Abstract
Background & Aims
The leukocyte composition of tumors is heterogeneous, as is the involvement of each leukocyte subset in promoting or restraining tumorigenesis. This heterogeneity reflects the tissue of origin, tumor stage, and the functional state of leukocyte activation, but its biological roots remain poorly understood. Since tumorigenesis is driven by various genetic events, we assessed the role of driver genes in shaping the profiles and the roles of leukocytes in tumorigenesis.
Methods
Mouse liver tumors were induced by hepatic overexpression of either MYC or the combination of myristoylated AKT and NRASV12 oncogenes via hydrodynamic transfection. A comparative, flow cytometry- and histology-based immunophenotyping of liver-infiltrating leukocytes was performed at various stages of liver tumorigenesis. The roles of the most abundant leukocyte subsets in tumorigenesis were addressed by immunodepletion. The contribution of liver injury was assessed by comparing the injury-inducing hydrodynamic transfection model to a model in which MYC is an inducible transgene.
Results
Myristoylated AKT and NRASV12 promoted a marked recruitment of CD11b+Ly6GhiLy6Cint neutrophils and CD11b+Ly6G−Ly6Chi monocytes to the liver, but their immunodepletion did not alter tumorigenesis. In contrast, despite minimal invasion by monocytes/neutrophils during MYC-driven tumorigenesis, immunodepletion of these cells reduced MYC tumor burden and extended survival. MYC-driven tumor-initiation was augmented specifically by Ly6C+ monocytes and their ability to promote liver injury.
Conclusions
Our results demonstrate that leukocyte profiles do not necessarily predict their involvement in tumorigenesis, that the functional role of leukocytes can be shaped by oncogenes, and that monocyte-dependent tissue injury selectively cooperates with MYC during tumorigenesis.
Keywords: oncogenes, MYC, AKT, RAS, tumor, inflammation, neutrophils, monocytes, liver injury
Introduction
While acquisition of driver genetic lesions is necessary to initiate and maintain tumors [1], the influence of tumor stroma on tumor development is now also widely appreciated [2, 3]. Tumor-infiltrating leukocytes play dual roles in cancer, with the potential to either restrain or facilitate tumorigenesis. Most solid tumors are infiltrated by leukocytes with diverse profiles [4], possibly reflecting the tissue of origin and tumor stage [5-7]. However, the precise role of driver genes in mediating leukocyte infiltration and function has been largely unexplored. Understanding the biological roots of this heterogeneity is important, because leukocyte subtype density and location within tumors can serve as a predictor of clinical outcomes and response to therapy [8].
Oncogenes can activate tumor cell-intrinsic transcriptional programs that elicit production of inflammatory mediators and promote myeloid cell recruitment [9-13]. For example, in the early stages of pancreatic β-cell carcinogenesis driven by MYC and BCL-XL co-expression, interleukin-1β-triggered angiogenesis is sustained by infiltrating mast cells [10, 13]. Similarly, mutant Kras-expression by pancreatic ductal epithelial cells sustains tumor growth by mediating myeloid cell recruitment and fostering an immunosuppressive microenvironment [11, 12]. However, these studies do not address the role of particular oncogenes in shaping of these immunological features, which may relate at least partially to the tissue and/or cell type being examined. For example, ibrutinib – purportedly through blockade of mast cell activation – suppresses growth of mutant Kras-driven pancreatic ductal adenocarcinoma [14].
It is also unclear the degree to which oncogene-driven inflammation interplays with extrinsic inflammation. Extrinsic inflammation caused by tissue injury or infection increases cancer risk and can be indispensable for tumorigenesis in some cases [5, 15, 16]. The link between inflammation and cancer is particularly evident in liver cancer [17], which typically develops in the setting of chronic viral hepatitis and cirrhosis of various etiologies [18]. These risk factors promote liver damage, and the liver regeneration response – thought to be mediated by inflammatory cells secreting soluble inflammatory mediators – drives compensatory hepatocyte proliferation and promotes tumorigenesis [19, 20].
To study oncogene-specific regulation of immune responses in liver tumors, we utilized mouse models for liver cancer driven by overexpression of MYC or the combination of myristoylated AKT1 (myrAKT) and NRASV12 oncogenes (AKT/RAS) [21, 22]. These models are amenable for comparative analysis, since both rely on the same method of hydrodynamic transfection of oncogenes and produce tumors in the same tissue type, with similar latency. Moreover, MYC, AKT, and RAS oncogenes have been implicated in human liver cancers. MYC is overexpressed in up to 70% of viral and alcohol-related human hepatocellular carcinomas (HCC) [23], the serine-threonine kinase AKT is activated in 23% of human HCCs [24], and the RAF/MEK/ERK pathway – which can be triggered by RAS – is activated in all human HCCs [25].
We performed a comparative, flow cytometry-based immunophenotyping of liver-infiltrating leukocytes at various stages of liver tumorigenesis driven by MYC and AKT/RAS, assessed the roles of the most abundant myeloid subsets by immunodepletion, and addressed the contribution of tissue injury to tumorigenesis.
Materials and methods
Animals and hydrodynamic transfection
Hydrodynamic injection [26] was performed on 6-8 weeks old female FVB mice from Jackson Laboratory (Bar Harbor, ME). Injections contained 1 μg of plasmid DNA/0.1 ml saline/gr mouse. pT3-EF1 α-MYC [22], or the combination of pT3-EF1 α-myrAKT1-HA and pT-CAGGS-NRASV12 [21] plasmid ratio to Sleeping Beauty transposase (pCMV-SB) [21] was 25:1 (w/w). The transgenic Tet-o-MYC/LAP-tTA (LT2-MYC) mice were described previously [27]. All animal studies conformed to National Institutes of Health guidelines and were approved by University of California Institutional Animal Care and Use Committee.
Flow cytometry
Livers were perfused with phosphate-buffered heparin, minced, and incubated in phosphate-buffered saline (PBS) containing 1 mg/ml Collagenase A (Roche) and 50 units/ml DNase I (Roche) for 30 min at 37°C. Digestion was quenched by adding FACS buffer (1 mM EDTA and 2% FBS in PBS) and single-cell suspensions were obtained by filtering through 70 μm cell strainers (BD Biosciences). Erythrocytes were removed by 5 min incubation in Pharm Lyse buffer (BD Biosciences). After washing in FACS buffer, <106 cells were incubated for 30 min on ice with rat anti-mouse CD16/CD32 mAb (Fc Block, BD Biosciences) diluted in PBS containing Live Dead Aqua (1:500, Life Technologies). Cells were then incubated in a mixture of fluorophore-conjugated mAbs (Supplementary Table 2) and fixed. Data was collected with LSR II (BD Biosciences) flow cytometer and analyzed using FlowJo software. Leukocytes were profiled based on the cell surface marker expression (Supplementary Table 1). The number of each leukocyte subset/mm2 tissue was calculated by combining the flow cytometry data (% of total CD45+ cells) with CD45 IHC staining index (# of CD45+ cells/mm2 tissue).
Immunohistochemistry, automated image acquisition, and analysis
Perfused livers were formalin-fixed, and paraffin-embedded. Liver tissue sections (5 μm thick) were stained with hematoxylin and eosin (H&E). For antigen retrieval, sections were microwaved for 10 min in 0.01 M Na-citrate buffer (pH 6.0) and endogenous peroxidase was blocked with 3% H2O2. Primary antibodies (rat monoclonal anti-CD45 (clone 30-F11), BD Pharmingen; rabbit monoclonal anti-MYC (clone Y69), Abcam; biotinlylated rat monoclonal anti-neutrophil (clone 7/4), Cedarlane) were applied for 2 h in blocking buffer (2.5% BSA, 5% normal goat serum in PBS), followed by species-appropriate secondary antibodies (Vectastain ABC kit) and DAB reagents (Vector Laboratories). Hematoxylin or methyl green was used for counterstaining. Images were acquired with Axiophot microscope (Zeiss) equipped with Leica DFC 420 C digital camera and Leica FireCam software (Leica Microsystems). Whole-slide digital images of CD45 immunostaining were captured with the Aperio ScanScope XT Slide Scanner (Aperio Technologies). Digital images were analyzed using Aperio ImageScope software.
Immunoblot
Livers were homogenized in the lysis buffer containing 50 mM HEPES-KOH pH 7.4, 130 mM NaCl, 1% Triton X-100, 1% SDS, 1% sodium deoxycholate, and complete protease inhibitor cocktail (Roche). Lysates were boiled for 5 min in Laemmli buffer (50 mM Tris-HCl pH 6.8, 12.5% glycerol, 2% SDS, 0.01% bromophenol blue, and 100 mM DTT), and proteins were electrophoresed and immunoblotted. Blots were probed with anti-MYC (clone Y69, Abcam) and anti-GAPDH (clone 6C5, EMD Millipore), followed by HRP-conjugated secondary antibodies and ECL reagent (Amersham).
Protein array and ELISA
Livers were lysed in PBS containing 1% Triton X-100 and complete protease inhibitor cocktail (Roche). The lysates (150 μg/sample) were used in Proteome Profiler Mouse Cytokine Array (R&D Systems) or in CCL2 and CXCL1 Quantikine ELISA (R&D Systems).
In vivo leukocyte depletion
Mice were injected intraperitoneally (I.P.) with 200 μl of antibodies (BioXCell) in saline solution. Gr-1 mAb or control rat IgG2b (clone LTF-2) were injected every three days for the total of four treatments at 250 μg/mouse; 500 μg/mouse of anti-Ly6G mAb (clone 1A8) or control rat IgG2a (clone 2A3) was injected triweekly for total of 9 treatments; 200 μg/mouse of anti-CD11b mAb (clone M1/70) or control rat IgG2b (clone LTF-2) was injected at triweekly for total of 7 treatments. Anti-CSF-1 mAb (clone 5A1) or control IgG1 (clone HRPN) were given once, in a single dose of 1 mg Ab/mouse.
Serum ALT measurement
Whole-blood samples were allowed to coagulate for 15 min at room temperature and centrifuged at 1,000 g at 4°C for 10 min to separate the serum. Aminotransferase levels were measured using Automated Diagnostic Analyzer (IDEXX Laboratories).
Statistical analyses
Data were analyzed using the Student's t-test to compare between two groups. Two-way ANOVA was used to compare among several groups, followed by the Tukey's post-hoc test (p = 0.05) for pairwise comparison.
Results
Inflammation during liver tumorigenesis induced by MYC and AKT/RAS
To address how oncogenes shape the leukocyte responses in the liver during tumorigenesis, we induced liver tumors by hydrodynamic transfection of mice with MYC or AKT/RAS [21, 22]. Hepatic overexpression of MYC results in highly aggressive, poorly differentiated liver tumors that resemble human hepatoblastomas [22, 27]. Overexpression of constitutively active myrAKT1 produces HCC with long latency [28], while NRASG12V does not give rise to liver tumors because of RAS-induced senescence that clears pre-malignant hepatocytes [29]. Nevertheless, NRAS synergizes with oncogenic AKT to produce tumors that resemble moderately differentiated HCC and cholangiocarcinoma [21].
Hydrodynamic tail-vein injection confirmed earlier reports of transient liver injury [30, 31] that healed by 6 days post-injection (dpi), while hydrodynamic transfection of MYC or AKT/RAS caused progressive neoplastic changes (Fig. 1A and Supplementary Fig. 1A-C). Hepatic steatosis was prominent in AKT/RAS livers, in line with previous reports of AKT-driven lipogenesis [28]. MYC and AKT/RAS tumors developed with similar latency, reaching terminal stages ~7 weeks post-transfection. Immunodetection of CD45 in the liver (Fig. 1A) revealed that oncogenes promoted a transient inflammation in the form of large leukocyte clusters at the early stages of tumorigenesis (3 dpi). This was more pronounced in AKT/RAS- than in MYC-transfected livers, and was minimal in saline-injected livers. Despite leukocyte clusters in both models, only AKT/RAS triggered a marked increase in leukocyte infiltration over saline control at 3 dpi (Fig. 1B). However, the leukocyte density returned to baseline by 14 dpi and was relatively constant throughout tumor progression in both models (Fig. 1B). Expression of oncogenes was detected as early as 3 dpi (Supplementary Fig. 1D), confirming efficient oncogene delivery and suggesting that early inflammation could be linked to oncogene activation.
Fig. 1. Inflammation during MYC- and AKT/RAS-driven liver tumorigenesis.
H&E and CD45 staining (brown) in perfused livers of mice hydrodynamically injected with saline, or with Sleeping Beauty transposon system carrying AKT/RAS or MYC. Scale bar=50 μm. (B) Quantification of CD45 immunostaining. Zero time point represents untreated livers. Data are displayed as mean ± SEM with n=3 mice per group. Significance (AKT/RAS 3 dpi vs normal liver) was determined by two-way ANOVA and Tukey's multiple comparisons post-hoc test (***p<0.001).
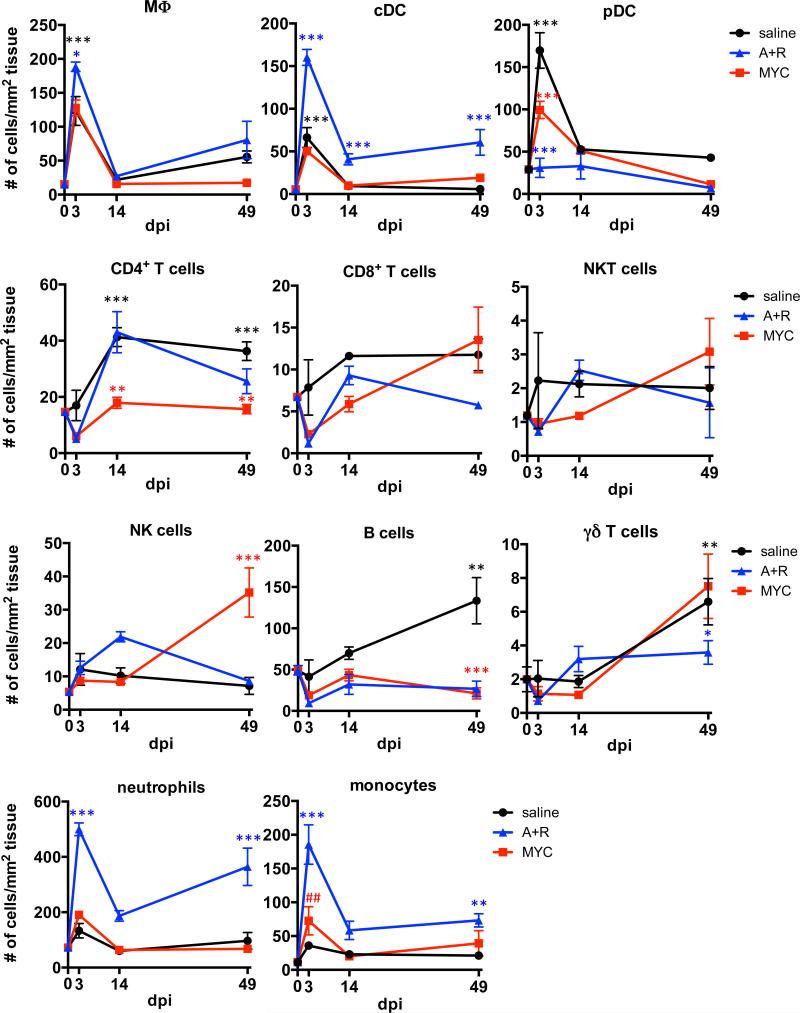
Distinct profiles of liver-infiltrating leukocytes during MYC- and AKT/RAS-driven tumorigenesis
To further characterize intrahepatic CD45+ leukocytes, single-cell suspensions from the perfused livers were stained with antibodies against various leukocyte lineage markers (Supplementary Table 1) and analyzed by polychromatic flow cytometry. The assay revealed dynamic changes in the leukocyte composition over the course or tumorigenesis (Fig. 2 and Supplementary Fig. 2A). Hydrodynamic injection of saline promoted a transient increase in macrophages, classical and plasmacytoid dendritic cells at 3 dpi, followed by the recruitment of CD4+ T cells, B cells, and γδ T cells at the later stages (Fig. 2). While oncogene-transfected livers shared certain trends with controls, including macrophage recruitment, several oncogene-specific changes were observed. Specifically, MYC-expressing livers failed to recruit CD4+ T lymphocytes, and terminal MYC tumors contained more NK cells than AKT/RAS tumors or the time-matched controls. MYC tumors also displayed a slight, yet significant increase in Ly6C+ monocyte recruitment at 3 dpi compared to untreated mice (p < 0.01).
Fig. 2. Distinct profiles of liver-infiltrating leukocytes during MYC- and AKT/RAS-driven tumorigenesis.
Immunophenotyping based on the expression of multiple lineage markers detected by flow cytometry. Oncogene-treatments were compared to time-matched saline controls (red asterisks for MYC, blue for AKT/RAS). Saline controls were compared to untreated (zero time point) samples to assess the effect of hydrodynamic transfection alone (black asterisks). Monocyte abundance in MYC-transfected livers compared to untreated (##p<0.01). Data are displayed as mean ± SEM with n=3 mice per group. Significance was determined by two-way ANOVA and Tukey's post-hoc test (*p<0.05, **p<0.01, ***p<0.001).
AKT/RAS promoted a marked recruitment of several myeloid cell populations into the livers at the early stages after transfection (3 dpi), including classical dendritic cells (CD11chiMHCII+), neutrophils (CD11b+Ly6G+Ly6Cint), and monocytes (CD11b+Ly6G−Ly6Chi) (Fig. 2). Although the effect was partially transient, all three of these populations were also increased at end-stage (49 dpi). Ly6G+ neutrophils represented the dominant immune population throughout AKT/RAS-driven tumorigenesis (Fig. 2 and Supplementary Fig. 2A), and were particularly prominent in AKT/RAS compared to MYC tumors (Fig. 3A). Transcript levels of monocyte/macrophage-derived cytokines, such as IL-6 and IL-1β, but not of TNFα, were higher in AKT/RAS- livers compared to MYC-transfected livers (Supplementary Fig. 2B). Immunostaining for 7/4 antigen, a differentiation marker associated specifically with neutrophils and monocytes [32], also corroborated that neutrophils and monocytes were more abundant in AKT/RAS livers (Fig. 3B).
Fig. 3. Marked Ly6G+ neutrophil and Ly6C+ monocyte infiltration and higher CXCL1 and CCL2 levels in the livers of AKT/RAS-transfected mice.
(A) Dot plots showing the gating of live/CD45+/CD3CD19CD49− cells for detection of neutrophils (CD11b+Ly6G+Ly6Cint) and monocytes (CD11b+Ly6G−Ly6Chi) in MYC and AKT/RAS tumors. (B) 7/4 antigen immunoreactivity (brown) in the livers of MYC and AKT/RAS mice at 3 dpi and in tumors (50 dpi). Scale bar=100 μm. (C) Heat map representing cytokine/chemokine expression in normal livers and MYC and AKT/RAS liver tumors, measured by protein array (n=1 sample per condition). (D) Quantitative ELISA measurements of CXCL1 and CCL2 in the livers of saline-injected, and AKT/RAS- or MYC-transfected mice. Data are displayed as mean ± SEM with n≥4 mice/group. Statistical significance was calculated using unpaired t-test.
To understand the difference in myeloid cell infiltration in the two models, we compared cytokine/chemokine expression in AKT/RAS and MYC tumors. A protein array identified several differentially expressed factors (Fig. 3C), including CCL2, a monocyte chemoattactant [33], and CXCL1, a neutrophil chemoattractant [34]. ELISA confirmed a transient induction of these chemokines by both oncogenes at the early stages of tumorigenesis (Fig. 3D) coinciding with inflammation (Fig. 1B). CXCL1 was 5-fold higher and CCL2 was 3-fold higher in AKT/RAS-compared to MYC-transfected livers, in line with a massive recruitment of neutrophils and monocytes specifically into AKT/RAS livers.
Gr-1+ myeloid cells selectively augment MYC-driven tumorigenesis
We postulated that neutrophils and monocytes, abundant in AKT/RAS-expressing livers, might regulate tumorigenesis in this model, especially given that Kras-driven pancreatic ductal adenocarcinoma has previously been linked with GM-CSF production and recruitment of immunosuppressive Gr-1+ (Ly6G/Ly6C) cells [11, 12]. Thus, we treated mice systemically with the anti-Gr-1 monoclonal antibody (mAb) RB6-8C5 to deplete leukocytes expressing Ly6G and/or Ly6C [35]. Flow cytometry analysis confirmed depletion of Ly6G+Ly6Cint neutrophils and Ly6G−Ly6Chi monocytes, but not Ly6G−Ly6C−MHCII+F4/80+ macrophages, from the livers of both MYC and AKT/RAS mice (Supplementary Fig. 3A). We observed no reduction in dendritic cells or lymphocyte populations by anti-Gr-1 treatment (data not shown).
Since the leukocyte depletion by anti-Gr-1 was transient (Supplementary Fig. 3B and C), we began Gr-1 treatments at −1, +4, and +16 days relative to hydrodynamic transfection to examine the role of Gr-1+ cells at different stages of tumorigenesis (Supplementary Fig. 3D). Unexpectedly, none of the treatment regimens altered the survival of AKT/RAS mice (Fig. 4A). In contrast, anti-Gr-1 mAb extended the survival of MYC mice when applied during the early stages of tumorigenesis (1 day before, or 4 days after hydrodynamic transfection) (Fig. 4B). No effect on survival was observed when Gr-1-depletion was performed at a later stage (16 dpi). Consistent with its effect on survival, anti-Gr-1 mAb reduced the burden of MYC tumors, but not that of AKT/RAS tumors, as determined by liver-to-body mass ratio (Fig. 4C and D). Additionally, the livers of anti-Gr-1-treated mice at 7 dpi contained fewer MYC-positive basophilic foci (Fig. 4E), suggesting that Gr-1+ cells cooperate with MYC during tumor initiation rather than progression. This decrease in the number of MYC-positive foci was not due to impaired transfection efficiency, since we saw no reduction in GFP transfection by anti-Gr-1 mAb treatment (Supplementary Fig. 4).
Fig. 4. Gr-1+ leukocytes augment MYC- but not AKT/RAS-driven tumorigenesis.
Survival of AKT/RAS (A) or MYC (B) mice treated with anti-Gr-1. Red triangles mark anti-Gr-1 treatments starting at −1, +4, or +16 dpi (log-rank test **p<0.01, *p<0.05; n≥3 mice/group). AKT/RAS (C) and MYC (D) tumor burden at 5 weeks. Anti-Gr-1 mAb treatment began at −1 dpi (*p<0.05, n≥3 mice/group). (E) Quantification of basophilic foci (arrows) in MYC-transfected livers at 7 dpi (*p<0.05, n=3). Representative H&E and MYC immunostaining are shown. Scale bar=200 μm. Data (C-E) are displayed as mean ± SEM with n≥3 mice/group. Statistical significance was calculated using unpaired t-test.
Thus, despite the dramatic infiltration of neutrophils and monocytes resulting from AKT/RAS transfection, these cells apparently played no role in early tumorigenesis. Instead, even though invasion into the tissue was minimal following MYC transfection, neutrophils and/or monocytes cooperated with MYC to enhance tumorigenesis in the liver.
Ly6C+ monocytes cooperate with MYC during tumorigenesis
We confirmed the selective involvement of myeloid cells in MYC-driven tumorigenesis through depletion of cells expressing CD11b (Fig. 5A and B). Surprisingly however, when we selectively depleted neutrophils using anti-Ly6G mAb (clone 1A8) (Supplementary Fig. 5A), we did not observe extended survival of MYC mice, or reduce tumor burden in either model (Fig. 5C and D), indicating that neutrophils were dispensable for tumorigenesis.
Fig. 5. Ly6C+ monocytes selectively enhance MYC-driven tumorigenesis.
Survival of anti-CD11b-treated MYC (A) or AKT/RAS (B) mice (log-rank **p<0.01; n≥5 mice/group). (C) Survival of anti-Ly6G-treated MYC mice (log-rank p=0.19; n≥5 mice/group). (D) Effect of anti-Ly6G on MYC (p=0.99) or AKT/RAS (p=0.27) tumor burden at 7 weeks after transfection. Data represent mean ± SEM with n≥3 mice/group. Statistical significance was calculated using unpaired t-test. Survival of anti-CSF-1-treated MYC (E) or AKT/RAS (F) mice (log-rank MYC p=0.16, n≥6 mice/group; AKT/RAS p=0.66, n≥4 mice/group). All antibody treatments started one day before oncogene transfection (red triangles). (G) Effect of depletional antibodies on MYC-driven tumorigenesis (MO-monocytes, NE-neutrophils, MΦ-macrophages).
During liver damage, recruited CD11b+Ly6Chi monocytes can differentiate into pro-inflammatory and pro-fibrogenic macrophages [36]. Tumor-associated macrophages have also been shown to derive from Ly6Chi monocytes [37]. We thus pre-treated mice with an anti-CSF-1 mAb to deprive macrophages of an important survival/recruitment factor [38-40], and significantly reduced the number of Ly6C−F4/80+MHCII+ macrophages in the livers at 3 dpi (Supplementary Fig. 5B). However, the treatment did not alter the survival MYC or AKT/RAS mice, suggesting that macrophages do not regulate early stages of tumorigenesis in either model (Fig. 5E and F). By excluding the role of neutrophils and macrophages (Fig. 5G), the results suggest that Ly6C+ inflammatory monocytes were the CD11b+Gr-1+ myeloid population responsible for augmenting tumorigenesis induced by MYC.
The role of tissue injury in monocyte-MYC cooperation
Ly6C+ monocytes can promote tumor growth by suppressing tumor-specific T lymphocytes [41]. However, systemic depletion of CD8+ T lymphocytes did not reverse the effect of anti-Gr1 mAb on survival of MYC mice (Supplementary Fig. 7A and B), indicating the involvement of an alternative mechanism. As anti-Gr-1 mAb extended survival only when administered in the early stages of MYC-induced tumorigenesis, we hypothesized that the liver injury induced by hydrodynamic transfection might relate to the role of monocytes in promoting tumor development. We thus turned to the LT2-MYC transgenic mouse model, allowing for doxycycline-regulated, hepatocyte-specific expression of MYC transgene [27]. LT2-MYC liver tumors are histologically similar to those induced by hydrodynamic transfection of MYC [22, 42]. The onset of hepatic MYC expression in LT2-MYC mice occurred ~20 days after doxycycline removal (Fig. 6A and B) and the MYC-positive foci (Fig. 6A) progressed into tumors, replacing most of the normal liver by day 60.
Fig. 6. Gr-1+ monocyte-dependent liver injury augments MYC-driven tumorigenesis.
(A) MYC immunoreactivity in LT2-MYC livers after doxycycline removal (days off Doxy). Scale bar=100 μm (B) Immunoblot detection of MYC and GAPDH in LT2-MYC liver lysates. (C) Survival of anti-Gr-1-treated, uninjured LT2-MYC mice (blue lines; log-rank p=0.6, n≥8 mice/group) and LT2-MYC mice hydrodynamically injected with saline (HDI) (red lines; log-rank *p=0.01, n≥11 mice/group). Ctrl IgG-treated LT2-MYC mice (Ctrl IgG) versus saline-injected LT2-MYC mice (HDI+Ctrl IgG) log-rank p=0.07. (D) Effect of anti-Gr-1 and anti-Ly6G treatment (started at -1 dpi) on serum ALT levels 3 days after control hydrodynamic transfection (ctrl HDT) or transfection of AKT/RAS (A+R HDT) or MYC (MYC HDT). Data represents mean ± SEM with n≥3 mice/group. Significance was determined using unpaired t-test (*p<0.05, **p<0.01).
To assess the role of Gr-1+ leukocytes in the early stages of tumorigenesis in LT2-MYC mice, we began anti-Gr-1 mAb treatment 18 days after doxycycline removal, and found no effect on survival (log-rank test p=0.6) (Fig. 6C). It has been demonstrated that hydrodynamic injection shortens the LT2-MYC tumor latency, likely due to the injury-triggered liver regeneration [43]. Indeed, hydrodynamic injection of LT2-MYC mice with saline (HDI) accelerated tumor progression, although this was not statistically significant (log-rank test p=0.07). Critically, we found that anti-Gr-1 mAb treatment partially reversed the effect of hydrodynamic injection and extended survival (log-rank test p=0.01) (Fig. 6C). Thus, Gr-1+ cells promoted MYC-driven tumorigenesis specifically in the context of liver injury.
Hydrodynamic injection promotes liver damage [44], and as expected, serum alanine transaminase (ALT) levels increased in the control-transfected mice at 3 dpi (Fig. 6D). AKT/RAS and MYC elicited a similar degree of liver injury, albeit higher than the control transfection (Fig. 6D), and also promoted hepatic apoptosis more so than the control (Supplementary Fig. 6). Anti-Gr1 mAb treatment reduced serum ALT levels by approximately 50% in the control- and oncogene-transfected groups (Fig. 6D). Liver damage, however, was neutrophil-independent, since anti-Ly6G mAb did not prevent the increase in serum ALT in any case (Fig. 6D). Together, these data demonstrate that AKT/RAS and MYC trigger a similar level of Ly6C+ monocyte-dependent liver injury, but that monocytes only augment tumorigenesis driven by MYC.
Discussion
Most solid tumors are infiltrated by diverse leukocytes whose type, density, and location within tumors can serve as a prognostic factor [8]. Here we report the changes in tissue-infiltrating leukocyte profiles during tumorigenesis driven by distinct oncogenes in a single tissue type. Activation of MYC or co-activation of AKT and RAS in the livers of mice promoted a varying degree of inflammation and infiltration of distinct leukocyte subsets, thereby demonstrating that the leukocyte composition of a tissue is shaped, at least in part, by tumor-driving genetic lesions. Our finding that Ly6C+ monocytes augment tumorigenesis driven by MYC was unexpected, considering the minimal hepatic infiltration of monocytes in this model. This suggests that the abundance of a given leukocyte subset in a pre-neoplastic tissue does not necessarily predict their role in tumorigenesis, and that their role is influenced by the type of tumor-initiating genetic lesion.
Myeloid cells, specifically neutrophils and monocytes, were markedly increased in the livers of AKT/RAS-transfected compared to MYC-transfected mice. Several oncoproteins, including MYC [10], RAS [9], RET [46] and EGFR [47] can activate tumor cell-intrinsic signaling pathways that drive production of pro-inflammatory cytokines and chemokines [5]. Loss of tumor suppressors p53, APC, or PTEN has also been implicated in regulation of tumor-associated inflammation [48, 49]. Here, we report that AKR/RAS co-activation in hepatocytes promotes an increase in CCL2 and CXCL1, and a massive recruitment of myeloid cells into the liver.
Recent work has demonstrated the functional significance of recruited Gr-1+ leukocytes for liver tumorigenesis at the early stages of oncogenic NRASG12V activation in hepatocytes [29]. In this model recruited Gr-1+ monocytes act in cooperation with CD4+ T lymphocytes to eliminate the RAS-expressing, pre-malignant senescent cells to restrain tumorigenesis [29]. Although we saw a similar massive recruitment of Ly6G+ and Ly6C+ leukocytes upon hepatic co-activation of AKT and RAS, we found that these cells were inconsequential for tumorigenesis. One possible explanation for why Ly6C+ monocytes were irrelevant during AKT/RAS tumor development is the suppression of RAS-driven senescence by AKT [50]. Indeed, previous studies have not detected senescence in the AKT/RAS model [21], thus potentially eliminating the role for Ly6C+ monocyte activity in senescence surveillance and tumorigenesis. Also, AKT/RAS signaling may confer hepatocytes with a superior proliferative capacity, allowing independence from the growth-promoting stimuli of the immune microenvironment. AKT-induced hepatic steatosis in AKT/RAS livers [28] may also drive hepatocyte proliferation independently of inflammation through oxidative stress-mediated JNK activation [51]. Thus, tumor-driving oncogenes are a critical determinant not only of the recruitment of myeloid cells to tissues, but also of their functional role.
While we found that monocytes play a role at the early stages of MYC-driven tumorigenesis, the precise timing of monocyte involvement and the molecular underpinnings of our observations remain unclear. Tumor-infiltrating myeloid cells promote tumorigenesis via multiple mechanisms [52, 53]. For example, they can sustain tumor cell survival and proliferation through secretion of pro-inflammatory cytokines [54], promote angiogenesis [55], blunt cytotoxic T cell activity by fostering an immunosuppressive microenvironment [11, 12], or prevent senescence [49]. Although we did not find a role for CD8+ T cells, future studies will be necessary to understand the mechanisms by which monocytes specifically enhance MYC-driven tumorigenesis.
The common causes of liver cancers in humans, including viral hepatitis, cirrhosis, alcoholic and toxin injury, promote liver damage and inflammation [56]. Comparing the injury-inducing hydrodynamic injection model to an inducible MYC overexpressing transgenic model, we found that MYC-driven tumor-initiation was augmented specifically by Ly6C+ monocytes recruited to the liver during injury. Since we found that both MYC and AKT/RAS promote similar levels of monocyte-dependent liver injury, it remains to be addressed why injury and/or monocytes selectively enhance MYC-driven tumor initiation. MYC tumors are poorly differentiated and resemble human hepatoblastomas, whereas AKT/RAS tumors resemble moderately differentiated HCC and cholangiocarcinoma [21, 22, 27]. These histological distinctions may make these two models inherently different in their susceptibility to immune regulation. Intriguingly, MYC is clinically associated with the injury-induced viral and alcohol-related HCC, but not with cryptogenic HCC [23], and MYC expression increases with hepatic injury in a chemically-induced liver cancer model [57]. These clinical observations correlate with our finding that MYC-driven liver tumorigenesis is injury dependent, and indicate that additional studies on the interplay between MYC-driven tumorigenesis, liver injury and monocytes are warranted.
Supplementary Material
Acknowledgements
We thank members of the Bishop laboratory for critical feedback; L.M. Coussens (Oregon Health and Science University, Portland, OR) for valuable discussion of preliminary results; L. Urisman and K. Jusewicz-Haidle (University of California, San Francisco, San Francisco, CA) for assistance with animal husbandry; and the UCSF Parnassus Flow Cytometry shared core resource, supported by Diabetes Research Center Grant (NIH P30 DK063720).
Financial support: This work was supported by UCSF/G.W. Hooper Research Foundation Endowment Funds and by NIH R01CA136606 grant from the National Institutes of Health to XC.
Abbreviations
- myrAKT
myristoylated AKT1
- AKT/RAS
myrAKT and NRAS
- HCC
hepatocellular carcinoma
- PBS
phosphate-buffered saline
- H&E
hematoxylin and eosin
- I.P.
intraperitoneal
- dpi
days post-injection
- mAb
monoclonal antibody
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The corresponding author V. Juric is currently an employee of Gilead Sciences. No potential conflicts of interest were disclosed by the other authors.
Author contributions: V.J. designed the study, acquired, analyzed and interpreted data, and prepared the manuscript; B.R. designed select experiments and analyzed data; K.E. and L.W. provided histopathological reviewing; X.C., L.C., and J.H. performed select experiments; V.J., B.R., and J.M.B. contributed to writing the manuscript.
References
- 1.Pagliarini R, Shao W, Sellers WR. Oncogene addiction: pathways of therapeutic response, resistance, and road maps toward a cure. EMBO Rep. 2015 doi: 10.15252/embr.201439949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 6.Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A. 2012;109:2796–2801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 9.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 10.Shchors K, Shchors E, Rostker F, Lawlor ER, Brown-Swigart L, Evan GI. The Myc-dependent angiogenic switch in tumors is mediated by interleukin 1beta. Genes Dev. 2006;20:2527–2538. doi: 10.1101/gad.1455706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–847. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 14.Masso-Valles D, Jauset T, Serrano E, Sodir NM, Pedersen K, Affara NI, et al. Ibrutinib exerts potent antifibrotic and antitumor activities in mouse models of pancreatic adenocarcinoma. Cancer Res. 2015;75:1675–1681. doi: 10.1158/0008-5472.CAN-14-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 16.de Martel C, Franceschi S. Infections and cancer: established associations and new hypotheses. Crit Rev Oncol Hematol. 2009;70:183–194. doi: 10.1016/j.critrevonc.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-[kappa]B functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 18.El-Serag HB. Epidemiology of Viral Hepatitis and Hepatocellular Carcinoma. Gastroenterology. 2012;142:1264–1273. e1261. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Sakurai T, Maeda S, Chang L, Karin M. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci U S A. 2006;103:10544–10551. doi: 10.1073/pnas.0603499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho C, Wang C, Mattu S, Destefanis G, Ladu S, Delogu S, et al. AKT and N-Ras co-activation in the mouse liver promotes rapid carcinogenesis via mTORC1, FOXM1/SKP2, and c-Myc pathways. Hepatology (Baltimore, Md) 2012;55:833–845. doi: 10.1002/hep.24736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tward AD, Jones KD, Yant S, Kay MA, Wang R, Bishop JM. Genomic progression in mouse models for liver tumors. Cold Spring Harb Symp Quant Biol. 2005;70:217–224. doi: 10.1101/sqb.2005.70.058. [DOI] [PubMed] [Google Scholar]
- 23.Schlaeger C, Longerich T, Schiller C, Bewerunge P, Mehrabi A, Toedt G, et al. Etiology-dependent molecular mechanisms in human hepatocarcinogenesis. Hepatology. 2008;47:511–520. doi: 10.1002/hep.22033. [DOI] [PubMed] [Google Scholar]
- 24.Boyault S, Rickman DS, de Reyniès A, Balabaud C, Rebouissou S, Jeannot E, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 25.Hwang YH, Choi JY, Kim S, Chung ES, Kim T, Koh SS, et al. Over-expression of c-raf-1 proto-oncogene in liver cirrhosis and hepatocellular carcinoma. Hepatology Research. 2004;29:113–121. doi: 10.1016/j.hepres.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Bell JB, Podetz-Pedersen KM, Aronovich EL, Belur LR, McIvor RS, Hackett PB. Preferential delivery of the Sleeping Beauty transposon system to livers of mice by hydrodynamic injection. Nature protocols. 2007;2:3153–3165. doi: 10.1038/nprot.2007.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shachaf CM, Kopelman AM, Arvanitis C, Karlsson A, Beer S, Mandl S, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431:1112–1117. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- 28.Calvisi DF, Wang C, Ho C, Ladu S, Lee SA, Mattu S, et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140:1071–1083. doi: 10.1053/j.gastro.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 30.Suda T, Gao X, Stolz DB, Liu D. Structural impact of hydrodynamic injection on mouse liver. Gene Ther. 2006;14:129–137. doi: 10.1038/sj.gt.3302865. [DOI] [PubMed] [Google Scholar]
- 31.Woodard LE, Hillman RT, Keravala A, Lee S, Calos MP. Effect of nuclear localization and hydrodynamic delivery-induced cell division on [phi]C31 integrase activity. Gene Ther. 2009;17:217–226. doi: 10.1038/gt.2009.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirsch S, Gordon S. Polymorphic expression of a neutrophil differentiation antigen revealed by monoclonal antibody 7/4. Immunogenetics. 1983;18:229–239. doi: 10.1007/BF00952962. [DOI] [PubMed] [Google Scholar]
- 33.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121:4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 35.Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- 36.Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261–274. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 37.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 38.Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 39.Ruffell B, Coussens LM. Macrophages and Therapeutic Resistance in Cancer. Cancer Cell. 2015;27:462–472. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chow EK-H, Fan L-l, Chen X, Bishop JM. Oncogene-specific formation of chemoresistant murine hepatic cancer stem cells. Hepatology. 2012;56:1331–1341. doi: 10.1002/hep.25776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woodard LE, Keravala A, Jung WE, Wapinski OL, Yang Q, Felsher DW, et al. Impact of hydrodynamic injection and phiC31 integrase on tumor latency in a mouse model of MYC-induced hepatocellular carcinoma. PLoS One. 2010;5:e11367. doi: 10.1371/journal.pone.0011367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 45.Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 46.Borrello MG, Alberti L, Fischer A, Degl'innocenti D, Ferrario C, Gariboldi M, et al. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci U S A. 2005;102:14825–14830. doi: 10.1073/pnas.0503039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu K, Shu HK. EGFR activation results in enhanced cyclooxygenase-2 expression through p38 mitogen-activated protein kinase-dependent activation of the Sp1/Sp3 transcription factors in human gliomas. Cancer Res. 2007;67:6121–6129. doi: 10.1158/0008-5472.CAN-07-0141. [DOI] [PubMed] [Google Scholar]
- 48.Yang L, Karin M. Roles of tumor suppressors in regulating tumor-associated inflammation. Cell Death Differ. 2014;21:1677–1686. doi: 10.1038/cdd.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Mitri D, Toso A, Chen JJ, Sarti M, Pinton S, Jost TR, et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature. 2014;515:134–137. doi: 10.1038/nature13638. [DOI] [PubMed] [Google Scholar]
- 50.Kennedy Alyssa L, Morton Jennifer P, Manoharan I, Nelson David M, Jamieson Nigel B, Pawlikowski Jeff S, et al. Activation of the PIK3CA/AKT Pathway Suppresses Senescence Induced by an Activated RAS Oncogene to Promote Tumorigenesis. Molecular Cell. 2011;42:36–49. doi: 10.1016/j.molcel.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 56.Sun B, Karin M. Inflammation and liver tumorigenesis. Front Med. 2013;7:242–254. doi: 10.1007/s11684-013-0256-4. [DOI] [PubMed] [Google Scholar]
- 57.Fang CH, Zhang GQ, Zhu XY, Gong JQ. Distribution of oval cells and c-myc mRNA expression in mouse hepatocarcinogenesis. Hepatobiliary Pancreat Dis Int. 2004;3:433–439. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.