Abstract
The impact of physical inactivity on heart failure (HF) mortality is unclear. We analyzed data from the HF Adherence and Retention Trial (HART) which enrolled 902 NYHA class II/III HF patients, with preserved or reduced ejection fraction, who were followed for 36 months. Based upon mean self-reported weekly exercise duration, patients were classified into inactive (0 min/week) and active (≥ 1 min/week) groups and then propensity-score matched according to 34 baseline covariates in 1:2 ratio. Sedentary activity was determined according to self-reported daily television screen time (<2 h/day, 2–4 h/day; >4 h/day). The primary outcome was all-cause death. Secondary outcomes were cardiac death and HF hospitalization. There were 196 inactive patients, of whom 171 were propensity matched to 342 active patients. Physical inactivity was associated with higher risk of all-cause death (HR, 2.01; CI, 1.47 – 3.00; P < 0.001) and cardiac death (HR, 2.01; CI, 1.28 – 3.17; P = 0.002), but no significant difference in HF hospitalization (P = 0.548). Modest exercise (1–89 min/week) was associated with a significant reduction in the rate of death (P = 0.003) and cardiac death (P = 0.050). Independent of exercise duration and baseline covariates, television screen time (>4 h/day versus <2 h/day) was associated with all-cause death (HR, 1.65; CI, 1.10 – 2.48; P = 0.016; incremental χ2= 6.05; P = 0.049). In conclusion, among symptomatic chronic HF patients, physical inactivity is associated with higher all-cause and cardiac mortality. Failure to exercise and television screen time are additive in their effects on mortality. Even modest exercise was associated with survival benefit.
Keywords: Physical activity, sedentary, heart failure, outcome
The impact of complete physical inactivity on various cardiovascular outcomes in healthy individuals without heart failure (HF) is well established.1 Two recent cohort studies have demonstrated that in subjects without established heart disease, physical inactivity and sedentary time were associated with new onset HF.2,3 The impact of physical inactivity, compared to modest activity, on HF mortality in patients with established HF is not well studied; this is particularly true for patients with HF with preserved ejection fraction (HFpEF). In this study, we investigated the relation between physical inactivity and risk of mortality and HF hospitalization in patients not engaged in cardiac rehabilitation or structured exercise training.
METHODS
We analyzed data from Heart Failure Adherence and Retention Trial (HART),4 which was a multi-hospital, partially blinded, behavioral efficacy randomized controlled trial, funded by the National Institutes of Health [HL065547]. HART assessed the impact of self-management counseling versus education alone on the primary outcome of death or HF related hospitalization in patients with symptomatic HF. Details of the trial were reported elsewhere.4,5 The study enrolled patients from 10 centers in the Chicago metropolitan area and was approved by the Institutional Review Board of each collaborating institution [NCT00018005].
Briefly, HART enrolled HF patients with New York Heart Association (NYHA) class II or III symptoms, having HF with reduced ejection fraction (HFrEF) or HFpEF. Reduced systolic function was defined as left ventricular ejection fraction ≤40%. Eligible patients had to have HF symptoms for no less than the prior 3 months and either (1) ejection fraction ≤40%; or (2) diuretic therapy for at least 3 months and one or more previous HF hospitalization. This was a null trial; it showed no significant impact of the self-management intervention, relative to the education-only control, on the composite endpoint of death or HF hospitalization and other HF outcomes.4 For the purpose of the current study, we assessed the impact of self-reported exercise and television screen time on HF outcomes over a median follow-up of 36 months.
No subjects in HART were enrolled in cardiac rehabilitation or a structured exercise training program. To assess patients’ inactivity level, a standardized questionnaire assessed daily exercise and duration of television watched at baseline and in follow-up visits at years 1, 2 and 3.4,5 Patients were asked the frequency/week and duration/episode of walking for exercise or performing other physical activities to improve fitness. Patients were also asked to report on the duration and frequency of television watching (Supplemental Table 1). Based on patients’ responses, the sum of weekly exercise duration (minutes/week) was calculated. Since exercise duration could have varied during the course of the study, particularly after HF hospitalizations, we analyzed it as a time-dependent variable, averaging duration reported in all visits preceding the first adverse event of death or HF hospitalization. Based on the calculated average exercise duration, we divided the cohort into 2 study groups: Inactive Group, not engaged in any exercise (0 min/week), and Active Group, engaged in ≥1 min/week of exercise. The Active Group was further classified into Partially Active (1–89 min/week) and Fully Active (≥ 90 min/week). Based on the self-reported time spent television watching, patients were categorized into 3 sedentary-time groups: <2 h/day, 2–4 h/day, and >4 h/day. Time spent in other sedentary activities, such as reading, computer work, or driving was not available for analysis.
During baseline and yearly visits, data were gathered on demographics, psychosocial characteristics, comorbidities, prescription medications, adherence, and NYHA class. Socioeconomic status was defined as low if the patient’s annual household income was <$30,000 or if the highest attained education level was high school or lower. Medication adherence for key HF medications was assessed as the proportion of pills consumed relative to the prescribed amount using electronic pill caps. The dosages of various loop diuretics were converted into furosemide dose equivalents using an established conversion guide (Supplemental Table 2). During each follow-up visit, the 6-minute walk distance was measured. Depression was defined by self-reported diagnosis or scoring ≥ 10 on the Geriatric Depression Screening scale.6 Chronic kidney disease was defined as glomerular filtration rate <60 ml/min/1.73m2 (Cockcroft-Gault formula) or dialysis therapy. Coronary artery disease was defined as a prior history of coronary revascularization or confirmed myocardial infarction. Quality of life was assessed using the Physical Function subscale of the 36-item Short-Form Health Survey (SF-36).7 We indexed the burden of 12 HF symptoms using the cardiopulmonary subscale of the HF Symptom Checklist (Supplemental Table 3).8
The primary outcome was all-cause death. Secondary outcomes were cardiac death and HF hospitalization. The median follow-up was 36 months (interquartile range, 27–36 months). Outcomes were determined by a blinded adjudication committee.4 All patients (or their family members) were contacted every 3 months by telephone to determine the occurrence of hospitalization or death. Reports of death were confirmed by medical records, death certificates, or queries from the Social Security Death Index. Cardiac death was defined as death caused by myocardial infarction, arrhythmias, or pump-failure. HF admissions were adjudicated by the presence of shortness of breath, peripheral edema, or chest radiographic evidence of pulmonary edema. HF admissions were confirmed if the patient responded to HF therapy or had a documented decrease in left ventricular function.
The chi-square test was used to compare dichotomous variables, which were expressed as numbers (percentages). The independent samples t-test was used to compare normally-distributed continuous variables, which were expressed as means ± standard deviations. The Wilcoxon test was used to compare skewed continuous data.
Since patients were not randomly assigned to physical activity groups, we matched patients according to their propensity to being physically inactive. A multivariate logistic regression model (propensity model) was fit to calculate the probability of being physically inactive based on 34 baseline variables listed in Table 1 (identified with †). The resultant probabilities were then transformed into propensity score logits [Ln 1/(1-probability)]. SF-36 physical function score was included in the propensity model to balance patient’s functional abilities in order to assess the effect of physical inactivity as a matter of lifestyle rather than physical incapacitation. Six-minute walk distance was not included in the propensity model due to collinearity with NYHA class and SF-36 physical function score. Each patient in the inactive group was then matched to 2 active patients (1:2 ratio) with a propensity score within a caliper width of 0.2 standard deviation of the propensity score logits.9 Matching was performed using an algorithm written in Python software - version 2.6.7 (Python Software Foundation, Python.org). Furthermore, a second propensity model was fit to calculate the probability of >4 h/day of sedentary time, based on 34 baseline variables listed in Table 1 (identified with †).
Table 1.
Baseline Characteristics of Physical Activity Groups
| Before Propensity Matching (N = 902) | After Propensity Matching (N = 513) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Covariates | Inactive (0 min/wk) N=196 |
Active (>0 min/wk) N=706 |
P value* | Inactive (0 min/wk) N=171 |
Active (>0 min/wk) N=342 |
P value* |
| Propensity score logit | −0.64 ± 0.43 | −0.98 ± 0.42 | <0.001 | −0.73 ± 0.38 | −0.75 ± 0.39 | 0.598 |
| Age (years)† | 65 ± 14 | 63 ± 13 | 0.149 | 64 ± 14 | 65 ± 14 | 0.882 |
| Men† | 95 (48%) | 380 (54%) | 0.184 | 85 (50%) | 167 (49%) | 0.851 |
| White† | 121 (62%) | 419 (59%) | 0.547 | 105 (61%) | 188 (55%) | 0.165 |
| Low socioeconomic status† | 143 (73%) | 426 (60%) | 0.001 | 122 (71%) | 234 (68%) | 0.498 |
| HFrEF† | 139 (71%) | 554 (78%) | 0.027 | 122 (71%) | 252 (74%) | 0.574 |
| NYHA class III (vs. class II)† | 83 (42%) | 202 (29%) | <0.001 | 63 (37%) | 131 (38%) | 0.748 |
| HART treatment arm† | 94 (48%) | 357 (51%) | 0.518 | 82 (48%) | 170 (50%) | 0.708 |
| Third heart sound† | 6 (3%) | 31 (4%) | 0.406 | 6 (4%) | 9 (3%) | 0.578 |
| Jugular venous distention† | 17 (9%) | 58 (8%) | 0.837 | 13 (8%) | 28 (8%) | 0.818 |
| Cardiopulmonary symptoms index† | 0.68 ± 0.57 | 0.56 ± 0.56 | 0.001 | 0.65 ± 0.56 | 0.69 ±0.62 | 0.786 |
| SF-36, physical function† | 38.4 ± 23.7 | 50.8 ± 24.6 | <0.001 | 40.9 ± 23.6 | 41.0 ± 22.3 | 0.716 |
| 6-minute walk distance (m) | 222 ± 134 | 268 ± 134 | <0.001 | 229 ± 132 | 228 ± 125 | 0.787 |
| Coronary artery disease† | 111 (57%) | 385 (55%) | 0.601 | 97 (57%) | 187 (55%) | 0.660 |
| Atrial fibrillation† | 75 (38%) | 286 (41%) | 0.570 | 68 (40%) | 132 (39%) | 0.798 |
| Hypertension† | 152 (78%) | 524 (74%) | 0.341 | 129 (75%) | 271 (79%) | 0.327 |
| Diabetes mellitus† | 92 (47%) | 270 (38%) | 0.028 | 76 (44%) | 150 (44%) | 0.900 |
| Tobacco use† | 22 (11%) | 63 (9%) | 0.329 | 19 (11%) | 36 (11%) | 0.840 |
| Chronic kidney disease† | 84 (43%) | 297 (42%) | 0.843 | 73 (43%) | 166 (49%) | 0.211 |
| Stroke† | 27 (14%) | 78 (11%) | 0.292 | 21 (12%) | 47 (14%) | 0.645 |
| Depression† | 84 (43%) | 254 (36%) | 0.078 | 72 (42%) | 142 (42%) | 0.899 |
| Chronic lung Disease† | 30 (15%) | 95 (13%) | 0.507 | 24 (14%) | 57 (17%) | 0.441 |
| Arthritis† | 110 (56%) | 333 (47%) | 0.027 | 92 (54%) | 181 (53%) | 0.851 |
| Body mass index (kg/m2)† | 32.0 ± 7.6 | 30.8 ± 7.7 | 0.061 | 31.5 ± 7.5 | 31.8 ± 8.3 | 0.382 |
| Hemoglobin (g/dL)† | 12.7 ± 1.6 | 13.1 ± 1.6 | 0.040 | 12.8 ± 1.5 | 12.7 ± 1.6 | 0.340 |
| Serum sodium (mmol/dL)† | 140.3 ± 3.1 | 139.9 ± 3.1 | 0.106 | 140.3 ± 3.2 | 140.3 ± 3.1 | 0.694 |
| Serum potassium (mEq/dL) | 4.58 ± 0.52 | 4.53 ± 0.53 | 0.397 | 4.56 ± 0.50 | 4.57 ± 0.52 | 0.921 |
| Serum albumin (g/dL)† | 4.10 ± 0.56 | 4.13 ± 0.44 | 0.102 | 4.13 ± 0.57 | 4.08 ± 0.40 | 0.661 |
| Serum total bilirubin (mg/dL)† | 0.66 ± 0.37 | 0.64 ±0.37 | 0.701 | 0.64 ± 0.33 | 0.64 ± 0.36 | 0.541 |
| Serum creatinine (mg/dL) | 2.03 ± 2.56 | 1.64 ± 1.53 | 0.042 | 1.97 ± 2.61 | 1.76 ± 1.77 | 0.840 |
| Sedentary television watching (h/day) | 0.011 | 0.740 | ||||
| < 2 | 50 (26%) | 221 (31%) | 44 (26%) | 92 (27%) | ||
| 2 – 4 | 65 (33%) | 273 (39%) | 57 (33%) | 122 (36%) | ||
| > 4 | 81 (41%) | 212 (30%) | 70 (41%) | 128 (37%) | ||
| Medical Treatment | ||||||
| Medication adherence (%)† | 72 ± 40 | 64 ± 58 | 0.009 | 57 ± 39 | 53 ± 39 | 0.404 |
| ACEi/ARB use† | 156 (80%) | 617 (87%) | 0.006 | 144 (84%) | 279 (82%) | 0.460 |
| β-Blocker use† | 126 (64%) | 510 (72%) | 0.031 | 114 (67%) | 215 (63%) | 0.397 |
| Spironolactone use† | 47 (24%) | 195 (28%) | 0.309 | 46 (27%) | 80 (23%) | 0.384 |
| Loop diuretic (mg/day)† | 76 ± 71 | 58 ± 56 | 0.003 | 73 ± 68 | 63 ± 60 | 0.148 |
| Thiazide 2nd diuretic use† | 10 (5%) | 42 (6%) | 0.653 | 9 (5%) | 23 (7%) | 0.519 |
| Statin use† | 89 (45%) | 363 (51%) | 0.137 | 81 (47%) | 164 (48%) | 0.901 |
| Aspirin use† | 89 (45%) | 333 (47%) | 0.662 | 80 (47%) | 158 (46%) | 0.900 |
HART, Heart Failure Adherence and Retention Trial; NYHA, New York Heart Association; HFrEF, heart failure with reduced ejection fraction; SF-36, 36-item short form questionnaire of the medical outcome study; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker
Continuous data were presented as mean ± standard deviation and dichotomous data were presented as numbers (%)
t-test (normally distributed continuous variables), X2 test (dichotomous data), Wilcoxon test (skewed data)
covariates accounted for in the propensity-score matching
Bold P values denote statistical significance
Kaplan-Meier curves and the log-rank test were used to compare time to event occurrence. Risk was expressed as hazard ratio (HR) and 95% confidence interval (CI), calculated using univariate and multivariate Cox-regression models. To confirm the findings of the propensity-matched analyses, we analyzed outcomes in the entire cohort using multivariate Cox proportional-hazards models, adjusting for the calculated propensity scores. The risk (HR, CI) of adverse outcomes with graded levels of exercise (inactive; partially active; fully active) and television screen time (<2 h/day; 2–4 h/day; >4 h/day) was analyzed using inverse probability-weighted Cox-regression models. The inverse probability weighting factors employed were 1/probability, for the inactive and sedentary groups, and 1/(1-probability), for the remainder of the samples. The proportional hazards assumption with respect to Cox-regression modeling was confirmed using “log minus log” survival plots. The Mantel-Haenszel extension of the chi-square test for trend was used to demonstrate stepwise increase in event rates.
As an exploratory analysis, we studied within the propensity-matched cohort the impact of physical inactivity on the primary outcome within the pre-specified subgroups of gender, age, ethnicity (White vs. others), NYHA class (II vs. III), HFrEF vs. HFpEF, coronary disease status, chronic kidney disease status, and obesity (body mass index ≥30 Kg/m2). We also used multivariate Cox-regression modeling to test for an interaction between physical inactivity and subgroup strata and adjusting for covariates with >10% post-matching absolute standardized difference. We then confirmed the findings from the subgroup analyses in the entire cohort using multivariate Cox regression models, adjusted for the calculated propensity score logits.
Two-tailed P values <0.05 were considered significant. The PASW 18.0 software (SPSS, Inc. - Chicago, IL) and SAS 9.3 (SAS Institute - Cary, NC) were used for statistical analyses.
Based on the observed rate of death in HART (20.8%), we calculated, post-hoc, that the available propensity-matched cohort provided the study with 83% power to detect an increase in the rate of all-cause mortality by two-thirds (log-rank test, two-tailed α=0.05).
RESULTS
Physical activity data were available for all 902 (100%) subjects enrolled in HART. The median exercise time in the entire cohort was 60 min/week (interquartile range, 7.5–143 min/week). A total of 196 (22%) patients were classified as inactive [0 min/week] and 706 (78%) were classified as active [≥1 min/week]. The median exercise time among active patients was 90 min/week (interquartile range, 36–172 min/week). The baseline characteristics of the study groups are summarized in Table 1. Notably, inactive patients were more likely to be of lower socioeconomic status, less likely to have HFrEF, more likely to have NYHA class III symptoms, more symptomatic, lower physical function (SF-36), more obese, and less adherent to HF medications. They also covered shorter 6-minute walk distance, used higher doses of loop diuretics, and spent more time watching television. Patients in the active group were further classified into partially active [1–89 min/week, n=341 (38%)] and fully active [≥90 min/week, n=365 (40%)]. The baseline characteristics of patients classified according to three levels of exercise essentially mirrored those in the 2-level groups, as shown in Supplemental Table 4.
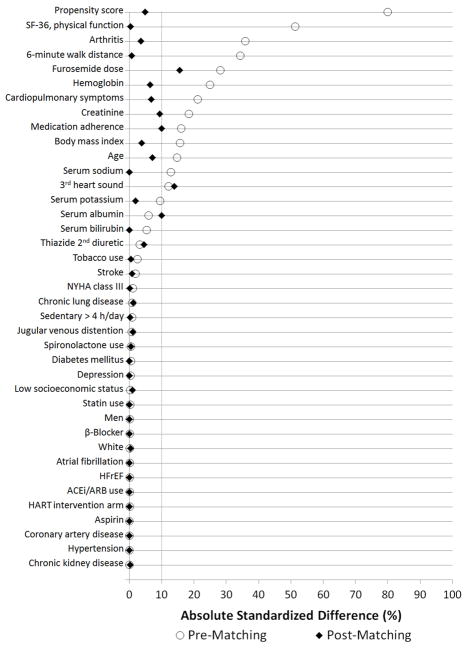
In unadjusted analyses in the entire cohort, inactive patients had a statistically significant increase in the rates of death and cardiac death, while the rate of HF hospitalization was not significantly different, as shown in Table 2. The means of the computed propensity-score logits were significantly different between the active and inactive groups (Table 1). A total of 171 (87%) patients in the inactive groups were successfully matched in 1:2 ratio to patients in the active group, resulting in a propensity-matched cohort of 513 patients (171 inactive, 342 active). After matching, there was no significant difference in the mean propensity-score between the matched groups and the balance between the study groups markedly improved, as none of the baseline characteristics were significantly different (Table 1). The absolute standardized differences between the propensity-matched groups was <10% for all baseline covariates, except for loop diuretic dose and 3rd heart sound, as illustrated in Figure 1.
Table 2.
Impact of Physical Inactivity on Heart Failure Outcomes
| Crude Even Rates | Unadjusted Risk | Adjusted Risk* | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Events | Total | Inactive | Active | HR (CI) | P value† | HR (CI) | P value§ |
| Entire Cohort, N=902 | |||||||
| Death | 188 (21%) | 72 (37%) | 116 (16%) | 2.69 (2.00–3.61) | <0.001 | 2.09 (1.53–2.86) | <0.001 |
| Cardiac death | 120 (13%) | 44 (22%) | 76 (11%) | 2.48 (1.71–3.59) | <0.001 | 1.92 (1.30–2.85) | 0.001 |
| HF hospitalization | 210 (23%) | 46 (23%) | 164 (23%) | 1.20 (0.86–1.66) | 0.284 | 0.85 (0.61–1.20) | 0.362 |
|
| |||||||
| PS Matched Cohort, N=513 | |||||||
| Death | 122 (24%) | 58 (34%) | 64 (19%) | 2.01 (1.47–3.00) | <0.001 | 2.00 (1.40–2.86) | <0.001 |
| Cardiac death | 75 (15%) | 35 (20%) | 40 (12%) | 2.01 (1.28–3.17) | 0.002 | 1.91 (1.21–3.02) | 0.005 |
| HF hospitalization | 133 (26%) | 38 (22%) | 95 (28%) | 0.89 (0.61–1.30) | 0.548 | 0.81 (0.55–1.18) | 0.264 |
HF, heart failure; HR, hazard ratio (inactive vs. active); CI, 95% confidence interval; PS, propensity score
HR and CI were derived from Cox proportional hazard models.
in the entire cohort analyses, adjustments were for the propensity scores; in the propensity-matched cohort analyses, adjustments were for covariates with >10% absolute standardized difference between the propensity-matched groups (loop diuretic dose and 3rd heart sound).
log-rank test;
Cox proportional hazard models;
Bold P values denote statistical significance
Figure 1.
Absolute Standardized Differences in Baseline Covariates between Physically Inactive and Physically Active Patients Pre and Post Propensity Score Matching
HFrEF, heart failure with reduced ejection fraction; SF-36, 36-item short-form questionnaire of the medical outcome study; NYHA, New York Heart Association; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; HART, Heart Failure Adherence and Retention Trial
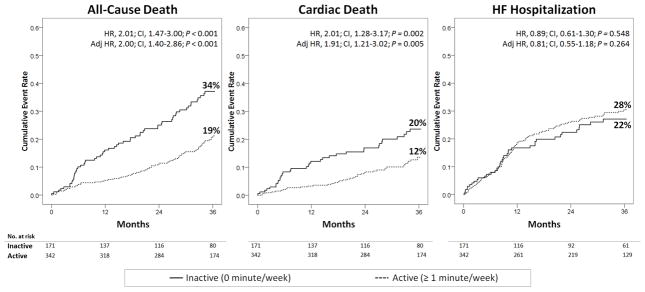
In the propensity-matched cohort, presented in Table 2 and Figure 2, physical inactivity was associated with a statistically significant increase in the rates of death and cardiac death, but there was no significant difference in the rates of HF hospitalization. These findings were consistent after adjusting for sub-optimally matched covariates (loop diuretic dose and 3rd heart sound). The findings of the propensity-matched analyses were confirmed in the entire cohort, after adjusting for propensity-score logits (Table 2).
Figure 2.
Impact of Physical Inactivity on Heart Failure Outcomes in the Propensity-Matched Cohort
HR, hazard ratio; CI, 95% confidence interval; Adj HR, hazard ratio adjusted for covariates with >10% post-matching absolute standardized difference (3rd heart sounds and loop diuretic dose).
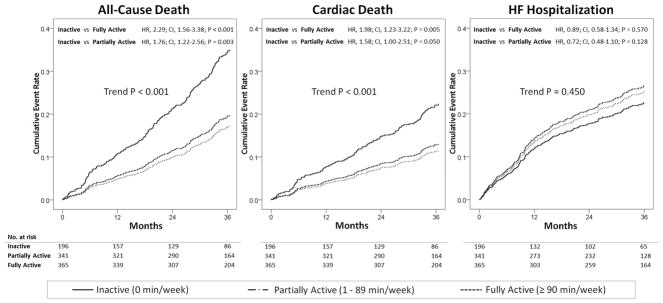
Since exercise training has been shown to improve patients’ outcomes,10,11 we sought to discern whether difference in outcome between active and inactive patients is derived from improved outcome among highly active patients versus poor outcome among inactive patients. Using inverse probability-weighted Cox-regression modeling over the entire cohort (n=902), we compared the cumulative risk of adverse outcomes among inactive patients to partially active (1–89 min/week) and fully active (≥90 min/week) patients. As presented in Figure 3, inactive patients were observed to have a significant increase in the risk of all-cause death and cardiac death compared to partially active and fully active subjects. The risk of all-cause death and cardiac death increased in stepwise patterns with decreasing activity level. However, the difference in the risk of HF hospitalization was not statistically different (Figure 3).
Figure 3.
Impact of Various Level of Physical Inactivity on Heart Failure Outcomes
Survival plots derived from inverse probability weighted Cox regression models fitted in the entire cohort.
HR, hazard ratio; CI, 95% confidence interval
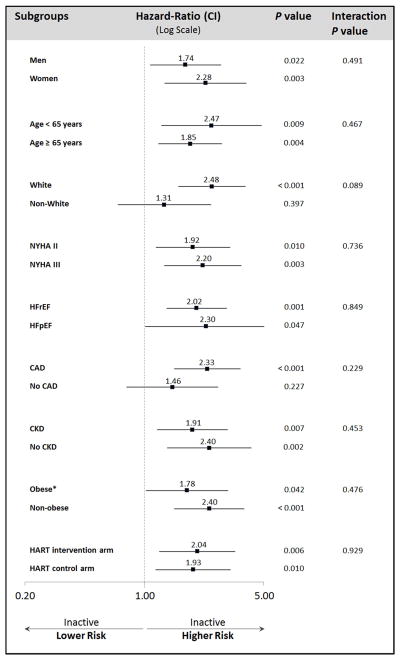
In subgroup analyses in the propensity-matched cohort (Figure 4), all hazard ratios for physical inactivity were consistently >1.0, indicating increased risk of death. Further, there was no statistically significant interaction between physical inactivity and any of the subgroup strata, indicating that physical inactivity was associated with similar increased risk of death across all subgroups. The findings of subgroups analyses were confirmed in the entire cohort, adjusting for propensity-score logits.
Figure 4.
Impact of Physical Inactivity on All-Cause Death by Subgroups of the Propensity-Matched Cohort
HR, hazard ratio; CI, 95% confidence interval; NYHA, New Your Heart Association classification; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; CAD, coronary artery disease; CKD, chronic kidney disease (glomerular filtration rate <60 ml/min/1.73m2 or dialysis)
* Obesity was defined as body mass index ≥30 kg/m2
HR, CI, and P values were derived from multivariate Cox-regression models in the propensity-matched cohort and adjusted for covariates with >10% post-matching absolute standardized difference (3rd heart sounds and loop diuretic dose).
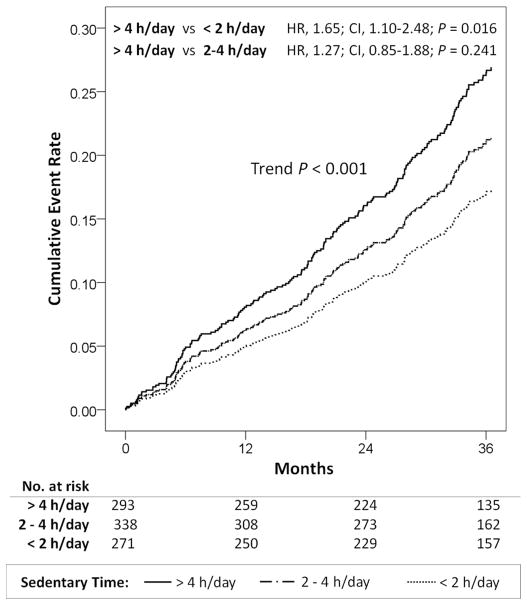
The baseline characteristics of the study cohort categorized according to daily sedentary time are detailed in Supplemental Table 5. We calculated the propensity scores (probabilities) of spending >4 h/day watching television. Using inverse probability-weighted Cox-regression modeling, increasing sedentary time was associated with a stepwise increase in the risk of all-cause death (trend P < 0.001), with statistically significant increase in risk between patients spending >4 h/day versus <2 h/day in sedentary time, after adjusting for total weekly exercise duration (Figure 5). Increasing sedentary time provided incremental prognostic value for all-cause death beyond weekly exercise time (Δχ2=6.106; P=0.013). Sedentary time was significantly associated with cardiac death (P=0.007; trend P=0.013) in univariate inverse-probability weighted Cox-regression analysis, but not after adjusting for exercise time. Sedentary time was not significantly associated with HF hospitalizations (P=0.257).
Figure 5. Impact of Sedentary Television Screen Time on All-Cause Mortality.
Survival plots derived from inverse probability weighted Cox regression models fitted in the entire cohort.
HR, hazard ratio; CI, 95% confidence interval
DISCUSSION
These analyses show that physical inactivity in patients with chronic HF was associated with nearly twice the risk of all-cause death and cardiac death. Even modest leisure exercise was associated with significantly reduced risk compared to complete physical inactivity. Moreover, television screen time was associated with incremental risk of all-cause death, above and beyond exercise duration and a broad range of sociodemographic and clinical covariates. Physical activity appears to be beneficial not only in HFrEF, but also in HFpEF patients. To our knowledge, this is the first study to demonstrate survival benefit with physical activity in HFpEF.
Most data demonstrating the benefit of exercise training have been derived from single-center studies and meta-analyses, which are subject to publication bias.12–15 A major multicenter randomized controlled trial, the HF-ACTION, showed that, compared to usual care, exercise training in patients with chronic HFrEF resulted in a nonsignificant reduction in the composite endpoint of all-cause mortality or hospitalization in the intention-to-treat analysis.10 An observational analysis of patients enrolled in the exercise training arm of HF-ACTION demonstrated that modest to moderate levels (3–7 MET-h/week) of exercise training were needed to observe a clinical benefit compared to patients not following their exercise prescription (but possibly engaged in other casual or leisure physical activities).11 Therefore, HF-ACTION and other single-center trials do not fully address the impact of voluntary commitment to exercise or sedentary behavior on HF outcomes. Moreover, the impact of physical inactivity on HF mortality remains unclear, since the control patients in the published literature, including HF-ACTION, have been involved in some degree of physical activity. In this investigation, we demonstrated a profound adverse impact of physical inactivity on mortality in patients with HF, irrespective of ejection fraction. Even modest exercise, such as walking, was associated with a significant improvement in outcome. Encouragement for HF patients to exercise either with cardiac rehabilitation or otherwise, should be part of routine care. Realistic, modest, and sustainable exercise levels are associated with profound survival benefits.
Most data addressing the value of exercise training in HF were derived from patients with HFrEF. To date, there has not been an outcome evaluation of exercise training in the growing HFpEF population. A few studies have demonstrated the value of exercise training in patients with HFpEF in improving important surrogate measures, such as peak oxygen consumption, exercise capacity, arterial stiffness, and health-related quality of life.16–18 Our investigation demonstrated that, compared to HFrEF subjects, HFpEF patients are similarly impacted by physical inactivity. This suggests the need for large controlled outcome studies evaluating the safety and efficacy of exercise training in HFpEF patients.
It is possible that the increased event rates observed in the inactive group were produced by unmeasured confounders leading to unrecognized reverse causality bias, such that sicker patients (with higher mortality risk) were the most inactive. This explanation is unlikely since our propensity-matching yielded study-groups that were well-balanced in terms of a wide range of plausible confounders, including HF severity, medical comorbidities, SF-36 physical function score, and 6-minute walk distance, body mass index, arthritis, and depression. For added rigor, we performed post-matching adjustment for covariates with residual difference between the matched groups and confirmed the findings over the entire cohort.
Physical inactivity was associated with increased risk of all-cause death and cardiac death but not HF hospitalization. There are two plausible explanations for this finding. First, inactive patients were less likely to be hospitalized due to increased competing risk of death. Second, one can speculate that active patients may have been more aware of deterioration in their physical functioning due to worsening HF, thus prompting medical attention and subsequent hospitalizations which may have negated any beneficial effect of exercise. The latter explanation is speculative and deserves study.
Beyond physical activity time, sedentary behavior defined as television screen time has been shown to be associated with adverse cardiovascular outcomes.19–22 In a recent cohort-study of 82,695 men aged ≥45 years with no established heart disease, Young et al. demonstrated that physical inactivity and sedentary behavior were associated with new onset HF.3 We established that in patients with pre-existing HF, sedentary behavior, represented by television screen time, was independently and incrementally predictive of all-cause mortality above and beyond a broad-range of baseline characteristics and exercise time. This finding expands our awareness of the detrimental effect of inactivity in HF, which should include both lack of exercise and extended sedentary activities.
The non-randomized design of our study is an obvious limitation. However, the propensity-matching analyses guarded against confounding in lieu of a physical activity trial which could have ethical challenges associated with randomizing patients to a non-exercise condition. We lacked data on implantable devices and plasma B-type natriuretic peptide levels; these are potentially important covariates. The study questionnaire lacked data on physical activity intensity and did not query sedentary behaviors other than television screen time.
Supplementary Material
Acknowledgments
The authors sincerely thank Guillaume Lambert, PhD for his contribution to the propensity-score matching of the study groups.
Funding: The Heart Failure Adherence and Retention Trial (NCT00018005) was funded by the National Heart, Lung, and Blood Institute (HL065547). This study is part of the Rush Center for Urban Health Equity, which is funded by the National Institute for Heart Lung and Blood (NHLBI), grant number 1P50HL105189-01.
Footnotes
Conflict of Interests: The authors have no relevant conflicts to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Proper KI, Singh AS, van Mechelen W, Chinapaw MJ. Sedentary behaviors and health outcomes among adults: a systematic review of prospective studies. Am J Prev Med. 2011;40:174–182. doi: 10.1016/j.amepre.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Andersen K, Mariosa D, Adami HO, Held C, Ingelsson E, Lagerros YT, Nyren O, Ye W, Bellocco R, Sundstrom J. Dose-response relationship of total and leisure time physical activity to risk of heart failure: a prospective cohort study. Circ Heart Fail. 2014;7:701–708. doi: 10.1161/CIRCHEARTFAILURE.113.001010. [DOI] [PubMed] [Google Scholar]
- 3.Young DR, Reynolds K, Sidell M, Brar S, Ghai NR, Sternfeld B, Jacobsen SJ, Slezak JM, Caan B, Quinn VP. Effects of physical activity and sedentary time on the risk of heart failure. Circ Heart Fail. 2014;7:21–27. doi: 10.1161/CIRCHEARTFAILURE.113.000529. [DOI] [PubMed] [Google Scholar]
- 4.Powell L, Calvin J, Jr, Richardson D, Janssen I, Mendes de Leon C, Flynn K. Self-management Counseling in Patients With Heart Failure: The Heart Failure Adherence and Retention Randomized Behavioral Trial. JAMA. 2010;304:1331–1338. doi: 10.1001/jama.2010.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powell L, Calvin J, Jr, Mendes de Leon C, Richardson D, Grady K, Flynn K, Rucker-Whitaker C, Janssen I, Kravitz G, Eaton C Heart Failure ART Investigators. The Heart Failure Adherence and Retention Trial (HART): design and rationale. Am Heart J. 2008;156:452–460. doi: 10.1016/j.ahj.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 7.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 8.Grady KL, Meyer PM, Dressler D, Mattea A, Chillcott S, Loo A, White-Williams C, Todd B, Ormaza S, Kaan A, Costanzo MR, Piccione W. Longitudinal change in quality of life and impact on survival after left ventricular assist device implantation. Ann Thorac Surg. 2004;77:1321–1327. doi: 10.1016/j.athoracsur.2003.09.089. [DOI] [PubMed] [Google Scholar]
- 9.Heinze G, Juni P. An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J. 2011;32:1704–1708. doi: 10.1093/eurheartj/ehr031. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor CM, Whellan DJ, Lee K, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pin IL Investigators ftH-A. Efficacy and Safety of Exercise Training in Patients With Chronic Heart Failure: HF-ACTION Randomized Controlled Trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keteyian SJ, Leifer ES, Houston-Miller N, Kraus WE, Brawner CA, O’Connor CM, Whellan DJ, Cooper LS, Fleg JL, Kitzman DW, Cohen-Solal A, Blumenthal JA, Rendall DS, Pina IL, Investigators H-A. Relation between volume of exercise and clinical outcomes in patients with heart failure. J Am Coll Cardiol. 2012;60:1899–1905. doi: 10.1016/j.jacc.2012.08.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Ismail H, McFarlane JR, Nojoumian AH, Dieberg G, Smart NA. Clinical Outcomes and Cardiovascular Responses to Different Exercise Training Intensities in Patients With Heart Failure. J Am Coll Cardiol HF. 2013;1:514–522. doi: 10.1016/j.jchf.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99:1173–1182. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 15.Belardinelli R, Georgiou D, Cianci G, Purcaro A. 10-year exercise training in chronic heart failure: a randomized controlled trial. J Am Coll Cardiol. 2012;60:1521–1528. doi: 10.1016/j.jacc.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 16.Edelmann F, Gelbrich G, Dungen HD, Frohling S, Wachter R, Stahrenberg R, Binder L, Topper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Loffler M, Hasenfuss G, Halle M, Pieske B. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 17.Taylor RS, Davies EJ, Dalal HM, Davis R, Doherty P, Cooper C, Holland DJ, Jolly K, Smart NA. Effects of exercise training for heart failure with preserved ejection fraction: a systematic review and meta-analysis of comparative studies. Int J Cardiol. 2012;162:6–13. doi: 10.1016/j.ijcard.2012.05.070. [DOI] [PubMed] [Google Scholar]
- 18.Kitzman DW, Brubaker PH, Herrington DM, Morgan TM, Stewart KP, Hundley WG, Abdelhamed A, Haykowsky MJ. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. J Am Coll Cardiol. 2013;62:584–592. doi: 10.1016/j.jacc.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford ES, Caspersen CJ. Sedentary behaviour and cardiovascular disease: a review of prospective studies. Int J Epidemiol. 2012;41:1338–1353. doi: 10.1093/ije/dys078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorp AA, Owen N, Neuhaus M, Dunstan DW. Sedentary behaviors and subsequent health outcomes in adults a systematic review of longitudinal studies, 1996–2011. Am J Prev Med. 2011;41:207–215. doi: 10.1016/j.amepre.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Stamatakis E, Hamer M, Dunstan DW. Screen-based entertainment time, all-cause mortality, and cardiovascular events: population-based study with ongoing mortality and hospital events follow-up. J Am Coll Cardiol. 2011;57:292–299. doi: 10.1016/j.jacc.2010.05.065. [DOI] [PubMed] [Google Scholar]
- 22.Nang EE, Salim A, Wu Y, Tai ES, Lee J, Van Dam RM. Television screen time, but not computer use and reading time, is associated with cardio-metabolic biomarkers in a multiethnic Asian population: a cross-sectional study. Int J Behav Nutr Phys Act. 2013;10:70. doi: 10.1186/1479-5868-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.