Abstract
Several animal models have evaluated the effect of stress on voluntary ethanol intake with mixed results. The experiments reported here examined the effects of different stressors on voluntary ethanol consumption in dependent and nondependent adult male C57BL/6J mice. In Experiment 1, restraint, forced swim, and social defeat stress procedures all tended to reduce ethanol intake in nondependent mice regardless of whether the stress experience occurred 1 h or 4 h prior to ethanol access. The reduction in ethanol consumption was most robust following restraint stress. Experiment 2 examined the effects of forced swim stress and social defeat stress on drinking in a dependence model that involved repeated cycles of chronic intermittent ethanol (CIE) exposure. Repeated exposure to forced swim stress prior to intervening test drinking periods that followed repeated cycles of CIE exposure further increased ethanol consumption in CIE-exposed mice while not altering intake in nondependent mice. In contrast, repeated exposure to the social defeat stressor in a similar manner reduced ethanol consumption in CIE-exposed mice while not altering drinking in nondependent mice. Results from Experiment 3 confirmed this selective effect of forced swim stress increasing ethanol consumption in mice with a history of CIE exposure, and also demonstrated that enhanced drinking is only observed when the forced swim stressor is administered during each test drinking week, but not if it is applied only during the final test week. Collectively, these studies point to a unique interaction between repeated stress experience and CIE exposure, and also suggest that such an effect depends on the nature of the stressor. Future studies will need to further explore the generalizability of these results, as well as mechanisms underlying the ability of forced swim stress to selectively further enhance ethanol consumption in dependent (CIE-exposed) mice but not alter intake in nondependent animals.
Keywords: alcohol, ethanol dependence, stress, mice
Introduction
Stress has been extensively implicated as a factor that may lead to ethanol (alcohol) drinking and induce relapse in abstinent alcoholics. Studies involving animal models and clinical investigations have indicated that the relationship between stress and ethanol consumption is complex and depends on a large number of variables (Becker, Lopez, & Doremus-Fitzwater, 2011; Spanagel, Noori, & Heilig, 2014). From a clinical perspective, the tension-reduction hypothesis postulates that people seek to consume ethanol for its sedative or anxiolytic effects (Brady & Sonne, 1999; Cappell & Greeley, 1987; Pohorecky, 1991; Sayette, 1999; Uhart & Wand, 2009). Further, as many individuals suffering with alcohol-use disorder (AUD) invariably experience several episodes of abstinence, stress associated with withdrawal may perpetuate excessive drinking motivated by attempts to reduce or avoid withdrawal-related distress (Becker, 2008, 2013, 2014; Heilig, Egli, Crabbe, & Becker; 2010; Koob, 2003, 2014; Koob & Le Moal, 2008). On the other hand, ethanol is known to serve as a stressor itself. For example, alcoholics undergoing treatment to maintain abstinence often present with alterations in hypothalamic-pituitary-adrenal axis (HPA) function (Adinoff et al., 1996; Costa et al., 1996; Lovallo, Dickensheets, Myers, Thomas, & Nixon, 2000; Marchesi, Chiodera, Ampollini, Volpi, & Coiro, 1997; Uhart & Wand, 2009). These alterations in baseline HPA axis activity along with enhanced reactivity to stress may trigger relapse and further augment drinking. Alcoholics frequently report that they resumed ethanol drinking after a stressful episode, and changes in the HPA axis have been related to ethanol craving (O’Malley, Krishnan-Sarin, Farren, Sinha, & Kreek, 2002; Sinha & O’Malley, 1999).
The preclinical literature concerning ethanol-stress interactions consists of numerous studies with mixed results. Some studies indicate that stress increases ethanol intake while others indicate that ethanol intake is reduced or unaltered by stress experience (reviewed by Becker et al., 2011; Spanagel et al., 2014). Methodological differences among the studies likely account for the variety of outcomes – many of these studies differ in the nature of stressor used, schedule of stress administration in relation to ethanol access, or amount/duration of prior ethanol intake (Becker et al., 2011; Pohorecky, 1990; Sillaber & Henniger, 2004; Spanagel et al., 2014).
Several preclinical studies have evaluated the effect of stress on relapse using operant conditioning models of reinstatement. In this case, stress is demonstrated to induce ethanol-seeking behavior when rodents are tested under extinction conditions (Lê & Shaham, 2002; Lê et al., 1999; Lê et al., 1998). Stress has also been shown to enhance cue-induced reinstatement in both rats and mice (Liu & Weiss, 2002). Furthermore, and of relevance for the present study, the effect of stress on ethanol-seeking behavior is magnified in ethanol-dependent rats compared to nondependent animals (Gehlert et al., 2007; Liu & Weiss, 2002; Sommer et al., 2008). In these studies, it may be argued that ethanol withdrawal-related stress enhances the effect of an acute stressor to trigger ethanol seeking. However, it is important to note that in these reinstatement studies, animals are not provided the opportunity to consume ethanol following stress exposure (i.e., testing is conducted under extinction conditions). Indeed, little is known about the effect of stress on voluntary ethanol intake in ethanol-dependent rodents. Numerous studies conducted with rats (Brown, Jackson, & Stephens, 1998; Gilpin, Richardson, Lumeng, & Koob, 2008; Gilpin et al., 2009; O’Dell, Roberts, Smith, & Koob, 2004; Roberts, Cole, & Koob, 1996; Roberts, Heyser, Cole, Griffin, & Koob, 2000; Valdez et al., 2002) and mice (Becker & Lopez, 2004; Finn et al., 2007; Griffin, Lopez, & Becker, 2009; Griffin, Lopez, Yanke, Middaugh, & Becker, 2009; Lopez & Becker, 2005) have shown increases in ethanol intake in subjects that experienced repeated episodes of chronic ethanol intoxication followed by periods of withdrawal. The studies presented in the current report aim to evaluate the effect of stress on ethanol consumption within the context of dependence. Using a mouse model of dependence and relapse drinking, the hypothesis guiding these experiments is that ethanol-dependent mice will not only exhibit the expected increase in ethanol intake relative to nondependent mice, but they also will be more sensitive to the ability of stress to further increase voluntary ethanol consumption.
Methods
Subjects
Adult male C57BL/6 mice purchased from Jackson Laboratories (Bar Harbor, ME) were individually housed with free access to food (Harlan Teklad, Madison, WI) and tap water throughout all phases of the experiments. Body weights were recorded weekly during ethanol drinking periods or daily during chronic intermittent ethanol (CIE) or air inhalation exposure (detailed below). In the experiment involving social defeat stress, adult male CD1 mice from Charles River Laboratories (Raleigh, NC) served as aggressors (described below). Mice were housed in a temperature- and humidity-controlled animal facility under a modified 12-h light/dark cycle (lights on at 2:00 AM). All procedures were approved by the Institutional Animal Care and Use Committee and followed the NIH Guide for the Care and Use of Laboratory Animals (8th edition, National Research Council, 2011).
Study Designs
Experiment 1: Effect of exposure to different stressors on voluntary ethanol intake in nondependent mice
Mice (n = 9–10/group) were allowed to drink ethanol (15% v/v vs. water) in their home cage using a limited-access (2 h/day) procedure described below. After 6 weeks of voluntary ethanol intake (baseline), mice were separated into seven groups balanced on the basis of intake level during the last baseline week. Mice in the no-stress (noSTS) control group continued to drink ethanol as during baseline weeks. The rest of the groups were exposed to restraint stress (RS), social defeat (SD), or forced swim (FS) either 1 or 4 h before access to ethanol in their home cages for 5 consecutive days. These time intervals were chosen to evaluate ethanol intake near peak stress-induced physiological changes or at a more remote time point when the acute effects of stress, such as elevated corticosterone levels and reduced locomotor activity had subsided (Cabib, Kempf, Schleef, Mele, & Puglisi-Allegra, 1988; Hare, Beierle, Toufexis, Hammack, & Falls, 2014; Patchev & Patchev, 2006). The following week, all mice resumed ethanol intake without exposure to stress.
Experiment 2: Effect of exposure to different stressors on voluntary ethanol intake in ethanol-dependent and nondependent mice
Mice (n = 7–12/group) were allowed to drink ethanol in their home cages under limited-access (2 h/day) conditions. Once stable baseline intake was observed, mice were separated into CIE-exposed (dependent) and air-exposed (nondependent) groups, and these groups were further separated into three stress conditions: no-stress (noSTS), social defeat (SD), and forced swim (FS). Mice were separated into these groups based on their intake level during the last week of baseline intake. Seventy-two hours after each CIE (or air) exposure cycle, stress procedures were administered 4 h before each daily drinking test session. This schedule of CIE (or air) exposure followed by 5 days of ethanol intake after stress or no stress exposure was repeated four times (Test cycles 1–4). Details regarding CIE (or air) exposure and stress procedures are presented below.
Experiment 3: Effect of forced swim stress on voluntary ethanol intake in ethanol-dependent and nondependent mice
This experiment followed similar procedures described for Experiment 2. Once stable baseline ethanol intake under limited-access conditions was established, mice (n = 8–10/group) were separated into CIE and air-control (CTL) groups, and further separated on the basis of stress condition. One group of CIE and CTL mice (FS4 groups) received forced swim (FS) stress exposure 4 h prior to test drinking sessions that followed each of the four weekly CIE (or air) exposure cycles. A second cohort of CIE and CTL mice (FS1 groups) received FS stress 4 h prior to drinking sessions, but only following the last (4th) CIE/air exposure cycle. The remaining CIE and CTL mice (noSTS groups) did not receive FS stress at any time during the study.
Limited-access ethanol drinking procedure
Mice had access to ethanol 5 days/week (Mon–Fri) starting at 30 min before the start of the dark cycle (1:30 PM). Two 15-mL graduated tubes containing either 15% v/v ethanol or tap water were introduced in the home cage replacing the regular water bottle. Two hours later, the graduated tubes were removed and the regular water bottle was made available again. The ethanol solution was prepared fresh every day by mixing 190 proof ethanol with deionized water. The amount of ethanol consumed by each mouse was converted to g/kg based on the volume of ethanol consumed (± 0.1 mL) and body weight (± 0.1 g).
Stress procedures
Restraint
Flat-bottomed restrainers for mice (1.5-in diameter × 4-in long; Plas Labs, Lansing, MI) were used for this procedure. Mice were placed head-first into plastic cylinders and secured with a divider to hold the mouse as far forward as possible in the restraint. After 30 min of restraint, mice were returned to their home cages.
Forced Swim
Mice experienced a 10-min inescapable swim test in a glass cylinder (20-cm diameter × 40-cm tall) filled with 23–25 °C water.
Social Defeat
This stressor consisted of placing a mouse (intruder) in the home cage of a CD1 male mouse. CD1 mice were singly housed as “residents” for at least 4 days without bedding changes prior to introducing the intruder mouse (C57BL/6J) to the cage. The social defeat procedure consisted of three phases: priming, defeat, and threat of defeat. For the priming phase, the intruder mouse was placed in a protective cage situated within the CD1 resident mouse cage for 5 min. In the second phase, the protective screen was removed, and the intruder interacted with the resident. This defeat phase was terminated with the display of a prototypic defeat posture for 3 sec or after 5-min exposure to the resident mouse, as previously detailed (Miczek, 1991). In mice, the upright defeat posture with retracted ears, limp forelimbs, and audible squeals upon approach by the aggressive stimulus animal are clear signs of defeat (Miczek, Thompson, & Shuster, 1982). Finally, the intruder was covered by a protective cage for the threat of defeat phase and remained in the resident cage for 20 min before being returned to its home cage. Each mouse was exposed to social defeat with a different CD1 mouse each day. All CD1 mice were ethanol-naïve and were not evaluated for ethanol intake after these stress procedures.
All the stress procedures were conducted in a room separate from the colony room where mice were housed.
Chronic intermittent ethanol (CIE) exposure
Mice in the CIE group received CIE vapor exposure in inhalation chambers (16 h/day for 4 days) while control mice (CTL) were similarly handled, but exposed to air in control chambers. CIE exposure was administered in inhalation chambers according to procedures previously described (Becker & Lopez, 2004; Griffin, Lopez, & Becker, 2009; Griffin, Lopez, Yanke, et al., 2009; Lopez & Becker, 2005). Briefly, mice were placed in Plexiglas® inhalation chambers (60 × 36 × 60 cm) and exposed to ethanol vapor at levels set to yield stable blood ethanol concentrations (BEC) in C57BL/6J mice in the range of 175–225 mg/dL. Housing conditions were similar to those in the colony room. Blood samples (40 μL) were obtained from the retro-orbital sinus with heparinized capillary tubes during each CIE cycle of exposure. Blood samples were centrifuged and plasma was processed in an Analox Instrument analyzer (Lunenburg, MA), with blood ethanol levels expressed as mg/dL.
Data analysis
Data were analyzed by ANOVA, with significant main effect or interactions (p < 0.05) further analyzed using the Newman-Keuls method for post hoc comparisons.
Results
Experiment 1: Effect of different stressors on voluntary ethanol intake in nondependent mice
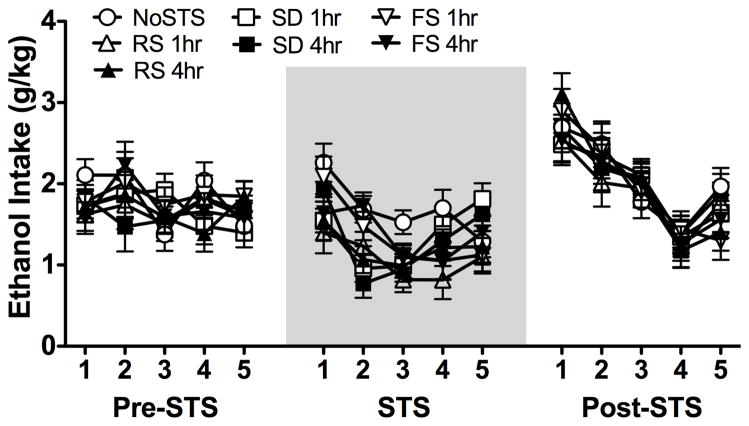
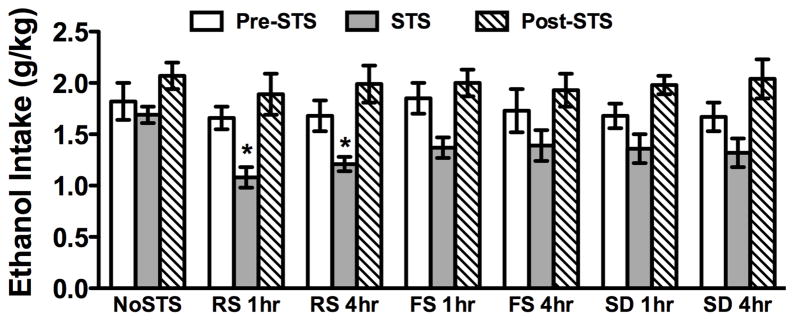
Preliminary analyses of ethanol intake included Stress Group as a between-subjects factor and Week and Days as within-subjects factors. The three-way ANOVA indicated significant main effects of Week [F(2,12) = 72.22; p < 0.001] and Days [F(4,24) = 38.36; p < 0.001], along with an interaction between these factors [F(8,48) = 16.34; p < 0.001]. However, there was no main effect of Stress Group or an interaction between stress experience and these variables (Fig. 1). Data also were averaged over 5 days for the last week of baseline (Pre-STS), the week of stress exposure (STS), and the week following stress exposure (Post-STS). Analysis of these data revealed a significant main effect of Week [F(2,120) = 72.31; p < 0.001], with post hoc comparisons indicating that ethanol intake was significantly lower for all groups during the week of stress exposure compared to before and after stress administration (Fig. 2). Ethanol consumption did not differ across groups either the week prior to or the week following stress exposure. A follow-up one-way ANOVA to compare all stress groups during the stress exposure week indicated a significant effect of Stress Group [F(6,60) = 2.71; p < 0.025], with restraint stress, either 1 or 4 h before drinking, producing a significant decrease in ethanol intake compared to the control NoSTS condition. Forced swim and social defeat stress experience also reduced ethanol consumption relative to the NoSTS group, but these differences were not statistically significant.
Fig. 1.
Ethanol intake (g/kg) for mice that experienced restraint stress (RS), forced swim (FS), social defeat (SD), 1 or 4 h before drinking, or no stress (noSTS) presented as daily intake for the 5 days before (Pre-STS), the 5 days of stress or no stress exposure (STS), and the 5 days following the stress experience (Post-STS). Data are mean ± SEM.
Fig. 2.
Ethanol intake (g/kg) for mice that experienced restraint stress (RS), forced swim (FS), social defeat (SD), 1 or 4 h before drinking, or no stress (noSTS). Data are averaged across 5 days before (Pre-STS), the 5 days of stress or no stress exposure (STS), and the 5 days following the stress experience (Post-STS). Data are mean ± SEM. * indicates a significant difference from NoSTS group.
Experiment 2: Effect of different stressors on voluntary ethanol intake in ethanol-dependent and nondependent mice
Analysis of blood ethanol concentrations (BECs) indicated a significant effect of CIE Cycle [F(3,108) = 19.17; p < 0.001] due to higher BECs during the third cycle of CIE exposure compared to all other exposure cycles (mean ± SEM mg/dL for cycles 1–4: 166.8 ± 5.8, 185.4 ± 10.3, 251.8 ± 5.8, and 176.7 ± 12.4, respectively). However, BECs did not differ between the different stress groups and there was no significant interaction between Stress and CIE exposure cycle.
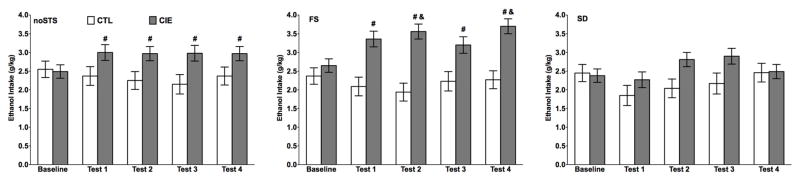
Preliminary analyses of ethanol consumption indicated that there were no differences in intake across days within each phase of the experiment. Therefore, data were collapsed for the last 5 days of baseline and the 5 days of drinking in each test cycle. The overall ANOVA, with Group (CTL, CIE) and Stress (noSTS, FS, and SD) as between-subjects factors and Test Cycle (Baseline, Test 1–4) as a repeated measure indicated a significant main effect of Group [F(1,52) = 26.37, p < 0.0001] and a significant interaction between Group and Test Cycle [F(4,208) = 7.38, p < 0.0001]. Post hoc analyses based on these effects indicated that CIE mice consumed more ethanol during Test Cycles 1–4 compared to their own baseline and CTL mice in the same test cycle. The ANOVA also indicate that the interaction between Group and Stress was near significance [F(2,53) = 2.85, p = 0.06].
Separate ANOVAs including Group (CTL, CIE) and Test Cycle (Baseline, Test Cycles 1–4) were also conducted for each stress procedure independently. Analysis of ethanol intake for mice that did not experience stress indicated a significant main effect of Group [F(1,18) = 8.76, p < 0.01] and a Group × Test Cycle interaction [F(4,72) = 2.68, p < 0.05] (Fig. 3). Post hoc comparisons indicated that while CTL and CIE mice had similar levels of intake during baseline, CIE mice evidenced higher intake levels than CTL mice during all test cycles. The post hoc evaluation also indicated that CTL mice maintained a stable level of intake across baseline and all test cycles while CIE mice consumed more ethanol during all test cycles compared to their baseline level of intake (p values < 0.05). Analysis of intake of mice that experienced FS stress indicated a significant main effect of Group [F(1,17) = 27.71, p < 0.001] and an interaction between Group and Test Cycle [F(4,68) = 5.35, p < 0.001]. Post hoc comparisons indicated that CIE mice consumed higher amounts of ethanol than CTL mice during all testing cycles. Also, CIE mice that experienced FS stress consumed more ethanol during all test cycles compared to their own baseline level of intake. In contrast, FS stress did not affect intake of CTL mice, which maintained a stable level of intake from baseline (Fig. 3). Analysis of ethanol intake for mice that experienced SD stress did not reveal significant effects of Group, Test Cycle, or the interaction between these factors. In this case, exposure to SD stress not only failed to alter drinking in CTL mice, but this stressor also prevented the expected increase in voluntary ethanol intake in mice that experienced CIE exposure (Fig. 3).
Fig. 3.
Ethanol intake (g/kg) for CIE and CTL mice that experienced forced swim (FS), social defeat (SD), or no stress (noSTS) during each test cycle (Test 1–4). Data are averaged for the last 5 days of baseline and the 5 days of each test period. Data are mean ± SEM. # indicates a significant difference from the CTL group in the same test cycle and from their own baseline. & indicates a significant difference from CIE-noSTS group in the same test cycle.
Experiment 3: Effect of forced swim stress on voluntary ethanol intake in ethanol-dependent and nondependent mice
ANOVA of BECs achieved via CIE exposure indicated a significant effect of CIE Cycle [F(3,66) = 12.62; p < 0.001] due to higher BEC values during the third and fourth cycles of CIE exposure compared to the first two cycles of CIE exposure (mean ± SEM mg/dL for cycles 1–4: 179.1 ± 10.8, 203.7 ± 7.6, 238.5 ± 10.4, and 244.5 ± 6.4, respectively). BEC did not differ between the FS and noSTS groups, and there was no significant interaction between Stress and CIE cycle.
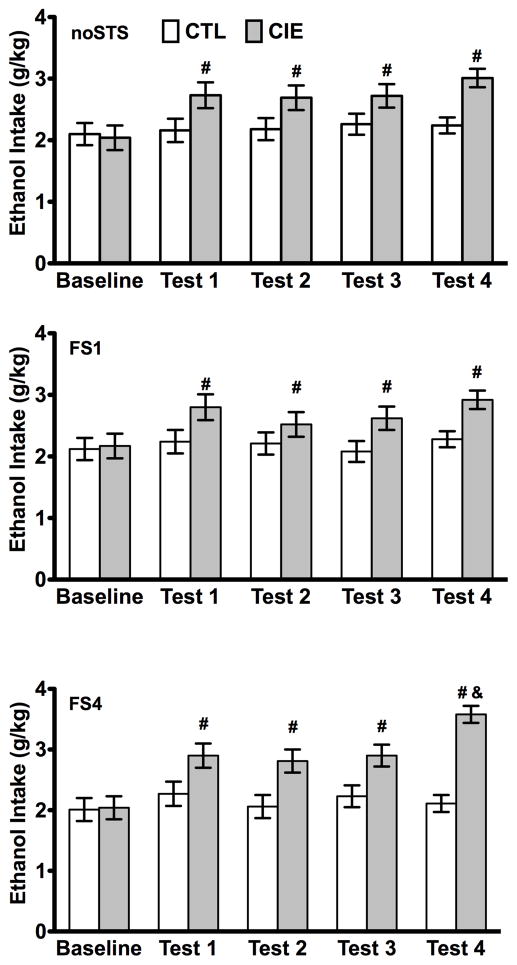
As in previous experiments, preliminary analyses indicated that there were no differences in ethanol intake across days within each phase of the experiment. Therefore, data were collapsed for the last 5 days of baseline and the 5 days of drinking in each test cycle. The overall ANOVA, with Group (CTL, CIE) and Stress (noSTS, FS1, FS4) as between-subjects factors and Test Cycle (Baseline, Test 1–4) as a repeated measure indicated significant main effects of Group [F(1,48) = 28.20, p < 0.001] and Test Cycle [F(4,192) = 13.55, p < 0.001], and a significant Group × Test Cycle interaction [F(4,192) = 7.90, p < 0.001] (Fig. 4). Post hoc analyses indicated that while CTL mice maintained a stable level of intake from baseline through all test cycles, CIE mice consumed more ethanol than CTL mice during the test cycles and significantly more than their own baseline level of intake (p values < 0.05). The three-way interaction between Group, Stress, and Test Cycle was not significant.
Fig. 4.
Ethanol intake (g/kg) for CIE and CTL mice that experienced forced swim stress only during Test 4 (FS1), during every test cycle (FS4), or did not receive stress (noSTS) before drinking. Data are mean ± SEM. # indicates a significant difference from the CTL group in the same test cycle and from their own baseline. & indicates a significant difference from CIE-noSTS group in the same test cycle.
Follow-up analyses conducted separately for each test cycle indicated that, as expected, there was no effect of Group or Stress during Baseline intake. However, for Test Cycles 1, 2, and 3, ANOVAs indicated significant main effects of Group due to significantly higher intake in CIE versus CTL mice [F(1,48) = 12.80, F(1,48) = 11.59, and F(1,48) = 14.75, respectively; all p values < 0.01]. For Test Cycle 4, ANOVA indicated a significant main effect of Group [F(1,48) = 71.25, p < 0.001], as well as a significant Group × Stress interaction [F(2,48) = 5.18, p < 0.01]. Post hoc comparisons indicated that each CIE group consumed more ethanol than its corresponding CTL group within the same stress condition (p values < 0.05). In addition, CIE mice that experienced FS stress during every test cycle (CIE FS4) consumed significantly more ethanol than CIE mice that did not experience stress (CIE noSTS) or those that experienced FS stress only during the last testing cycle (CIE FS1) (p < 0.05) (Fig. 4).
Discussion
It is generally recognized that experience with stressful events may promote increased ethanol intake as well as provoke relapse in abstinent alcoholics. The experiments presented here evaluated the effects of various stressors on voluntary ethanol consumption in dependent and nondependent mice. In general, exposure to various stress procedures did not significantly influence ethanol consumption under limited-access conditions in nondependent mice. In contrast, exposure to some, but not all, stress procedures potentiated escalation of ethanol intake that is typically observed after repeated cycles of CIE. Collectively, these results suggest that a history of chronic ethanol exposure that ordinarily engenders increased ethanol consumption influences the ability of some stressors to further modulate drinking.
Results from Experiment 1 indicated that administration of three different stressors (restraint, forced swim, and social defeat) had little influence on voluntary ethanol intake in nondependent mice. Overall, ethanol intake was lower on days that mice experienced stress, with a significant reduction occurring when mice experienced restraint stress before their daily drinking sessions. In accordance with these findings, previous studies in rats (Chester, Blose, Zweifel, & Froehlich, 2004) and mice (Chester, de Paula Barrenha, DeMaria, & Finegan, 2006) have also reported decreases in ethanol intake following restraint stress. The few studies that reported increases in ethanol consumption after exposure to restraint stress were conducted in rats that experienced chronic stress during early development and had free access to ethanol throughout the day (Ploj, Roman, & Nylander, 2003; Roman, Ploj, & Nylander, 2004). Forced swim and social defeat stressors have previously been shown to produce a delayed effect on ethanol intake (Lowery, Sparrow, Breese, Knapp, & Thiele, 2008; Sillaber et al., 2002). For example, increased ethanol intake was observed several weeks after the experience of social defeat stress (Sillaber et al., 2002). A recent report indicated that mice subjected to a more intense regimen of social defeat show a significant decrease in ethanol intake during the days of stress experience but a significant increase in ethanol consumption the week immediately after the stress exposure was terminated (Norman et al., 2015). This ‘delayed’ or ‘rebound’ effect was not observed in the present experiment with any of the stress procedures examined. Several factors may contribute to the discordant results, including differences in the strain of mice used, intensity of the stress experience, and duration of access to ethanol in the home cage (Becker et al., 2011).
Because the majority of previous studies that evaluated effects of stress on ethanol intake measured consumption under unlimited-access conditions (24-h), it is difficult to determine at what point in time stress-induced changes in ethanol intake occurred, and whether these changes resulted in significant increases or decreases in blood ethanol levels. The present experiment evaluated the effects of stress exposure on ethanol consumption under limited-access conditions, and evaluated whether a relatively short (1-h) or more extended (4-h) delay in access to ethanol following the stress procedure influenced the study outcome. However, even when access to ethanol was delayed beyond the peak physiological effects of the various stressors (Patchev & Patchev, 2006), restraint, forced swim, and social defeat stress procedures all reduced ethanol consumption in nondependent mice. This effect was most robust following restraint stress. Thus, elevated circulating levels of corticosterone and reduced general locomotion cannot explain the decrease in ethanol consumption since these effects resolved when ethanol was made available for consumption 4 h following the stress exposure (Cabib et al., 1988; Hare, Beierle, Toufexis, Hammack, & Falls, 2014).
Results from the second experiment confirmed findings from Experiment 1 in that neither forced swim nor social defeat stress procedures significantly altered ethanol intake in nondependent mice. In contrast, ethanol consumption was significantly altered by stress in CIE-exposed mice, but the effect depended on the stress procedure. That is, forced swim stress further augmented elevated ethanol consumption in CIE-exposed mice while social defeat stress exposure tended to reduce intake in dependent mice. In fact, experience with social defeat stress appeared to block escalation of drinking typically observed in CIE-exposed mice relative to nondependent mice in this model (Becker & Lopez, 2004; Griffin, Lopez, & Becker, 2009; Griffin, Lopez, Yanke, et al., 2009; Lopez & Becker, 2005). Given that forced swim stress did not significantly alter ethanol intake in nondependent mice, results from this experiment suggest that experience with this stressor interacts in a unique manner with CIE exposure to produce further escalation of drinking. At present, it is not known why a similar effect was not observed with a different stress procedure (social defeat stress). To our knowledge, this is the first study to examine the effects of these stress procedures on drinking in this mouse model of dependence. Notably, differences in the effects of forced swim stress versus social defeat stress on voluntary drinking in dependent mice cannot be explained by differences in the level of ethanol intoxication experienced during CIE exposure. All stressed and non-stressed mice experienced the same level of ethanol intoxication (BECs) during repeated cycles of CIE exposure.
Results from Experiment 3 confirmed the finding that repeated exposure to forced swim stress during intervening weeks of CIE exposure resulted in a further elevation of ethanol intake in CIE-exposed mice without influencing drinking in nondependent mice. Furthermore, this effect was only observed when forced swim stress was administered after each CIE exposure cycle (prior to drinking sessions during each test cycle). In contrast, mice that experienced forced swim stress only after the last CIE exposure cycle did not exhibit enhanced drinking beyond the level produced by CIE exposure alone. This suggests that experience with forced swim stress during early test periods following repeated CIE exposure cycles may facilitate the development of escalated drinking in this dependence model. Of note, forced swim stress experience did not significantly alter ethanol consumption in the nondependent (air-exposed) mice. In contrast, forced swim stress resulted in a 0.5–0.75 g/kg increase in consumption over the amount consumed by CIE-exposed mice that did not receive the stress exposure (Experiments 2 and 3). Although blood ethanol levels were not measured in the present studies, our previous work indicates that this increased amount of ethanol intake (consumed within 2 h) results in significantly elevated blood and brain ethanol levels (Griffin, Lopez, Yanke, et al., 2009; Becker & Lopez, 2004). Thus, this stress-induced enhancement of drinking in mice with a history of CIE exposure is of physiological relevance and likely represents an important contributing factor in the addiction process.
It is possible that stress associated with repeated cycles of CIE exposure may be a significant factor that contributes to escalated drinking in dependent animals (Vendruscolo et al., 2012). Indeed, the experience of repeated bouts of chronic ethanol exposure and withdrawal is known to serve as a potent stressor (Becker, 2008; Koob & Le Moal, 2008) that, in addition, alters subsequent stress responsiveness (Gehlert et al., 2007; Sommer et al., 2008). In this vein, it may be that exposure to forced swim stress amplifies the contribution of stress factors that impact motivation to drink. Similar to results presented here, a previous study indicated that forced swim stress induced a significant increase in ethanol consumption, but only in rats that experienced chronic ethanol inhalation exposure (Sommer et al., 2008). This study also suggested that this effect of enhanced drinking in dependent rats might be linked to changes in CRF signaling in the amygdala (Sommer et al., 2008).
It is unclear why forced swim stress appears particularly effective in further enhancing ethanol consumption in dependent mice. It is interesting that the nature of the forced swim procedure allows for evaluation of the behavioral response to this stress challenge, representing an opportunity to further explore mechanisms underlying the differential effect of forced swim stress on voluntary ethanol intake in ethanol-dependent compared to nondependent mice. We recently observed that CIE-exposed C57BL/6J mice exhibit an altered behavioral coping response to forced swim stress (Doremus-Fitzwater & Becker, 2010). More specifically, compared to nondependent mice, dependent mice displayed reduced immobility (increased struggling behavior) in the face of this inescapable stress situation when tested 3 days or 1 week after a final cycle of CIE exposure (Doremus-Fitzwater & Becker, 2010). While it is unclear whether this altered stress response is related to ethanol drinking, the relationship between coping behavior during the forced swim challenge and ethanol intake has been examined in other studies. For instance, rats selectively bred for high susceptibility to forced swim (i.e., high immobility) have been shown to drink more ethanol under non-stress situations (Bertholomey et al., 2011). However, P rats (selectively bred for high ethanol preference) demonstrate less immobility than non-preferring (NP) rats in the forced swim test, indicating that there is no simple genetic link between the two behaviors (Bertholomey et al., 2011; Godfrey, Froehlich, Stewart, Li, & Murphy, 1997; Viglinskaya et al., 1995). Another study reported that rats that were chronically exposed to ethanol displayed greater immobility during forced swim stress (Walker et al., 2010). Thus, it is difficult to predict whether altered behavioral coping response to this stressor relates to its ability to modulate ethanol consumption.
Interestingly, we also recently found that the ability of CIE exposure to increase struggling behavior in the forced swim procedure could be recapitulated in nondependent mice following central administration (intracerebroventricular) of CRF in a dose-related manner (unpublished data). Thus, activation of CRF within brain stress circuitry resulting from repeated forced swim stress challenge may be an important contributing factor in enhancing ethanol consumption in animals with a history of CIE exposure. This is in agreement with a large body of literature that indicates chronic ethanol exposure engages the CRF system in extrahypothalamic circuitry to increase motivation to consume ethanol (Becker, 2012; Heilig & Koob, 2007; Koob, 2014). In this vein, congruent with the findings of others using rat (Funk, Zorrilla, Lee, Rice, & Koob, 2007; Gehlert et al., 2007; Gilpin et al., 2008; Sommer et al., 2008) and mouse (Chu, Koob, Cole, Zorrilla, & Roberts, 2007) models of dependence, our laboratory recently found a CRF1 receptor antagonist to block escalation of drinking in CIE-exposed mice without altering intake in nondependent mice (unpublished data). Collectively, these findings suggest that engagement of CRF activity following combined forced swim stress and CIE exposure may mediate further escalation of drinking. The reason why such an effect was not observed with another stressor (social defeat) is unclear at present and will require further investigation.
In sum, results from the present set of experiments indicate that while a number of different stressors tend to reduce ethanol consumption in nondependent mice, repeated exposure to forced swim stress in the context of dependence further enhances escalation of drinking ordinarily observed following repeated cycles of CIE exposure. This effect of forced swim stress to selectively increase drinking in mice with a history of CIE exposure was not observed when a different stressor (repeated social defeat) was used. Future studies will need to further explore this apparent selective effect of forced swim stress in this CIE model of dependence and relapse drinking, as well as examine mechanisms underlying the ability of certain stressors to further augment excessive levels of ethanol intake associated with dependence.
Highlights.
Restraint, forced swim, and social defeat stress reduced ethanol intake in nondependent mice.
Chronic intermittent ethanol (CIE) exposure increased voluntary ethanol intake.
Social defeat stress reduced ethanol intake in CIE-exposed mice.
Forced swim stress enhanced drinking in CIE-exposed mice.
Acknowledgments
Supported by NIAAA grants U01 AA020929 (MFL), U01 AA014095 (HCB), P50 AA010761 (HCB), T32 AA007474 (RIA), F32 AA023700 (RIA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adinoff B, Anton R, Linnoila M, Guidotti A, Nemeroff CB, Bissette G. Cerebrospinal fluid concentrations of corticotropin-releasing hormone (CRH) and diazepam-binding inhibitor (DBI) during alcohol withdrawal and abstinence. Neuropsychopharmacology. 1996;15:288–295. doi: 10.1016/0893-133X(95)00212-V. [DOI] [PubMed] [Google Scholar]
- Becker HC. Alcohol dependence, withdrawal and relapse. Alcohol Research & Health. 2008;31:348–361. [PMC free article] [PubMed] [Google Scholar]
- Becker HC. Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Research. 2012;34:448–458. [PMC free article] [PubMed] [Google Scholar]
- Becker HC. Animal models of excessive alcohol consumption in rodents. Current Topics in Behavioral Neurosciences. 2013;13:355–377. doi: 10.1007/7854_2012_203. [DOI] [PubMed] [Google Scholar]
- Becker HC. Alcohol Dependence, Withdrawal, and Relapse. In: Noronha A, Cui C, Harris RA, Crabbe JC, editors. Neurobiology of Alcohol Dependence. Waltham, MA: Academic Press (Elsevier); 2014. pp. 377–410. [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcoholism: Clinical and Experimental Research. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl) 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholomey ML, West CH, Jensen ML, Li TK, Stewart RB, Weiss JM, et al. Genetic propensities to increase ethanol intake in response to stress: studies with selectively bred swim test susceptible (SUS), alcohol-preferring (P), and non-preferring (NP) lines of rats. Psychopharmacology (Berl) 2011;218:157–167. doi: 10.1007/s00213-011-2381-6. [DOI] [PubMed] [Google Scholar]
- Brady KT, Sonne SC. The role of stress in alcohol use, alcoholism treatment, and relapse. Alcohol Research & Health. 1999;23:263–271. [PMC free article] [PubMed] [Google Scholar]
- Brown G, Jackson A, Stephens DN. Effects of repeated withdrawal from chronic ethanol on oral self-administration of ethanol on a progressive ratio schedule. Behavioural Pharmacology. 1998;9:149–161. [PubMed] [Google Scholar]
- Cabib S, Kempf E, Schleef C, Mele A, Puglisi-Allegra S. Different effects of acute and chronic stress on two dopamine-mediated behaviors in the mouse. Physiology & Behavior. 1988;43:223–227. doi: 10.1016/0031-9384(88)90242-9. [DOI] [PubMed] [Google Scholar]
- Cappell H, Greeley J. Alcohol and tension reduction: An update on research and theory. In: Blane HT, Leonard KE, editors. Psychological Theories of drinking and alcoholism. New York: Guilford Press; 1987. pp. 15–54. [Google Scholar]
- Chester JA, Blose AM, Zweifel M, Froehlich JC. Effects of stress on alcohol consumption in rats selectively bred for high or low alcohol drinking. Alcoholism: Clinical and Experimental Research. 2004;28:385–393. doi: 10.1097/01.alc.0000117830.54371.7a. [DOI] [PubMed] [Google Scholar]
- Chester JA, de Paula Barrenha G, DeMaria A, Finegan A. Different effects of stress on alcohol drinking behaviour in male and female mice selectively bred for high alcohol preference. Alcohol and Alcoholism. 2006;41:44–53. doi: 10.1093/alcalc/agh242. [DOI] [PubMed] [Google Scholar]
- Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacology, Biochemistry, and Behavior. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Bono G, Martignoni E, Merlo P, Sances G, Nappi G. An assessment of hypothalamo-pituitary-adrenal axis functioning in non-depressed, early abstinent alcoholics. Psychoneuroendocrinology. 1996;21:263–275. doi: 10.1016/0306-4530(96)00001-7. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Becker HC. Effects of ethanol dependence on ethanol intake and behavior in the forced swim test in male C57BL/6J mice. Alcoholism: Clinical and Experimental Research. 2010;34:200A. [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, et al. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcoholism: Clinical and Experimental Research. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biological Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Lê AD, Hipskind PA, Hamdouchi C, et al. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. The Journal of Neuroscience. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Lumeng L, Koob GF. Dependence-induced alcohol drinking by alcohol-preferring (P) rats and outbred Wistar rats. Alcoholism: Clinical and Experimental Research. 2008;32:1688–1696. doi: 10.1111/j.1530-0277.2008.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, Richardson HN. Operant behavior and alcohol levels in blood and brain of alcohol-dependent rats. Alcoholism: Clinical and Experimental Research. 2009;33:2113–2123. doi: 10.1111/j.1530-0277.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey CD, Froehlich JC, Stewart RB, Li TK, Murphy JM. Comparison of rats selectively bred for high and low ethanol intake in a forced-swim-test model of depression: effects of desipramine. Physiology & Behavior. 1997;62:729–733. doi: 10.1016/s0031-9384(97)00171-6. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcoholism: Clinical and Experimental Research. 2009;33:1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl) 2009;201:569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare BD, Beierle JA, Toufexis DJ, Hammack SE, Falls WA. Exercise-associated changes in the corticosterone response to acute restraint stress: evidence for increased adrenal sensitivity and reduced corticosterone response duration. Neuropsychopharmacology. 2014;39:1262–1269. doi: 10.1038/npp.2013.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addiction Biology. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends in Neurosciences. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcoholism: Clinical and Experimental Research. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurocircuitry of alcohol addiction: synthesis from animal models. Handbook of Clinical Neurology. 2014;125:33–54. doi: 10.1016/B978-0-444-62619-6.00003-3. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annual Review of Psychology. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Lê AD, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacology & Therapeutics. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Lê AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Lê AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology. 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. The Journal of Neuroscience. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcoholism: Clinical & Experimental Research. 2000;24:651–658. [PubMed] [Google Scholar]
- Lowery EG, Sparrow AM, Breese GR, Knapp DJ, Thiele TE. The CRF-1 receptor antagonist, CP-154,526, attenuates stress-induced increases in ethanol consumption by BALB/cJ mice. Alcoholism: Clinical and Experimental Research. 2008;32:240–248. doi: 10.1111/j.1530-0277.2007.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi C, Chiodera P, Ampollini P, Volpi R, Coiro V. Beta-endorphin, adrenocorticotropic hormone and cortisol secretion in abstinent alcoholics. Psychiatry Research. 1997;72:187–194. doi: 10.1016/s0165-1781(97)00101-7. [DOI] [PubMed] [Google Scholar]
- Miczek KA. Tolerance to the analgesic, but not discriminative stimulus effects of morphine after brief social defeat in rats. Psychopharmacology (Berl) 1991;104:181–186. doi: 10.1007/BF02244176. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Thompson ML, Shuster L. Opioid-like analgesia in defeated mice. Science. 1982;215:1520–1522. doi: 10.1126/science.7199758. [DOI] [PubMed] [Google Scholar]
- Norman KJ, Seiden JA, Klickstein JA, Han X, Hwa LS, DeBold JF, et al. Social stress and escalated drug self-administration in mice I. Alcohol and corticosterone. Psychopharmacology (Berl) 2015;232:991–1001. doi: 10.1007/s00213-014-3733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcoholism: Clinical and Experimental Research. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Patchev AV. Experimental models of stress. Dialogues in Clinical Neurosciences. 2006;8:417–432. doi: 10.31887/DCNS.2006.8.4/vpatchev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploj K, Roman E, Nylander I. Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neuroscience. 2003;121:787–799. doi: 10.1016/s0306-4522(03)00499-8. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Interaction of ethanol and stress: research with experimental animals--an update. Alcohol and Alcoholism. 1990;25:263–276. doi: 10.1093/oxfordjournals.alcalc.a045000. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Stress and alcohol interaction: an update of human research. Alcoholism: Clinical & Experimental Research. 1991;15:438–459. doi: 10.1111/j.1530-0277.1991.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcoholism: Clinical and Experimental Research. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Roman E, Ploj K, Nylander I. Maternal separation has no effect on voluntary ethanol intake in female Wistar rats. Alcohol. 2004;33:31–39. doi: 10.1016/j.alcohol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Sayette MA. Does drinking reduce stress? Alcohol Research & Health. 1999;23:250–255. [PMC free article] [PubMed] [Google Scholar]
- Sillaber I, Henniger MS. Stress and alcohol drinking. Annals of Medicine. 2004;36:596–605. doi: 10.1080/07853890410018862. [DOI] [PubMed] [Google Scholar]
- Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgänsberger W, Wurst W, et al. Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science. 2002;296:931–933. doi: 10.1126/science.1069836. [DOI] [PubMed] [Google Scholar]
- Sinha R, O’Malley SS. Craving for alcohol: findings from the clinic and the laboratory. Alcohol and Alcoholism. 1999;34:223–230. doi: 10.1093/alcalc/34.2.223. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, et al. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biological Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Noori HR, Heilig M. Stress and alcohol interactions: animal studies and clinical significance. Trends in Neurosciences. 2014;37:219–227. doi: 10.1016/j.tins.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Uhart M, Wand GS. Stress, alcohol and drug interaction: an update of human research. Addiction Biology. 2009;14:43–64. doi: 10.1111/j.1369-1600.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, et al. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcoholism: Clinical and Experimental Research. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. The Journal of Neuroscience. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viglinskaya IV, Overstreet DH, Kashevskaya OP, Badishtov BA, Kampov-Polevoy AB, Seredenin SB, et al. To drink or not to drink: tests of anxiety and immobility in alcohol-preferring and alcohol-nonpreferring rat strains. Physiology & Behavior. 1995;57:937–941. doi: 10.1016/0031-9384(94)00368-f. [DOI] [PubMed] [Google Scholar]
- Walker BM, Drimmer DA, Walker JL, Liu T, Mathé AA, Ehlers CL. Effects of prolonged ethanol vapor exposure on forced swim behavior, and neuropeptide Y and corticotropin-releasing factor levels in rat brains. Alcohol. 2010;44:487–493. doi: 10.1016/j.alcohol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]