young mice
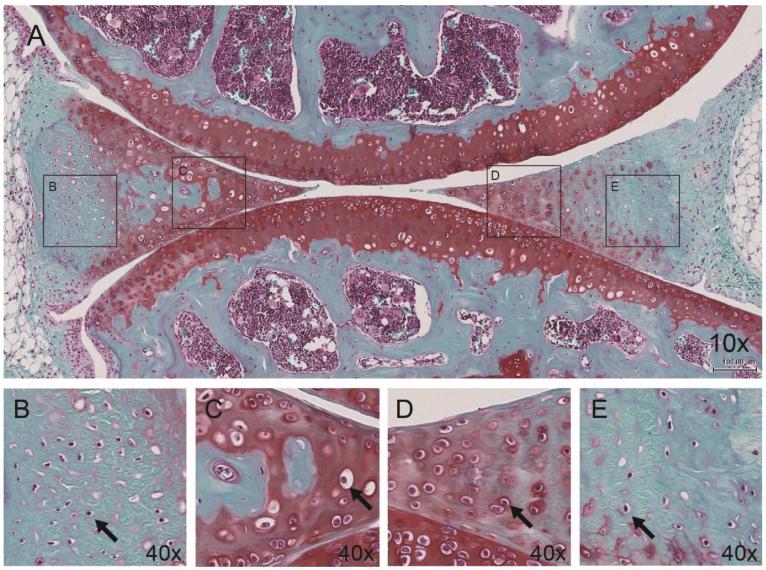
Mature C57BL/6J mice at 6 and 12 months of age were examined to identify histological features of normal murine menisci. In these mice, the surface structure was smooth and intact with no or minimal fibrillation or undulation. Cellularity was defined by regions in murine menisci (Fig. 5A). The vascular (outer) regions contained fusiform cells, resembling fibroblast-like cells (Fig. 5B, E). The inner regions contained round and ovoid-shaped cells, resembling the so-called fibrochondrocytes (Fig. 5C, D). These fibrochondrocytes were also found along the superficial zone in the anterior horn, but not in the posterior. In addition, small cyst-like cavities and small ossicles were occasionally observed in the anterior horns of normal mice menisci.
Figure 5.
Normal pattern of matrix staining and cellularity in young mice (6 months). A very similar pattern was found in 12-month-old mice. A) Minimal to no staining in the outer region and moderate staining in the inner region diffused in the superficial and deep zone. Outer meniscal cells resemble (B, E) fibroblast cells (black arrows) and inner meniscal cells resemble (C, D) fibrochondrocytes (black arrows).
In normal, young mice, a strong correlation between cellular phenotype and Safranin O staining was observed. The matrix surrounding fibrochondrocytes (avascular and superficial zone) were usually homogeneously stained with Safranin O of moderate intensity (Fig. 5C, D). In the vascular regions, only the localized pericellular matrix surrounding fibroblast-like cells were faintly stained by Safranin O while the more distant areas away from the cell were not stained (Fig. 5B, E). Therefore, a normal profile for Safranin O in murine menisci was defined by homogenous staining in avascular and superficial zones with vascular regions devoid of stain. Note, in some 6-month-old mice, this Safranin O staining profile was less defined and more diffuse throughout the menisci. In addition, in some young mice, hypertrophic cells were observed in the avascular zone of the anterior horn.
Analysis of menisci in aged mice
The new histological grading system detected moderate meniscal degeneration (Grade 2) by 18 months of age (Fig. 4A). Therefore, age-related changes in murine menisci were identified by examining the histology of 18- to 36-month old mice with no or minimal signs of OA in articular cartilage in comparison to younger mice (6–12 months). In this younger age group, the articular cartilage was relatively intact with low OARSI scores (Fig. 4C).
The aged mice showed mild to moderate levels of meniscal structural changes that include surface fibrillations and undulations. Fibrillations were defined as fraying at the surface while undulations were wave-like or ripple formations at the surface (Fig. 6A, B). These structural changes increased in severity with age. In aged mice, the surface regions became fibrillated and undulated with faint or no Safranin O staining (Fig. 6B).
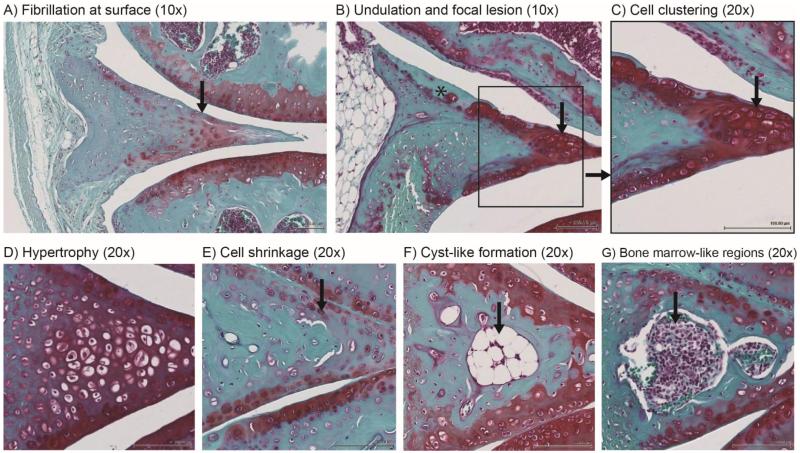
Figure 6.
Age-related abnormal changes to tissue structure, cells and extracellular matrix in aged mice (24 to 36 months old). Structural changes include (A) fibrillation (indicated by arrow) and (B) undulation mainly at the femoral aspect surface (asterisk indicates surface defects with faint or no Safranin O staining). Hypercellular changes included (C) cell clustering (shown in inset and indicated by arrow) and (D) hypertrophy. Hypocellularity was observed through (E) reduced cell density and cell shrinkage (indicated by arrow). (F) Cyst-like formation (indicated by arrow) and (G) bone marrow-like regions were also observed more frequently in aged mice (indicated by arrow).
Compared to young mice, menisci in aged mice also showed a decrease in Safranin O staining intensity in the avascular region and superficial zones. This pattern in Safranin O staining correlated with abnormal phenotypic change mainly in the fibrochondrocytes located in the avascular and superficial zones. Abnormal cell patterns included hypertrophy, cell shrinkage, and cell clustering (Fig 6C-E). In addition, there were formations of large, irregular cyst-like cavities that are surrounded by structures with a bony spicule appearance (Fig. 6F) and bone marrow-like regions (Fig. 6G). The cavities and ossicles were larger in size in aged mice compared to those observed in young mice. The frequency of the cavities and ossicles were counted with results demonstrating age-dependency for both (Fig. 4D). Less significant changes were found within the posterior horn of menisci though changes in cell size, morphology, and density were most apparent with advanced aged mice.
Correlation of changes in cartilage and menisci in normal aging mice
Most changes in articular cartilage occurred at the femoral articular surface in the region covered by the anterior meniscus. Changes in both cartilage and anterior meniscus appeared to be similar and occurred in parallel (See Supplementary Fig. 1A). These changes included alterations in Safranin O distribution, cell reduction, and cell clustering/proliferation. However, the meniscus appeared to better maintain its structure, Safranin O distribution, and cell arrangement compared to articular cartilage. In the 30- and 36-month-old group, most cases showed that articular cartilage underwent more accelerated changes compared to meniscus. This was mainly identified by a greater reduction in Safranin O stain at the femoral surface indicative of focal lesions and degeneration (See Supplementary Fig. 1A). However, a few cases showed a greater reduction in Safranin O staining of the meniscus (See Supplementary Fig. 1B).
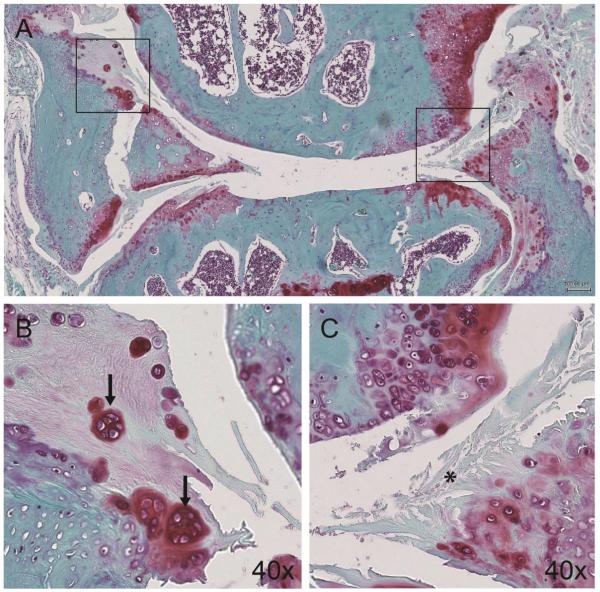
In the 30- and 36-month-old group, a few mice spontaneously developed OA characterized by severe degeneration of articular cartilage as well as meniscus (Fig. 7A). Both the articular cartilage and meniscus showed an abundant loss of tissue at the superficial zone with fibrillations that extended into the deep zone. This group showed extensive variations in Safranin O staining intensity and distribution. Cellular changes included reduced cell density in many areas as well as the presence of cell clusters, a hallmark of OA that forms in the fibrillated clefts of the superficial zone (Fig. 7B, C). Only in these specimens was cell death (denoted by empty lacuna and pyknotic nuclei) visually prominent in the menisci.
Figure 7.
(A) OA manifestation in the meniscus and articular cartilage (36 months) characterized by (B) cell clusters (black arrow) and (C) severe disruption of meniscal tissue (asterisk) and articular cartilage.
Analysis of menisci in surgical OA model
OA was surgically induced by DMM close to the anterior horn (Fig. 2). Most of the DMM-related changes presented as an early tissue response within the first 2 weeks after surgery with features not seen in the normal mice or mice with aging-related spontaneous OA. Cells appeared to infiltrate from the synovium through the injury site and spread throughout the tissue as early as 2-weeks post-surgery. Starting at 4-weeks, abnormal histological features similar to age-related changes were observed, including formation of large cyst-like cavities and ossicles. These changes in the cells and extracellular matrix occurred while the meniscus structure and surface remained relatively intact. However, by 12-weeks post-destabilization, all DMM mice developed full-blown OA, defined by severe articular cartilage damage. The histological scores for menisci in the DMM mice did not change significantly over time from 2 to 12 weeks (Fig. 4B).
DISCUSSION
The role of meniscal injuries as well as partial or total meniscectomy in the development OA is well established [13, 39-42]. However, the mechanistic relationship between age-associated meniscal degradation and OA development is not well understood. Better understanding of meniscal pathophysiology requires mechanistic studies to identify early degradative changes most suitable in animal models. Animal studies in mice could potentially identify key biological processes, which may also be involved in human OA, thus leading to improved therapeutic strategies to treat and repair meniscal injuries as well as to prevent and treat OA in the knee.
A prerequisite for mechanistic studies is a grading system that allows quantification of histopathological changes in murine menisci. Here, we have developed and validated a grading system for mouse menisci that evaluates aging-associated or experimental OA-related changes. We used C57BL/6J transgenic mice from age 6 to 36 months as this strain of mice most commonly used in studies of joint aging and OA [25, 26, 28, 30, 43, 44]. At 6 months, these mice reach skeletal maturity and exhibit healthy normal physiology. Therefore these mice are used to characterize the healthy, normal meniscus state. These mice can spontaneously develop OA in cartilage with the meniscus showing minimal signs of degeneration. Hence, a DMM mice model was used to induce severe degeneration in menisci for histological assessment and to determine the effects of an unstable meniscus on the development of OA.
There are obvious differences between human and murine menisci, notably their distinct Safranin O staining profiles. In human menisci, young healthy tissues show little to no Safranin O staining, which then increases with age and degradation [34]. In murine menisci, Safranin O staining is present in both young and old mice, though staining intensity and distribution varies with age and degradation. In young, healthy mice, Safranin O staining is concentrated mainly at the superficial zone in the anterior meniscus. In the posterior meniscus, Safranin O staining appears mainly in the inner region. In aged mice, the distribution of Safranin O staining appears disrupted with reduced intensity of staining, which could indicate early signs of degradation. In general, menisci in OA-affected mouse knees tend to have much less Safranin O staining compared to human OA menisci.
In murine menisci, there is a strong correlation between cell type and Safranin O staining which is also age-dependent. The rounded ‘fibrochondrocytes’ reside in areas with dense Safranin O staining, while only the pericellular matrix is stained around the fusiform fibroblast-like cells. In aged mice, strong staining intensity usually correlates with abnormal age-related cellular changes in cell size, morphology, and distribution (e.g., cell shrinkage and cell clustering) (Fig. 6). These changes can occur with the articular cartilage surfaces remaining relatively intact. The relationship between meniscus and articular cartilage was also observed in the Safranin O staining profiles, which show a strong correlation between the intensity of staining in articular cartilage and meniscus.
In mice with OA-like cartilage changes, the menisci were severely degraded with numerous and deep fibrillations at the surface and loss of tissue structure. Besides the obvious structural changes, OA-related cellular changes in meniscus include cell clustering in the fibrillated clefts of the superficial zone, which is almost identical to those seen in articular cartilage [26, 27, 38, 45]. The differences between age-associated vs OA-associated cell clustering are unknown.
The DMM mice represent secondary OA induced by injury and abnormal mechanical loading [33]. All of these mice developed an injury-repair response that appeared to take place in three stages over the course of 12-weeks post DMM surgery [46-51]. A large infiltration of cells at the injury site from the synovium indicated an acute inflammatory response, which was absent in mice with normal knees and aging-related OA [33, 52]. The inflammation was present already at 2-weeks and persisted through 12-weeks post-destabilization. It spread throughout the tissue, indicating a pathologic healing process. Cellular abnormalities, including the formation of cyst-like cavities and ossicles as well as changes in Safranin O staining were noted as early as 4 weeks post-surgery. These changes are presumed to be part of an injury-induced repair response associated with abnormal cell proliferation and matrix deposition [46, 47]. Ultimately, this led to the third stage defined by severe meniscal degradation, which evidently influenced the state of articular cartilage. At 12-weeks post-destabilization, all DMM mice developed full-blown OA, defined by severe degradation of articular cartilage as well as meniscus.
Meniscal surfaces often remained intact while distinct age-related changes in Safranin O staining and cellularity were observed within the tissue substance. This indicates that degeneration of menisci possibly initiates within the tissue substance before detection of changes in the surface. This phenomenon was more evident in the DMM model in which dramatic changes in the cells and extracellular matrix occurred with most of the surface structure remaining intact. This result was in direct contrast to degradation of articular cartilage, where changes progressed from the surface to the mid and deep zones. In general, the anterior menisci appeared to undergo more change in cellular and matrix components than the posterior menisci. In the DMM mice, the prominent changes in the anterior segments were also likely due to the location of the surgical injury in the medial meniscus, as shown in the sections (Fig. 2). Meniscal surface fibrillations were first seen at the inner rim, which progresses to loss of meniscal tissue, mainly in the avascular region. Signs of aging and degeneration included formation of cyst-like cavities, inflammation, bony spicules, and bone marrow-like regions. We also observed the presence of hypertrophic-like single cells in regions intensely stained with Safranin O, indicative of abnormal proliferation and hypertrophic differentiation.
A limitation of this study is that only one strain of mice was included in the histological analysis. C57BL/6J transgenic strain is commonly used to study joint aging and OA, as it is known to spontaneously develop OA with aging [19, 33]. Therefore, the present study was developed to quantitatively assess age-related degenerative changes in menisci that accompany OA development. Analysis of knee joints from other mice strains that do not develop OA with aging may reveal differences between aging and osteoarthritic meniscal degeneration. This study also examines only the anterior and posterior locations of the menisci for convenience in establishing a standard protocol for histological analysis of menisci.
In summary, we present a comprehensive meniscus grading system for mouse models undergoing normal aging, aging-related and surgical OA. Previous studies used individual histological grading systems for mouse articular cartilage to evaluate mechanistic studies of articular cartilage pathophysiology [19]. With a standard grading system, outcomes of similar studies can be directly compared to identify mechanisms of meniscal pathophysiology. This system may be useful to characterize the molecular mechanisms that are reflected in the reported age-related changes in the structure, cells, and matrix. This system can also be used to establish the temporal relationship of and identify cause and effect mechanisms of age-associated changes in the menisci. This system was developed to serve as a standardized technique and tool for further studies in murine meniscal pathophysiology models. Further development of this system could integrate evaluations of all joint tissues, including synovium, ligaments, bone, and cartilage regions covered or not covered by the meniscus.
Supplementary Material
Supplementary Figure 1. Spatial and temporal relationship between articular cartilage and meniscus presented in two cases (boxed in black). (A) Most cases showed greater reduction in Safranin O staining in articular cartilage. (B) Fewer cases showed greater reduction in meniscus.
Supplementary Table 1. Validation of reliability and reproducibility was quantified by ICC for the histological system evaluating both the anterior and posterior meniscus.
ACKNOWLEDGMENTS
Grant support: This study was supported by NIH grant AG007996.
Funding support: The authors wish to acknowledge support from Donald and Darlene Shiley and from the Shaffer Family Foundation
Footnotes
Author contributions: Study design: DD, SG, ML, JK, HO. Data acquisition: JK, HO, MO. Data analysis and interpretation: JK, SG, ML, DD. Manuscript preparation and approval: JK, ML, DD. We thank James Koziol for his help with the statistical analysis.
Competing interests: None.
Ethics approval: This study was conducted with the approval of the Institutional Animal Care and Use Committee at The Scripps Research Institute.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jeanie Kwok, Materials Science and Engineering Program, Department of Mechanical and Aerospace Engineering, University of California, San Diego jckwok@ucsd.edu.
Hiroyuki Onuma, St. Marianna University School of Medicine, Miyamae-ku, Kawasaki, Kanagawa, Japan herrihero@marianna-u.ac.jp.
Merissa Olmer, Department of Molecular and Experimental Medicine, The Scripps Research Institute molmer@scripps.edu.
Martin K. Lotz, Department of Molecular and Experimental Medicine, The Scripps Research Institute mlotz@scripps.edu.
Shawn P. Grogan, Shiley Center for Orthopaedic Research and Education at Scripps Clinic Grogan.Shawn@scrippshealth.org.
Darryl D. D’Lima, Shiley Center for Orthopaedic Research and Education at Scripps Clinic 11025 North Torrey Pines Road, Suite 200, La Jolla, CA 92037.
REFERENCES
- 1.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987;30:914–918. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 3.Aspden R, Yarker Y, Hukins D. Collagen orientations in the meniscus of the knee joint. J Anat. 1985;140:371. [PMC free article] [PubMed] [Google Scholar]
- 4.Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop Relat Res. 1990;252:19–31. [PubMed] [Google Scholar]
- 5.Proctor C, Schmidt M, Whipple R, Kelly M, Mow V. Material properties of the normal medial bovine meniscus. J Orthop Res. 1989;7:771–782. doi: 10.1002/jor.1100070602. [DOI] [PubMed] [Google Scholar]
- 6.Seedhom B. Transmission of the Load in the Knee Joint with Special Reference to the Role of the Menisci Part I: Anatomy, Analysis and Apparatus. Eng Med. 1979;8:207–219. [Google Scholar]
- 7.Seedhom B. Loadbearing function of the menisci. Physiotherapy. 1976;62:223–223. [PubMed] [Google Scholar]
- 8.Tissakht M, Ahmed A. Tensile stress-strain characteristics of the human meniscal material. J Biomech. 1995;28:411–422. doi: 10.1016/0021-9290(94)00081-e. [DOI] [PubMed] [Google Scholar]
- 9.Walker PS, Erkiuan MJ. The role of the menisci in force transmission across the knee. Clin Orthop Relat Res. 1975;109:184–192. doi: 10.1097/00003086-197506000-00027. [DOI] [PubMed] [Google Scholar]
- 10.Englund M. The role of the meniscus in osteoarthritis genesis. Med Clin North Am. 2009;93:37–43. x. doi: 10.1016/j.mcna.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Englund M, Guermazi A, Lohmander LS. The meniscus in knee osteoarthritis. Rheum Dis Clin North Am. 2009;35:579–590. doi: 10.1016/j.rdc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Englund M, Guermazi A, Lohmander SL. The role of the meniscus in knee osteoarthritis: a cause or consequence? Radiol Clin North Am. 2009;47:703–712. doi: 10.1016/j.rcl.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Englund M, Roos EM, Lohmander LS. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: a sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum. 2003;48:2178–2187. doi: 10.1002/art.11088. [DOI] [PubMed] [Google Scholar]
- 14.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 15.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3:261–267. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 16.Garrett WE, Jr, Swiontkowski MF, Weinstein JN, Callaghan J, Rosier RN, Berry DJ, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88:660–667. doi: 10.2106/JBJS.E.01208. [DOI] [PubMed] [Google Scholar]
- 17.Cook JL, Kuroki K, Visco D, Pelletier JP, Schulz L, Lafeber FP. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the dog. Osteoarthritis Cartilage. 2010;18(Suppl 3):S66–79. doi: 10.1016/j.joca.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Gerwin N, Bendele AM, Glasson S, Carlson CS. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage. 2010;18(Suppl 3):S24–34. doi: 10.1016/j.joca.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 19.Glasson S, Chambers M, Van Den Berg W, Little C. The OARSI histopathology initiative–recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18:S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Kraus VB, Huebner JL, DeGroot J, Bendele A. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthritis Cartilage. 2010;18(Suppl 3):S35–52. doi: 10.1016/j.joca.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laverty S, Girard CA, Williams JM, Hunziker EB, Pritzker KP. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rabbit. Osteoarthritis Cartilage. 2010;18(Suppl 3):S53–65. doi: 10.1016/j.joca.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 22.Little CB, Smith MM, Cake MA, Read RA, Murphy MJ, Barry FP. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in sheep and goats. Osteoarthritis Cartilage. 2010;18(Suppl 3):S80–92. doi: 10.1016/j.joca.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 23.McIlwraith CW, Frisbie DD, Kawcak CE, Fuller CJ, Hurtig M, Cruz A. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the horse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S93–105. doi: 10.1016/j.joca.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 24.Helminen HJ, Saamanen AM, Salminen H, Hyttinen MM. Transgenic mouse models for studying the role of cartilage macromolecules in osteoarthritis. Rheumatology (Oxford) 2002;41:848–856. doi: 10.1093/rheumatology/41.8.848. [DOI] [PubMed] [Google Scholar]
- 25.Akasaki Y, Hasegawa A, Saito M, Asahara H, Iwamoto Y, Lotz M. Dysregulated FOXO transcription factors in articular cartilage in aging and osteoarthritis. Osteoarthritis Cartilage. 2014;22:162–170. doi: 10.1016/j.joca.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caramés B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lotz MK, Otsuki S, Grogan SP, Sah R, Terkeltaub R, D'Lima D. Cartilage cell clusters. Arthritis Rheum. 2010;62:2206–2218. doi: 10.1002/art.27528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otsuki S, Taniguchi N, Grogan SP, D'Lima D, Kinoshita M, Lotz M. Expression of novel extracellular sulfatases Sulf-1 and Sulf-2 in normal and osteoarthritic articular cartilage. Arthritis Res Ther. 2008;10:R61. doi: 10.1186/ar2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taniguchi N, Caramés B, Kawakami Y, Amendt BA, Komiya S, Lotz M. Chromatin protein HMGB2 regulates articular cartilage surface maintenance via β-catenin pathway. Proc Natl Acad Sci U S A. 2009;106:16817–16822. doi: 10.1073/pnas.0904414106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taniguchi N, Caramés B, Ronfani L, Ulmer U, Komiya S, Bianchi ME, et al. Aging-related loss of the chromatin protein HMGB2 in articular cartilage is linked to reduced cellularity and osteoarthritis. Proc Natl Acad Sci U S A. 2009;106:1181–1186. doi: 10.1073/pnas.0806062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sokoloff L. Natural history of degenerative joint disease in small laboratory animals. I. Pathological anatomy of degenerative joint disease in mice. AMA Arch Pathol. 1956;62:118–128. [PubMed] [Google Scholar]
- 32.Silberberg M, Silberberg R. Age changes of bones and joints in various strains of mice. Am J Anat. 1941;68:69–95. [Google Scholar]
- 33.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Pauli C, Grogan SP, Patil S, Otsuki S, Hasegawa A, Koziol J, et al. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis Cartilage. 2011;19:1132–1141. doi: 10.1016/j.joca.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alirezaei M, Kemball C, Flynn C, Wood M, Whitton J, Kiosses W. Short-term fasting induces profound neuronal autophagy. Autophagy. 2010;6:702–710. doi: 10.4161/auto.6.6.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shrout P, Fleiss J. Intraclass correlations: uses in assessing rater reliability. Psychol. Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 38.Lotz M, Loeser RF. Effects of aging on articular cartilage homeostasis. Bone. 2012;51:241–248. doi: 10.1016/j.bone.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen P, Denham R, Swan A. Late degenerative changes after meniscectomy. Factors affecting the knee after operation. J Bone Joint Surg Br. 1984;66:666–671. doi: 10.1302/0301-620X.66B5.6548755. [DOI] [PubMed] [Google Scholar]
- 40.Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum. 2004;50:2811–2819. doi: 10.1002/art.20489. [DOI] [PubMed] [Google Scholar]
- 41.Fairbank T. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30:664–670. [PubMed] [Google Scholar]
- 42.Roos H, Laurén M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41:687–693. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 43.Stoop R, Van Der Kraan PM, Buma P, Hollander AP, Billinghurst RC, Poole AR, et al. Type II collagen degradation in spontaneous osteoarthritis in C57BL/6 and BALB/c mice. Arthritis & Rheumatism. 1999;42:2381–2389. doi: 10.1002/1529-0131(199911)42:11<2381::AID-ANR17>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 44.Caramés B, Hasegawa A, Taniguchi N, Miyaki S, Blanco FJ, Lotz M. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Annals of the Rheumatic Diseases. 2012;71:575–581. doi: 10.1136/annrheumdis-2011-200557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grogan SP, Miyaki S, Asahara H, D'Lima DD, Lotz MK. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11:R85. doi: 10.1186/ar2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buckwalter JA, Brown TD. Joint injury, repair, and remodeling: roles in post-traumatic osteoarthritis. Clin Orthop Relat Res. 2004;423:7–16. [PubMed] [Google Scholar]
- 47.Colwell CW, Jr, D'Lima DD, Hoenecke HR, Fronek J, Pulido P, Morris BA, et al. In vivo changes after mechanical injury. Clin Orthop Relat Res. 2001;391:S116–S123. doi: 10.1097/00003086-200110001-00012. [DOI] [PubMed] [Google Scholar]
- 48.D'Lima D, Hermida J, Hashimoto S, Colwell C, Lotz M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006;54:1814–1821. doi: 10.1002/art.21874. [DOI] [PubMed] [Google Scholar]
- 49.D'Lima DD, Hashimoto S, Chen PC, Colwell CW, Jr, Lotz MK. Impact of mechanical trauma on matrix and cells. Clin Orthop Relat Res. 2001;391:S90–S99. doi: 10.1097/00003086-200110001-00009. [DOI] [PubMed] [Google Scholar]
- 50.Lotz M. Cytokines in cartilage injury and repair. Clin Orthop Relat Res. 2001;391:S108–115. doi: 10.1097/00003086-200110001-00011. [DOI] [PubMed] [Google Scholar]
- 51.McKinley TO, Borrelli J, Jr, D’Lima DD, Furman BD, Giannoudis PV. Basic science of intraarticular fractures and posttraumatic osteoarthritis. J Orthop Trauma. 2010;24:567. doi: 10.1097/BOT.0b013e3181ed298d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Spatial and temporal relationship between articular cartilage and meniscus presented in two cases (boxed in black). (A) Most cases showed greater reduction in Safranin O staining in articular cartilage. (B) Fewer cases showed greater reduction in meniscus.
Supplementary Table 1. Validation of reliability and reproducibility was quantified by ICC for the histological system evaluating both the anterior and posterior meniscus.