Abstract
Purpose
We compared all-cause and human immunodeficiency virus (HIV) mortality in a population-based, HIV-infected cohort.
Methods
Using records of people diagnosed with HIV during 2000–2009 from the Florida Enhanced HIV/Acquired Immunodeficiency Syndrome (AIDS) Reporting System, we conducted a proportional hazards analysis for all-cause mortality and a competing risk analysis for HIV mortality through 2011 controlling for individual level factors, neighborhood poverty, and rural/urban status and stratifying by concurrent AIDS status (AIDS within 3 months of HIV diagnosis).
Results
Of 59,880 HIV-infected people, 32.2% had concurrent AIDS, and 19.3% died. Adjusting for period of diagnosis, age group, sex, country of birth, HIV transmission mode, area level poverty and rural/urban status, non-Hispanic Black (NHB) and Hispanic people had an elevated adjusted hazards ratio (aHR) for HIV mortality relative to non-Hispanic whites (NHB concurrent AIDS: aHR 1.34, 95% CI 1.23–1.47; NHB without concurrent AIDS: aHR 1.41, 95% CI 1.26–1.57; Hispanic concurrent AIDS: aHR 1.18, 95% CI 1.05–1.32; Hispanic without concurrent AIDS: aHR 1.18, 95% CI 1.03–1.36).
Conclusions
Considering competing causes of death, NHB and Hispanic people had a higher risk of HIV mortality even among those without concurrent AIDS, indicating a need to identify and address barriers to HIV care in these populations.
Keywords: human immunodeficiency virus, acquired immunodeficiency syndrome, mortality, racial disparities, competing risks models
INTRODUCTION
Non-Hispanic Blacks (NHBs) and Hispanics continue to be disproportionately affected by the human immunodeficiency virus (HIV) epidemic in the United States (US). In 2012 the age-adjusted HIV mortality rate among NHBs was 9.8 per 100,000 compared with 2.2 for Hispanics and 1.0 for NHWs [1]. Antiretroviral treatments significantly improve life expectancy; currently a 20 year-old person infected with HIV who is taking combination antiretroviral therapy has a life expectancy of about 50 additional years [2–3]. However, non-whites have had a persistently lower life expectancy than whites, which may be due to differences in socioeconomic status (SES) and access to care [3–5].
Long-term survival with HIV infection is dependent on early diagnosis, linkage to and retention in care, and adherence to treatment [6–7]. In the US, of the estimated 1.2 million people living with HIV in 2011, 14% were not yet diagnosed, 60% did not obtain medical care, and 63% were not prescribed antiretroviral therapy during the prior 12 months [6]. The percentage of NHBs not virally suppressed was estimated at 72%, higher than the 68% among NHWs and 69% among Hispanics although the differences were not statistically significant [6]. Florida has been particularly affected by the HIV epidemic. An estimated 99,209 people in the state were living with diagnosed HIV infection in 2012 for a prevalence of 599 per 100,000, the third highest in the US [8]. There also are large racial/ethnic disparities in Florida with an estimated HIV prevalence of 1,978.8 per 100,000 among NHBs, 558.9 among Hispanics, and 288.5 among NHWs [8]. A recent report on the continuum of HIV care during 2014 in Florida indicated that NHBs and Hispanics were less likely to have a suppressed viral load than NHWs (53%, 61% and 66% respectively) [9]. A study of all-cause survival among Floridians diagnosed with HIV infection from 1993–2004 found that survival was significantly lower among NHBs compared with NHWs [10]. The objective of the present study was to compare all-cause and HIV mortality by race/ethnicity and estimate the role of neighborhood poverty and rural/urban residence in survival disparities among people diagnosed with HIV infection in Florida.
METHODS
Data included de-identified records of Florida residents who were reported to the Florida Department of Health (DOH) Enhanced HIV/AIDS Reporting System (eHARS) during 2000– 2009 with an HIV diagnosis meeting the Centers for Disease Control and Prevention (CDC) surveillance case definition [11–12]. Individual-level variables included month and year of HIV and AIDS diagnoses (if applicable); age at HIV diagnosis; sex at birth; race/ethnicity; country of birth; HIV transmission mode; and number months between HIV diagnosis and death or December 2011, whichever came first. Race/ethnicity data were classified into 4 groups: NHBs, NHWs, Hispanics, and “others” which included multiracial, Asian, American Indian, and Native Hawaiian/Pacific Islanders.
Vital status and cause of death
Deaths and underlying causes (UCs) through December 2011 were ascertained by matching eHARS records with Florida DOH Vital Records and the National Death Index. Causes and significant conditions were coded with the International Classification of Diseases, Tenth Revision (ICD-10) [13]. UCs were classified into two groups based on the ICD codes: HIV/AIDS and all others. The HIV/AIDS group included deaths with an UC due to HIV disease (B20-B24), laboratory evidence of HIV infection (R75), and UCs of death suggestive of HIV disease due to an apparent underreporting of HIV as an UC (see Appendix A). These included codes for which the World Health Organization recommends use of HIV disease codes (B20-B24) in a person with HIV infection [14].
Area-level factors
Five-year estimates of poverty data from the 2007–2011 American Community Survey were obtained from the US Census Bureau [15] and linked using the zip code tabulation area (ZCTA). The Census Bureau reports data by ZCTA, which approximates zip codes and is built by aggregating Census Bureau blocks based on the zip code of addresses in these blocks [16]. Records of diagnosed HIV cases with missing or non-existing zip codes were excluded from the analysis. In addition, records of cases diagnosed in a correctional facility were excluded because the inmates’ care is unrelated to characteristics of the surrounding ZCTA. Rural/urban status of the zip code was based on categorization C of Version 2.0 Rural-Urban Commuting Area (RUCA) data codes [17–18].
Analyses
Observations were classified as cases having concurrent AIDS or cases without concurrent AIDS at the time of HIV diagnosis, because survival of people without concurrent AIDS is likely to be more strongly related to linkage, retention, and adherence to HIV care and treatment. Concurrent AIDS was defined as an AIDS diagnosis within 3 months of the HIV diagnosis based on the CDC recommendation that people start HIV care by 3 months [19].
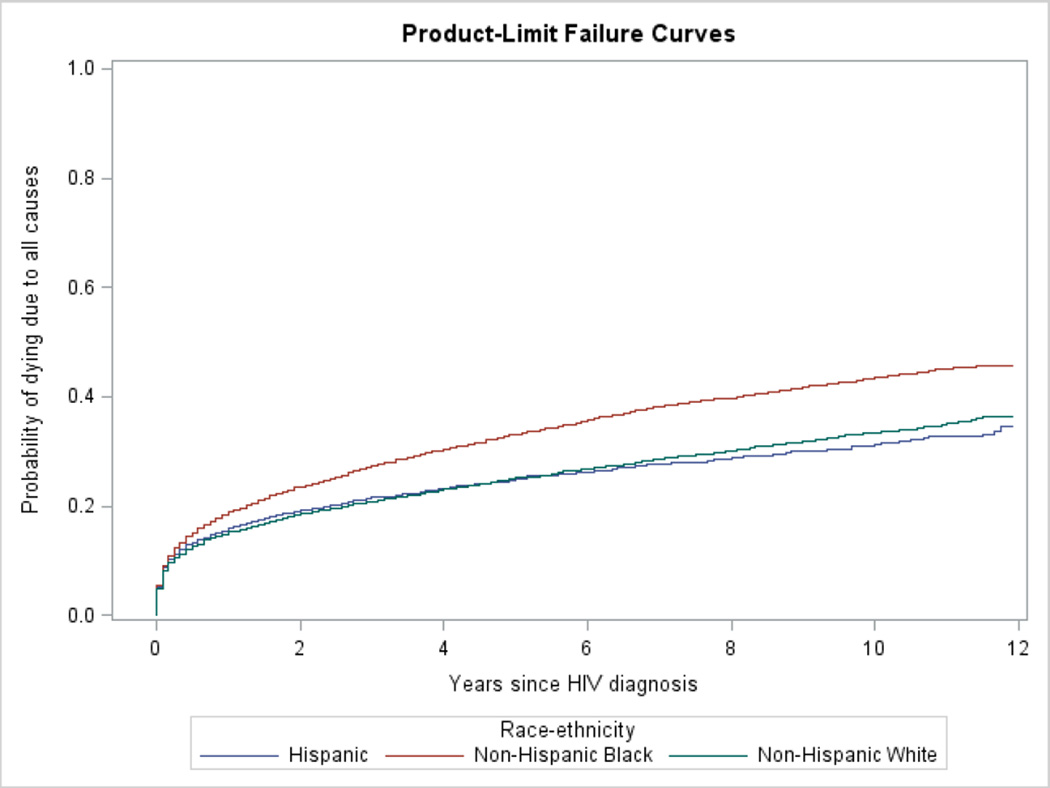
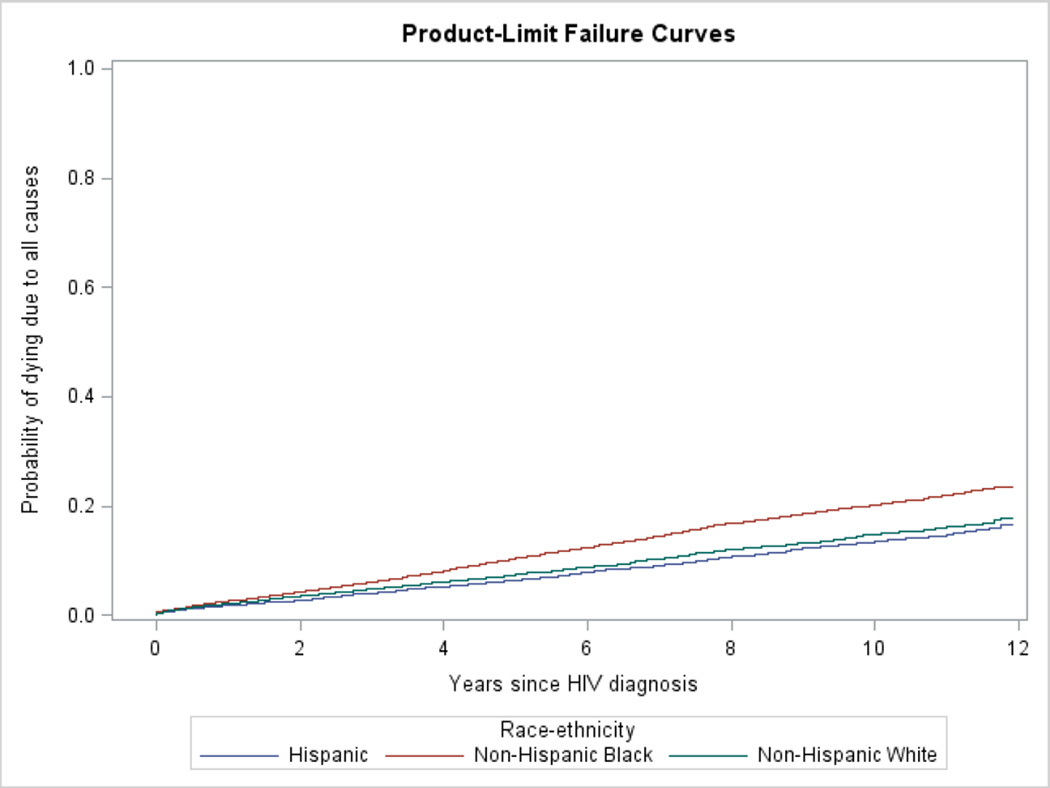
Analyses were stratified by concurrent AIDS diagnosis status. Associations between race and potential predictors of survival (all categorical) were tested using the chi-square test. Kaplan-Meier survival curves for all-cause mortality for each racial/ethnic group (NHB, NHW, and Hispanic) were generated. The assumption of proportional hazards required for Cox regression models was examined using log-negative-log curves and the correlation of Schoenfeld residuals with time and log of time for all variables [20]. Significant and meaningful correlations were defined as those greater than 0.3 [21], but there were none greater than 0.1. All-cause survival curves were run as failure curves for Figure 1 so that they could be easily compared with the cumulative incidence functions for HIV death.
Figure 1.
Unadjusted failure curves for death due to all causes by race/ethnicity among those with and without concurrent AIDS diagnosis
A. Those with concurrent AIDS at time of HIV diagnosis
B. Those without concurrent AIDS at time of HIV diagnosis
Cox proportional hazards models were performed using PROC PHREG to examine the association between race/ethnicity and all-cause mortality, controlling for individual level factors, area-level poverty, and rural/urban status. Multicollinearity was assessed by checking the variance inflation factor with PROC REG and vital status as a dependent variable, and a cutoff of 10 as indicating a potential problem [22].
In analyzing deaths due to HIV, a competing risk of death framework was used because death from HIV can be preceded by death from another cause [23]. If the risk of these competing causes were higher in a particular racial/ethnic group than in another, the apparent risk of HIV would be lower than it actually is. In assessing competing causes of death, cumulative incidence functions were generated for HIV death for each racial/ethnic group, and subdistribution proportional hazard models (a method developed by Fine and Gray [24]) were performed using a SAS macro developed by Kohl and Heinze [25]. Subdistribution models were chosen because they do not require the assumption that competing risks are independent and because the models can be used to compare the cumulative incidence for different groups such as race/ethnicity [23,26]. Models included individual level factors, rural/urban residence, and area-level poverty. In the all-cause and HIV-mortality analyses, a SAS random statement was used to account for area-level clustering at the ZCTA level, and two-way interactions of all variables with race/ethnicity were examined to determine if any were significant and if they changed the parameter estimates of the race/ethnicity variable. We conducted all analyses using SAS 9.4 [27]. The institutional review boards of the Florida Department of Health and Florida International University approved the study protocol.
RESULTS
Of the 67,033 people diagnosed and reported with HIV infection 2000–2009, 2,744 (4.1%) were diagnosed in a correctional facility, 2,780 (4.1%) had a missing or invalid zip code, and 246 (0.37%) had an incomplete or missing HIV diagnosis or death date. These people were excluded, leaving 61,263 people. Of these remaining people, 1,383 (2.3%) were in the “other” racial/ethnic category including 1,082 multiracial people, 219 Asians, 55 American Indians, and 27 Native Hawaiian/Pacific Islanders. Because of the heterogeneity of this group, they were excluded from further analysis, leaving 59,880 as the final study population. The distribution of demographic factors by race/ethnicity is described in Table 1. There was no significant difference in the percentage of cases that were concurrent AIDS cases between any of the racial/ethnic groups.
Table 1.
Characteristics of people diagnosed with HIV infection, Florida, 2000–2009 by race/ethnicity
| Characteristic | Entire cohort, n (%) | Non-Hispanic black, n (%) |
Non-Hispanic white, n (%) |
Hispanic, n (%) |
|---|---|---|---|---|
| Total | 59,880 | 30,968 | 16,766 | 12,146 |
| Period of diagnosis | ||||
| 2000–2001 | 14,174 (23.7) | 7,975 (25.8)* | 3,667 (21.9) | 2,532 (20.9) |
| 2002–2003 | 12,969 (21.7) | 6,951 (22.5) | 3,437 (20.5) | 2,581 (21.3) |
| 2004–2005 | 11,405 (19.1) | 5,634 (18.2) | 3,338 (19.9) | 2,433 (20.0) |
| 2006–2007 | 10,933 (18.3) | 5,169 (16.7) | 3,408 (20.3) | 2,356 (19.4) |
| 2008–2009 | 10,399 (17.4) | 5,239 (16.9) | 2,916 (17.4) | 2,244 (18.5) |
| Age group at diagnosis |
||||
| < 20 years | 2,252 (3.8) | 1,674 (5.4)* | 256 (1.5) | 322 (2.7)* |
| 20–39 years | 29,273 (48.9) | 14,930 (48.2) | 7,753 (46.2) | 6,590 (54.3) |
| 40–59 years | 25,295 (42.2) | 12,619 (40.8) | 8,014 (47.8) | 4,662 (38.4) |
| 60 years or older | 3,060 (5.1) | 1,745 (5.6) | 743 (4.4) | 572 (4.7) |
| Sex at birth | ||||
| Female | 18,750 (31.3) | 13,522 (43.7)* | 2,761 (16.5) | 2,467 (20.3)* |
| Male | 41,130 (68.7) | 17,446 (56.3) | 14,005 (83.5) | 9,679 (79.7) |
| Born in the United States |
||||
| Yes | 44,303 (74.0) | 24,237 (78.3)* | 15,174 (90.5) | 4,892 (40.3)* |
| No | 15,577 (26.0) | 6,731 (21.7) | 1,592 (9.5) | 7,254 (59.7) |
| Mode of transmission | ||||
| MSM | 23,912 (39.9) | 6,676 (21.6)* | 10,862 (64.8) | 6,374 (52.5)* |
| MSM and IDU | 1,597 (2.7) | 527 (1.7) | 738 (4.4) | 332 (2.7) |
| IDU | 4,545 (7.6) | 2,426 (7.8) | 1,264 (7.5) | 855 (7.0) |
| Heterosexual | 21,888 (36.6) | 16,443 (53.1) | 2,358 (14.1) | 3,087 (25.4) |
| Other/unknown | 7,938 (13.3) | 4,896 (15.8) | 1,544 (9.2) | 1,498 (12.3) |
| Concurrent AIDSa | ||||
| Yes | 19,278 (32.2) | 10,139 (32.7) | 5,309 (31.7) | 3,830 (31.5) |
| No | 40,602 (67.8) | 20,829 (67.3) | 11,457 (68.3) | 8,316 (68.5) |
| Vital status and cause of death at end of study |
||||
| Alive | 48,333 (80.7) | 23,987 (77.5)* | 14,035 (83.7) | 10,311 (84.9)* |
| HIV death | 7,488 (12.5) | 4,614 (14.9) | 1,662 (9.9) | 1,212 (10.0) |
| Other cause of death | 4,059 (6.8) | 2,367 (7.6) | 1,069 (6.4) | 623 (5.1) |
| Percent of ZCTA households below poverty line |
||||
| 0–8.6 | 4,076 (6.8) | 1,023 (3.3)* | 2,269 (13.5) | 784 (6.5)* |
| 8.7–12.9 | 9,928 (16.6) | 2,862 (9.2) | 4,624 (27.6) | 2,442 (20.1) |
| 13–19.3 | 17,187 (28.7) | 7,519 (24.3) | 5,513 (32.9) | 4,155 (34.2) |
| 19.4 or more | 28,689 (47.9) | 19,564 (63.2) | 4,360 (26.0) | 4,765 (39.2) |
| Rural/urban status of ZCTA |
||||
| Rural | 1,722 (2.9) | 831 (2.7)* | 653 (3.9) | 238 (2.0)* |
| Urban | 58,158 (97.1) | 30,137 (97.3) | 16,113 (96.1) | 11,908 (98.0) |
Note: HIV = human immunodeficiency virus; MSM = men who have sex with men; IDU = injection drug use; AIDS = acquired immunodeficiency syndrome; ZCTA = ZIP code tabulation area.
p<0.01 for group compared with non-Hispanic white
Diagnosed with AIDS within 3 months of HIV diagnosis.
All-cause mortality
Of cases meeting the inclusion criteria, 11,547 (19.3%) died. Of the concurrent AIDS group, 33.5% (6,449/19,278) died, and the crude rate was 6.10 per 100 person-years. In the nonconcurrent AIDS group, 12.6% (5,098/40,602) died for a crude rate of 1.90 per 100 person-years (data not in table). Figure 1 depicts the failure curves. The crude NHB:NHW hazards ratio (HR) were elevated in both the concurrent AIDS (1.38, 95% CI 1.30–1.46), and nonconcurrent AIDS groups (1.41, 95% CI 1.32–1.51). The crude Hispanic:NHW HR was not significant (0.96, 95% CI 0.89–1.04) in the concurrent AIDS group but was in the nonconcurrent AIDS group (0.89, 95% CI 0.81–0.98). There was no evidence of multicollinearity, and no significant violation of the assumption of proportionality.
In Cox proportional hazards models adjusting for individual level variables, area-level poverty, and rural/urban status, NHBs had an elevated adjusted hazards ratio (aHR) for all-cause mortality relative to NHWs among those with concurrent AIDS (aHR 1.23, 95% CI 1.15–1.32) and without concurrent AIDS (aHR 1.21, 95% CI 1.12–1.31) (Table 2). There was no statistically significant difference between NHWs and Hispanics. For those with concurrent and without concurrent AIDS, the following variables had an elevated aHR: earlier periods of diagnosis, increasing age, male sex at birth, US nativity, injection drug use (IDU) or other/unknown mode of transmission, and living in a neighborhood with the highest quartile of poverty. Among those with concurrent AIDS, there was an age-race/ethnicity interaction in that there was an increase with age in the Hispanic:NHW aHR (aHR 0.93, 95% CI 0.80–1.08 for ages 20–39 years; aHR 1.12, 95% CI 1.00–1.25 for ages 40–59; and aHR 1.40, 95% CI 1.09–1.80 for ages 60 and older) (data not in table). The NHB:NHW aHR decreased slightly with age (aHR 1.30, 95% CI: 1.17–1.46 for ages 20–39 years; aHR 1.20, 95% CI 1.10–1.31 for ages 40–59, and aHR 1.18, 95% CI 0.97–1.43 for 60 and older). Among those without concurrent AIDS, there was a sex-race/ethnicity interaction such that there was an elevated NHB:NHW aHR for males (1.28; 1.17–1.41) but not females. There was a transmission mode-race/ethnicity interaction in that the NHB:NHW aHR was significant for the MSM (aHR 1.48, 95% CI 1.30–1.67) but not for other modes of transmission. There was also a poverty-race/ethnicity interaction in which there was a significant Hispanic:NHW aHR for the highest quartile of poverty only (1.19, 95% CI 1.03–1.38).
Table 2.
Adjusted hazard ratios and 95% confidence intervals for all-cause and HIV-related death for cases diagnosed with HIV 2000–2009, Florida, with and without concurrent AIDS at time of HIV diagnosis
| All-cause death adjusted hazard ratios (95% CI)a |
HIV-related death adjusted hazard ratios (95% CI) |
|||
|---|---|---|---|---|
| Characteristic | Concurrent AIDSb | No concurrent AIDS | Concurrent AIDS | No concurrent AIDS |
| Race | ||||
| Non-Hispanic black | 1.23 (1.15–1.32) | 1.21 (1.12–1.31) | 1.34 (1.23–1.47) | 1.41 (1.26–1.57) |
| Hispanic | 1.08 (0.99–1.18) | 1.01 (0.91–1.12) | 1.18 (1.05–1.32) | 1.18 (1.03–1.36) |
| Non–Hispanic white | Ref. | Ref. | Ref. | Ref. |
| Period of diagnosis | ||||
| 2000–2001 | 1.54 (1.39–1.71) | 1.60 (1.41–1.81) | 1.82 (1.60–2.08) | 1.95 (1.62–2.36) |
| 2002–2003 | 1.44 (1.29–1.60) | 1.42 (1.26–1.60) | 1.70 (1.49–1.94) | 1.67 (1.38–2.02) |
| 2004–2005 | 1.27 (1.14–1.42) | 1.22 (1.07–1.39) | 1.48 (1.29–1.69) | 1.35 (1.10–1.64) |
| 2006–2007 | 1.23 (1.10–1.37) | 1.09 (0.94–1.27) | 1.37 (1.19–1.59) | 1.15 (0.93–1.41) |
| 2008–2009 | Ref. | Ref. | Ref. | Ref. |
| Age group at diagnosis | ||||
| < 20 years | 0.15 (0.11–0.20) | 0.11 (0.08–0.13) | 0.27 (0.19–0.39) | 0.21 (0.16–0.28) |
| 20–39 years | 0.34 (0.31–0.37) | 0.19 (0.17–0.21) | 0.52 (0.46–0.58) | 0.33 (0.29–0.38) |
| 40–59 years | 0.51 (0.47–0.55) | 0.39 (0.36–0.42) | 0.66 (0.59–0.74) | 0.57 (0.49–0.66) |
| 60 years or older | Ref. | Ref. | Ref. | Ref. |
| Sex at birth | ||||
| Female | Ref. | Ref. | Ref. | Ref. |
| Male | 1.09 (1.03–1.15) | 1.26 (1.16–1.37) | 1.02 (0.94–1.10) | 1.13 (1.00–1.24) |
| Born in the United States | ||||
| Yes | 1.35 (1.27–1.44) | 1.45 (1.35–1.56) | 1.48 (1.36–1.61) | 1.55 (1.39–1.72) |
| No | Ref. | Ref. | Ref. | Ref. |
| Mode of transmission | ||||
| MSM | 0.90 (0.83–0.97) | 0.70 (0.63–0.78) | 0.96 (0.87–1.05) | 0.74 (0.65–0.83) |
| MSM and IDU | 0.96 (0.81–1.13) | 1.05 (0.89–1.23) | 1.11 (0.90–1.37) | 1.07 (0.86–1.34) |
| IDU | 1.36 (1.25–1.48) | 1.52 (1.37–1.68) | 1.42 (1.27–1.59) | 1.33 (1.18–1.51) |
| Heterosexual | Ref. | Ref. | Ref. | Ref. |
| Other/unknown | 1.77 (1.64–1.97) | 1.41 (1.26–1.57) | 1.61 (1.47–1.77) | 1.13 (1.00–1.27) |
| Percent of ZCTA households below poverty line |
||||
| 0–8.6 | Ref. | Ref. | Ref. | Ref. |
| 8.7–12.9 | 1.03 (0.90–1.18) | 0.92 (0.79–1.06) | 0.93 (0.79–1.10) | 1.02 (0.82–1.27) |
| 13–19.3 | 1.09 (0.96–1.24) | 1.08 (0.95–1.24) | 1.06 (0.90–1.24) | 1.28 (1.05–1.58) |
| 19.4 or more | 1.32 (1.16–1.49) | 1.24 (1.10–1.40) | 1.15 (0.99–1.34) | 1.41 (1.15–1.72) |
| Rural/urban status of ZCTA | ||||
| Rural | 1.11 (0.74–1.28) | 1.11 (0.96–1.28) | 1.25 (1.05–1.49) | 0.98 (0.78–1.29) |
| Urban | Ref. | Ref. | Ref. | Ref. |
Note: HIV = human immunodeficiency virus; AIDS = acquired immunodeficiency syndrome; Ref.= referent group; MSM = men who have sex with men; IDU = injection drug use; ZCTA = ZIP code tabulation area.
Wald Robust Confidence Limits
Diagnosed with AIDS within 3 months of HIV diagnosis
HIV mortality
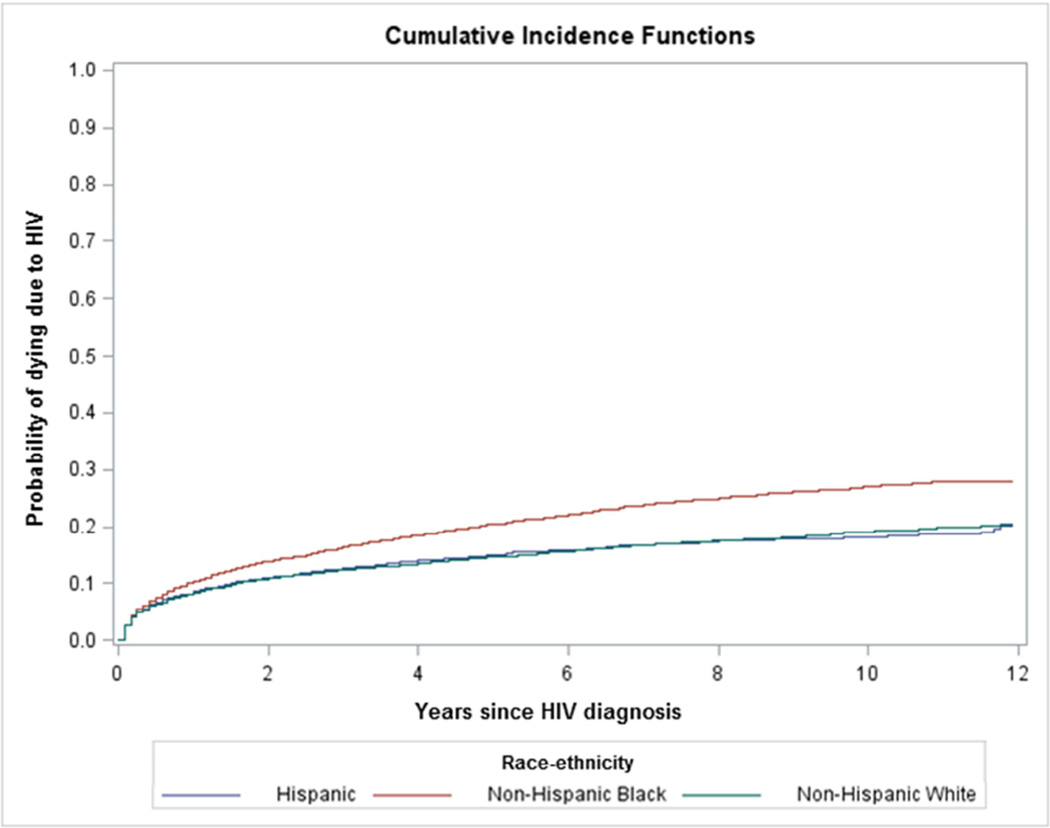
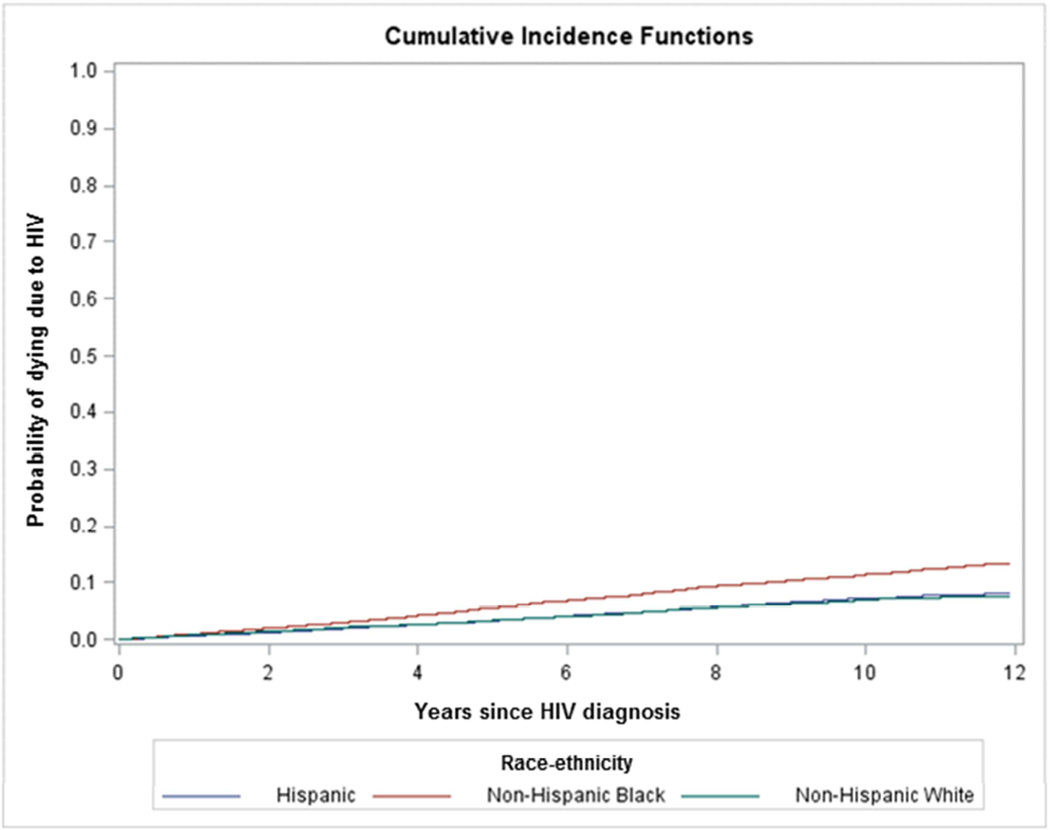
There were 4,849 deaths due to HIV in the concurrent AIDS group and a rate of 4.45 per 100 person-years. There were 2,883 HIV deaths in the nonconcurrent AIDS group and a rate of 1.04 per 100 person-years. The cumulative incidence curves by race/ethnicity are depicted in figure 2. The crude HR for NHBs relative to NHWs was significantly elevated for both groups (HR 1.46, 95% CI 1.35–1.58; HR 1.69, 95% CI 1.53–1.86 respectively) (Data not in table). There was no statistically significant difference between Hispanics and NHWs in the unadjusted HR for those with and without concurrent AIDS (HR 0.99, 95% CI 0.90–1.10; HR 1.02, 95% CI 0.90–1.16 respectively).
Figure 2.
Cumulative incidence curves for death due to HIV by race/ethnicity among those with and without concurrent AIDS diagnosis
A. Those with concurrent AIDS at time of HIV diagnosis
B. Those without concurrent AIDS at time of HIV diagnosis
In the competing risks models adjusting for individual level variables, area level poverty, and rural/urban status, NHBs had an elevated aHR for HIV mortality relative to NHWs among those with and without concurrent AIDS (aHR 1.34, 95% CI 1.23–1.47; aHR 1.41, 95% CI 1.26–1.57 respectively) (Table 2). Unlike with all-cause mortality, Hispanics had an elevated aHR for HIV mortality in both groups (aHR 1.18, 95% CI 1.05–1.32; aHR 1.18, 95% CI 1.03–1.36 respectively). In the modeling process, the aHR for Hispanics relative to NHWs became significant only after adding US nativity, and stratified analysis indicated that US-born Hispanics, the minority of Hispanics, had a higher aHR than foreign-born Hispanics for concurrent cases (1.24; 95% CI 1.04–1.48) and nonconcurrent cases (1.37; 95% CI 1.10–1.69). In the full model, factors with an elevated aHR for HIV mortality were similar to those for all-cause mortality except among those with concurrent AIDS, for whom there was a significantly elevated aHR for rural residence and there were no significant differences between males and females, MSM and heterosexual transmission modes, or by neighborhood poverty levels. Among those without concurrent AIDS, there was a higher aHR for the highest two poverty quartiles relative to the lowest quartile. For noncurrent AIDS, there was a sex-race/ethnicity interaction such that the NHB:NHW aHR and the Hispanic:NHW aHR, were significant for males but not females (NHB:NHW aHR 1.56, 95% CI 1.37–1.79 vs. 1.16, 95% CI 0.96–1.39 and Hispanic:NHW aHR 1.27, 95% CI 1.08–1.48 vs. 1.01, 95% CI 0.78–1.31). There was also a US birth-race/ethnicity interaction such that there was an elevated aHR for foreign born Hispanics (2.01, 95% CI 1.27–3.18) relative to foreign-born NHWs but not US-born Hispanics relative to US-born NHWs (1.08, 95% CI 0.92–1.28). Finally, there was a transmission mode-race/ethnicity interaction such that the NHB:NHW aHR was significant for the MSM group (aHR 1.71; 95% CI 1.45–2.03) and MSM/IDU group (aHR 2.25; 95% CI 1.37–3.69) but not the IDU and other groups, and the Hispanic:NHW aHR was significant for the MSM/IDU group (aHR 2.19; 95% CI 1.21.3.98) but not the other transmission modes.
DISCUSSION
Our study had four main findings. First, relative to NHWs, NHBs had a higher aHR for HIV and all-cause mortality among people with and without concurrent AIDS. Second, relative to NHWs, Hispanics with and without concurrent AIDS had a significantly higher aHR for HIV mortality, but not for all causes. Third, people who were born in the US had a higher aHR for both HIV and all-cause mortality. Fourth, area poverty was associated with an elevated aHR for all-cause mortality among those with and without concurrent AIDS and for HIV mortality among those without concurrent AIDS.
That NHBs had an elevated HR for all-cause mortality is consistent with an earlier study using surveillance data in Florida for people diagnosed with AIDS 1993–2004 [10]. The present study indicates that these racial/ethnic survival disparities are persisting and that they also exist for people without concurrent AIDS. The current study is also consistent with a study of relative survival of people diagnosed with HIV in 33 states from 1996–2003 [28]. In that study the relative risk of excess death within 5 years of HIV diagnosis (including those with and without concurrent AIDS) was significantly higher for NHBs relative to NHWs [28]. Similarly, a study of three-year survival of people diagnosed with HIV during 1996–2001 in 25 states found that the relative risk of death after controlling for individual factors was significantly higher for NHBs relative to NHWs for both those with and without concurrent AIDS [29]. The higher NHB:NHW hazards ratio among MSM and not among other modes of transmission suggests that engagement in the continuum of HIV care may be particularly difficult for NHB MSM compared with NHW MSM.
Because NHBs in the US have a lower life expectancy on average than NHWs and have higher death rates from other causes such as heart disease, homicide, and diabetes [30], the competing risk analysis is particularly important to assess disparities related to HIV death. We found that NHBs had a significantly higher aHR for HIV mortality relative to NHWs among those with and without concurrent AIDS. We identified one other study that considered competing risks in assessing HIV-related mortality. It reported an elevated, but not statistically significant, HR (1.18; 95% 0.97–1.42) for non-white race [31]. However, the males in that study were all MSM, and few were non-white. Two previous studies, one using US data, and one using New York City data, did not consider competing causes of death and found that HIV/AIDS mortality rates were significantly higher for blacks relative to whites [5,32]. Those findings and our findings that NHBs had a higher aHR even among those without concurrent AIDS suggest that NHBs are particularly disadvantaged in the HIV care continuum after diagnosis. The finding among nonconcurrent cases that the disparity in HIV mortality was present for males and not for females suggests that the barriers to care and treatment are even greater for male NHBs. From our study we cannot determine if the disparities are arising from issues with linkage to care, retention in care, or adherence. It is imperative that this be investigated so that appropriate interventions can be implemented to address these problems. It is notable that racial disparities were very similar for those with concurrent AIDS; this could also be due to differences in adherence or quality of care. While both NHBs and NHWs in this group present with HIV at a relatively late stage (AIDS), it is possible that the NHBs within this group had even later diagnosis. Among concurrent cases with CD4 counts (84.2%), the median CD4 count for NHBs (63; interquartile range 20–152) was slightly lower than that of NHWs (84; interquartile range 20–163) (Wilcoxon Rank Sum test P<0.0001).
The second major finding was that there was an elevated aHR for Hispanics relative to NHWs for HIV mortality but not all-cause mortality among those with and without concurrent AIDS. The all-cause mortality findings are similar to those of an earlier study in Florida that found no difference in survival after AIDS diagnosis between Hispanics and NHWs [10]. The race/poverty interaction for Hispanics suggests that there is a notable disparity in all-cause mortality for those without concurrent AIDS that are living in the neighborhoods with the highest quartile of poverty. With respect to HIV mortality in the current study there was almost no difference in the crude HIV mortality HR for Hispanics. However, when adjusting for U.S. birth, Hispanics had an elevated HR. The disparity between foreign-born Hispanics and foreign-born NHWs may be due to differences in documentation status as well as different educational and socioeconomic backgrounds; foreign-born NHWs were predominately from resource-rich countries while foreign-born Hispanics were predominately from resource-poor countries.
Among the nonconcurrent cases, there was a Hispanic:NHW disparity for foreign born but not US born. Additionally, the race/sex interaction suggested that the Hispanic:NHW disparity was primarily among men. A study of national HIV surveillance data from 25 states found no difference in three-year survival after HIV diagnosis in 1996–2001 between Hispanics and NHWs [29]. A second study with similar data found lower life expectancy for Hispanic males compared with NHW males after HIV diagnosis, but not for females [4]. We found no population-based study that assessed HIV mortality considering competing causes that reported results for Hispanics. However, a study in New York City found no difference in the HIV-cause-specific HR between Hispanics and NHWs [32]. Our results suggest that although the aHR for Hispanics was smaller than that for NHBs, there may be problems related to linkage to care, care and treatment and adherence among Hispanics, particularly for males. Another study suggests that Hispanics relative to NHWs are losing more years of life expectancy due to delays in starting HAART and also discontinuing treatment [33].
Since being born in the US was one of the strongest factors associated with all-cause and HIV-related mortality, it was important to control for this in our analysis because a sizeable proportion of Hispanics (59.7%) and NHBs (21.7%) in the cohort were born outside the US. There was a small, statistically significant difference between the percentage of US-born people who had concurrent AIDS (33.8%) compared to the people born outside the US (31.6%) (P<0.0001). This difference is in contrast to a national study that found that foreign-born people were more likely to have a late diagnosis of HIV (as defined by diagnosis of AIDS within 12 months of HIV infection) (41.8 vs. 31.1%) [34]. In that study, though, the largest percentage of people born outside the continental US was from Central America. In our study the largest percentage of foreign-born people was from Haiti (28.9%) followed by Cuba (12.6%). The apparent benefit of being born outside of the US on survival seems at odds with the small difference in late diagnosis. One possible explanation is that we have incomplete ascertainment of mortality among people born outside of the US who return to their country of birth.
The fourth finding was that the highest relative to the lowest quartile of poverty was associated with an elevated HR for all causes for those with and without concurrent AIDS and for HIV death for those without concurrent AIDS. Other studies have found that mortality was higher for people in areas with low SES for all causes [28] and HIV [5]. Importantly, for those without concurrent AIDS, HIV mortality was associated with poverty, suggesting that people living in impoverished areas may be having greater difficulties getting linked to care, obtaining care and treatment, and/or adhering to therapy.
The study has three major limitations. First, we had no individual-level measure of SES. It is likely that those living in ZCTAs with higher levels of poverty were more likely to have lower incomes than those living in ZCTAs with lower levels of poverty. Without individual-level socioeconomic measurements, we cannot fully control for the effects of low individual-level SES. A second limitation is that cause of death may have been misclassified. This would not affect the all-cause mortality analyses, but it is possible that attributing a death to HIV infection may vary by race of the decedent. Variations by race in attributing a cause of death have been reported in studies of suicide and heart disease mortality [35–37]. However, a study in Florida found that while about 9.1% of deaths due to HIV were underreported, there was no significant difference by race [38]. Third, we had no CD4 count/percent values for a test within 3 months of HIV diagnosis for 15.8% of the people with concurrent AIDS and 83.5% for people without concurrent AIDS. Given the large percentage of missing values particularly for those with HIV only, we could not perform imputation. Although HIV disease stage was partially addressed by stratifying by concurrent AIDS diagnosis status, if there were racial/ethnic differences in CD4 count/percent among those without concurrent AIDS, some of the observed racial/ethnic disparities in survival could have been related to late diagnosis.
Our study also has several important strengths. Based on our research, it is one of the first population-based studies that assesses racial/ethnic disparities in HIV mortality in the US using a competing risks framework, which is very important given higher mortality rates among NHBs relative to NHWs for other causes of death. Second, our study is population-based and provides evidence of ongoing disparities in HIV-related survival for NHBs as well as Hispanics.
Conclusions
In conclusion, we found NHBs diagnosed with and without concurrent AIDS had higher hazards of all-cause mortality and HIV mortality. Hispanics were also disadvantaged with respect to HIV mortality, but only after controlling for US nativity. These disparities exist years after widespread HAART availability. Given the higher incidence of HIV in both of these populations relative to NHWs, it is critical that the reasons for these disparities be elucidated and the disparities addressed.
Acknowledgments
The project described was supported by Award Number R01MD004002 from the National Institute on Minority Health and Health Disparities (NIMHD) and Award Number F31DA037790 from the National Institute on Drug Abuse (NIDA) at the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Minority Health and Health Disparities, the National Institute on Drug Abuse, or the National Institutes of Health. The authors would like to acknowledge the assistance of Khaleeq Lutfi, MPH, in dataset preparation and Tracina Bush, BS, for assistance in reviewing the National Death Index matches.
List of abbreviations
- AIDS
Acquired immunodeficiency syndrome
- aHR
Adjusted hazards ratio
- CDC
Centers for Disease Control and Prevention
- DOH
Department of Health (DOH)
- eHARS
Enhanced HIV/AIDS Reporting System
- HR
Hazards ratio
- HAART
Highly active antiretroviral therapy
- HIV
Human immunodeficiency virus
- ICD-10
International Classification of Diseases, Tenth Revision
- IDU
Injection drug use
- MSM
Men who have sex with men
- NHB
Non-Hispanic black
- NHW
Non-Hispanic white
- 95%CI
95% confidence interval
- RUCA
Rural-urban commuting area
- SES
Socioeconomic status
- UC
Underlying cause
- US
United States
- ZCTA
ZIP code tabulation area
Appendix A
List of all underlying cause of death International Classification of Diseases, Tenth Revision (ICD-10) codes that were used for classifying cause of death as an human immunodeficiency virus (HIV) death
HIV disease (B20-B24)
Laboratory evidence of HIV infection (R75)
Codes for which the World Health Organization recommends use of HIV disease codes (B20-B24) in a person with HIV infection: C46, C81-96 for neoplasms and A00-B19, B25-B49, B58-B64, B99, and J12-J18 for infections with the following exceptions: A00, A05.1, A20-A23, A27, A33-39, A70, A75-A79, A80, A81.0, A81.1, A82-86, A91-92, A95, A96.0-A96.2, A98, B03-06, B16-17.1, B26, B50-57, B90-92, B94.0, B94.1, B94.2.
Unspecific immunodeficiency (D84.9)
Unspecified disorder of immune system (D89.9)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Center for Health Statistics. Detailed Tables for the National Vital Statistics Report (NSR) [Accessed April 27, 2015];Deaths: Final Data for. 2012 Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr63/nvsr63_09.pdf.
- 2.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the Gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison KM, Song R, Zhang X. Life expectancy after HIV diagnosis based on national HIV surveillance data from 25 states, United States. J Acquir Immune Defic Syndr. 2010;53(1):124–130. doi: 10.1097/QAI.0b013e3181b563e7. [DOI] [PubMed] [Google Scholar]
- 5.Rubin MS, Colen CG, Link BG. Examination of inequalities in HIV/AIDS mortality in the United States from a fundamental cause perspective. Am J Pub Health. 2010;100(6):1053–1059. doi: 10.2105/AJPH.2009.170241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley H, Hall HI, Wolitski RJ, Van Handel MM, Stone AE, LaFlam M, et al. Vital Signs: HIV diagnosis, care and treatment among persons living with HIV—United States, 2011. MMWR. 2014;63(47):1113–1117. [PMC free article] [PubMed] [Google Scholar]
- 7.Horstmann E, Brown J, Islam F, Buck J, Agins BD. Retaining HIV-infected patients in care: where are we? Where do we go from here? Clin Infect Dis. 2010;50(5):752–761. doi: 10.1086/649933. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. [Accessed April 27, 2015];HIV Surveillance Report. 2013 vo. 25 Available at: http://www.cdc.gov/hiv/library/reports/surveillance/2013/surveillance_Report_vol_25.html. Published February 2015. [Google Scholar]
- 9.Maddox L, Poschman K. [Retrieved January 15, 2016];The Continuum of Care Florida 2014. 2015 Oct 15; Available at http://www.floridahealth.gov/diseases-and-conditions/aids/surveillance/_documents/hiv-aids-slide-sets/2014/florida-continuumofhivcare-2014b.pdf.
- 10.Trepka MJ, Niyonsenga T, Maddox L, Lieb S, Lutfi K, Pavlova-McCalla E. Community poverty and trends in racial/ethnic survival disparities among people diagnosed with AIDS in Florida, 1993–2004. Am J Public Health. 2013;103(4):717–726. doi: 10.2105/AJPH.2012.300930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider E, Whitmore S, Glynn KM, Dominguez K, Mitsch A, McKenna MT, et al. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged< 18 months and for HIV infection and AIDS among children aged 18 months to< 13 years—United States, 2008. MMWR Recomm Rep. 2008;57(RR10):1–12. [PubMed] [Google Scholar]
- 12.Fleming PL, Ward JW, Janssen RS, De Cock KM, Valdiserri RO, Gayle HD, et al. Guidelines for national human immunodeficiency virus case surveillance, including monitoring for human immunodeficiency virus infection and acquired immunodeficiency syndrome. MMWR Recomm Rep. 1999;48(RR13):1–28. [PubMed] [Google Scholar]
- 13.World Health Organization. [Accessed April 27, 2015];International Classification of Diseases (ICD) 2014 Available at: http://www.who.int/classifications/icd/en/
- 14.UNAIDS/WHO Working Group on Global HIV/AIDS and STI Surveillance. [Accessed April 27, 2015];Guidelines for HIV mortality measurement. Available at: http://www.ncbi.nlm.nih.gov/books/NBK236997/pdf/TOC.pdf.
- 15.US Census Bureau. American FactFinder. Washington, DC: Government Printing Office; [Accessed April 27, 2015]. No date. Available at: http://factfinder2.census.gov/faces/nav/jsf/pages/index.xhtml. [Google Scholar]
- 16.US Census Bureau. ZIP Code™ tabulation areas (ZCTA™) Washington, DC: Government Printing Office; [Accessed April 27, 2015]. no date. Available at: http://www.census.gov/geo/reference/zctas.html. [Google Scholar]
- 17.Hart LG, Larson EH, Lishner DM. Rural Definitions for Health Policy and Research. Am J Public Health. 2005;95(7):1149–1155. doi: 10.2105/AJPH.2004.042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WWAMI Rural Health Research Center. [Accessed April 27, 2015];Rural Urban Commuting Areas (RUCA) no date. Available at: http://depts.washington.edu/uwruca/ruca-uses.php.
- 19.Centers for Disease Control and Prevention, Health Resources and Services Administration, National Institutes of Health, American Academy of HIV Medicine, Association of Nurses in AIDS Care, International Association of Providers of AIDS Care, the National Minority AIDS Council; Urban Coalition for HIV/AIDS Prevention Services. [Accessed April 27, 2015];Recommendations for HIV Prevention with Adults and Adolescents with HIV in the United States, 2014. 2014 Available from: URL: http://stacks.cdc.gov/view/cdc/26062.
- 20.Allison PD. Survival Analysis Using SAS A Practical Guide. 2nd. Cary, North Carolina: SAS Institute Inc; 2010. [Google Scholar]
- 21.Hinkle DE, Wiersma W, Jurs SG. Applied Statistics for the Behavioral Sciences. 5th. Boston: Houghton Mifflin; 2003. [Google Scholar]
- 22.Kleinbaum DG, Kupper LL, Nizam A, Rosenberg E. Applied Regression Analysis and Other Multivariable Methods. 5th. Boston: Cengage Publishing; 2013. pp. 358–372. [Google Scholar]
- 23.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 25.Kohl M, Heinze G. [Accessed April 27, 2015];%PSHREG: A SAS® macro for proportional and nonproportional subdistribution hazards regression for survival analyses with competing risk data. Technical Report 06/2014. http://www.meduniwien.ac.at/user/georg.heinze/pshreg/techrep.pdf.
- 26.Dignam JJ, Zhang Q, Kocherginsky MN. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18(8):2301–2308. doi: 10.1158/1078-0432.CCR-11-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.SAS [computer program] Version 9.4. Cary, NC: SAS Institute; 2010. [Google Scholar]
- 28.McDavid Harrison K, Ling Q, Song R, Hall HI. County-level socioeconomic status and survival after HIV diagnosis, United States. Ann Epidemiol. 2008;18(12):919–927. doi: 10.1016/j.annepidem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Hall HI, McDavid K, Ling Q, Sloggett A. Determinants of progression to AIDS or death after HIV diagnosis, United States, 1996–2001. Ann Epidemiol. 2006;16(11):824–833. doi: 10.1016/j.annepidem.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Kochanek KD, Arias E, Anderson RN. How did cause of death contribute to racial differences in life expectancy in the United States in 2010. NCHS Data Brief. 2013;125:1–8. [PubMed] [Google Scholar]
- 31.Wada N, Jacobson LP, Cohen M, French A, Phair J, Muñoz A. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984–2008. Am J Epidemiol. 2013;177(2):116–125. doi: 10.1093/aje/kws321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145(6):397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 33.Losina E, Schackman BR, Sadownik SN, Gebo KA, Walensky RP, Chiosi JJ, et al. Racial and sex disparities in life expectancy losses among HIV-infected persons in the United States: impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clin Infect Dis. 2009;49(10):1570–1578. doi: 10.1086/644772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prosser AT, Tang T, Hall HI. HIV in persons born outside the United States, 2007–2010. JAMA. 2012;308(6):601–607. doi: 10.1001/jama.2012.9046. [DOI] [PubMed] [Google Scholar]
- 35.Coady SA, Sorlie PD, Cooper LS, Folsom AR, Rosamond WD, Conwill DE. Validation of death certificate diagnosis for coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. J Clin Epidemiol. 2001;54(1):40–50. doi: 10.1016/s0895-4356(00)00272-9. [DOI] [PubMed] [Google Scholar]
- 36.Rockett IR, Samora JB, Coben JH. The black-white suicide paradox: possible effects of misclassification. Soc Sci Med. 2006;63(8):2165–2175. doi: 10.1016/j.socscimed.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 37.Rockett IRH, Wang S, Stack S, De Leo D, Frost JL, Ducatman AM, et al. Race/ethnicity and potential suicide misclassification: window on a minority suicide paradox? BMC Psychiatry. 2010;10:35. doi: 10.1186/1471-244X-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trepka MJ, Sheehan DM, Fennie KP, Niyonsenga T, Lieb S, Maddox LM. Completeness of HIV reporting on death certificates for Floridians reported with HIV infection, 2000–2011. AIDS Care. 2015 Aug 14;:1–6. doi: 10.1080/09540121.2015.1069786. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]