Abstract
Background & Aims
Despite an allocation system designed to give deceased-donor livers to the sickest patients, many transplantable livers are declined by U.S. transplant centers. It is unknown whether centers vary in their propensities to decline organs for the highest-priority patients, and how these decisions directly impact patient outcomes.
Methods
We analyzed Organ Procurement and Transplantation Network (OPTN) data from 5/1/07-6/17/13, and included all adult liver-alone waitlist candidates offered an organ that was ultimately transplanted. We evaluated acceptance rates of liver offers for the highest-ranked patients and their subsequent waitlist mortality.
Results
Of the 23,740 unique organ offers, 8,882 (37.4%) were accepted for the first-ranked patient. Despite adjusting for organ quality and recipient severity of illness, transplant centers within and across geographic regions varied strikingly (p<0.001) in the percentage of organ offers they accepted for the highest-priority patients. Among all patients ranked first on waitlists, the adjusted center-specific organ acceptance rates ranged from 15.7% to 58.1%. In multivariable models, there was a 27% increased odds of waitlist mortality for every 5% absolute decrease in a center’s adjusted organ offer acceptance rate (adjusted OR: 1.27, 95% CI: 1.20–1.32). However, the absolute difference in median 5-year adjusted graft survival was 4% between livers accepted for the first-ranked patient, compared to those declined and transplanted at a lower position.
Discussion
There is marked variability in center practices regarding accepting livers allocated to the highest-priority patients. Center-level decisions to decline organs substantially increased patients’ odds of dying on the waitlist without a transplant.
Keywords: organ offer, decline, waitlist mortality
INTRODUCTION
Prioritization on the liver transplant waitlist follows an urgency-based (i.e., ‘sickest-first’) model. Urgency-based prioritization aligns with the ‘Final Rule’ proposed by the US Department of Health and Human Services in 1998.1 Since 2002, the Model for End-Stage Liver Disease (MELD) score, an objective measure that strongly predicts the risk of death on the waitlist,2 has been used to prioritize patients and allocate organs. All patients actively listed are determined by a center as being well enough for transplantation at the time of an organ offer. With few exceptions, organs are first offered based on the MELD score, first locally among liver transplant programs within the 58 donor service areas, then regionally to a greater number of programs among the 11 United Network for Organ Sharing (UNOS) regions, and then nationally to all programs.
Despite MELD-based allocation3, geographic differences in waitlist mortality rates exist.4–9 These differences have been attributed to variations in organ supply as compared to waitlist demand. Proposals to redraw lines of organ distribution in order to improve equity are under review, and have generated considerable discussion5, including by members of Congress whose support is divided by geography rather than political party lines.10,11 However in all of these discussions, little attention has been given to an alternative potential source of differences in waitlist mortality: differential utilization of available organs by transplant centers. Previous data has suggested that transplant centers differ in average “quality” of the liver allografts transplanted, with higher-volume centers and centers with more local competition using higher-risk organs.12 Furthermore, six liver transplant centers (out of nearly 110 in the US) utilize 64% of all nationally placed allografts (livers transplanted outside of the UNOS region of procurement).13 An explanation for this is that many factors beyond the organ that is being allocated also may influence the decision to accept an offer.14
Despite our current knowledge of organ utilization among centers, there are limited data defining how often organ offers are accepted for those sickest patients most in need (the highest-priority patients), how often organs are declined by centers, how centers differ in their acceptance behavior, and how these behaviors influence the probability a patient will be transplanted successfully, die after transplant, or die on the waitlist.15,16 We sought to evaluate: 1) within and across-region center variability in center acceptance patterns for livers offered to the highest-prioritized patients; 2) factors associated with offer acceptance; and 3) the association between center behavior on patient outcomes.
METHODS
US organ allocation system
The organ transplant system in the US is managed by UNOS. For the purposes of organ donation and allocation, the US is divided geographically at two levels. There are 11 ‘regions’, with each region (except for region 9), encompassing multiple adjacent states (e.g., region 2 includes Pennsylvania, Delaware, New Jersey, Maryland, Washington D.C., and West Virginia). Second, regions are subdivided into 58 smaller geographic units (donor service areas). Donor service areas vary in land area and population size, but are the local unit by which organs are procured and allocated, with one organ procurement organization in each donor service area responsible for procuring donor organs. For example, in Region 2, there are five donor service areas that do not necessarily follow state boundaries. With certain exceptions, organs are first offered to patients in the same donor service areas as the donor. If not accept for patients in the same donor service area, the organ is next offered to patients in the same region. It must be noted that although the performance of transplant centers in the US is closely tracked, with potential penalties for outcomes that are lower than expected, these do not include any penalties for declining organ offers for a given patient.
Match run waitlist mechanics
Each time a deceased-donor liver becomes available, UNOS ranks all patients eligible to receive the organ based on the donor and potential recipient’s blood type, the potential recipient’s geographic location, most recent MELD score, and potential recipient’s willingness to accept organs from donors with certain characteristics (e.g., is the patient willing to accept an organ from a donor with hepatitis C, or a donor over a certain age). The organ is then offered to the center at which the highest-ranked patient is waitlisted. This is referred to as the match run. With few exceptions, organ offers follow a sequential process (Supplementary Figures 1 and 2). A center, and specifically the on-call transplant surgeon, could decline an offered organ for several reasons: donor quality (e.g., donor age), recipient clinical status, donor-recipient size mismatch, or the opinion that a patient with lower priority has a greater risk of death. When an organ offer is declined for the highest-ranked patient, that patient will either be transplanted after a future offer is accepted, or die without being transplanted (with or without receiving subsequent offers). The rank list is dynamic, changing daily as new patients are added, existing patients removed, and MELD scores are updated. As such, the decision to decline an organ offer for the highest ranked patient does not assure that the same patient will be ranked at or near the top when the next organ is available.
Study sample
Using data from the Organ Procurement and Transplantation Network (OPTN)/UNOS, this study evaluated match runs for livers from May 1, 2007, the first date of available match-run data, through June 17, 2013. Match-run data were only available for livers that were ultimately transplanted.
We excluded match runs in which the highest-ranked patient was <18 years of age or a multiorgan transplant candidate; the organ offer was bypassed to a lower-ranked patient (e.g., directed donation where the donor’s next-of-kin specify their recipient of choice); or critical donor data (e.g., age) were missing. Transplant centers with patients ranked first on a match run <20 times/year were excluded because their acceptance patterns could be artificially variable due to small sample sizes (6/110 transplant centers with a total of 305 match runs with a patient ranked first during the study period).
Outcomes
In analyses evaluating center variability in organ offer acceptance rates, the outcome was whether the offer was accepted for a given patient. The first-ranked patient for each offer was the unit of analysis, aggregated by center to calculate that center’s acceptance rate (Supplementary Figure 2). For models assessing waitlist mortality, the outcome was waitlist removal for death or clinical deterioration, which included dying on the waitlist, or removal for being ‘too sick to transplant,’ or for ‘other’ reasons with a known date of death within 90 days of removal, based on Social Security Death Master File data.4,17 Waitlist removal was modeled as a binary outcome given the short time interval from initial organ offer to waitlist removal date (median 10 days; 72.9% of removals for death or clinical deterioration occurring within 30 days of an organ offer being declined, and 81.5% within 60 days) between being ranked first and subsequent outcomes of death or transplantation (the outcome in >95% patients ranked first).17,18 Lastly, we evaluated the graft-specific outcome of graft failure (recipient death or retransplantation19).
Covariates
Recipient covariates included: age, sex, allocation MELD score (the higher value of the calculated or exception MELD score), race/ethnicity20, previous liver transplantation, and receipt of hepatocellular carcinoma (HCC) or other exception points (which increase a patient’s priority beyond their calculated MELD score20). Donor covariates included: sex, height, weight, age, race/ethnicity, cause of death, donation after cardiac death status (in contrast to standard brain-dead donors, these grafts have more ischemic injury and inferior outcomes21–23), and share type. Share type refers to the geographic location of the donor with regards to the patient offered the organ: a) donor service area (local unit of organ allocation); b) statewide-share; or 3) regional-share (same UNOS region). Local transplant-center density was modeled as the number of liver transplant centers within a donor service area. We did not adjust for blood type in organ acceptance models as donor organs are only offered to waitlist patients of the same (or compatible for recipient blood group AB or B under specific circumstances).
Statistical analysis
Center acceptance rates
Mixed-effects logistic regression models with patient- and center-level random intercepts were used to quantify and test for variability in acceptance rates across transplant centers after adjustment for recipient and donor characteristics among all included match runs. Individual patients are clustered (or, grouped) within transplant centers, which could lead to correlated outcomes of patients within those clusters. In contrast to a traditional fixed effect (i.e., gender, age, race, ethnicity) that has levels that are of primary interest and would be used again if the study were repeated, random effects can be viewed as selecting from a much larger set of levels.24 In the case of this study, centers serve as the random effects, because one can view the centers in this study as being selected from the set of all transplant centers.
In the case of this study, the primary models are multi-level, because there are two levels of clustering: patients within centers; and individual patients themselves, because patients could have more than one organ offer, and thus, acceptance decisions could be correlated among patients within centers, and among individual patients with multiple offers.24 In these mixed-effects models, the null hypothesis was that there was no residual heterogeneity (e.g., after accounting for all of the covariates in the model, the acceptance rates among centers are statistically not different from one another). A p-value <0.05 suggested that there were significant differences in organ offer acceptance rates after adjusting for the observed covariates in the model.25 We conducted a hypothesis test that the standard deviation of the center-level random intercepts was equal to 0 using a likelihood ratio test, comparing a model that included both patient- and center-level random intercepts to a model that included only patient-level random intercepts. Due to the limitations of this approach26, we also examined whether the 95% confidence interval for the random intercepts' standard deviation excluded the null-hypothesized value of 0. Adjusted acceptance rates were then calculated using the output of the multivariable model (post-estimation commands in Stata 13.0) that standardized each center’s observed acceptance rate to the expected rate based on the model.27 Primary analyses included Status-1 patients (acute liver failure in the absence of chronic liver disease20), and focused on patients ranked first on a match run, with secondary analyses including patients ranked second or third.
Waitlist mortality
We fit mixed-effects logistic regression models to assess the association between center acceptance patterns and waitlist mortality for first-ranked patients. The primary exposure was the adjusted acceptance rate (calculated above) for the given transplant center for each patient. The outcome of waitlist mortality (waitlist removal for death or clinical deterioration) was determined at the patient level. To account for correlation due to clustering within centers and donor service areas, the model included center-level and donor service area-level random intercepts. Primary waitlist mortality models excluded Status-1 patients, who by definition have a different baseline risk of dying on the waitlist compared to patients with chronic liver disease28–32; in secondary analyses these patients were included and an additional covariate (status-1 yes/no) was included. After fitting these models, we calculated adjusted center-level waitlist mortality rates using post-estimation methods described above.
Sensitivity and secondary analyses
A series of sensitivity analyses were conducted to assess the robustness of the primary analyses of center acceptance rates: 1) inclusion of only one match run per patient to assess for potential bias from within-patient clustering not accounted for by multi-level modeling; 2) including only organ offers for brain-dead donors <50 years of age (‘ideal donors’15) or the lowest 25th percentile (highest graft ‘quality’) of donors based on donor risk index (DRI)33 to determine if acceptance rates are influenced by donor quality not accounted for in multivariable models; and 3) exclusion of patients receiving exception points who are less acutely ill than patients with similar calculated MELD scores (patients can receive additional MELD points, exception points, to increase their waitlist priority). Adjusted center-specific acceptance rates were calculated for patients ranked second and third on match runs, while waitlist mortality models were fit only for second-ranked patients given similar results to the primary mortality analyses. Lastly, adjusted graft survival rates were used to compare donor liver ‘quality’ for accepted vs. declined organs using Cox models, stratified by transplanted center. These models adjusted only for recipient covariates significantly associated with graft survival in this cohort (pre-transplant location of home versus hospital versus intensive care unit, functional status using the Karnofsky score, laboratory MELD score, HCC, need for dialysis prior to transplantation, age, etiology of liver disease, and race/ethnicity) leaving donor factors/graft quality as the main determinant of outcomes.
There was no funding source for this study.
RESULTS
From May 1, 2007 through June 17, 2013, 23,740 match runs met the study criteria, with 13,255 unique patients ranked first on ≥1 match run (Supplementary Figure 3). The median highest-ranked patient was representative of the general liver waitlist: median age: 54 (IQR: 47–60) years; 62% male; 70% white, 12.2% Hispanic, and 11.2% black.34 The most common etiologies of liver disease were hepatitis C (37%), non-alcoholic steatohepatitis (15%), or alcohol-induced liver disease (15%); 16% had increased prioritization based on receiving exception points for HCC35. The median match MELD score was 30 (IQR: 23–40). Of the 23,740 match runs, the organ offer was accepted for the highest-ranked patient in 37.4% of cases. Among the 13,255 unique first-ranked patients, the initial offer to that patient was accepted 47.5% of the time. Overall, 65% (15,386/23,740) of included organ offers were transplanted into a patient ranked in the top three of the match run. Among these recipients, the median (IQR) match MELD scores were highest at position 1 (29, IQR: 23–40), subsequently decreased at positions 2(25, IQR: 22–33) and 3 (25, IQR: 22–31), and were stable at positions 4 (25, IQR: 22–30) and 5 (25, IQR: 22–29).
Among organ offers declined for patients ranked first that were subsequently transplanted into a lower-ranked patient, 93.7% were transplanted in the same OPTN/UNOS region, 56.9% in the same donor service area, and 29.5% at the same transplant center. When restricted to donor service areas with the greatest center density (>3 centers), 70.2% of declined livers were subsequently transplanted in the same donor service area, while only 19.9% were transplanted at the same center. Among organ offers declined for the first-ranked patient and subsequently transplanted into a lower-ranked patient, it was more likely to be used for the patient ranked second when used at the same transplant center (39.4%) compared to the same donor service area (33.8%) or same UNOS region (29.4%). Furthermore, when the organ offer was declined for the patient ranked first and subsequently transplanted into a lower ranked patient at the same transplant center, it was significantly (p<0.001) more likely to be used for the patient ranked second in single-center donor service areas compared to those with greater transplant center density: 52.7% (single-center), 40.1% (2 centers), 35.4% (3 centers), 27.2% (≥4 centers).
Among patients whose initial first-rank offer was declined, <60% had a subsequent offer while ranked first. Yet, 71.7% of patients whose initial first-ranked offer was declined were subsequently transplanted, with 42.0% of these patients being transplanted within 3 days of their initial decline.
Within-region variability
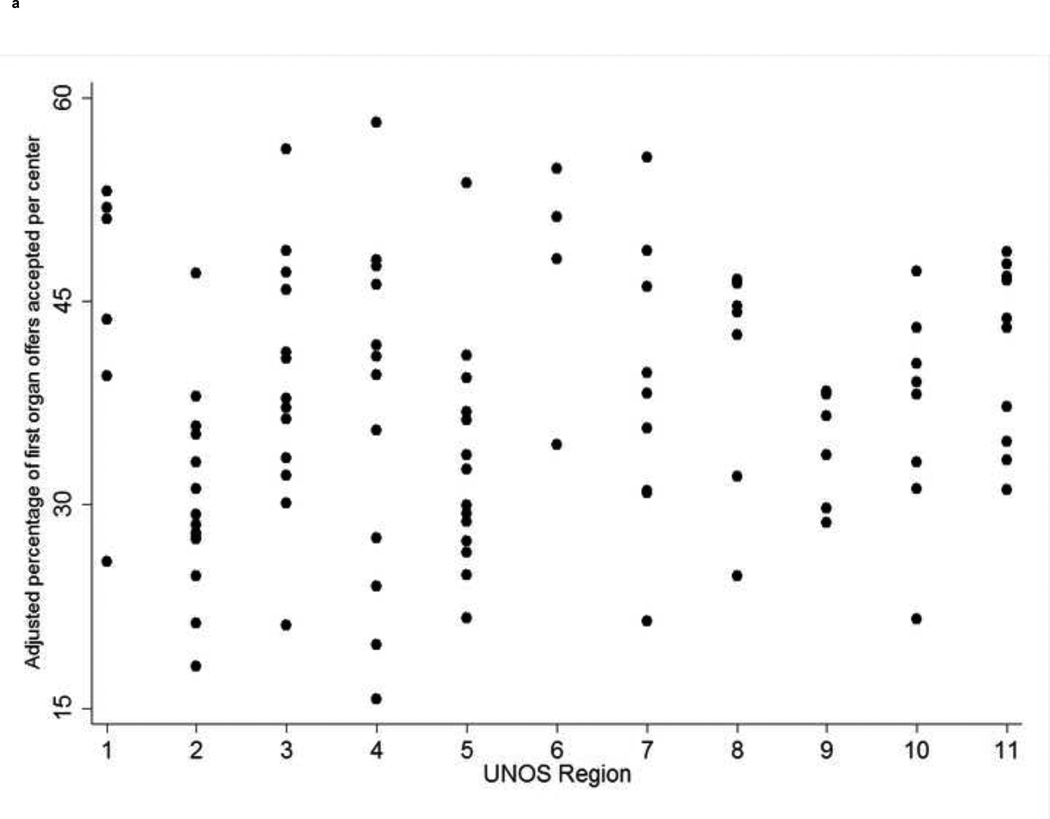
Despite adjustments for donor, recipient, and center characteristics, there was significant among-center variability (p<0.001) in center acceptance within each region, and across all 11 UNOS regions (Figure 1a; Table 1). Significant among-center differences were evident with exclusion of Status-1 patients (Supplementary Figure 4), and in donor service areas with multiple liver transplant centers (Supplementary Figure 5). Adjusted center-specific acceptance rates at each of the top three positions were strongly correlated (p<0.001 for all correlations; Supplementary Figure 6). For example, centers’ acceptance rates at the first and second positions were correlated at r=0.81.
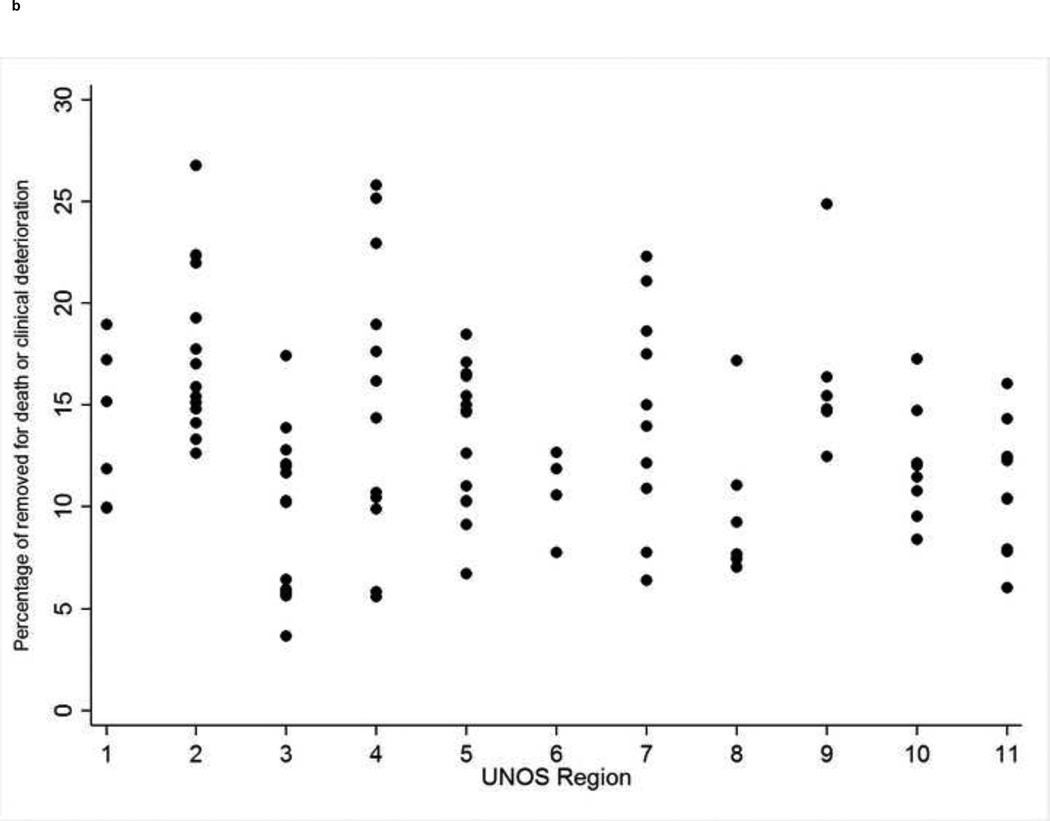
Figure 1. (four panels): Among-center differences in organ offer acceptance rates and waitlist mortality and the association between organ acceptance and waitlist mortality.
- UNOS=United Network for Organ Sharing
- *Footnote
- Excluding Status-1 patients
- UNOS=United Network for Organ Sharing
-
Figure Legend
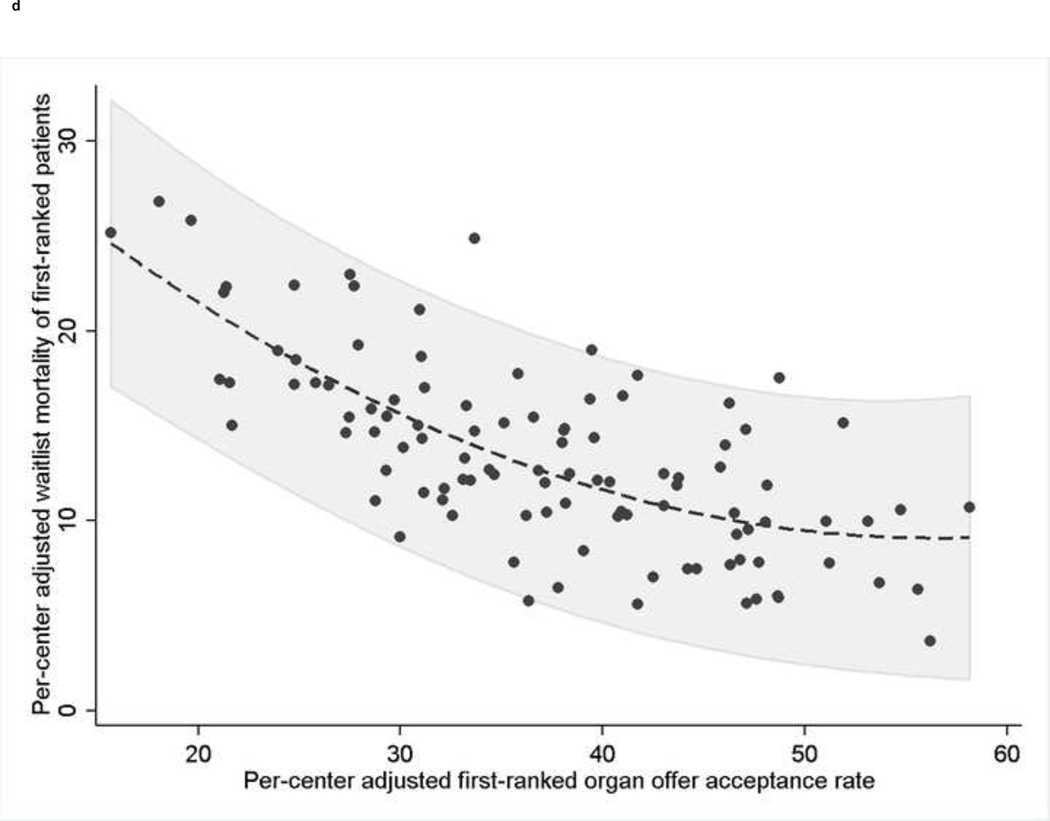
- *Footnote: * Each dot represents an individual transplant center. The dashed line represents the best-fit quadratic line of waitlist mortality regressed on the acceptance rate due to the non-linear relationship between center acceptance rate and center waitlist mortality rate. The area shaded in gray represents the 95% confidence interval of the standard error of the predicted waitlist mortality regressed on the acceptance rate, rather than the standard error of the mean. Correlation coefficient=−0.7, p<0.001.
Table 1.
Multivariable mixed-effects model evaluating factors associated with accepting an organ offer for a patient ranked first on a match run from 5/1/07–6/17/13, n=23,740*
| Variable | Multivariable odds ratio* | P-value |
|---|---|---|
| Recipient factors | ||
| Age at match run† | 1.02 (1.01–1.03) | 0.007 |
| Male sex | 1.17 (1.10–1.25) | <0.001 |
| HCC exception points | 1.18 (1.07–1.30) | 0.001 |
| ‘Other’ exception points‡ | 0.87 (0.77–0.98) | 0.03 |
| Status 1 candidate | 0.50 (0.45–0.55) | <0.001 |
| Donor factors | ||
| Age† | 0.88 (0.87–0.89) | <0.001 |
| Cause of death | <0.001 | |
| Stroke | 1 | |
| Head trauma | 1.34 (1.23–1.46) | |
| Anoxia | 1.28 (1.17–1.39) | |
| Brain tumor | 0.73 (0.46–1.15) | |
| Other | 1.07 (0.86–1.33) | |
| DCD donor | 0.26 (0.22–0.31) | <0.001 |
| Weight** | 0.95 (0.94–0.96) | <0.001 |
| Organ share type | <0.001 | |
| Local OPO | 1 | |
| Regional share | 0.59 (0.54–0.65) | |
| Geographic factors | ||
| OPO competition | <0.001 | |
| 1 center OPO | 1 | |
| 2 center OPO | 0.72 (0.55–0.94) | |
| 3 center OPO | 0.67 (0.47–0.95) | |
| ≥4 centers/OPO | 0.42 (0.30–0.59) | |
| UNOS region | 0.004 | |
| 1 | 1 | |
| 2 | 0.41 (0.26–0.64) | |
| 3 | 0.48 (0.30–0.78) | |
| 4 | 0.68 (0.43–1.07) | |
| 5 | 0.45 (0.28–0.71) | |
| 6 | 0.84 (0.45–1.55) | |
| 7 | 0.60 (0.38–0.96) | |
| 8 | 0.47 (0.28–0.81) | |
| 9 | 0.67 (0.39–1.16) | |
| 10 | 0.41 (0.24–0.70) | |
| 11 | 0.53 (0.32–0.88) |
Model was mixed-effects logistic regression model with individual center-level random intercepts. The p<0.001 indicated that there was residual heterogeneity between transplant centers after accounting for the patient demographic and geographic factors in the multivariable model based on the test of random center effects. We also examined the 95% confidence interval for the random intercept’s standard deviation, which was (0.34, 0.47). The null-hypothesized value of 0 was far outside this confidence interval, bolstering our confidence in the hypothesis test. Other covariates forced into the model but not presented because they were not significant were: increasing match MELD score at match run (p=0.28), recipient race/ethnicity (p=0.79), donor race/ethnicity (p=0.11), re-transplant candidate (p=0.29); and donor height (p=0.06). Donor sex was not associated with organ offer acceptance in univariable models (p=0.34), and was not included in multivariable models
Per 5-year increase in age
Other exception points included increased prioritization granted for patients for conditions other than HCC, such as hepatopulmonary syndrome, recurrent cholangitis, complications of portal hypertension, etc.
Per 5-kg increase in weight
HCC=hepatocellular carcinoma; DCD=donation after cardiac death; OPO=organ procurement organization; UNOS=United Network for Organ Sharing; MELD=Model for End-Stage Liver Disease
Factors associated with acceptance
In multivariable models, several donor, recipient, and geographic factors were associated with organ offer acceptance. Donation after cardiac death donors, regional organ offers, and Status 1 were the donor and recipient factors with the strongest negative associations with offer acceptance (Table 1). There was a significant association between being prioritized based on exception points and having an organ offer accepted. Compared to patients who did not receive exception points, those with HCC exception points were more likely to have an organ offer accepted, while those with ‘other’ non-HCC exception points were less likely (Table 1).
Local transplant center density was significantly associated with organ offer acceptance patterns (p<0.001). Significant differences (p<0.001) were observed before and after adjustment for donor and recipient factors (Table 1)—adjusted acceptance rates: 44.9% (single-center OPO, of which there were 18 adult liver transplant centers without another adult liver transplant center in their donor service area); 38.3% (2-center OPO); 33.9% (3-center OPO); 31.5% (≥4-center OPO). Transplant-center volume was not associated with organ offer acceptance (p=0.71).
Among patients ranked first on a subsequent match run after having their initial first-ranked offer declined, the organ quality of the subsequent first-ranked offer was ‘better’ (lower DRI) in 54% of cases (median DRI difference of second and first offers: −0.054, 95% CI: −0.44, 0.31). There were no significant differences based on local transplant center density (p=0.32). The organ offer was accepted for these patients in 31.2% of cases.
Association between center acceptance patterns and patient survival
There was significant among-center variability in waitlist removal for death or clinical deterioration, ranging from 3.7% to 26.8% across all US centers (p<0.001; Figure 1b). When centers were aggregated into UNOS regions, the absolute difference in region-specific waitlist mortality between the lowest (region 8: 8.6%) and highest (region 2: 17.4%) region was smaller than the absolute difference in adjusted center waitlist mortality between the center with the lowest and highest adjusted waitlist mortality within 10 of the UNOS regions (Supplementary Table 2).
Short-term mortality among patients ranked first whose initial organ offer was declined differed for patients with and without HCC exception points. Excluding patients with non-HCC exceptions, 27.2% of patients ranked first due to calculated MELD scores were removed for death or clinical deterioration after having their initial first-ranked organ offer refused, compared to 2.5% (p<0.001) of HCC patients.
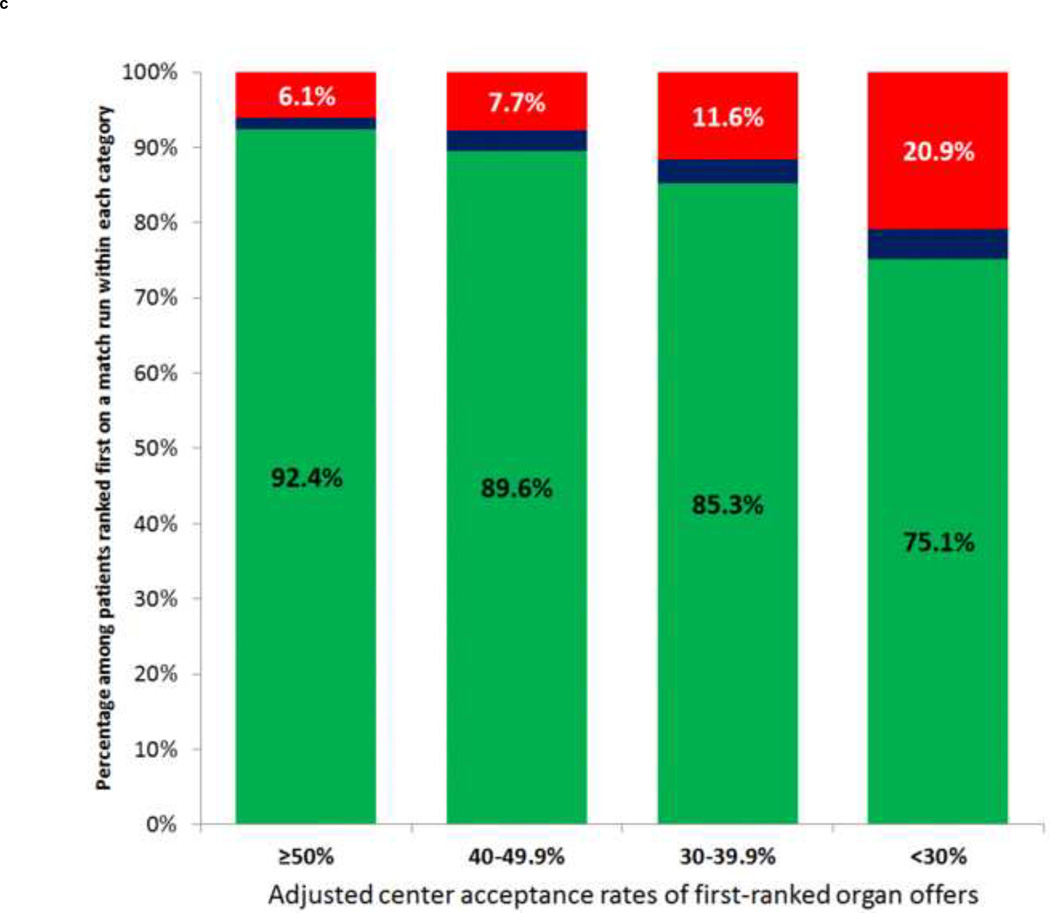
Unadjusted rates of waitlist removal for death or clinical deterioration were significantly higher for first-ranked patients waitlisted at transplant centers with lower organ acceptance rates (p<0.001; Figure 1c). In multivariable models that modeled adjusted center acceptance rates as a continuous exposure, decreasing organ acceptance rates were associated with significantly increased risk of waitlist removal for death or clinical deterioration, with a 27% increased odds of waitlist mortality for every 5% decrease in a centers’ adjusted acceptance rates (OR: 1.27, 95% CI: 1.20–1.32; Supplementary Table 1). In centers with adjusted organ acceptance rates ≥50%, the adjusted estimated waitlist mortality of the highest-ranked patients was 5.4%, compared to 17.9% in centers that accepted fewer than 30% of offers for firstranked patients (Table 2). There was a strong inverse correlation between a center’s adjusted first-ranked acceptance rate and the adjusted center-specific waitlist mortality of first-ranked patients (r=−0.7, p<0.001; Figure 1d). However, the overall post-transplant unadjusted 1-, 3, and 5-year patient survival of all deceased-donor liver transplants recipients during the study period, stratified by adjusted organ offer acceptance rates at a given center, were similar: <30%: 88.3%, 79.7%, and 73.0%; 30–39.9%: 89.6%, 80.7%, and 74.4%; 40–49.9%: 89.8%, 81.4%, and 74.4%; and ≥50%: 90.2%, 81.6%, and 75.%, respectively.
Table 2.
Unadjusted and adjusted waitlist outcomes among all non-Status 1 patients ranked first on at least one match run based on center acceptance patterns for first-ranked organ offers from 5/1/07–6/17/13, n=11,533*
| Adjusted percentage of first-ranked offers accepted at center |
Centers | Patients | Waitlist mortality, No. (%)† |
Transplanted, No. (%)* |
Estimated adjusted waitlist mortality rates (95% CI)‡ |
|---|---|---|---|---|---|
| ≥50% | 9 | 1,002 | 61 (6.1) | 926 (92.4) | 5.4% (3.5–7.3%) |
| 40–49.9% | 31 | 4,392 | 338 (7.7) | 3,937 (89.6) | 8.4% (7.2–9.6%) |
| 30–39.9% | 37 | 3,889 | 451 (11.6) | 3,316 (85.3) | 12.0% (10.5–13.4%) |
| <30% | 27 | 2,250 | 471 (20.9) | 1,689 (75.1) | 17.9% (15.6–20.2%) |
Similar among-category comparisons were seen with inclusion of Status 1 patients, although the percentage dying on the waitlist within each category was marginally higher (data not shown). The sum of the waitlist mortality and transplanted categories is <100%, as a small fraction of patients either had a condition that improved, were removed for “other” unspecified reasons, or refused transplantation.
Waitlist mortality defined as removed from waitlist for death or clinical deterioration. P<0.001 for chi-square test comparing proportion of patients removed from waitlists for death or clinical deterioration and for the proportion transplanted.
Estimated adjusted mortality rates calculated as predictive margins based on multilevel logistic regression model, with binary outcome of died (yes/no), with post-estimation marginal means calculated based on the adjusted odds ratio for waitlist removal for death or clinical deterioration based on a center’s adjusted accepted rate, with waitlist mortality also adjusted for covariates in Supplementary Table 1 (recipient race/ethnicity, recipient age, sex, UNOS region, match MELD score, OPO competition, status 1 recipient status, and receipt of HCC and/or non-HCC exception points). P<0.001 for test of associated between categorical variable of adjusted center acceptance rate and waitlist mortality in multivariable mixed-effects logistic regression model.
These predictive margins correlated with these respective multivariable odds ratios evaluating the risk of waitlist removal for death or clinical deterioration, with ≥50% as the reference: 40–49.9%=1.64 (95% CI: 1.09–2.42); 30–39.9%=2.47 (95% CI: 1.63–3.76); <30%=4.13 (95% CI: 2.67–6.38).
UNOS=United Network for Organ Sharing; MELD=Model for End-Stage Liver Disease; OPO=organ procurement organization; HCC=hepatocellular carcinoma
Secondary and sensitivity analyses
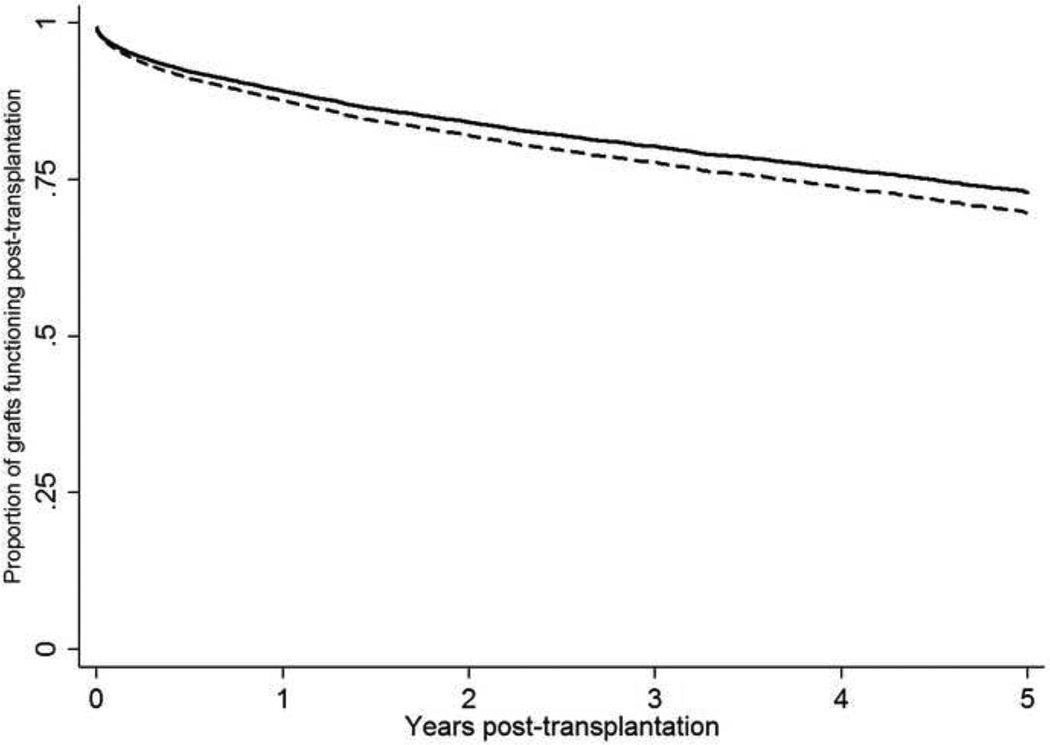
The DRI of organ offers accepted in the first position were statistically significantly lower (‘better quality’) than those that were declined, but these differences were not clinically meaningful (Table 3), based on the original DRI data published by Feng et al.33 There was a statistically increased risk of adjusted graft failure (defined as death or need for retransplantation) among livers declined for the highest-ranked patient, but the absolute difference in median 5-year adjusted graft survival was 4% between livers accepted for the first-ranked patient, compared to those declined and transplanted at a lower position (Figure 2). We were unable to obtain a confidence interval for the difference in the adjusted survival rate, but the confidence intervals for the unadjusted survival rates were quite narrow (95% CI for unadjusted 5-year graft survival: 70.6–73.2% for livers transplanted in the first position; 69.1–71.1% for livers transplanted below first position). All sensitivity analyses produced similar results regarding center-level variability (Supplementary Figures 7–11). Waitlist mortality analyses were similar among second-ranked patients (Supplementary Figure 4). Lastly, among first-ranked patients whose initial organ offer was declined who subsequently died on the waitlist, the time from declining the organ offer to death did not differ among centers with the highest and lowest acceptance rates (Supplementary Table 4). Inclusion of transplant centers (n=6) with small sample sizes did not change the results of the primary analyses. Donor-recipient size mismatch did not appear to explain why organ offers were frequently declined, as the recipient-to-donor height and weight differentials were not clinically meaningfully different between organ offers that were accepted or declined. More importantly, the distribution of organ offers declined for apparent size mismatch was similar among centers. Finally, the association between having an organ offer declined and subsequent survival was unlikely related to unmeasured center-level factors and appeared to be related directly to having an organ offer declined. In analyses restricted to first-ranked patients whose initial organ offer was declined (excluding those who were removed from the waitlist for death or clinical deterioration within 14 days of having the organ offer declined), matched to lower-ranked patients at the same transplant center transplanted with that liver (n=3,229 pairs of patients), the hazard for death in stratified multivariable Cox models was significantly higher for first-ranked patients who had the organ offer declined (HR: 1.86, 95% CI: 1.58–2.19). The 1-, 3-, and 5-year survival from the date of the match run for a first-ranked patient for whom the liver was declined was: 78.7% (77.0–80.4%), 70.9% (69.8–72.8%), and 64.9% (62.6–67.2%), respectively for first-ranked patients with the organ offer declined, and 90.8% (89.8–91.8%), 81.2% (79.7–82.6%), and 74.4% (72.5–76.2%) for the lower-ranked patient at the same center who received the same liver. Inclusion of number of new listings per center and percentage of new listings that had exception points did not alter the findings of among-center variability. To consider the potential for refusal rates to be influenced by the center’s recent graft and/or patient survival, we included a binary indicator of whether a center was cited for graft and/or patient survival rates that was significantly below the expected rates in the previous 6-month reporting period. There was no statistical association between low performance and organ acceptance rates the following period (p=0.65).
Table 3.
Graft quality and unadjusted graft outcomes of organ offers, stratified by adjusted center acceptance and offer acceptance versus decline for first-ranked patients*
| Center acceptance rate category† |
Organ disposition | DRI, median (IQR) | Unadjusted graft survival | ||
|---|---|---|---|---|---|
| 1-year | 3-year | 5-year | |||
| ≥50% | Accepted; transplanted into first-ranked patient | 1.29 (1.09–1.56) | 88.5 (86.1–90.5) | 78.9 (75.7–81.7) | 72.6 (68.9–76.1) |
| Declined; transplanted into lower-ranked patient | 1.42 (1.16–1.70) | 90.1 (87.1–92.4) | 79.8 (75.5–83.4) | 72.6 (67.1–77.3) | |
| 40–49.9% | Accepted; transplanted into first-ranked patient | 1.31 (1.12–1.59) | 88.0 (86.8–89.1) | 78.8 (77.2–80.3) | 71.9 (69.8–73.8) |
| Declined; transplanted into lower-ranked patient | 1.47 (1.21–1.79) | 88.1 (87.0–89.2) | 78.3 (76.7–79.7) | 70.9 (68.9–72.8) | |
| 30–39.9% | Accepted; transplanted into first-ranked patient | 1.34 (1.15–1.63) | 87.0 (85.7–88.2) | 78.2 (76.5–79.8) | 71.7 (69.5–73.8) |
| Declined; transplanted into lower-ranked patient | 1.50 (1.22–1.84) | 87.8 (86.9–88.7) | 78.1 (76.9–79.3) | 69.8 (68.1–71.4) | |
| <30% | Accepted; transplanted into first-ranked patient | 1.37 (1.15–1.68) | 87.4 (85.3–89.2) | 79.3 (76.7–81.7) | 72.2 (68.4–75.5) |
| Declined; transplanted into lower-ranked patient | 1.47 (1.20–1.80) | 87.5 (86.5–88.5) | 77.0 (75.6–78.4) | 69.7 (67.7–71.5) | |
Graft quality defined by donor risk index (DRI), as described by Feng et al.20 The DRI accounts for donor variables known to be associated with graft survival, but includes cold ischemic time, which is not determined until after a liver has been transplanted.
Center acceptance rate category based on a center’s adjusted organ offer acceptance rate for organs offers to patients ranked first on a match run, described in the Methods section, and Table 2.
Figure 2.
- *Footnote: Graft survival curves adjusted for recipient characteristics at transplantation. Adjusted hazard ratio for graft survival when organ offer accepted at first position: 0.89, 95% CI: 0.81–0.98. Curves based on grafts with available graft survival data (21,935/23,740 overall grafts)
- Figure legend:
──── Organ offer accepted for patients ranked first on match run – – – – Organ offer declined for patients ranked first on match run
| Organ offer | Years post-transplantation | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Accepted | 13,765 | 10,685 | 8,048 | 5,785 | 3,781 | 2,081 |
| Declined | 8,170 | 6,398 | 4,832 | 3,457 | 2,187 | 1,181 |
DISCUSSION
In this analysis of deceased-donor liver transplant organ offers from 2007–2013, we demonstrate wide variability in center behavior regarding accepting liver offers for the sickest patients, despite accounting for donor and recipient characteristics, local transplant-center density, and center size. Organs that were declined had only slightly inferior characteristics and outcomes to those that were accepted. Most importantly, the decision of whether to accept or decline an organ, largely explained by center-specific variability, substantially increased a patient’s odds of dying on the waitlist without a transplant. For the individual patient at the top of the waitlist, these data suggest that when an organ offer is made, it should be accepted because having an organ offer declined significantly increases one’s odds of dying. Furthermore, patients at centers with lower acceptance rates have significantly higher risks of dying without a transplant, which is an important consideration going forward from the perspectives of patients, referring providers, and insurers.
The MELD allocation system is designed to provide access to livers for those at greatest risk of waitlist mortality. Under the Final Rule, transplant centers have a mandate to transplant the sickest patients with the highest waitlist priority.1 Once passed over, many of the highest-ranked patients do not survive long enough to be ranked first again. Although some of the declined organ offers could be attributable to centers attempting to use extended criteria grafts for patients with lower MELD scores, this would not explain the among-center differences in analyses restricted to the highest-quality grafts. If the liver transplant community continues to operate under an urgency-based model, then efforts to minimize center variability appear necessary if we wish to diminish waitlist mortality and provide equitable access to lifesaving transplants. If transplant physicians strictly followed sickest-first principles, the issue of variable organ offer acceptance rates as a mediator of waitlist mortality would go away. If differences still persisted, only then should we determine if policies such as redistricting or changing the exception point system could help to minimize waitlist mortality.
Although it is unrealistic to expect uniform organ offer acceptance patterns among transplant centers, these data would suggest the need for development of standards of organ offer acceptance (i.e., a standardized acceptable range of organ offer acceptance), coupled with a standard center acceptance metric that would be publicly available. Such efforts could potentially decrease waitlist mortality by ensuring appropriate organ offer acceptance rates for the sickest patients, while also adding transparency to a process that historically has not been frequently discussed.
The strong association between center-specific practice patterns and patient survival suggests a potential causal relationship between organ offer acceptance behavior and patient outcomes. First, mechanistically, patients at the top of the waitlist, especially those without exception points, are acutely ill. As a result, having an organ offer declined during the narrow time window that a high MELD patient is at the top of the waitlist would be expected to lead to subsequent death if the patient does not receive a subsequent offer (>40% of patients ranked first whose initial first-ranked organ offer was declined never were ranked first on a subsequent match run). Second, the associations between center-specific organ offer acceptance rates and patient survival were robust to multivariable analyses, and persisted when organ acceptance rates were measured as either a continuous and categorical exposure. Third, first-ranked patients whose initial organ offer was declined had nearly twice the hazard of death in multivariable models that adjusted for patient characteristics, when compared to lower-ranked patients at the same center transplanted with that liver. This suggests that mortality was related to having an organ offer declined, rather than unmeasured center-level factors.
This study has potential implications for broader policy changes that would broadly share organs over greater distances in the US. First, these data demonstrate that geography alone is not the major determinant of waitlist survival. Among-center variability in waitlist mortality within distinct regions is as large, if not larger, than the among-region differences in waitlist mortality. As such, potential changes in redistribution may not reduce disparities in survival. Second, simulations of the impact of policy changes in liver allocation and distribution are limited, because the software used assumes that centers exhibit, “the same organ acceptance behavior,” which these data clearly show is not the case.36 Third, increased transplant-center density was associated with reduced odds of accepting an organ offer, which could be exacerbated under redistricting proposals that would create four or eight regions of organ distribution.5,6
This study had several strengths. It evaluated over 23,000 match runs that included over 13,000 unique patients. Further, our findings remained robust throughout multiple sensitivity analyses that were restricted to the best potential donors, the sickest non-exception recipients, as well as second- and third-ranked patients.
This study had limitations with regards to knowing the specific reason why a transplant center declined an organ offer. Each time an organ offer is declined, a transplant center must submit the reason for refusal (refusal code) to UNOS. Although the OPTN/UNOS dataset contains these refusal codes, they have been shown to be invalid, and thus do not allow for ascertainment of the true reason for decline.15 Specifically, 55% of ‘high-quality’ liver offers based on donor age and medical criteria received a refusal code of “organ quality” or “donor age.”15 Our own internal analysis validated these findings (data not shown). However, future work that seeks to understand the reasons for graft refusals is critical in order to better understand the center variability we have highlighted. Second, by the nature of available match run data, all organs considered in these analyses were of high enough quality to ultimately be transplanted. Any small unmeasured differences in organs would not explain the degree of among-center differences. Also, it is estimated that approximately 10% of all donor livers are procured and discarded.37 Although this may introduce some degree of survivor bias, this is unlikely to have impacted the among-center comparisons. Third, the time from organ decline to waitlist mortality did not differ based on center acceptance patterns, suggesting that declines in low-accepting centers are unlikely to be due to differences in patients that were too sick to transplant. Fourth, although donor-recipient size mismatch may have contributed to organ declines, this represented a small fraction of cases, and did not appear to explain among-center differences in acceptance rates. Fifth, the UNOS match-run dataset only included data on the match MELD score at the time of an organ offer (the MELD score used to rank a patient, accounting for exception points), and not the calculated MELD score, or its individual components.
In conclusion, there is substantial variability in center behavior towards accepting livers allocated to patients with the highest priority. These differences in behavior are not clearly explained by different patient and donor characteristics, and vary substantially among centers within the same geographic area. These differences in behavior are strongly correlated with a patient’s probability of dying on the waitlist without a transplant, and highlight how current center practices are not aligned with the underlying principles of MELD-based allocation. Further exploration and standardization of center-level differences in decision-making are needed to improve the stewardship of organ offers.
Supplementary Material
Acknowledgments
Dr. Gilroy is a member of the UNOS Liver and Intestinal Organ Transplantation Committee. This work represents the view of Dr. Gilroy and his co-authors, and does not necessarily reflect the views of the UNOS Liver and Intestinal Organ Transplantation Committee. Dr. Halpern is a member of the SRTR Technical Advisory Committee. This work represents the view of Dr. Halpern and his coauthors, and does not necessarily reflect the views of the SRTR Technical Advisory Committee.
We list the relevant grant information below:
This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, the Center for Medicare and Medicaid Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
David Goldberg: K08 DK098272
Ronac Mamtani: K23 CA187185
Frank Scott: K08 DK095951
Abbreviations
- OPTN
Organ Procurement and Transplantation Network
- MELD
Model for End-Stage Liver Disease
- UNOS
United Network for Organ Sharing
- HCC
Hepatocellular carcinoma
- DRI
Donor risk index
- LSAM
Liver Simulated Allocation Model
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Conflicts of Interest: The authors of this manuscript have no conflicts of interest to disclose as it pertains to this manuscript.
Author’s contributions
David S. Goldberg, MD, MSCE: Study concept and design, acquisition of the data, analysis and interpretation of data, statistical analysis, drafting of the manuscript, and critical revision of the manuscript
Benjamin French, PhD: Study concept and design, analysis and interpretation of data, statistical analysis, and critical revision of the manuscript
James D. Lewis, MD, MSCE: Study concept and design, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript
Frank I Scott, MD, MSCE: Analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript
Ronac Mamtani, MD, MSCE: Analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript
Richard Gilroy, MD: Study concept and design, analysis and interpretation of data, and critical revision of the manuscript
Scott D. Halpern, MD, PhD: Study concept and design, analysis and interpretation of data, and critical revision of the manuscript
Peter L, Abt, MD: Study concept and design, acquisition of the data, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript
The study protocol and final version was approved by HRSA.
References
- 1.Institute of Medicine. Organ Procurement and Transplantation: Assessing Current Policies and the Potential Impact of the DHHS Final Rule. 1999 [PubMed] [Google Scholar]
- 2.Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 3.Sharma P, Schaubel DE, Gong Q, Guidinger M, Merion RM. End-stage liver disease candidates at the highest model for end-stage liver disease scores have higher wait-list mortality than status-1A candidates. Hepatology. 2012;55(1):192–198. doi: 10.1002/hep.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg DS, French B, Forde KA, et al. Association of distance from a transplant center with access to waitlist placement, receipt of liver transplantation, and survival among US veterans. Jama. 2014;311(12):1234–1243. doi: 10.1001/jama.2014.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.OPTN/UNOS Liver and Intestinal Organ Transplantation Committee. Redesigning Liver Distribution to Reduce Variation in Access to Liver Transplantation. 2014 [Google Scholar]
- 6.Gentry SE, Massie AB, Cheek SW, et al. Addressing geographic disparities in liver transplantation through redistricting. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(8):2052–2058. doi: 10.1111/ajt.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zorzi D, Rastellini C, Freeman DH, Elias G, Duchini A, Cicalese L. Increase in Mortality Rate of Liver Transplant Candidates Residing in Specific Geographic Areas: Analysis of UNOS Data. Am J Transplant. 2012;12(8):2188–2197. doi: 10.1111/j.1600-6143.2012.04083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeh H, Smoot E, Schoenfeld DA, Markmann JF. Geographic inequity in access to livers for transplantation. Transplantation. 2011;91(4):479–486. doi: 10.1097/TP.0b013e3182066275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuttle-Newhall JE, Rutledge R, Johnson M, Fair J. A statewide, population-based, time series analysis of access to liver transplantation. Transplantation. 1997;63(2):255–262. doi: 10.1097/00007890-199701270-00014. [DOI] [PubMed] [Google Scholar]
- 10.S F. Dozens of lawmakers call for liver ‘redistricting’ plan. [December 17, 2014];2014 [Google Scholar]
- 11.A M. Liver transplant reallocation plan on hold. [December 17, 2014];2014 http://www.khi.org/news/2014/sep/26/liver-transplant-reallocation-plan-hold/ [Google Scholar]
- 12.Volk ML, Reichert HA, Lok AS, Hayward RA. Variation in organ quality between liver transplant centers. Am J Transplant. 2011;11(5):958–964. doi: 10.1111/j.1600-6143.2011.03487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai JC, Roberts JP, Vittinghoff E, Terrault NA, Feng S. Patient, center and geographic characteristics of nationally placed livers. Am J Transplant. 2012;12(4):947–953. doi: 10.1111/j.1600-6143.2011.03962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnier KE, Cox JC, McIntyre C, Ruhil R, Sadiraj V, Turgeon N. Transplantation at the nexus of behavioral economics and health care delivery. Am J Transplant. 2013;13(1):31–35. doi: 10.1111/j.1600-6143.2012.04343.x. [DOI] [PubMed] [Google Scholar]
- 15.Lai JC, Feng S, Roberts JP. An examination of liver offers to candidates on the liver transplant wait-list. Gastroenterology. 2012;143(5):1261–1265. doi: 10.1053/j.gastro.2012.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai JC, Feng S, Vittinghoff E, Roberts JP. Offer patterns of nationally placed livers by donation service area. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2013;19(4):404–410. doi: 10.1002/lt.23604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg D, French B, Abt P, Feng S, Cameron AM. Increasing disparity in waitlist mortality rates with increased model for end-stage liver disease scores for candidates with hepatocellular carcinoma versus candidates without hepatocellular carcinoma. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2012;18(4):434–443. doi: 10.1002/lt.23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg DS, Reese PP, Amaral S, Abt PL. Reframing the impact of combined heart-liver allocation on liver transplant wait-list candidates. Liver Transpl. 2014;20(11):1356–1364. doi: 10.1002/lt.23957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg DS, French B, Abt PL, Olthoff K, Shaked A. Superior survival using living donors and donor-recipient matching using a novel living donor risk index. Hepatology (Baltimore, Md.) 2014;60(5):1717–1726. doi: 10.1002/hep.27307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.OPTN/UNOS Policies and Bylaws [Google Scholar]
- 21.Abt PL, Desai NM, Crawford MD, et al. Survival following liver transplantation from non-heart-beating donors. Annals of surgery. 2004;239(1):87–92. doi: 10.1097/01.sla.0000103063.82181.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen AM, Kim WR, Xiong H, et al. Survival of recipients of livers from donation after circulatory death who are relisted and undergo retransplant for graft failure. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(5):1120–1128. doi: 10.1111/ajt.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selck FW, Grossman EB, Ratner LE, Renz JF. Utilization, outcomes, and retransplantation of liver allografts from donation after cardiac death: implications for further expansion of the deceased-donor pool. Annals of surgery. 2008;248(4):599–607. doi: 10.1097/SLA.0b013e31818a080e. [DOI] [PubMed] [Google Scholar]
- 24.Experimental Design and Analysis. [Accessed November 11, 2014];2014 http://www.stat.cmu.edu/~hseltman/309/Book/Book.pdf. [Google Scholar]
- 25.Goldberg DS, French B, Abt PL, Gilroy RK. Increasing the Number of Organ Transplants in the United States by Optimizing Donor Authorization Rates. Am J Transplant. 2015 doi: 10.1111/ajt.13362. [DOI] [PubMed] [Google Scholar]
- 26.Stram DO, Lee JW. Variance components testing in the longitudinal mixed effects model. Biometrics. 1994;50(4):1171–1177. [PubMed] [Google Scholar]
- 27.Normand S-LT, Shahian DM. Statistical and Clinical Aspects of Hospital Outcomes Profiling. 2007:206–226. [Google Scholar]
- 28.Lee WM. Acute liver failure in the United States. Semin Liver Dis. 2003;23(3):217–226. doi: 10.1055/s-2003-42641. [DOI] [PubMed] [Google Scholar]
- 29.Schiodt FV, Atillasoy E, Shakil AO, et al. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transpl Surg. 1999;5(1):29–34. doi: 10.1002/lt.500050102. [DOI] [PubMed] [Google Scholar]
- 30.Hoofnagle JH, Carithers RL, Jr, Shapiro C, Ascher N. Fulminant hepatic failure: summary of a workshop. Hepatology. 1995;21(1):240–252. [PubMed] [Google Scholar]
- 31.O'Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342(8866):273–275. doi: 10.1016/0140-6736(93)91818-7. [DOI] [PubMed] [Google Scholar]
- 32.O'Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97(2):439–445. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 33.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6(4):783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg D, French B, Thomasson A, Reddy KR, Halpern SD. Waitlist survival of patients with primary sclerosing cholangitis in the model for end-stage liver disease era. Liver Transpl. 2011;17(11):1355–1363. doi: 10.1002/lt.22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.OPTN UNOS Allocation Policy 3.6. 2013 [Google Scholar]
- 36.Scientific Registry of Transplant Recipients. [Accessed July 6, 2015];Liver Simulated Allocation Model User's Guide. Version. 2014 http://srtr.org/sam/content/LSAM.pdf. [Google Scholar]
- 37.Massie AB, Chow EK, Wickliffe CE, et al. Early changes in liver distribution following implementation of Share 35. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15(3):659–667. doi: 10.1111/ajt.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.