Abstract
Objective
Noninvasive brain stimulation (NIBS) can augment functional recovery following stroke; however, the technique lacks regulatory approval. Low enrollment in NIBS clinical trials is a key roadblock. Here, we pursued evidence to support the prevailing opinion that enrollment in trials of NIBS is even lower than enrollment in trials of invasive, deep brain stimulation (DBS).
Methods
We compared 2 clinical trials in stroke conducted within a single urban hospital system, one employing NIBS and the other using DBS, (1) to identify specific criteria that generate low enrollment rates for NIBS and (2) to devise strategies to increase recruitment with guidance from DBS.
Results
Notably, we found that enrollment in the NIBS case study was 5 times lower (2.8%) than the DBS trial (14.5%)(χ2 = 20.815, P < .0001). Although the number of candidates who met the inclusion criteria was not different (χ2 = .04, P < .841), exclusion rates differed significantly between the 2 studies (χ2 = 21.354, P < .0001). Beyond lack of interest, higher exclusion rates in the NIBS study were largely due to exclusion criteria that were not present in the DBS study, including restrictions for recurrent strokes, seizures, and medications.
Conclusions
Based on our findings, we conclude and suggest that by (1) establishing criteria specific to each NIBS modality, (2) adjusting exclusion criteria based on guidance from DBS, and (3) including patients with common contraindications based on a probability of risk, we may increase enrollment and hence significantly impact the feasibility and generalizability of NIBS paradigms, particularly in stroke.
Keywords: tDCS, rehabilitation, TMS, DBS, clinical trial, patient recruitment
Introduction
Noninvasive brain stimulation (NIBS) has become a popular method to augment plasticity expressed during recovery in patients with stroke.1–4 NIBS is able to safely modulate such cortical plasticity through currents applied over targeted regions of the brain5–7 and has been proven to be particularly advantageous for rehabilitation because it is relatively inexpensive and easy to administer.8 However, despite decade-long investigations,1,6,9–14 no NIBS modality is clinically approved for stroke rehabilitation.
A primary roadblock to clinical approval is the lack of demonstrated efficacy in pivotal large-scale phase III clinical trials. Understandably, a crucial reason for this is that evidence describing the efficacy of NIBS has been mixed, with reports citing inconsistent responses.15–19 Further, while large-scale trials would be needed to generate class A or level I quality of evidence, currently, NIBS studies suffer from limited patient enrollment.19,20 For example, in 2014, Anjos et al21 reported that only 4.7% of screened patients were enrolled (enrollment rate) in their NIBS clinical trial for stroke rehabilitation. In addition, we have noted that, in general, percent enrollment in NIBS trials in stroke over the past decade has varied from 5% to 45% and the number of patients enrolled is typically between 5 and 50.12,21–35 Thus, while large sample sizes would help account for inherent patient variability in stroke and allow for stratified patient subset analysis, to date, this has yet to be fully realized.
Given the reported challenge of patient recruitment for clinical trials in stroke,36–39 it is not surprising that enrollment rates for the study of NIBS in stroke are alarmingly low. However, when compared to other invasive stimulation modalities, an even more surprising paradox is revealed. Specifically, enrollment for the study of NIBS in stroke is even lower than that across trials of invasive stimulation, such as deep brain stimulation (DBS) for movement disorders.28,29,34,40 For example, current work suggests an average enrollment rate of 40%–91% for DBS trials with 70–200 patients per trial.40–44 This difference between recruitment in stimulation modalities is staggering because NIBS is by definition nonsurgical, safer, and simpler than invasive stimulation and stroke is a more prevalent cause of disability. Therefore, besides concerns for approval, this paradox raises serious ethical concerns regarding the clinical utility of NIBS for stroke. In particular, given this paradox, we pose the question: are there possible reasons for poor enrollment for studies of NIBS in stroke in comparison to invasive modalities, such as DBS?
Aims
To address this question directly, here, our primary aim was to examine the paradox of patient enrollment and to answer whether NIBS is indeed more restrictive than DBS in the same neurological population of stroke. We also aimed to evaluate whether inclusion/exclusion criteria create lower enrollment rates for NIBS in comparison to DBS.
To address our aims, we chose to compare enrollment rates and rationale for patient exclusion between 2 clinical trials being conducted at the Cleveland Clinic: (1) a NIBS trial aimed at facilitating rehabilitative outcomes of the paretic upper limb and (2) a DBS trial for poststroke thalamic pain. We chose to compare only these 2 trials for several reasons. Our primary goal was to compare enrollment of NIBS to a DBS trial utilizing the same patient population of stroke, but we could not identify any active clinical trials using DBS to facilitate rehabilitative outcomes of the paretic upper limb in stroke (as indicated by clinicaltrials.gov). Second, institutional policies could affect recruitment rates; therefore, by utilizing ongoing, rather than retrospective, clinical trials at the same center, we aimed to ensure a comparable demographic pool and recruitment efforts.
By using such a unique comparison, we sought to address whether in trying to ensure safety we have become so restrictive that we limit the generalizability of NIBS in stroke. In a much broader sense, we aimed to learn strategies to recruit patients for testing the effects of NIBS with guidance from DBS.
Methods
Case Study 1: NIBS for Stroke Rehabilitation
The NIBS study involved a single-center, randomized pilot clinical trial design, where patients were assigned to receive transcranial direct current stimulation (tDCS) or sham tDCS. While tDCS was applied during rehabilitation of the paretic upper limb with the intent of augmenting therapeutic benefit, transcranial magnetic stimulation (TMS) was utilized for evaluating plasticity (NCT01539096). Details of this trial are provided in Plow et al.17 This trial was chosen because it represents the most common indication for use of NIBS in stroke, affecting rehabilitative outcomes of the paretic upper limb. Inclusion and exclusion criteria were based on published recommendations for TMS,45 tDCS,18 and magnetic resonance imaging (MRI)46 (Table 1).
Table 1.
Inclusion and exclusion criteria for the NIBS and DBS clinical trials for poststroke rehabilitation
| NIBS case study | DBS case study |
|---|---|
| Inclusion criteria | |
|
|
| Exclusion criteria | |
|
|
Abbreviations: DBS, deep brain stimulation; MRI, magnetic resonance imaging; NIBS, noninvasive brain stimulation; TPS, thalamic pain syndrome.
Patients were recruited and screened from a large urban hospital system using medical record review from databases, community outreach via support groups and advertisements, and referrals from providers. To streamline recruitment, all identified patients were prescreened by staff through a brief medical chart review to determine preliminary eligibility. Patients who did not present any apparent exclusion/inclusion restrictions were classified as “candidates.” Reasons for failure to meet inclusion criteria, or exclusion due to contraindications were noted sequentially throughout the enrollment process (Table 1).
Case Study 2: DBS for Poststroke Pain
As stated in the section “Aims,” we chose to compare our NIBS trial aimed at facilitating rehabilitative outcomes of the paretic upper limb to a single-center pilot, first-in-man, crossover DBS trial for poststroke thalamic pain also conducted at the Cleveland Clinic (NCT01072656).47 As a crossover design, patients enrolled in this trial underwent both on-stimulation and off-stimulation phases. Inclusion and exclusion criteria for the DBS study were established based on safety of DBS implantation and target patient populations48 (Table 1). Patients were recruited via referrals from the Cleveland Clinic, Northeast Ohio, and other states, and through voluntary interest, where patients sought information about the study (clinicaltrials.gov, etc.) and expressed interest to the study team or to their treating physicians. Patients for both trials were enrolled following written informed consent, and the Institutional Review Board of the Cleveland Clinic approved the respective protocols.
Data Analysis
To understand whether specific screening criteria generated the paradox, we first compared enrollment between the NIBS and the DBS trials by contrasting frequencies of inclusion and exclusion criteria. For each, the total candidate pool was assessed sequentially for reasons for failure of inclusion, or for reasons of exclusion. Frequency of each criterion was then defined as the percentage of the total candidate population for each study, where final comparisons were computed using a χ2 test (SPSS; IBM Inc., Chicago, IL) with significance set at a P value less than .05.
Results
Medical Chart Review and Patient Prescreening
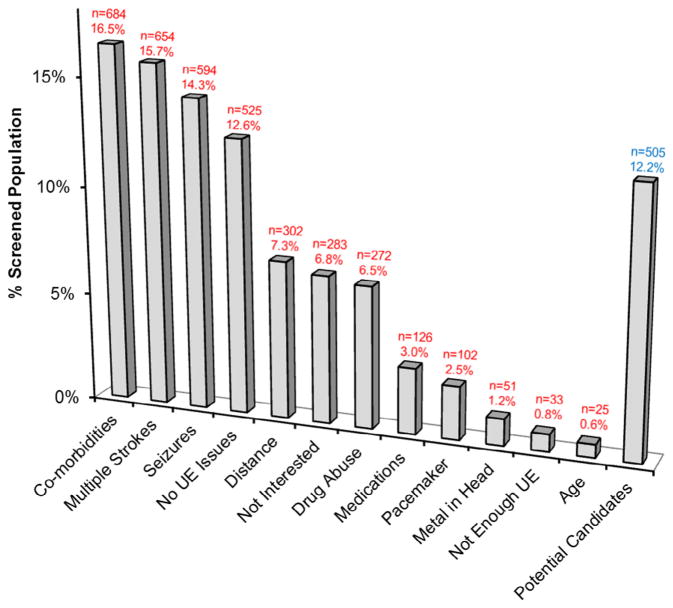
A total of 5192 medical charts were reviewed for the NIBS case study. Of these, records of 4156 patients were studied as the remaining patients were either deceased or did not present with a confirmed stroke. Only 505 patients were considered potential candidates, which represented 12.2% of the total 4156 records. From the review of charts, patients were most commonly excluded for comorbidities (16.5%, n = 684), multiple or recurrent strokes (15.7%, n = 654), and a history of seizures (14.3%, n = 594) (Fig 1). The most commonly noted comorbidities that were exclusionary for the NIBS case study were dementia (n = 103), congestive heart failure (n = 92), and cancer (n = 70). This initial analysis was only available for the NIBS trial because medical charts were not reviewed to identify candidates for the DBS trial.
Figure 1.
Prevalence of exclusion criteria in total prescreened patient population in the NIBS trial. Comorbidities, multiple strokes, and seizures were determined to be the most common exclusion criteria (n = 4156). Of the screened population, only 12.2% (n = 505) was determined to be potential study candidates. Abbreviation: UE, upper extremity.
Potential Candidate Population and Enrollment Rates
The average age (± standard deviation [SD]) of the 505 candidates for the NIBS study was 63.1 ± 13.7 years and 48.9% were female (n = 247). In contrast, for the DBS study, 69 patients were deemed candidates. Their average age (±SD) was 56.1 ± 28.8 years and 43.5% were female (n = 30).
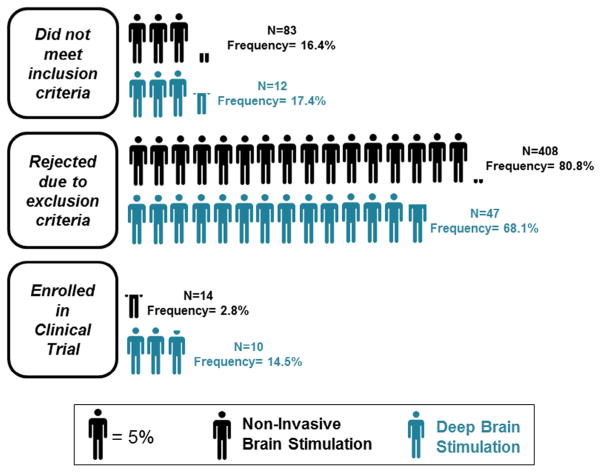
Out of the 505 candidates for the NIBS study, only 2.8% (n = 14) fulfilled the inclusion and exclusion criteria (Fig 2). This number represents only .3% of the total 4156 records that were studied. In contrast, of the 69 candidates in the DBS study, approximately 14.5% (n = 10) fulfilled the inclusion and exclusion criteria. Thus, taken collectively, we noted an approximate 5 times higher enrollment rate in the DBS study (14.5%) in comparison to the NIBS study (2.8%) (χ2 = 20.815, P < .0001). A disconcerting finding was that rates were more favorable for the DBS study even though the total number of candidates for the NIBS study was higher (n = 505) (Fig 2).
Figure 2.
Summary of included and excluded candidates for the NIBS and invasive DBS studies. Significantly higher enrollment rates were noted in the DBS study (n = 69) than in the NIBS study (n = 505) (χ(1) = 20.815; P < .0001). Abbreviations: DBS, deep brain stimulation; NIBS, noninvasive brain stimulation.
Frequency of Candidates Not Meeting Inclusion Criteria
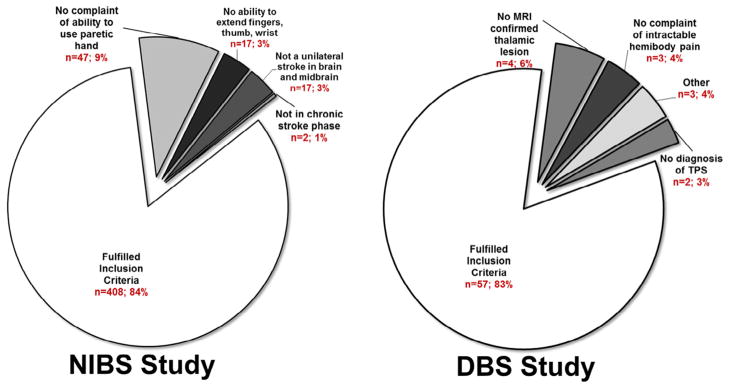
Even though the total number of candidates for the NIBS study was higher (n = 505) than for the DBS study (n = 69), the percentage of patients who did not meet inclusionary criteria in the NIBS study (16.4%, n = 83) and the DBS study (17.4%, n = 12) was not different (χ2 = .04, P < .841) (Fig 2). In the NIBS study, the majority failed to fulfill inclusionary prerequisites because they had no complaint of inability to use the paretic hand (9.2% of the 505 NIBS candidates, n = 47; Fig 3), whereas for the DBS study, the majority failed to fulfill inclusionary prerequisites because they lacked an MRI-confirmed thalamic stroke (5.8% of the 69 DBS candidates, n = 4; Fig 3).
Figure 3.
Percent of candidates that failed to meet inclusion criteria. Of the total number of potential candidates for each trial, the percentages of patients who were not included for the NIBS (left) and the DBS (right) trials for each respective inclusion criteria are noted here. Abbreviations: DBS, deep brain stimulation; MRI, magnetic resonance imaging; NIBS, noninvasive brain stimulation; TPS, thalamic pain syndrome.
Frequency of Candidates Possessing Exclusion Criteria
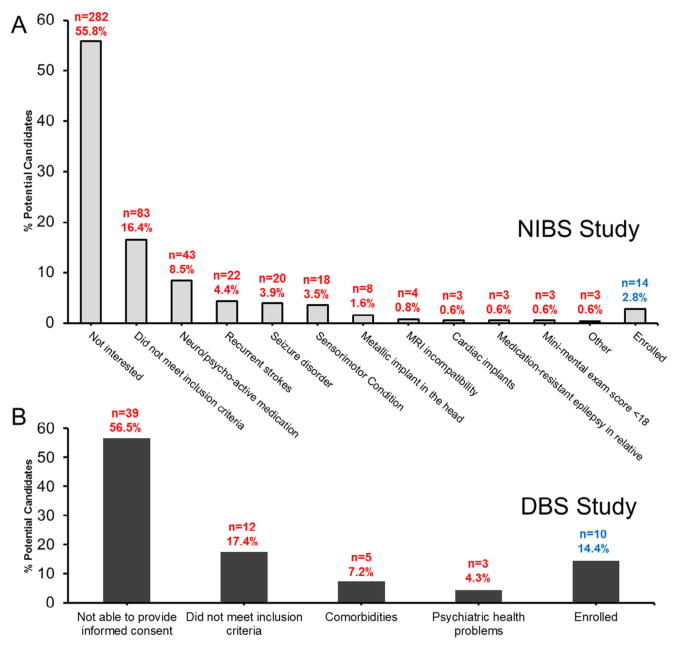
Unlike inclusion rates, exclusion rates differed significantly between the 2 studies (χ2 = 21.354, P < .0001). This was largely due to the difference between the number of exclusion criteria for the DBS study and the NIBS study (6 vs. 14) (Table 1). For the NIBS study, beyond lack of interest (55.8%), the main reasons for exclusion were contraindicated medications (~8.5%), recurrent strokes (4.4%), history of seizures (3.9%), and other conditions affecting the sensorimotor systems (3.5%) (Fig 4). In contrast, for the DBS study, the main reason for exclusion besides the inability to provide informed consent was comorbidities (7.2%, Fig 4); the most common comorbidity was coagulation complications (~3%).
Figure 4.
Percentage of candidates who possessed exclusion criteria and did not fulfill inclusion criteria. Percent population of patients who were excluded for the (A) NIBS and (B) DBS trials for each respective exclusion criteria. The number of patients meeting each criteria (n) and the percentage of the total potential candidate population are shown for each study. Abbreviations: DBS, deep brain stimulation; NIBS, noninvasive brain stimulation.
Discussion
Improving Enrollment for NIBS Studies in Stroke: Learning from DBS Studies
To the best of our knowledge, the present study is the first comparison of a NIBS and a DBS trial in stroke. In general, we noted that enrollment rates were higher in the DBS study in comparison to the NIBS study, despite a significant difference in the number of initial candidates (Fig 2). Our results are perplexing considering that NIBS is safer and simpler than DBS and that NIBS was utilized for upper limb paresis, a condition that is more prevalent (~60%–80%49) than the indication for DBS in the present study, thalamic pain syndrome, which carries an incidence of ~1%–8% in stroke.50 Given such results, we hypothesize that enrollment for NIBS is poor in stroke due to a modality mismatch that is specific to stroke. Specifically, the nature of the population per se presents inherent comorbidities or incompatibilities that are considered potential contraindications to the application of NIBS in stroke but are not relevant or exclusionary toward the use DBS. Based on our results, these include metallic implants in the head, seizures, neuro- or psychoactive medications, and comorbidities (Fig 4).45,51,52 Therefore, in this first step, we highlight how an enrollment paradox emerges with the use of 2 forms of stimulation in similar neurological populations.
Recurrent Stroke
Recurrent strokes currently remain an exclusion criterion for the majority of NIBS studies. While the rationale for this exclusion at present remains unclear, it is likely utilized because the benefit of NIBS may be reduced if multiple areas and/or hemispheres are affected. Thus, recurrent strokes pose a contraindication because they can introduce greater heterogeneity in clinical research.
However, while homogenous samples can improve statistical power, they limit generalizability. For example, recurrent strokes currently have an incidence of more than 30%52 and account for a majority of exclusions in NIBS trials.21 In line with the rationale of the exclusion criteria, though, it may still be possible for patients with multiple strokes to be considered candidates if subsequent strokes occurred within the same territory and caused similar lesion and/or corticospinal tract damage. Indeed, as multiple groups53–55 have demonstrated the predictive role of corticospinal tracts toward outcomes, if subsequent strokes were to damage tracts in the same hemisphere, it is possible that heterogeneity could still remain minimum.
Neuro- and Psychoactive Medications
Neuro- and psychoactive medications serve as an exclusion criterion in most NIBS studies that utilize TMS or repetitive transcranial magnetic stimulation (rTMS). This is primarily because such medications pose as a potential hazard for lowering seizure threshold,45 although evidence for such a claim in stroke is still not available (see the section “Metallic Implant Contraindications”).
Our NIBS trial excluded ~8.5% of patients based on intake of neuro- or psychoactive medications (Fig 4, A), the majority of which included antianxiety, antiepileptic, and antidepression medications. This number does not even account for ~3% (n = 126) that were excluded out of the original 4156 patients while determining candidacy (Fig 1). However, if guidelines were adjusted for medications based on data from hallmark publications in healthy individuals, this could allow for higher inclusion rates. For example, guidelines could be adjusted based on the work by Alper et al, which evaluated the incidence of seizure in FDA Phase II and III trials on psychotropic drugs between 1985 and 2004.56–58 Based on Alper’s evaluation, exclusion criteria could target specific antidepressants and antianxiety and antipsychotic drugs, such as bupropion, alprazolam, and clozapine, that were more prone to induce seizures in the subject population. Guidelines could then be supplemented by publications indicating that patients on a neuroactive medication dosage higher than the World Health Organization defined daily dose are approximately 3 times more likely to have a seizure.57 Further, we suggest that it may be ideal to develop a database, similar to Get With The Guidelines-Stroke,59 to record the relation between the incidence of seizures and the intake of neuroactive medications in stroke.60 Therefore, statistical analyses, similar to those done in healthy individuals, could be used to determine if particular medications truly provide a higher risk of seizure.
Beyond changing the potential seizure threshold, it is important to note that such neuroactive medications can also alter cortical plasticity and thereby limit the efficacy and reliability of NIBS tools.61–63 For example, work across healthy individuals and neurological diseases suggests that most neuroactive medications can change motor excitability thresholds, intracortical inhibition/facilitation, and spinal excitability.61,62,64–67 Thus, while particular neuroactive medications may have low potential for altering seizure threshold, it is still likely that they would affect the long-term effects and outcomes of NIBS modalities. One possible solution to address this potential confound would be to enroll patients under the premise that they would be required to maintain the same dose and intake regime of his or her neuroactive medications throughout the study.
Metallic Implant Contraindications
Metallic implants serve as a contraindication to brain stimulation because they can influence the electric/magnetic fields of the delivered modality, thereby affecting efficacy. In the case of DBS, though, neurosurgeons can often circumvent the region of implanted object, such as metal plates, to position electrodes. However, NIBS can present more of a challenge because stimulation is not as focused and metal implants can affect current flow and intensity.68 To understand this confound, modeling studies are necessary, else all implants will continue to be considered incompatible. In addition to modeling, safety testing can help determine the likelihood of heating and displacement of metals, such as first reported in the case of titanium plates.69 Another important point to consider may be whether implants found safe at 3-T strength of MRI would be considered compatible with NIBS because modalities deliver transient pulses at ~2 T.
History of Seizures
History of seizure remains an exclusion criterion for NIBS studies utilizing TMS and rTMS to prevent recurring, potentially harmful, seizure events. Specifically, because both TMS and rTMS deliver pulses that can alter inhibitory and excitatory synaptic activity in the cortex, such techniques can induce seizure events, albeit with relatively low risk (~.3%–1%).45 In fact, most case reports of TMS/rTMS-induced seizure have occurred in patients predisposed to recurring seizures (e.g., history of seizure and neuroactive medication),45 although reports of seizure have still been noted in patients without these predispositions.
Seizures occur in approximately 8%–10% of patients after stroke.70 However, a study done by De Reuck et al70 has suggested that risk varies by etiology. For example, stroke patients with an intracranial hemorrhage have a less than 4.7% chance of developing a seizure 15 days after stroke. Of patients developing a seizure 25 months after stroke, only 2.1% develop reoccurring seizure activity.70 In addition, patients who develop a seizure at the time of stroke onset are less likely to have recurrent seizures, and thus may present with less of a risk. Therefore, as stroke etiology and postictal duration may relate to the likelihood of recurrent seizures, varying criteria by these guidelines could facilitate inclusion in NIBS studies in stroke. This change is further supported by evidence that, while seizures may occur with stimulation of motor cortices, the event does not predispose the individual to develop epilepsy subsequently.71
Finally, it is important to note that over the last 15–20 years of research using rTMS/TMS in stroke, to our knowledge, only 5 seizure incidents that have occurred in a stroke patient following stimulation have been documented.72–76 Therefore, the risk of seizure appears to be extremely low in patients with stroke when the appropriate safety guidelines are maintained.45
Comorbidities
As it has been demonstrated that the majority of the stroke population is older than 50 years old, and cardiovascular conditions are primary risk factors for stroke,52 comorbidities are unavoidable. Given this, it is not surprising that comorbidities were the most common reason for initial exclusion among stroke patients who were screened for the NIBS (16.5%, Fig 1) or DBS trial (7.2%, Fig 4).
Modifying criteria to accommodate patients with comorbidities could present a challenge. For instance, some of the most common comorbidities (e.g., dementia and cancer) are the rationale for exclusion because they can directly interfere with rehabilitation. As such, including those who present with exclusionary comorbidities is challenging because cognitive impairment or fatigue could affect their ability to participate in rehabilitation and even exaggerate safety risks. Rather, it is plausible that on a patient-by-patient basis, seeking approval and advice from primary care physicians and/or other relevant specialists (e.g., neurologists and oncologists) involved in patient care could facilitate decision making.
Improving Enrollment for NIBS Studies in Stroke: Other Considerations
Beyond specific exclusion criteria, it cannot be overlooked that 55.8% of screened patients for the NIBS study were excluded due to lack of interest. We submit that such a high rate of lack of interest exists for several reasons. First, the clinical trial study design may significantly influence interest. For example, in the DBS trial, candidates were always guaranteed to receive DBS whether right after enrollment or following crossing over to the second phase of the study. In contrast, in the NIBS trial, patients may or may not have received stimulation. There was a 50% chance of being allocated to the sham stimulation group that would have never received real NIBS stimulation. Thus, patients may have been hesitant to participate and get a placebo treatment.
Second, we cannot discount a possible role for the method of patient recruitment. For the present NIBS study, the majority of patients were identified using medical chart review and then were contacted to determine interest and eligibility. This is in contrast to other studies, such as the DBS study presented here, that have utilized patient referrals from physicians or were conducted in close proximity to or within the departments of neurosurgery.60,77 Thus, patients who had been recommended by their physician may be more inclined to participate in a clinical trial.
Third, the patient’s ability to travel to the study site and perceived level of disability may also contribute. For instance, the present NIBS study only dispersed a small remuneration to the patient for completing the trial and did not cover costs of travel. In contrast, other studies, such as the present DBS trial, may have been able to provide full or substantial coverage for travel and lodging through either insurance or study funds. Further, if patients may have felt more disabled when they failed to respond to treatments, they could have been more inclined to seek information and routes to participate in research studies. Finally, we submit that surgical appeal may have also contributed to a patient’s lack of interest in the present NIBS study.
Assumptions and Limitations
Our case study comparison to understand barriers to patient recruitment carries inherent concerns. First, NIBS and DBS operate by different mechanisms. To address this, we chose to compare enrollment with a DBS trial in the population of stroke. Even though the NIBS trial investigated upper limb deficit whereas the DBS study included thalamic pain syndrome, upper limb deficits are also common in the latter subset (>50%). Second, we must acknowledge that prescreening in the DBS study was not utilized. Rather, patients were recruited primarily from physician referrals and thus we were unable to identify the specific number of patients contacted. Taken collectively, as discussed in the section “Improving Enrollment for NIBS Studies in Stroke: Other Considerations,” the use of such a recruitment method for the DBS trial could have also influenced our observed results. For instance, if patients were recruited during clinical visits to their physician, they may have been more inclined to agree to participate. In contrast, because patients for the NIBS trial were prescreened, identified from medical charts, and were contacted via phone calls, they may have been less willing to agree to participate. Finally, we cannot discount that the noted recruitment rate in the present study (2.8%, Fig 2) is lower than that of previously published studies. However, our noted exclusion criteria—seizures, multiple strokes—are uniformly incorporated across all present NIBS studies. Thus, even if our success rate for the NIBS had matched prior studies, the recruitment rate would still be 2 times lower than that which has been previously shown for DBS in pain and Parkinson’s disease.
Conclusion: Advancing toward Regulatory Approval for NIBS
NIBS carries tremendous promise to improve the state of rehabilitative services in stroke and ultimately mitigates associated disability. However, a major roadblock is poor enrollment in clinical trials, which depletes the quality of evidence of efficacy and stalls regulatory approval. Moving forward, we suggest that reducing or modifying exclusion criteria with guidance from other stimulation modalities, such as DBS, would substantially improve enrollment and hence the chances of gaining high-quality evidence from large-scale phase III clinical trials. With this, we would mitigate the “barriers to feasibility” hindering the clinical approval for NIBS in stroke rehabilitation.
Acknowledgments
This study was supported by grants from the National Institutes of Health (NIH) and the American Heart Association to E.P. (1K01HD069504 and 13BGIA17120055), and a grant from the NIH to A.M. (DOD006469A).
The authors wish to acknowledge researchers within the American Society for Neurorehabilitation and the Society for Neuroscience for conversations who added intellectual value to the manuscript.
Footnotes
Conflicts of interest: A.M. discloses that he was an Intelect medical advisory board member, consultant, and shareholder; obtained distribution rights from intellectual property for ATI, Enspire, and Cardionomics; and was a consultant for Functional Neurostimulation and Deep Brain Innovations. A.B.C. reports that she received a travel award from Pfizer/BMS and Boehringer.
References
- 1.Hummel F, Celnik P, Giraux P, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- 2.Rossi S, Rossini PM. TMS in cognitive plasticity and the potential for rehabilitation. Trends Cogn Sci. 2004;8:273–279. doi: 10.1016/j.tics.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Floel A, Cohen LG. Recovery of function in humans: cortical stimulation and pharmacological treatments after stroke. Neurobiol Dis. 2010;37:243–251. doi: 10.1016/j.nbd.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webster BR, Celnik PA, Cohen LG. Noninvasive brain stimulation in stroke rehabilitation. NeuroRx. 2006;3:474–481. doi: 10.1016/j.nurx.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Pino G, Pellegrino G, Assenza G, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. 2014;10:597–608. doi: 10.1038/nrneurol.2014.162. [DOI] [PubMed] [Google Scholar]
- 6.Liew SL, Santarnecchi E, Buch ER, et al. Non-invasive brain stimulation in neurorehabilitation: local and distant effects for motor recovery. Front Hum Neurosci. 2014;8:378. doi: 10.3389/fnhum.2014.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandrini M, Cohen LG. Noninvasive brain stimulation in neurorehabilitation. Handb Clin Neurol. 2013;116:499–524. doi: 10.1016/B978-0-444-53497-2.00040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 2007;3:383–393. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- 9.Hummel FC, Celnik P, Pascual-Leone A, et al. Controversy: noninvasive and invasive cortical stimulation show efficacy in treating stroke patients. Brain Stimul. 2008;1:370–382. doi: 10.1016/j.brs.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Bolognini N, Vallar G, Casati C, et al. Neurophysiological and behavioral effects of tDCS combined with constraint-induced movement therapy in poststroke patients. Neurorehabil Neural Repair. 2011;25:819–829. doi: 10.1177/1545968311411056. [DOI] [PubMed] [Google Scholar]
- 11.Talelli P, Greenwood RJ, Rothwell JC. Exploring theta burst stimulation as an intervention to improve motor recovery in chronic stroke. Clin Neurophysiol. 2007;118:333–342. doi: 10.1016/j.clinph.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Talelli P, Wallace A, Dileone M, et al. Theta burst stimulation in the rehabilitation of the upper limb: a semirandomized, placebo-controlled trial in chronic stroke patients. Neurorehabil Neural Repair. 2012;26:976–987. doi: 10.1177/1545968312437940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butefisch CM, Khurana V, Kopylev L, et al. Enhancing encoding of a motor memory in the primary motor cortex by cortical stimulation. J Neurophysiol. 2004;91:2110–2116. doi: 10.1152/jn.01038.2003. [DOI] [PubMed] [Google Scholar]
- 14.Zimerman M, Heise KF, Hoppe J, et al. Modulation of training by single-session transcranial direct current stimulation to the intact motor cortex enhances motor skill acquisition of the paretic hand. Stroke. 2012;43:2185–2191. doi: 10.1161/STROKEAHA.111.645382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plow EB, Carey JR, Nudo RJ, et al. Invasive cortical stimulation to promote recovery of function after stroke: a critical appraisal. Stroke. 2009;40:1926–1931. doi: 10.1161/STROKEAHA.108.540823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plow EB, Cunningham DA, Varnerin N, et al. Rethinking stimulation of the brain in stroke rehabilitation: why higher motor areas might be better alternatives for patients with greater impairments. Neuroscientist. 2015;21:225–240. doi: 10.1177/1073858414537381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plow EB, Cunningham DA, Beall E, et al. Effectiveness and neural mechanisms associated with tDCS delivered to premotor cortex in stroke rehabilitation: study protocol for a randomized controlled trial. Trials. 2013;14:331. doi: 10.1186/1745-6215-14-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Lefaucheur JP, Andre-Obadia N, Antal A, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) Clin Neurophysiol. 2014;125:2150–2206. doi: 10.1016/j.clinph.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Nouri S, Cramer SC. Anatomy and physiology predict response to motor cortex stimulation after stroke. Neurology. 2011;77:1076–1083. doi: 10.1212/WNL.0b013e31822e1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anjos SM, Cohen LG, Sterr A, et al. Translational neurorehabilitation research in the third world: what barriers to trial participation can teach us. Stroke. 2014;45:1495–1497. doi: 10.1161/STROKEAHA.113.003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geroin C, Picelli A, Munari D, et al. Combined transcranial direct current stimulation and robot-assisted gait training in patients with chronic stroke: a preliminary comparison. Clin Rehabil. 2011;25:537–548. doi: 10.1177/0269215510389497. [DOI] [PubMed] [Google Scholar]
- 23.Kim DY, Lim JY, Kang EK, et al. Effect of transcranial direct current stimulation on motor recovery in patients with subacute stroke. Am J Phys Med Rehabil. 2010;89:879–886. doi: 10.1097/PHM.0b013e3181f70aa7. [DOI] [PubMed] [Google Scholar]
- 24.Lee SJ, Chun MH. Combination transcranial direct current stimulation and virtual reality therapy for upper extremity training in patients with subacute stroke. Arch Phys Med Rehabil. 2014;95:431–438. doi: 10.1016/j.apmr.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 25.Wu D, Qian L, Zorowitz RD, et al. Effects on decreasing upper-limb poststroke muscle tone using transcranial direct current stimulation: a randomized sham-controlled study. Arch Phys Med Rehabil. 2013;94:1–8. doi: 10.1016/j.apmr.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 26.You DS, Kim DY, Chun MH, et al. Cathodal transcranial direct current stimulation of the right Wernicke’s area improves comprehension in subacute stroke patients. Brain Lang. 2011;119:1–5. doi: 10.1016/j.bandl.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Lefebvre S, Thonnard JL, Laloux P, et al. Single session of dual-tDCS transiently improves precision grip and dexterity of the paretic hand after stroke. Neurorehabil Neural Repair. 2014;28:100–110. doi: 10.1177/1545968313478485. [DOI] [PubMed] [Google Scholar]
- 28.Pomeroy VM, Cloud G, Tallis RC, et al. Transcranial magnetic stimulation and muscle contraction to enhance stroke recovery: a randomized proof-of-principle and feasibility investigation. Neurorehabil Neural Repair. 2007;21:509–517. doi: 10.1177/1545968307300418. [DOI] [PubMed] [Google Scholar]
- 29.Seniow J, Bilik M, Lesniak M, et al. Transcranial magnetic stimulation combined with physiotherapy in rehabilitation of poststroke hemiparesis: a randomized, double-blind, placebo-controlled study. Neurorehabil Neural Repair. 2012;26:1072–1079. doi: 10.1177/1545968312445635. [DOI] [PubMed] [Google Scholar]
- 30.Higgins J, Koski L, Xie H. Combining rTMS and task-oriented training in the rehabilitation of the arm after stroke: a pilot randomized controlled trial. Stroke Res Treat. 2013;2013:539146. doi: 10.1155/2013/539146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang WH, Kim YH, Yoo WK, et al. rTMS with motor training modulates cortico-basal ganglia-thalamocortical circuits in stroke patients. Restor Neurol Neurosci. 2012;30:179–189. doi: 10.3233/RNN-2012-110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khedr EM, Etraby AE, Hemeda M, et al. Long-term effect of repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Acta Neurol Scand. 2010;121:30–37. doi: 10.1111/j.1600-0404.2009.01195.x. [DOI] [PubMed] [Google Scholar]
- 33.Ackerley SJ, Stinear CM, Barber PA, et al. Combining theta burst stimulation with training after subcortical stroke. Stroke. 2010;41:1568–1572. doi: 10.1161/STROKEAHA.110.583278. [DOI] [PubMed] [Google Scholar]
- 34.Hsu WY, Cheng CH, Liao KK, et al. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: a meta-analysis. Stroke. 2012;43:1849–1857. doi: 10.1161/STROKEAHA.111.649756. [DOI] [PubMed] [Google Scholar]
- 35.Koch G, Bonni S, Giacobbe V, et al. theta-burst stimulation of the left hemisphere accelerates recovery of hemispatial neglect. Neurology. 2012;78:24–30. doi: 10.1212/WNL.0b013e31823ed08f. [DOI] [PubMed] [Google Scholar]
- 36.Alexandrov AV. Slow recruitment in clinical trials: failure is not an option! International Journal of Stroke. 2006;1:160. doi: 10.1111/j.1747-4949.2006.00046.x. [DOI] [PubMed] [Google Scholar]
- 37.Lloyd G, Dean CM, Ada L. Issues in recruiting community-dwelling stroke survivors to clinical trials: the AMBULATE trial. Contemp Clin Trials. 2010;31:289–292. doi: 10.1016/j.cct.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Cheeran B, Cohen L, Dobkin B, et al. The future of restorative neurosciences in stroke: driving the translational research pipeline from basic science to rehabilitation of people after stroke. Neurorehabil Neural Repair. 2009;23:97–107. doi: 10.1177/1545968308326636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blanton S, Morris DM, Prettyman MG, et al. Lessons learned in participant recruitment and retention: the EXCITE trial. Phys Ther. 2006;86:1520–1533. doi: 10.2522/ptj.20060091. [DOI] [PubMed] [Google Scholar]
- 40.Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 41.Morrell MJ Group RNSSiES. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 42.Daniels C, Krack P, Volkmann J, et al. Risk factors for executive dysfunction after subthalamic nucleus stimulation in Parkinson’s disease. Mov Disord. 2010;25:1583–1589. doi: 10.1002/mds.23078. [DOI] [PubMed] [Google Scholar]
- 43.Abboud H, Floden D, Thompson NR, et al. Impact of mild cognitive impairment on outcome following deep brain stimulation surgery for Parkinson’s disease. Parkinsonism Relat Disord. 2015;21:249–253. doi: 10.1016/j.parkreldis.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 44.Weiss D, Walach M, Meisner C, et al. Nigral stimulation for resistant axial motor impairment in Parkinson’s disease? A randomized controlled trial. Brain. 2013;136:2098–2108. doi: 10.1093/brain/awt122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossi S, Hallett M, Rossini PM, et al. Safety of TMSCG. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shellock FG. 2014 MRIsafety.com.
- 47.Machado AG, Baker KB, Plow E, et al. Cerebral stimulation for the affective component of neuropathic pain. Neuromodulation. 2013;16:514–518. doi: 10.1111/j.1525-1403.2012.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plow EB, Malone DA, Machado A. Deep brain stimulation of the ventral striatum/anterior limb of the internal capsule in thalamic pain syndrome: study protocol for a pilot randomized controlled trial. Trials. 2013;14:241. doi: 10.1186/1745-6215-14-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Broeks JG, Lankhorst GJ, Rumping K, et al. The long-term outcome of arm function after stroke: results of a follow-up study. Disabil Rehabil. 1999;21:357–364. doi: 10.1080/096382899297459. [DOI] [PubMed] [Google Scholar]
- 50.Kim JS. Post-stroke pain. Expert Rev Neurother. 2009;9:711–721. doi: 10.1586/ern.09.19. [DOI] [PubMed] [Google Scholar]
- 51.Costello AH. Guidance for industry and Food and Drug Administration staff: class II special controls guidance document: repetitive transcranial magnetic stimulation (rTMS) systems. Services USDoHaH; 2011. [Google Scholar]
- 52.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allendorfer JB, Storrs JM, Szaflarski JP. Changes in white matter integrity follow excitatory rTMS treatment of post-stroke aphasia. Restor Neurol Neurosci. 2012;30:103–113. doi: 10.3233/RNN-2011-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Groisser BN, Copen WA, Singhal AB, et al. Corticospinal tract diffusion abnormalities early after stroke predict motor outcome. Neurorehabil Neural Repair. 2014;28:751–760. doi: 10.1177/1545968314521896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lindenberg R, Zhu LL, Ruber T, et al. Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Hum Brain Mapp. 2012;33:1040–1051. doi: 10.1002/hbm.21266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coupland C, Dhiman P, Barton G, et al. A study of the safety and harms of antidepressant drugs for older people: a cohort study using a large primary care database. Health Technol Assess. 2011;15:1–218. doi: 10.3310/hta15280. [DOI] [PubMed] [Google Scholar]
- 57.Montgomery SA. Antidepressants and seizures: emphasis on newer agents and clinical implications. Int J Clin Pract. 2005;59:1435–1440. doi: 10.1111/j.1368-5031.2005.00731.x. [DOI] [PubMed] [Google Scholar]
- 58.Alper K, Schwartz KA, Kolts RL, et al. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62:345–354. doi: 10.1016/j.biopsych.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 59.American Heart Association. Get With The Guidelines-Stroke. Dallas TX: 2014. [Accessed January 26, 2016]. Available at: http://www.heart.org/HEARTORG/HealthcareResearch/GetWithTheGuidelines/Get-With-The-Guidelines-Stroke_UCM_306098_SubHomePage.jsp. [Google Scholar]
- 60.Potter-Baker KA, Bonnett CE, Chabra P, et al. A game of hide and seek: is it possible to recruit more patients for NIBS studies in stroke? J Neurol Sci. 2015;358:472–474. doi: 10.1016/j.jns.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115:1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 62.Ziemann U, Lonnecker S, Steinhoff BJ, et al. Effects of antiepileptic drugs on motor cortex excitabilitv in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- 63.Ziemann U, Muellbacher W, Hallett M, et al. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124:1171–1181. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]
- 64.Barry MD, Bunday KL, Chen R, et al. Selective effects of baclofen on use-dependent modulation of GABAB inhibition after tetraplegia. J Neurosci. 2013;33:12898–12907. doi: 10.1523/JNEUROSCI.1552-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Florian J, Muller-Dahlhaus M, Liu Y, et al. Inhibitory circuits and the nature of their interactions in the human motor cortex a pharmacological TMS study. J Physiol. 2008;586:495–514. doi: 10.1113/jphysiol.2007.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Inghilleri M, Berardelli A, Marchetti P, et al. Effects of diazepam, baclofen and thiopental on the silent period evoked by transcranial magnetic stimulation in humans. Exp Brain Res. 1996;109:467–472. doi: 10.1007/BF00229631. [DOI] [PubMed] [Google Scholar]
- 67.Kahkonen S, Ilmoniemi R. Transcranial magnetic stimulation: applications for neuropsychopharmacology. J Psychopharmacol. 2004;18:257–261. doi: 10.1177/0269881104042631. [DOI] [PubMed] [Google Scholar]
- 68.Datta A, Bikson M, Fregni F. Transcranial direct current stimulation in patients with skull defects and skull plates: high-resolution computational FEM study of factors altering cortical current flow. Neuroimage. 2010;52:1268–1278. doi: 10.1016/j.neuroimage.2010.04.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rotenberg A. Safety of 1 Hz repetitive transcranial magnetic stimulation (rTMS) in patients with titanium skull plates. Clin Neurophysiol. 2009;120:1415–1417. doi: 10.1016/j.clinph.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 70.De Reuck J, Sieben A, Van Maele G. Characteristics and outcomes of patients with seizures according to the time of onset in relation to stroke. Eur Neurol. 2008;59:225–228. doi: 10.1159/000115635. [DOI] [PubMed] [Google Scholar]
- 71.Henderson JM, Heit G, Fisher RS. Recurrent seizures related to motor cortex stimulator programming. Neuromodulation. 2010;13:37–43. doi: 10.1111/j.1525-1403.2009.00256.x. [DOI] [PubMed] [Google Scholar]
- 72.Agosta S, Galante E, Ferraro F, et al. Report of a delayed seizure after low frequency repetitive transcranial magnetic stimulation in a chronic stroke patient. Clin Neurophysiol. 2015 doi: 10.1016/j.clinph.2014.11.029. pii: S1388–2457(15)00731-2. [DOI] [PubMed] [Google Scholar]
- 73.Harrington RM, Chan E, Turkeltaub PE, et al. Simple partial status epilepticus one-day post single-pulse TMS to the affected hemisphere in a participant with chronic stroke. Brain Stimul. 2015;8:682–683. doi: 10.1016/j.brs.2015.01.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Homberg V, Netz J. Generalised seizures induced by transcranial magnetic stimulation of motor cortex. Lancet. 1989;2:1223. doi: 10.1016/s0140-6736(89)91835-7. [DOI] [PubMed] [Google Scholar]
- 75.Fauth C, Meyer B-U, Prosiegel M, et al. Seizure induction and magnetic brain stimulation after stroke. Lancet. 1992;339:362. doi: 10.1016/0140-6736(92)91678-2. [DOI] [PubMed] [Google Scholar]
- 76.Kumar N, Padma Srivastava MV, Verma R, et al. Can low-frequency repetitive transcranial magnetic stimulation precipitate a late-onset seizure in a stroke patient? Clin Neurophysiol. 2015 doi: 10.1016/j.clinph.2015.06.033. pii: S1388–2457(15)00727-0. [DOI] [PubMed] [Google Scholar]
- 77.Plow EB, Machado A. Invasive neurostimulation in stroke rehabilitation. Neurother. 2014;11:572–582. doi: 10.1007/s13311-013-0245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]