Abstract
Fetal Alcohol Spectrum Disorders (FASD) are characterized by deficits in working memory, response inhibition, and behavioral flexibility. However, the combination and severity of impairments are highly dependent upon maternal ethanol consumption patterns, which creates a complex variety of manifestations. Rodent models have been essential in identifying behavioral endpoints of prenatal alcohol exposure (PAE). However, experimental model outcomes are extremely diverse based on level, pattern, timing, and method of ethanol exposure, as well as the behavioral domain assayed and paradigm used. Therefore, comparisons across studies are difficult and there is currently no clear comprehensive behavioral phenotype of PAE. This lack of defined cognitive and behavioral phenotype is a contributing factor to the difficulty in identifying FASD individuals. The current review aims to critically examine preclinical behavioral outcomes in the social, cognitive, and affective domains in terms of the PAE paradigm, with a special emphasis on dose, timing, and delivery, to establish a working model of behavioral impairment. In addition, this review identifies gaps in our current knowledge and proposes future areas of research that will advance knowledge in the field of PAE outcomes. Understanding the complex behavioral phenotype, which results from diverse ethanol consumption will allow for development of better diagnostic tools and more critical evaluation of potential treatments for FASD.
Keywords: executive function, social behavior, spatial and temporal memory, anxiety and motor learning, preclinical models
Introduction
Beginning with the first reports that prenatal alcohol exposure (PAE) could have severe and long-lasting consequences on the neurobehavior of offspring (Jones, 1975; Jones & Smith, 1973), there has been a consistent focus on identifying how alcohol affects the developing fetus and delineating the spectrum of behavioral changes. Although there is an increasing awareness that high levels of alcohol consumption during pregnancy can impair growth, cognition, and social behavior of the child, PAE remains one of the most common developmental insults (Day et al., 2002; Green et al., 2009; Thomas, Kelly, Mattson, & Riley, 1998). Recent reports suggest that as many as one-third of women drink at some time during pregnancy, and between 5–10% report binge drinking incidents (Ethen et al., 2009). There is a growing consensus that even moderate alcohol intake during pregnancy, which is the more common pattern, can lead to lasting cognitive impairments even when growth and morphological changes are absent. These impairments, which fall under the category of Fetal Alcohol Spectrum Disorder (FASD), may not be evident until early adolescence and are characterized by impairments in working memory, response inhibition, and behavioral flexibility (Green et al., 2009; Mattson, Goodman, Caine, Delis, & Riley, 1999; Streissguth et al., 1991).
Rodent models have become an important tool for studying the effects of alcohol on development at all levels, particularly as studies in human patients and rodent models suggest a congruent effect of blood alcohol concentration (BAC) on behavioral outcomes across species (Driscoll, Streissguth, & Riley, 1990). Rodent models are an indispensable tool for studying causal factors of prenatal exposure effects, for several reasons: 1) inbred strains of mice and rats are genetically homogenous populations, with which the issue of genetic heterogeneity in epidemiological studies can be largely circumvented, making it more feasible to parcel out impacts of environmental factors; 2) environmental insults, such as alcohol, can be strictly timed and given in exact quantities, enabling the discoveries of sensitive time windows and threshold of harmful doses; 3) the impacts of environmental factors can be tested on the behavioral level, as well as on neuroanatomical, neurochemical, and neuronal levels, enabling the discovery of the effects of certain environmental insults on the whole organism; and 4) cross-species comparisons ensure that results are applicable and generalizable in different species. These studies have identified several factors that are involved in the impact of PAE including method of delivery, level of exposure, pattern of exposure, and timing of exposure during development.
Here we review the available literature on the behavioral impacts of PAE in rodent models, from early studies to the most recently published, with the goal of providing a comprehensive behavioral phenotype spanning the social, affective, and cognitive domains (Fig. 1). Although alcohol was recognized as a teratogen in 1973, it was not until 2002 that the CDC began developing diagnostic guidelines for FAS and FASD (Williams, Smith, Committee on Substance Abuse, 2015). However, in 2015, in a cohort of foster children who were referenced to a mental health clinic for behavioral disorders, 86.7% were either previously undiagnosed or misdiagnosed (Chasnoff, Wells, & King, 2015). FASD is commonly misdiagnosed with other conditions such as attention deficit hyperactivity disorder (ADHD) or Autism Spectrum Disorder, because behavioral profiles can be similar (Bishop, Gahagan, & Lord, 2007; Peadon & Elliott, 2010). However, it should be noted that ADHD has been estimated to be co-morbid with FASD in up to 94% of individuals (Peadon & Elliott, 2010). Being able to fully characterize the FASD behavioral and cognitive phenotype based on approximate exposure level and timing would greatly improve diagnosis and therefore treatment, particularly early intervention. Therefore, this review seeks to highlight areas in which more concentrated research using rodent models is needed to fill in the missing framework.
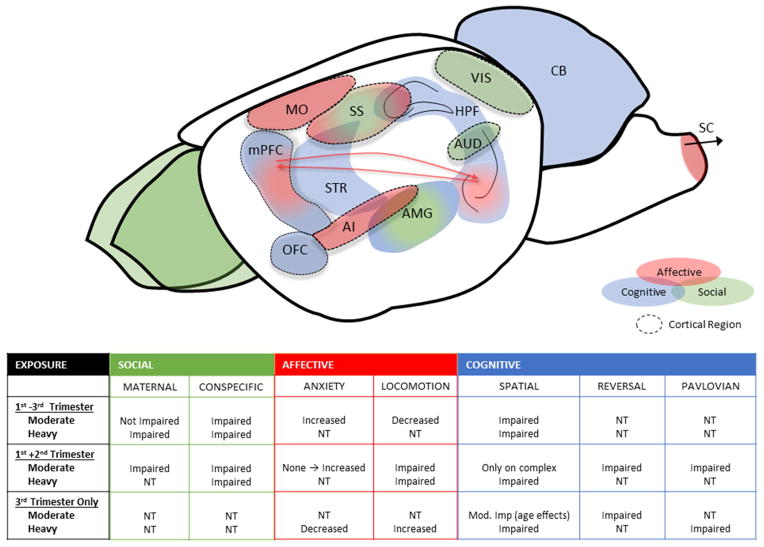
Fig. 1. Regions of interest for behavioral domains and summary of behavioral effects in rodent models of PAE.
Primary areas of the rodent brain that have been implicated in social, affective, and cognitive behaviors across species are reviewed in Figs. 2–7. These regions may make unique targets for PAE, especially regions implicated in more than one class of behaviors (indicated by double-color shading). The tables summarize the overall conclusion of the effect of PAE on behavioral domains reviewed, based on the three most prominent exposure timelines utilized. NT = not tested, MO = primary motor area, SS = somatosensory area, VIS = primary visual cortex, AUD = auditory cortex, AI = agranular insular cortex, OFC = orbital frontal cortex, mPFC = medial prefrontal cortex, SC = spinal cord, CB = cerebellum, HPF = hippocampal formation, STR = striatum, AMG = amygdala.
Timing, dose, and delivery
Rodent studies have aimed to replicate prenatal exposures in humans using a variety of delivery methods including intraperitoneal (i.p.) injection, oral gavage, intragastric gavage, liquid diets, voluntary drinking, limited-access models, and vaporized alcohol inhalation (for a comprehensive review see Patten, Fontaine, & Christie, 2014). These techniques have been used to produce a wide range of doses as measured by Blood Alcohol Concentration (BAC). The question of what is considered heavy versus moderate exposure in humans and rodents is still not widely agreed upon, due to differences in alcohol metabolism, and the effects that pattern of exposure, coupled with delivery method, have on BAC (for a full discussion, see Valenzuela, Morton, Diaz, & Topper, 2012). For example, in rodents, BACs at the U.S. legal intoxicating limit (80 mg/dL) are considered low exposure; however, in clinical research this is a moderate to high dose, if it occurs repeatedly. To make conclusions drawn in this review more easily comparable to clinical literature, we have broadly categorized the reviewed studies into heavy exposure (>100 mg/dL) and more moderate exposures (<100 mg/dL). However, it should be stressed that studies categorized as moderate in the current review, often result in BACs at or below 80 mg/dL. Therefore, specific BACs are reported in figures, where available.
Finally, these methods and levels of exposure have been delivered across gestation and early neonatal development in various regimens. Mammalian neural development can be split into six distinct steps: neural genesis, migration, glial proliferation, axon/dendrite proliferation, synaptogenesis, pruning/cell death, and myelination (West, 1987). Developmental time points across species are equated using these processes. For humans, the first trimester corresponds to gastrulation, the second to proliferation and migration, and the third to brain growth spurt and differentiation. Pruning and myelination occur after birth and throughout development. To ease comparisons between studies and species, the experiments discussed will be characterized by the equivalent time points in rodent gestational days (GD) or postnatal days (PD). This will broadly divide exposure windows into first-trimester (GD1–10), second-trimester (GD11–21), or third-trimester (PD0–12) equivalents (specific details regarding method and timing are given in figures). The third-trimester equivalent poses unique challenges to the PAE field, as it occurs postnatally. The largest difference is that pups are exposed directly to alcohol and not first-pass metabolites, as would occur during gestational exposure. Even with these differences, the third-trimester equivalent cannot be discounted in rodent models because human mothers continue to ingest alcohol during this developmental time window (Ethen et al., 2009).
Specific effects should, therefore, be seen based on the timing of PAE insult. For example, exposure during proliferation would result in an abnormal number of neurons, exposure during migration would result in differential placement, while disrupted axonogenesis would result in aberrant synapses and possibly differential signaling. However, most PAE models span several days, or weeks, of development when multiple developmental processes are occurring simultaneously. This means that numerous circuits and regions may be affected by a single model.
While these models have been developed to try to closely model drinking patterns in humans, this design makes it difficult to parse how a diffuse alcohol exposure results in a specific behavioral outcome. Further, the method of alcohol administration becomes increasingly important, as an administration by i.p. injection or gavage will result in rapid peak BACs and may cause a different effect on the neuronal circuitry, and therefore behavior, compared to a method yielding a lower BAC over a prolonged time course. Therefore, we take special consideration when reviewing studies using varying exposure paradigms. It should also be noted that even if the alcohol exposure does not occur during the peak of a region’s development, this does not mean the region will be unaffected. For example, the bulk of hippocampal dentate gyrus development occurs postnatally (Bayer, Altman, Russo, & Zhang, 1993); however, gestational exposures affect behaviors mediated by this region (see below). This may mean connecting regions may be affected, which may influence the region of interest itself.
For these reasons, determining the causes of regional dysfunction after PAE continues to be a challenge. Complicating this picture, PAE is a global insult, affecting multiple systems developing at the time of insult. Therefore, in this review we do not examine the structural changes that have been investigated after PAE. While this is a key in determining how PAE affects the system, results are difficult to interpret without a clear behavioral outcome, which we develop here.
Effects on social behavior
One of the most well-studied outcomes of PAE in preclinical models is the alterations in a wide range of social behaviors. Due to the high level of control and ability to monitor across lifespan, animal models of PAE create a window to the complex mechanisms mediating social interactions, both between pups and dams and between conspecifics in adolescence and adulthood.
Maternal interaction has been studied from both the dams’ and the pups’ perspectives. Pups have limited behavioral repertoires, so studies focus on ultrasonic vocalizations (USV). On the other hand, dams’ behaviors are measured based on positive and negative interaction with her pups or nursing behaviors. In particular, arched-back nursing is a strong focus as it is thought to be the most active nursing posture, and therefore, the most nurturing. Measures of conspecific interactions are more straightforward. The majority of the studies reviewed look at play behaviors between littermates, or cross-fostered pups, with several studies particularly focusing on aggressive play behaviors such as wrestling and biting, which are considered negative interactions. In addition, avoidance and approach behaviors have also been utilized as measures of willingness to interact socially, while social learning using recognition of a familiar conspecific has also been studied. Finally, only a few studies have looked at mating behaviors and preference for same-sex conspecifics.
Regions of interest
The presented behavioral results pose interesting questions about the effects of PAE on the brain regions thought to mediate social behaviors. While fronto-cortical regions are implicated in social interaction behaviors, the sensory systems, somatosensory area, and amygdala appear to be the most influential regions (Baron-Cohen et al., 1999). Lesion studies give us insights into which regions are important in particular behaviors and which ones may be affected by PAE. Lesions of either the parietal cortex (including the somatosensory cortex) or the amygdala decrease play behavior in adulthood, with lesions earlier in the neonatal period producing longer lasting effects (Daenen, Wolterink, Gerrits, & Van Ree, 2002; Panksepp, Normansell, Cox, & Siviy, 1994). In addition, lesion of the amygdala alter adult social interactions such as anogenital sniffing and approach behaviors (Diergaarde, Gerrits, Stuy, Spruijt, & van Ree, 2004). Decreased play behaviors are congruent with high-dose PAE, but not more moderate doses (reviewed below), suggesting both alcohol dose and withdrawal effects likely have different effects on the social brain structures. In addition, sexually dimorphic social-learning results suggest that PAE acts differently on brain regions between sexes, possibly affecting the female somatosensory cortex and male hippocampal memory functions.
Maternal-pup interactions
With any developmental exposure there is concern for altered maternal interactions, due to effects of exposure on either the dam or pup. Literature on the most severe human form of PAE (fetal alcohol syndrome [FAS]) reports disrupted sleep, nursing, and feeding, as well as increases in irritability in infants, which may lead to altered maternal interactions (Martin, Martin, Streissguth, & Lund, 1979; Ouellette, Rosett, Rosman, & Weiner, 1977). Importantly, outcomes are more negative in offspring if proper maternal care is not received during critical perinatal periods (Gershon, Sudheimer, Tirouvanziam, Williams, & O’Hara, 2013; Kim, McHale, Wayne Osgood, & Crouter, 2006). For example, deficits in reversal learning after PAE, which are discussed in the cognitive section, can be rescued by handling pups during the perinatal period, demonstrating the importance of early life environment in influencing effects of a PAE insult (Lee & Rabe, 1999). Therefore, studying maternal-infant interactions is critically important, but human studies are often complicated by co-drug exposures and other uncontrollable factors. Rodent models, therefore, are an important model for the study of social changes after PAE. Outcomes of maternal-pup interactions can be measured from two perspectives: the pup’s behavior and the dam’s behavior.
Overall, the literature demonstrates that dose and timing of PAE greatly influences outcome on pup behavior (Fig. 2). For example, vocalization of distress, as measured by USV after separation from a dam, a common way to measure pup behavior, is differentially affected depending on these factors. While a three-trimester high-dose exposure has been shown to increase USV, a moderate dose over the same timespan did not (Marino, Cronise, Lugo, & Kelly, 2002; Ness & Franchina, 1990). Interestingly, a moderate first- and second-trimester exposure actually decreased USV (Wellmann, George, Brnouti, & Mooney, 2015). USV changes appear to be dependent upon dose as well as timing of exposure. Furthermore, a high-dose three-trimester exposure altered female pups’ ability to suckle (Barron, Kelly, & Riley, 1991). Combined alterations of vocalizations and feeding behaviors may be a contributing factor to low birth weights and growth rates seen in some studies. Together, these studies suggest that both high and moderate doses can affect pup behavior, but outcome depends on timing of exposure. This may be due to the timing of development of the regions involved in social interactions related to peak exposure.
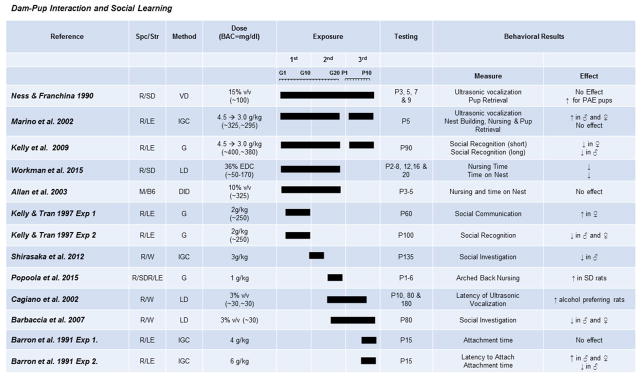
Fig. 2. The effects of prenatal exposure on dam-pup interactions and social learning.
Results of studies focused on maternal and neonatal behavior and learning of social stimuli are organized by exposure times (solid bars indicate exposure duration and timing). Species/Strains are indicated by R = rat, M = mouse, SD = Sprague-Dawley, LE = Long-Evans, W = Wistar, B6 = C57BL/6J. Methods include VD = voluntary drinking, IGC = intragastric cannula, G = oral gavage, LD = liquid diet, DID = drinking-in-the-dark (i.e., limited-access voluntary drinking). Dose indicates concentration of ethanol given with blood alcohol concentration (BAC) in parentheses in mg/dL when reported.
Maternal behavior appears to be most influenced by the intoxication level of the dam. Maternal care was unaltered to cross-fostered control or high three-trimester exposed pups, under a daily gavage paradigm, even with increased PAE pup USV (Marino et al., 2002). Intriguingly, however, in a liquid-diet paradigm, where both natural and cross-fostered dams drank throughout lactation, dams preferred to retrieve isolated control pups first (Ness & Franchina, 1990). These results suggest that intoxication of the dam, not altered pup behavior, appears to be the more influential variable in altered maternal care. The majority of studies confirm this hypothesis and do not show alterations in positive maternal care measures (e.g., licking, grooming, and retrieval) (Allan, Chynoweth, Tyler, & Caldwell, 2003; Popoola, Borrow, Sanders, Nizhnikov, & Cameron, 2015). Only one study found an increased proportion of dams engaging in negative pup-care behaviors (e.g., dragging pups). However, there were no alterations in positive care measures in this study (Workman, Raineki, Weinberg, & Galea, 2015). However, the increased observance of negative pup-care behaviors may be due to the extended observation time of the dam-pup interactions in this study, as negative care behaviors are rare occurrences and shorter observation times, like those in other studies, may not have been sufficient to observe any. The one maternal behavior that does seem to be affected by alcohol ingestion, but not necessarily alcohol presence, is active nursing. No studies were found that utilized a high-dose exposure, but moderate gestational exposures decreased time spent in nursing postures, with a higher BAC and longer exposure reducing the time more significantly (Popoola et al., 2015, Workman et al., 2015). In conclusion, studies suggest that, while nursing behaviors may be impaired by alcohol ingestion, maternal-pup interactions are not significantly affected unless alcohol is present in the system. This may be extendable to the clinical population where mothers continue to drink postpartum. Further studies will need to be done in rodents to identify long-term consequences of decreased care by intoxicated dams.
Conspecific interactions
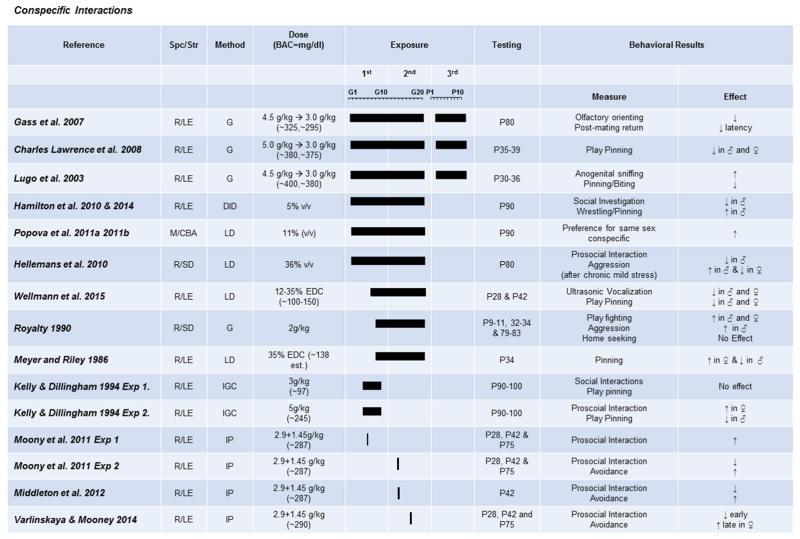
Many studies have looked at the effects of PAE on social interaction and play behaviors in adolescent and adult rodents (Fig. 3). The majority of rodent studies show that aggressive play is decreased in adolescent and adult males across PAE exposures (Charles Lawrence, Cale Bonner, Newson, & Kelly, 2008; Kelly & Dillingham, 1994; Lugo, Marino, Cronise, & Kelly, 2003; Meyer & Riley, 1986; Wellmann et al., 2015). However, some studies have shown increases in wrestling behaviors in males (Hamilton et al., 2010, 2014; Royalty, 1990). The reason for the different outcome is difficult to determine as similar paradigms, BAC, and age at testing have shown conflicting results. However, length of withdrawal period may be a critical factor in these effects. Studies showing decreases in aggressive play behaviors utilized either high-dose gavage or moderate liquid-diet paradigms, which may result in short withdrawal periods and more stable BACs, respectively. However, in the two studies showing increased aggressive play behaviors, one utilized a 4-h voluntary drinking-in-the-dark paradigm and the other a lower dose gavage (2 g/kg/day compared to 3–5 g/kg/day) (Charles Lawrence et al., 2008; Royalty, 1990). In these paradigms, the withdrawal periods are most likely much longer. Thus, alterations in aggressive play behaviors in males may be a combined result of alcohol exposure and withdrawal. Currently, there are no studies looking at the effects of repetitive withdrawal exposures during PAE on play behaviors. Therefore, this hypothesis makes for an intriguing future direction.
Fig. 3. The effects of prenatal exposure on conspecific behaviors.
Results of studies focused on conspecific interactions and sexual behavior are organized by exposure time (solid bars indicate exposure duration and timing) and dose. Species/Strains are indicated by R = rat, M = mouse, SD = Sprague-Dawley, LE = Long-Evans, W = Wistar, B6 = C57BL/6J. Methods include VD = voluntary drinking, IGC = intragastric cannula, G = oral gavage, LD = liquid diet, DID = drinking-in-the-dark (i.e., limited-access voluntary drinking). Dose indicates concentration of ethanol given with blood alcohol concentration (BAC) in parentheses in mg/dL when reported.
Interestingly, effects of PAE on female social behavior appear to be more varied. Studies show both increases and decreases in aggressive behaviors, using similar exposures and age of testing (Meyer & Riley, 1986; Royalty, 1990; Wellmann et al., 2015). Differences may be due to estrous cycle of the female at time of testing; however, none of the studies discussed here reported this variable. These studies clearly highlight the need to study both males and females due to sexually dimorphic effects on behavior as well as the need to carefully examine estrous cycle in behavioral experiments. Acute, heavy alcohol exposures further emphasize the differences in effects of PAE timing between sexes on social interaction. Single alcohol exposures on either GD7 or GD12 resulted in impairments in social motivation, social investigation, and decreased aggressive interactions most strongly in males and weakly, if at all, in females, while the same acute dose on GD15 resulted in impairments in social investigation during early adolescence (PD28) and decreased social motivation in females as age at testing increased (PD42–75) (Middleton, Varlinskaya, & Mooney, 2012; Mooney & Varlinskaya, 2011). Furthermore, a very short PAE during the first trimester (GD4–9) increased female social behavior but decreased male social behavior during adulthood (Kelly & Dillingham, 1994). These results clearly show sexually dimorphic interactions of PAE timing on social interaction deficits, as well as the ability of binge-like exposures to alter adult behavior, providing important insight for future clinical studies.
In addition to aggressive behaviors, PAE is also able to disrupt social learning processes (see Fig. 2). Social recognition of either a novel or conspecific juvenile has been shown to be extremely sensitive to developmental alcohol exposure. Both high- and moderate-dose three-trimester models impair recognition of a conspecific (Barbaccia et al., 2007; Kelly & Tran, 1997). While the deficit does not appear to be sexually dimorphic, the underlying cause of the impairment differs substantially. Males have been shown to be impaired on long-term memory of the conspecifics, whereas females are reported to have sensory encoding deficits (Kelly, Leggett, & Cronise, 2009). In males, these results may explain why there are alterations in interactions with female rats after PAE. For instance, PAE males do not learn female conspecifics over time (Shirasaka et al., 2012) and have changes in mating behaviors that suggest decreases in sexual motivation (Cagiano et al., 2002). For females, a change in sensory encoding may mean that females misinterpret social cues or are hyper-aware of social cues, which could lead to the somewhat contradictory observed increases in communication learning (Kelly & Tran, 1997). This deficit may also be a possible explanation for altered mating pacing behaviors (Gass, Jenkins, Marino, Lugo, & Kelly, 2007). There are very few studies examining mating behaviors after PAE to corroborate these findings. However, as many FASD individuals are accused of inappropriate social behaviors and sexual abuse (Streissguth et al., 2004), these deficits should be investigated in rodent models to determine if FASD individuals are truly unable to read social cues in this area.
Areas for future focus
Perhaps the most important development regarding PAE in the last decade was the growing understanding of the long-term effects of moderate exposures on a wide range of behaviors, including perinatal interaction and social learning, but there are still many avenues for future research as few of the above studies actually utilized a moderate exposure. In addition, most of the studies cited above focus on males, but there is clear evidence that shows female rodents have a different behavioral profile in social interactions. More work needs be done to elucidate the full female behavioral phenotype of PAE and to explain why sexually dimorphic behavioral changes occur under the same PAE paradigm.
Finally, these behavioral studies form a firm foundation to investigate regions of interest in social behaviors. It is, therefore, a wide open question: if and how, the activity and connectivity of the “social brain” (OFC, amygdala, and sensory systems) are altered to cause behavioral changes after PAE. Since these regions have also been implicated in stress processing, they may play a role in the link between stress and PAE behavioral outcome. PAE paired with a stress can exacerbate decreases in aggressive and affiliative play behaviors in male offspring and even alter male rat preference for female rats (Hellemans et al., 2010; Popova, Morozova, & Amstislavskaya, 2011; Popova, Morozova, & Naumenko, 2011). Combinations of exposures, such as stress and PAE, will provide more significance to interactions that occur within the human FASD population.
Learning, memory, and executive control (cognitive)
Although lacking in physical malformations seen in FAS, children with less severe FASD have learning, memory, and executive control alterations (Green et al., 2009; Kodituwakku, 2007; Kodituwakku, Kalberg, & May, 2001). However, the severity and type of impairments are highly varied. Here we aim to thoroughly and critically evaluate the literature in these domains to reach a consensus on what impairments occur after PAE in rodent models.
Regions of interest
The hippocampus is thought to play a major role in spatial and temporal learning and memory paradigms, including the Morris water maze and fear conditioning tasks (Jarrard, 1978; Logue, Paylor, & Wehner, 1997; Morris, Garrud, Rawlins, & O’Keefe, 1982). Due to the overwhelming behavioral effects of PAE on learning and memory reviewed below, the hippocampus is clearly affected by a wide range of doses and timings. The majority of organization and development of the dentate gyrus occurs in the first two postnatal weeks, which makes the hippocampus extremely vulnerable to stress and insults such as PAE during this time point (Lajud & Torner, 2015). This vulnerability to stress-induced effects makes consideration of administration method extremely important when studying processes that rely on the hippocampus. However, it may be that the hippocampus, in general, is especially vulnerable to PAE, and indeed, there has been extensive work done in this area determining how PAE alters hippocampal structure and leads to behavioral deficits. In particular, long-term potentiation (LTP), a hallmark measure of the synaptic plasticity that is thought to underlie learning and memory, has been consistently shown to be altered by PAE (Berman & Hannigan, 2000). Importantly, high levels of PAE are not required to impair LTP in the hippocampus, with even more moderate exposures leading to deficits (Valenzuela et al., 2012).
Pavlovian learning, including eye-blink conditioning (EBC) and avoidance learning, do not rely on the hippocampus but are also vulnerable targets of PAE insults. The cerebellum is the clear target when studying classical EBC (Kim & Thompson, 1997). All the studies reviewed here follow a third-trimester PAE exposure paradigm because the majority of the cerebellum’s maturation is neonatal (White & Sillitoe, 2013). The major cell types (Golgi cells, basket cells, stellate cells, and granule cells) all differentiate and develop in the 3 weeks following birth (Bayer et al., 1993). All of the literature reviewed below has neonatal exposure, theoretically directly influencing these cells. However, this does not mean gestational alcohol exposure cannot affect the cerebellum. The Purkinje cells are produced during mid-gestation (around GD14–15; Bayer et al., 1993). While most of this development occurs prenatally in humans, there is some evidence that some basket and stellate cells are generated after birth (Bayer et al., 1993). Of particular importance in non-spatial learning paradigms, which utilize foot shock, is the amygdala. Lesions of the amygdala inhibit avoidance learning (Poremba & Gabriel, 1997). Particularly, in humans, amygdala-striatal interactions were linked with formation of avoidant responses (Delgado, Jou, Ledoux, & Phelps, 2009). PAE may affect each of these systems and cause differential effects due to differences in developmental timing of these regions. While the striatum has a long development protracted across E15 to P3, the development of the amygdala is more contained during E12–19 (Bayer et al., 1993). Therefore, in learning and memory, there is a multitude of possible behavioral outcomes depending on timing and dose of PAE paradigm.
Finally, behavioral flexibility is well known to be mediated by discrete regions of the prefrontal cortex (PFC) (de Bruin, Sànchez-Santed, Heinsbroek, Donker, & Postmes, 1994). Lesions of the orbital frontal cortex (OFC) selectively impair reversal learning across species, while lesions of the medial PFC (mPFC) in rodents impairs rule-shifting behaviors (Bissonette et al., 2008; Brigman et al., 2013; Rudebeck, Saunders, Prescott, Chau, & Murray, 2013). It is thought that, while the mPFC plays a role in behavioral inhibition and error monitoring, the OFC plays a discrete role in value updating, which seems to be particularly important in reversal-learning behaviors (Bissonette, Powell, & Roesch, 2013; Rudebeck et al., 2013; Schoenbaum, Chiba, & Gallagher, 2000). The cortex, including the prefrontal cortex, develops during middle to late gestation, with more superficial layers developing from E16 to birth. However, the striatum is also involved in behavioral flexibility because of its role in reward behaviors (Yin et al., 2009). As previously mentioned, the striatum has an extended time of development; therefore, alterations in behaviors often associated with the prefrontal cortex are likely a result of effects on both the frontal cortex and striatum. However, the contribution to the changes in behavior may be different based on different exposure paradigms and may lead to slightly different outcome measures, e.g., changes in reward learning vs. changes in actual behavioral flexibility.
Collectively, the diverse set of regions that facilitate cognitive behaviors coupled with the diversity of PAE paradigms set the stage for extremely diverse outcomes. In addition, some regions play a role in multiple types of behaviors, leading to a possible diverse array of causes depending on the dose and timing of PAE. This may be one challenge in moving forward in defining structural and functional changes that lead to behavioral deficits, because of the complexity of dysfunctions that may lead to a particular behavioral outcome, coupled with the multitude of different behavioral phenotypes that interact.
Spatial learning and memory
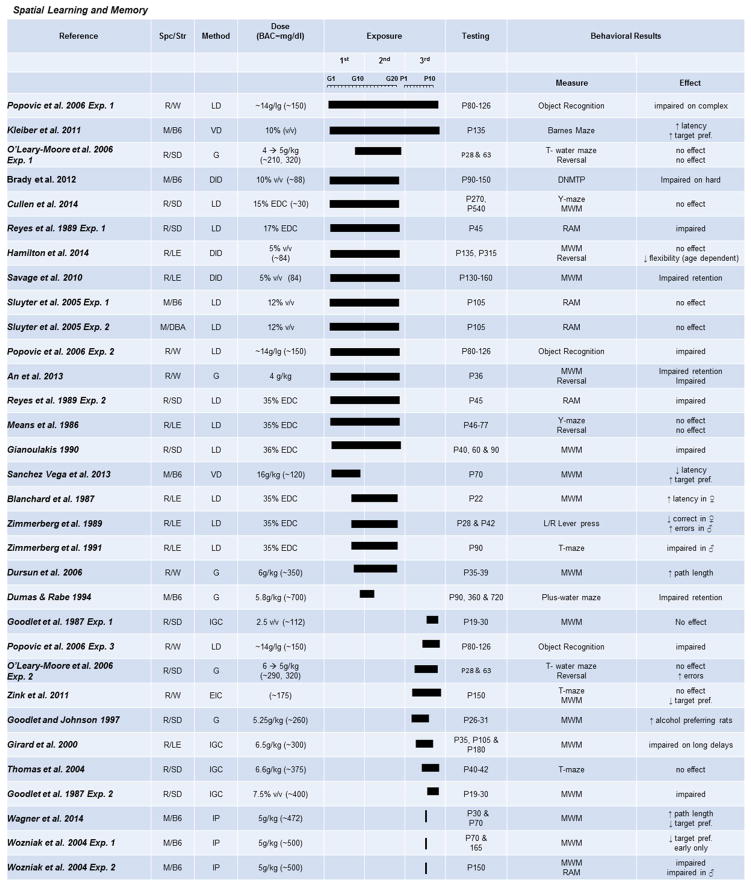
Impairments in spatial learning and memory are a well-documented consequence of PAE in preclinical models and are one of the most common outcome measures of PAE in rodent models (Fig. 4). The majority of the studies reviewed here were completed using the Morris water maze (MWM) task and measured both acquisition of a spatial memory and retention of the spatial memory during probe trials. There is a clear detrimental effect of PAE across a variety of doses, exposure times, and testing ages on acquisition during the MWM (An & Zhang, 2013; Dursun, Jakubowska-Doğru, & Uzbay, 2006; Gianoulakis, 1990; Girard, Xing, Ward, & Wainwright, 2000; Goodlett & Johnson, 1997; Means, Russ, Medlin, & Gray, 1986; Wagner, Zhou, & Goodlett, 2014; Wozniak et al., 2004). However, moderate doses, particularly during gestation, do not reliably impact learning and memory in either the MWM or Y-maze task (Cullen, Burne, Lavidis, & Moritz, 2014; Hamilton et al., 2014; Sanchez Vega, Chong, & Burne, 2013). However, with increasing demands on memory processes, rodents with only moderate PAE are revealed to have deficits in spatial memory (Brady, Allan, & Caldwell, 2012; Savage et al., 2010). This mirrors the result that stress exacerbates social interaction deficits. It leads to the interesting conclusion that individuals with FASD may not reveal deficits until significant stress or challenge is applied, further underlining the necessity of proper assessment tools in both clinical and preclinical settings. However, perhaps surprisingly, given results in social behavior studies, the majority of spatial learning studies show no sexual dimorphic pattern of deficits. Only one study has reported greater deficits in spatial learning in females than males (Blanchard, Riley, & Hannigan, 1987).
Fig. 4. The effects of prenatal exposure on spatial learning and memory tasks.
Results of studies focused on spatial learning and memory paradigms after PAE are organized by exposure time (solid bars indicate exposure duration and timing) and dose. Species/Strains indicated by R = rat, M = mouse, SD = Sprague-Dawley, LE = Long-Evans, W = Wistar, B6 = C57BL/6J. Methods include VD = voluntary drinking, IGC = intragastric cannula, G = oral gavage, LD = liquid diet, DID = drinking-in-the-dark (i.e., limited-access voluntary drinking). Dose indicates concentration of ethanol given with blood alcohol concentration (BAC) in parentheses in mg/dL when reported.
In contrast to acquisition of spatial memories, the impact on memory retention during MWM probe trials is more variable. Studies have reported either less retention (An & Zhang, 2013; Gianoulakis, 1990; Wagner et al., 2014; Wozniak et al., 2004) or no change compared to controls (Dursun et al., 2006; Goodlett & Johnson, 1997; Wagner et al., 2014; Wozniak et al., 2004). Only one study reports decreases in probe task memory without accompanying altered learning (Zink et al., 2011). Interestingly, a recent study reported increased time spent in the target quadrant on the probe trial by PAE. The authors hypothesized that this increase was due to either hyper-locomotion or increased reward learning, supported by findings that their PAE model also increased sucrose drinking during the first hour of a 2-bottle choice task (Sanchez Vega et al., 2013). It should also be noted that spatial memory deficits after PAE are not limited to the MWM but have also been reported on an operant left/right lever press task (Zimmerberg, Mattson, & Riley, 1989) and a T-maze alternation task (Zimmerberg, Sukel, & Stekler, 1991). As in the MWM, these findings are sensitive to both exposure window and age of testing, as impairments seen in young adults given three trimesters of exposure were not present in PAE rats exposed only during the third trimester (Zink et al., 2011). This result is especially intriguing, as the majority of hippocampal development is postnatal, and therefore, a third-trimester only model should greatly impact a spatial memory paradigm. This suggests that factors other than simple regional development are coming into play. To date, there is no clear pattern of dosage, timing of exposure, or age at testing to explain the differences in findings for memory retention. This suggests that results of memory retention after PAE may be a combined result of exposure window and study task design. Therefore, more controlled studies will be needed to access the validity of MWM memory retention findings.
Some investigators have proposed that memory deficits may be difficult to detect in adults but readily observable in young animals due to maturation and growth. Rodent literature does suggest that age ameliorates, but does not completely reverse, MWM learning and memory deficits (Gianoulakis, 1990; Wagner et al., 2014; Wozniak et al., 2004). Other spatial tasks, including the Barnes maze and can- task, also show learning and memory deficits that are dependent upon both task difficulty and age of the animal (Kleiber, Wright, & Singh, 2011; Popović, Caballero-Bleda, & Guerri, 2006). The more complex radial-arm maze task reveals deficits in juvenile mice (Reyes, Wolfe, & Savage, 1989), but not in 3-month-old mice (Sluyter, Jamot, Bertholet, & Crusio, 2005) with dose-dependent effects. It is clear that the extent of spatial memory deficits is dependent upon age of testing and dosage of PAE.
Pavlovian learning and memory
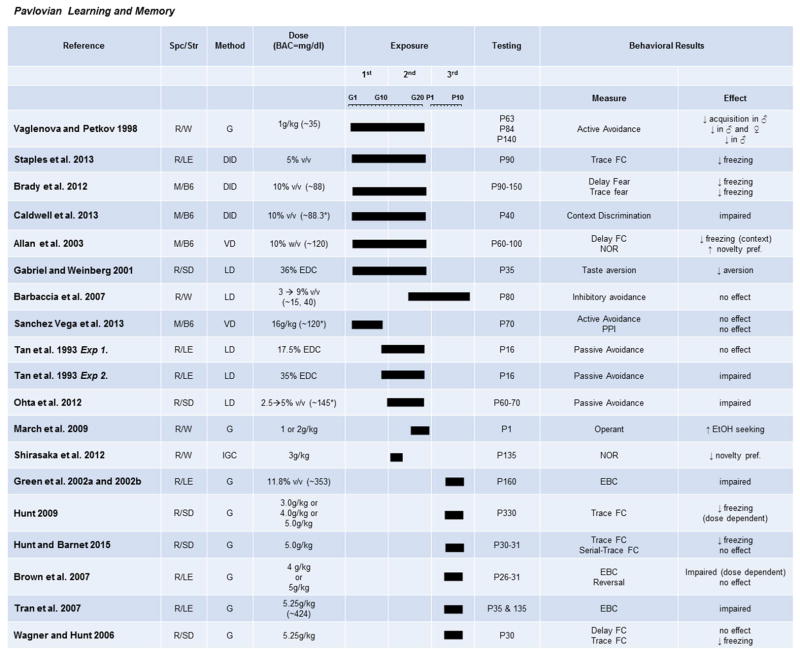
The effects of PAE on learning and memory have also been investigated using Pavlovian measures, including fear conditioning and approach tasks (Fig. 5). Fear conditioning paradigms test the ability to associate a conditioned stimulus (CS; in this case, a context) with an unconditioned stimulus (US), such as a mild foot shock. Freezing, a mouse’s natural fear response, is observed as a measure of whether the animal has learned or retained the association. Both high and moderate exposures impair hippocampal-dependent trace fear conditioning, resulting in decreased freezing of PAE rodents (Allan et al., 2003; Brady et al., 2012; Caldwell, Goggin, Tyler, & Allan, 2013; Hunt & Barnet, 2015; Hunt, Jacobson, & Torok, 2009; Wagner & Hunt, 2006). Interestingly, PAE rodents were not impaired on a trace-conditioning paradigm with both auditory and visual cues, further supporting the idea that deficits are not apparent under facilitative conditions (Hunt & Barnet, 2015). Less studied, but also highly hippocampal-dependent, delay fear conditioning, is impaired by a moderate gestational exposure (Brady et al., 2012), but not after a third trimester-only exposure (Hunt & Barnet, 2015). These conflicting results could suggest a limited window of vulnerability for this type of hippocampal-based learning, or they may point to differences as a result of age of animal at testing or different fear-conditioning paradigms. Either way, this area is one that would benefit from more research. Novel object recognition tasks, which are also mediated by the hippocampus, have also been shown to be impaired by both heavy and moderate PAE (Shirasaka et al., 2012).
Fig. 5. The effects of prenatal exposure on Pavlovian learning paradigms.
Results of studies focused on learning paradigms using approach/avoidance, fear learning, and eye-blink conditioning are organized by exposure timing (solid bars indicate exposure duration and timing) and dose. Species/Strains are indicated by R = rat, M = mouse, SD = Sprague-Dawley, LE = Long-Evans, W = Wistar, B6 = C57BL/6J. Methods include VD = voluntary drinking, IGC = intragastric cannula, G = oral gavage, LD = liquid diet, DID = drinking-in-the-dark (i.e., limited-access voluntary drinking). Dose indicates concentration of ethanol given with blood alcohol concentration (BAC) in parentheses in mg/dL when reported.
Prenatal exposures can also affect several forms of non-hippocampal-dependent Pavlovian learning paradigms. At high doses of ethanol during the third-trimester equivalent, eye-blink conditioning is impaired in juvenile and adult offspring (Green, Johnson, Goodlett, & Steinmetz, 2002; Green, Tran, Steinmetz, & Goodlett, 2002; Tran, Stanton, & Goodlett, 2007). Gestational exposures have not been tested, as this task is cerebellar dependent and the majority of cerebellar maturation occurs neonatally, during the third-trimester equivalent (Bayer et al., 1993, White & Sillitoe, 2013). While neonatal third-trimester exposures are logical, lower doses will be needed to examine effects that are more clinically relevant. Avoidance-learning paradigms, in which a behavioral response must be learned to avoid a stressor, are also altered by prenatal exposure. The latency to escape either an exposed area or a mild painful shock is significantly reduced in rats exposed during the second-trimester equivalent (Ohta, Sakata-Haga, & Fukui, 2012; Tan, Abel, & Berman, 1993), but is not affected after a first-trimester exposure (Sanchez Vega et al., 2013). Similarly, moderate exposure across the second- and third-trimester equivalent in rats impaired passive avoidance learning without affecting anxiety-like behaviors on the elevated plus maze (Barbaccia et al., 2007; Vaglenova & Petkov, 1998). These results conclusively show that learning in stressful situations is impaired in FASD, unless alcohol exposure is moderate and limited to the first trimester. This may be because regions involved in these behaviors, such as the amygdala, do not hit the peak of maturation until after the first trimester (Bayer et al., 1993), and that moderate alcohol exposure does not impair precursor development enough to result in an observable behavioral change in adulthood. Interestingly, it should be noted that moderate exposures may also alter taste preference and drive to work for reward (March, Abate, Spear, & Molina, 2009), which is congruent with possible changes in the striatum caused by PAE. However, few studies have addressed the response to taste and reward of appetitive stimuli, so decisive conclusions cannot be drawn. This may be a possible confound in many studies that have utilized food rewards and should be tested in future studies.
Reversal learning
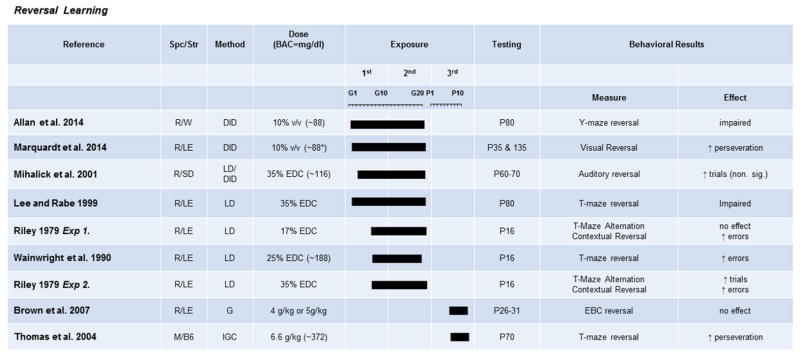
Although there is strong evidence that PAE can result in deficits in hippocampal-mediated learning and classical conditioned responses, clinical studies examining FASD have brought increasing focus on the effects of all levels of exposure on higher-order cognition mediated by the prefrontal cortex (Fig. 6). Behavioral flexibility has most widely been assessed in rodents using reversal-learning tasks that require a subject to first learn a stimulus-reward association, then, once the behavior is well-established, switch the reward association. It is well established that in spatial-reversal tasks (Y-maze, T-maze, MWM), rodents show impairment on reversal, even when there are no effects on acquisition of the initial spatial discrimination in both high- and moderate-dose paradigms (An & Zhang, 2013; Hamilton et al., 2014; Lee & Rabe, 1999; Riley, Lochry, Shapiro, & Baldwin, 1979; Thomas, Garrison, & O’Neill, 2004; Wainwright et al., 1990). Recently we have shown that reversal impairments are not confined to spatial learning, as a moderate exposure during the first two trimesters impairs reversal of visual stimuli in a touch-screen operant task (Marquadt, Sigdel, Caldwell, & Brigman, 2014).
Fig. 6. The effects of prenatal exposure on reversal learning.
Results of studies focused on paradigms measuring behavioral flexibility in operant or maze-based tasks are organized by exposure timing (solid bars indicate exposure duration and timing) and dose. Species/Strains indicated by R = rat, M = mouse, SD = Sprague-Dawley, LE = Long-Evans, W = Wistar, B6 = C57BL/6J. Methods include VD = voluntary drinking, IGC = intragastric cannula, G = oral gavage, LD = liquid diet, DID = drinking-in-the-dark (i.e., limited-access voluntary drinking). Dose indicates concentration of ethanol given with blood alcohol concentration (BAC) in parentheses in mg/dL when reported.
Other studies have reported no alterations in spatial reversal learning after PAE (Means et al., 1986; O’Leary-Moore, McMechan, Mathison, Berman, & Hannigan, 2006). These negative results may be due to a very mild deficit that is not captured in the data reported, as the only evidence for impaired reversal in another T-maze-based reversal task was increased perseverative errors (Thomas et al., 2004). In another modality, a tone-light eye-blink conditioning paradigm showed decreased eye-blink conditioning acquisition, but not impaired reversal (Brown, Calizo, Goodlett, & Stanton, 2007). This result may be due to the impairment in acquisition obscuring alterations in reversal learning, or may be because prefrontal cortical regions, thought to be important in reversal, do not play a large role in flexibly changing eye-blink conditioning contingencies. Similarly, acquisition of an auditory cued go/no go task was reported to be significantly impaired after PAE, while reversal was not (Mihalick, Crandall, Langlois, Krienke, & Dube, 2001), indicating again that reversal deficits may be obscured by initial learning problems. Together these studies suggest that not all processes are impaired after PAE when challenged to alter flexibly, and that some reversal deficits may be obscured by initial acquisition deficits or be hard to measure based on the paradigm chosen.
Areas for future focus
Deficits in spatial and learning memory are the most supported conclusions in the rodent PAE literature. However, this may be due to the overwhelming amount of literature published on this topic. The thing most striking about spatial memory, Pavlovian conditioning, and reversal learning is that deficits are exceedingly similar across ethanol doses and exposure times. While cognitive outcomes are clearly more severe with higher PAE, timing of exposure does not seem to be a large factor in determining outcome. Both social and affective behavioral outcomes are highly dose-, timing-, and sex-dependent. This leaves a great amount of research to be done in expanding the understanding of cognitive deficits to determine if they are truly caused by the same underlying mechanism.
With the increasing evidence that PAE in humans can result in wide-ranging alterations in executive control in the absence of gross morphological changes or learning deficits (Green et al., 2009; Mattson et al., 1999; Streissguth et al., 1991), future studies need to focus on more sensitive measures of cognition. Good examples are recent studies suggesting that task difficulty can play a crucial role in detecting deficits after PAE, with challenging task variants revealing differences that were not apparent in standard protocols (Brady et al., 2012; Savage, Becher, de la Torre, & Sutherland, 2002). It is increasingly important to use these sensitive measures, as moderate PAE paradigms can result in long-lasting physical and mechanistic changes, such as decreased synaptic plasticity (Sutherland, McDonald, & Savage, 1997) or alteration of receptors in the dentate gyrus (Brady et al., 2012).
Affective behaviors
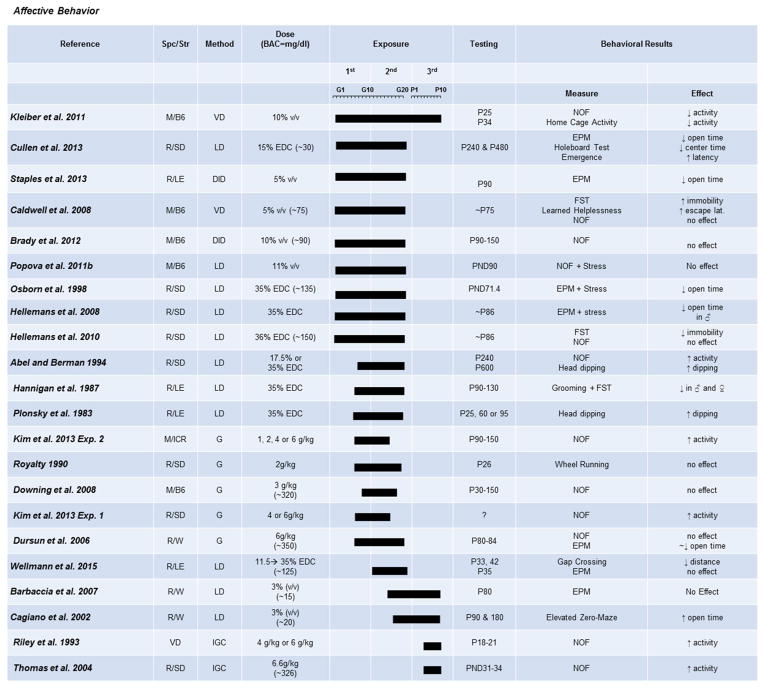
Although not as widely studied as the other domains, PAE is strongly implicated in anxiety-related disorders, and several studies have used preclinical models to examine the effects on anxiety- and depression-like behaviors (Fig. 7).
Fig. 7. The effects of prenatal exposure on affective behaviors.
Results of studies focused on paradigms measuring anxiety- and depression-like behavior are organized by exposure timing (solid bars indicate exposure duration and timing) and dose. Species/Strains indicated by R = rat, M = mouse, SD = Sprague-Dawley, LE = Long-Evans, W = Wistar, B6 = C57BL/6J. Methods include VD = voluntary drinking, IGC = intragastric cannula, G = oral gavage, LD = liquid diet, DID = drinking-in-the-dark (i.e., limited-access voluntary drinking). Dose indicates concentration of ethanol given with blood alcohol concentration (BAC) in parentheses in mg/dL when reported.
Regions of interest
The amygdala is implicated in the processing and display of acute fear responses, but not in the personality trait of “anxiousness” (Kalin, Shelton, Davidson, & Kelley, 2001). Instead, anxiety is thought to be a result of a higher-order cognitive process, which utilizes the anterior insula (Paulus & Stein, 2006). In support of this, anxiety-prone individuals show increased insular activation during emotion processing and unpleasant stimuli (Stein, Simmons, Feinstein, & Paulus, 2007). The anterior insula is considered a part of the cortex, and as such, all five cortical layers are not fully developed until late gestation in rodents. Therefore, depending on the timing of the PAE, different effects could be seen based on what layer was most affected during development (Bayer et al., 1993). However, due to the inherently difficult nature of defining anxiety, direct actions of PAE on the circuitry involved in these behaviors will be a difficult future task.
There are multiple possible epicenters for PAE to alter locomotion. The spinal cord, in what is referred to as “central pattern generators”, which power rhythmic movements such as walking, is a likely target (MacKay-Lyons, 2002). However, selective neonatal lesioning of the ventral hippocampus (VH) by ibotenic acid reliably produces hyperlocomotion in adult rodents and potentiates amphetamine-induced hyperlocomotion (Sams-Dodd, Lipska, & Weinberger, 1997; Wan, Giovanni, Kafka, & Corbett, 1996). Additionally, lesions in the mPFC alone show attenuated locomotion, while combined mPFC and VH lesions normalize locomotion in adulthood (Lipska, al-Amin, & Weinberger, 1998). Therefore, the mPFC and VH circuit may be of particular interest. Furthermore, sensory afferents, including those in the paws and whiskers or even somatosensory regions further upstream, may also be affected by PAE and alter locomotion. Therefore, changes in locomotion caused by PAE may not be easy to attribute to one region of interest.
Anxiety and locomotion
Many studies support the conclusion of persistent increases in anxiety-like behavior in males and females after PAE, measured by both the elevated-plus maze and time spent in an illuminated novel open field (Cullen, Burne, Lavidis, & Moritz, 2013; Dursun et al., 2006; Kleiber et al., 2011). Interestingly, it may be that moderate exposures are not enough to cause anxiety changes that are measurable on the elevated-plus maze (Barbaccia et al., 2007), but become evident on more stressful paradigms, such as gap crossing (Wellmann et al., 2015). As with the other domains, contrary findings have been reported, such as decreased anxiety on the zero maze using a moderate second- and third-trimester exposure (Cagiano et al., 2002). Differences in paradigms used to test anxiety-like behavior may contribute to these inconsistencies. The zero maze eliminates the ambiguous center square of the elevated-plus maze and could increase locomotion, which could consequently conceal alterations in anxiety, unless more stressful conditions existed. In accordance with this hypothesis, stress exacerbates anxiety-like behaviors in PAE mice (Hannigan, Blanchard, & Riley, 1987; Hellemans, Verma, Yoon, Yu, & Weinberg, 2008; Osborn, Kim, Steiger, Weinberg, 1998; Staples, Rosenberg, Allen, Porch, & Savage, 2013). However, one moderate first- and second-trimester study done in mice did not show an alteration in center time in an open-field paradigm after a mild restraint stress in adult male mice (Popova, Morozova, & Naumenko, 2011). This suggests that stress may need to reach a certain threshold before exacerbating deficits primed by PAE.
Numerous studies have looked at locomotion in an open-field paradigm because hyper- and hypo-locomotor activity could confound the interpretations of other behavioral tasks. In addition, FASD is often co-morbid or misdiagnosed with ADHD. Several studies have found that moderate and heavy PAE during gestation or the third trimester increases locomotor activity (Abel & Berman, 1994; Kim et al., 2013; Plonsky & Riley, 1983; Riley, Barron, Melcer, & Gonzalez, 1993; Thomas et al., 2004). However, not all high-dose paradigms were able to induce locomotor alterations (Downing, Balderrama-Durbin, Hayes, Johnson, & Gilliam, 2009; Royalty, 1990). This may be due to the slight difference in exposure windows, indicating that locomotor behavior may only be altered with an extremely high-dose PAE when not delivered during the peak vulnerability window, which appears to be late second trimester (around birth) and third trimester, which is in congruence with late hippocampal development. To support this, several studies using moderate doses, only given over the first and second trimesters, fail to see alterations in locomotion (Brady et al., 2012; Caldwell et al., 2008; Dursun et al., 2006; Hellemans et al., 2010). These studies suggest that changes in hyperactivity are not a general feature of FASD, but can be precipitated by particular dosages and timings of PAE. Whether the cause behind these changes in hyperactivity are the same as in ADHD is unclear from these studies, and should be investigated in the future. Unsurprisingly, stress exacerbates locomotor activity after mild stress in a moderate first- and second-trimester exposure (Hannigan et al., 1987, Hellemans et al., 2008), similar to anxiety measures. Finally, only one study reviewed found decreased locomotor behavior after a moderate three-trimester voluntary drinking model in both an open-field and familiar home-cage environment (Kleiber et al., 2011). This result is especially perplexing as two other studies done in mice, with similar BACs, but first- and second-trimester only exposures, do not show changes in locomotion (Brady et al., 2012; Caldwell et al., 2008). It may further suggest that locomotor behavioral outcome is highly sensitive to PAE timing.
Areas for future focus
The prevalence rate of depression is twice as high in individuals with FASD than in the general population (Cryan, Markou, & Lucki, 2002; Streissguth et al., 2004). However, surprisingly little work has been done in this area. Of the studies found, all used moderate drinking exposures throughout gestation. In both rats and mice, females showed higher levels of depressive-like behaviors in the forced-swim task and learned-helplessness paradigm (Caldwell et al., 2008; Hellemans et al., 2010; Wilcoxon, Kuo, Disterhoft, & Redei, 2005). However, males showed less depressive-like behaviors in adulthood (Hellemans et al., 2010). These results indicate that even at lower exposures, changes in depressive-like behaviors are evident, and they are sexually dimorphic. However, it must be kept in mind that these results may show such an effect because tasks used to measure these symptoms are also somewhat stressful and could therefore be illuminating an effect which is not immediately present. Therefore, the dosing effects, age, and stress effects on depressive-like behaviors in rodents after PAE is an area in need of research.
Conclusion
Not surprisingly, method of delivery, level of exposure, pattern of exposure, and timing of exposure during development affected behavioral outcome in PAE models. These factors appear to form a complex web of interaction that highly influences outcomes, which mirrors the often diverse manifestations of FASD in humans. In addition, the reviewed studies make it clear that age at testing and sex must also be considered, as complicated maturation and gender differences seem to play a role in certain behaviors. It is clear that while many studies find congruent results, a good study is carefully designed around these factors to create the optimal paradigm.
Ongoing research should focus on systematic examination of the effects of both timing of the exposure (early or late gestational, perinatal) and dose on behavioral outcome. Additionally, since the PAE literature points to a clear sexual dimorphism on most behavioral outcomes, continued work is needed to study these sex differences in PAE and their underlying mechanisms. With the increasing evidence that PAE in humans can result in wide-ranging alterations in executive control in the absence of gross morphological changes or learning deficits (Green et al., 2009; Mattson et al., 1999; Streissguth et al., 1991), future studies need to focus on more sensitive measures of cognition. A good example is recent studies suggesting that task difficulty can play a crucial role in detecting deficits after PAE, with challenging task variants revealing differences that were not apparent in standard protocols (Brady et al., 2012; Savage et al., 2002; Westergren, Rydenhag, Bassen, Archer, & Conradi, 1996). Finally, while numerous studies have examined either the effects of PAE on mechanisms that may underlie social, cognitive, and affective domains or the behavioral impact of these exposures, only recently have studies begun to systematically examine both morphological and functional changes and their impact on the behavioral phenotype. In order to provide potential avenues for therapeutic intervention, future work needs to closely follow this model in order to increase our understanding of how neurodevelopmental insult leads to behavioral deficits later in life.
In summary, it is our hope that this review has demonstrated that creating a valid animal model of PAE requires a deep understanding of the physiologies and behaviors of humans and the model species, and a carefully constructed methodology. Much research has yet to be done to systematically analyze the adverse effects of PAE on neurodevelopment and to determine how these factors interact with genetic factors to render susceptibility.
Highlights.
Development of a comprehensive rodent PAE behavioral phenotype from available literature.
Focus on social, cognitive and affective domain behavioral modifications after PAE.
Specially considers timing, dose and administration method effects of PAE on behavioral outcome.
Highlights current gaps in the literature that are needed for a full PAE behavioral phenotype.
Acknowledgments
JLB is supported by NIAAA grants 1K22-AA020303-01 and 1P50AA022534-01. KM is supported by NIAAA grant 5T32AA014127-13.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL, Berman RF. Long-term behavioral effects of prenatal alcohol exposure in rats. Neurotoxicology and Teratology. 1994;16:467–470. doi: 10.1016/0892-0362(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Allan AM, Chynoweth J, Tyler LA, Caldwell KK. A mouse model of prenatal ethanol exposure using a voluntary drinking paradigm. Alcoholism: Clinical and Experimental Research. 2003;27:2009–2016. doi: 10.1097/01.ALC.0000100940.95053.72. [DOI] [PubMed] [Google Scholar]
- An L, Zhang T. Spatial cognition and sexually dimorphic synaptic plasticity balance impairment in rats with chronic prenatal ethanol exposure. Behavioural Brain Research. 2013;256:564–574. doi: 10.1016/j.bbr.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Scaccianoce S, Del Bianco P, Campolongo P, Trezza V, Tattoli M, et al. Cognitive impairment and increased brain neurosteroids in adult rats perinatally exposed to low millimolar blood alcohol concentrations. Psychoneuroendocrinology. 2007;32:931–942. doi: 10.1016/j.psyneuen.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, et al. Social intelligence in the normal and autistic brain: an fMRI study. The European Journal of Neuroscience. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Barron S, Kelly SJ, Riley EP. Neonatal alcohol exposure alters suckling behavior in neonatal rat pups. Pharmacology, Biochemistry, and Behavior. 1991;39:423–427. doi: 10.1016/0091-3057(91)90202-d. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10:94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Bishop S, Gahagan S, Lord C. Re-examining the core features of autism: a comparison of autism spectrum disorder and fetal alcohol spectrum disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2007;48:1111–1121. doi: 10.1111/j.1469-7610.2007.01782.x. [DOI] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. The Journal of Neuroscience. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Powell EM, Roesch MR. Neural structures underlying set-shifting: roles of medial prefrontal cortex and anterior cingulate cortex. Behavioural Brain Research. 2013;250:91–101. doi: 10.1016/j.bbr.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard BA, Riley EP, Hannigan JH. Deficits on a spatial navigation task following prenatal exposure to ethanol. Neurotoxicology and Teratology. 1987;9:253–258. doi: 10.1016/0892-0362(87)90010-9. [DOI] [PubMed] [Google Scholar]
- Brady ML, Allan AM, Caldwell KK. A limited access mouse model of prenatal alcohol exposure that produces long-lasting deficits in hippocampal-dependent learning and memory. Alcoholism: Clinical and Experimental Research. 2012;36:457–466. doi: 10.1111/j.1530-0277.2011.01644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Daut RA, Wright T, Gunduz-Cinar O, Graybeal C, Davis MI, et al. GluN2B in corticostriatal circuits governs choice learning and choice shifting. Nature Neuroscience. 2013;16:1101–1110. doi: 10.1038/nn.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KL, Calizo LH, Goodlett CR, Stanton ME. Neonatal alcohol exposure impairs acquisition of eyeblink conditioned responses during discrimination learning and reversal in weanling rats. Developmental Psychobiology. 2007;49:243–257. doi: 10.1002/dev.20178. [DOI] [PubMed] [Google Scholar]
- Cagiano R, Cassano T, Coluccia A, Gaetani S, Giustino A, Steardo L, et al. Genetic factors involved in the effects of developmental low-level alcohol induced behavioral alterations in rats. Neuropsychopharmacology. 2002;26:191–203. doi: 10.1016/S0893-133X(01)00306-2. [DOI] [PubMed] [Google Scholar]
- Caldwell KK, Goggin SL, Tyler CR, Allan AM. Prenatal alcohol exposure is associated with altered subcellular distribution of glucocorticoid and mineralocorticoid receptors in the adolescent mouse hippocampal formation. Alcoholism: Clinical and Experimental Research. 2013;38:392–400. doi: 10.1111/acer.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell KK, Sheema S, Paz RD, Samudio-Ruiz SL, Laughlin MH, Spence NE, et al. Fetal alcohol spectrum disorder-associated depression: evidence for reductions in the levels of brain-derived neurotrophic factor in a mouse model. Pharmacology, Biochemistry, and Behavior. 2008;90:614–624. doi: 10.1016/j.pbb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles Lawrence R, Cale Bonner H, Newsom RJ, Kelly SJ. Effects of alcohol exposure during development on play behavior and c-Fos expression in response to play behavior. Behavioural Brain Research. 2008;188:209–218. doi: 10.1016/j.bbr.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasnoff IJ, Wells AM, King L. Misdiagnosis and missed diagnoses in foster and adopted children with prenatal alcohol exposure. Pediatrics. 2015;135:264–270. doi: 10.1542/peds.2014-2171. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends in Pharmacological Sciences. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Cullen CL, Burne TH, Lavidis NA, Moritz KM. Low dose prenatal ethanol exposure induces anxiety-like behaviour and alters dendritic morphology in the basolateral amygdala of rat offspring. PloS One. 2013;8:e54924. doi: 10.1371/journal.pone.0054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen CL, Burne TH, Lavidis NA, Moritz KM. Low dose prenatal alcohol exposure does not impair spatial learning and memory in two tests in adult and aged rats. PloS One. 2014;9:e101482. doi: 10.1371/journal.pone.0101482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daenen EW, Wolterink G, Gerrits MA, Van Ree JM. The effects of neonatal lesions in the amygdala or ventral hippocampus on social behaviour later in life. Behavioural Brain Research. 2002;136:571–582. doi: 10.1016/s0166-4328(02)00223-1. [DOI] [PubMed] [Google Scholar]
- Day NL, Leech SL, Richardson GA, Cornelius MD, Robles N, Larkby C. Prenatal alcohol exposure predicts continued deficits in offspring size at 14 years of age. Alcoholism: Clinical and Experimental Research. 2002;26:1584–1591. doi: 10.1097/01.ALC.0000034036.75248.D9. [DOI] [PubMed] [Google Scholar]
- de Bruin JP, Sànchez-Santed F, Heinsbroek RP, Donker A, Postmes P. A behavioural analysis of rats with damage to the medial prefrontal cortex using the Morris water maze: evidence for behavioural flexibility, but not for impaired spatial navigation. Brain Research. 1994;652:323–333. doi: 10.1016/0006-8993(94)90243-7. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Jou RL, Ledoux JE, Phelps EA. Avoiding negative outcomes: tracking the mechanisms of avoidance learning in humans during fear conditioning. Frontiers in Behavioral Neuroscience. 2009;3:33. doi: 10.3389/neuro.08.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Gerrits MA, Stuy A, Spruijt BM, van Ree JM. Neonatal amygdala lesions and juvenile isolation in the rat: differential effects on locomotor and social behavior later in life. Behavioral Neuroscience. 2004;118:298–305. doi: 10.1037/0735-7044.118.2.298. [DOI] [PubMed] [Google Scholar]
- Downing C, Balderrama-Durbin C, Hayes J, Johnson TE, Gilliam D. No effect of prenatal alcohol exposure on activity in three inbred strains of mice. Alcohol and Alcoholism. 2009;44:25–33. doi: 10.1093/alcalc/agn082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll CD, Streissguth AP, Riley EP. Prenatal alcohol exposure: comparability of effects in humans and animal models. Neurotoxicology and Teratology. 1990;12:231–237. doi: 10.1016/0892-0362(90)90094-s. [DOI] [PubMed] [Google Scholar]
- Dursun I, Jakubowska-Doğru E, Uzbay T. Effects of prenatal exposure to alcohol on activity, anxiety, motor coordination, and memory in young adult Wistar rats. Pharmacology, Biochemistry, and Behavior. 2006;85:345–355. doi: 10.1016/j.pbb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Ethen MK, Ramadhani TA, Scheuerle AE, Canfield MA, Wyszynski DF, Druschel CM, et al. Alcohol consumption by women before and during pregnancy. Maternal and Child Health Journal. 2009;13:274–285. doi: 10.1007/s10995-008-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Jenkins WJ, Marino MD, Lugo JN, Jr, Kelly SJ. Alcohol exposure during development: analysis of effects on female sexual behavior. Alcoholism: Clinical and Experimental Research. 2007;31:2065–2072. doi: 10.1111/j.1530-0277.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- Gershon A, Sudheimer K, Tirouvanziam R, Williams LM, O’Hara R. The long-term impact of early adversity on late-life psychiatric disorders. Current Psychiatry Reports. 2013;15:352. doi: 10.1007/s11920-013-0352-9. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Rats exposed prenatally to alcohol exhibit impairment in spatial navigation test. Behavioural Brain Research. 1990;36:217–228. doi: 10.1016/0166-4328(90)90060-r. [DOI] [PubMed] [Google Scholar]
- Girard TA, Xing HC, Ward GR, Wainwright PE. Early postnatal ethanol exposure has long-term effects on the performance of male rats in a delayed matching-to-place task in the Morris water maze. Alcoholism: Clinical and Experimental Research. 2000;24:300–306. [PubMed] [Google Scholar]
- Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicology and Teratology. 1997;19:435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- Green CR, Mihic AM, Nikkel SM, Stade BC, Rasmussen C, Munoz DP, et al. Executive function deficits in children with fetal alcohol spectrum disorders (FASD) measured using the Cambridge Neuropsychological Tests Automated Battery (CANTAB) Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2009;50:688–697. doi: 10.1111/j.1469-7610.2008.01990.x. [DOI] [PubMed] [Google Scholar]
- Green JT, Johnson TB, Goodlett CR, Steinmetz JE. Eyeblink classical conditioning and interpositus nucleus activity are disrupted in adult rats exposed to ethanol as neonates. Learning & Memory. 2002;9:304–320. doi: 10.1101/lm.47602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JT, Tran T, Steinmetz JE, Goodlett CR. Neonatal ethanol produces cerebellar deep nuclear cell loss and correlated disruption of eyeblink conditioning in adult rats. Brain Research. 2002;956:302–311. doi: 10.1016/s0006-8993(02)03561-8. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Rice JP, Johnson TE, Candelaria-Cook FT, Maes LI, et al. Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: relationship to structural plasticity and immediate early gene expression in frontal cortex. Behavioural Brain Research. 2010;207:290–304. doi: 10.1016/j.bbr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, Magcalas CM, Barto D, Bird CW, Rodriguez CI, Fink BC, et al. Moderate prenatal alcohol exposure and quantification of social behavior in adult rats. Journal of Visualized Experiments. 2014 doi: 10.3791/52407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan JH, Blanchard BA, Riley EP. Altered grooming responses to stress in rats exposed prenatally to ethanol. Behavioral and Neural Biology. 1987;47:173–185. doi: 10.1016/s0163-1047(87)90299-8. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Verma P, Yoon E, Yu W, Weinberg J. Prenatal alcohol exposure increases vulnerability to stress and anxiety-like disorders in adulthood. Annals of the New York Academy of Sciences. 2008;1144:154–175. doi: 10.1196/annals.1418.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Verma P, Yoon E, Yu WK, Young AH, Weinberg J. Prenatal alcohol exposure and chronic mild stress differentially alter depressive- and anxiety-like behaviors in male and female offspring. Alcoholism: Clinical and Experimental Research. 2010;34:633–645. doi: 10.1111/j.1530-0277.2009.01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PS, Barnet RC. An animal model of fetal alcohol spectrum disorder: Trace conditioning as a window to inform memory deficits and intervention tactics. Physiology & Behavior. 2015;148:36–44. doi: 10.1016/j.physbeh.2014.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PS, Jacobson SE, Torok EJ. Deficits in trace fear conditioning in a rat model of fetal alcohol exposure: dose-response and timing effects. Alcohol. 2009;43:465–474. doi: 10.1016/j.alcohol.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE. Selective hippocampal lesions: differential effects on performance by rats of a spatial task with preoperative versus postoperative training. Journal of Comparative and Physiological Psychology. 1978;92:1119–1127. doi: 10.1037/h0077516. [DOI] [PubMed] [Google Scholar]
- Jones KL. The fetal alcohol syndrome. Addictive Diseases. 1975;2:79–88. [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;302:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Kelley AE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. The Journal of Neuroscience. 2001;21:2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Dillingham RR. Sexually dimorphic effects of perinatal alcohol exposure on social interactions and amygdala DNA and DOPAC concentrations. Neurotoxicology and Teratology. 1994;16:377–384. doi: 10.1016/0892-0362(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Leggett DC, Cronise K. Sexually dimorphic effects of alcohol exposure during development on the processing of social cues. Alcohol and Alcoholism. 2009;44:555–560. doi: 10.1093/alcalc/agp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Tran TD. Alcohol exposure during development alters social recognition and social communication in rats. Neurotoxicology and Teratology. 1997;19:383–389. doi: 10.1016/s0892-0362(97)00064-0. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Thompson RF. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends in Neurosciences. 1997;20:177–181. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- Kim JY, McHale SM, Wayne Osgood D, Crouter AC. Longitudinal course and family correlates of sibling relationships from childhood through adolescence. Child Development. 2006;77:1746–1761. doi: 10.1111/j.1467-8624.2006.00971.x. [DOI] [PubMed] [Google Scholar]
- Kim P, Park JH, Choi CS, Choi I, Joo SH, Kim MK, et al. Effects of ethanol exposure during early pregnancy in hyperactive, inattentive and impulsive behaviors and MeCP2 expression in rodent offspring. Neurochemical Research. 2013;38:620–631. doi: 10.1007/s11064-012-0960-5. [DOI] [PubMed] [Google Scholar]
- Kleiber ML, Wright E, Singh SM. Maternal voluntary drinking in C57BL/6J mice: advancing a model for fetal alcohol spectrum disorders. Behavioural Brain Research. 2011;223:376–387. doi: 10.1016/j.bbr.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: a review. Neuroscience and Biobehavioral Reviews. 2007;31:192–201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Kalberg W, May PA. The effects of prenatal alcohol exposure on executive functioning. Alcohol Research & Health. 2001;25:192–198. [PMC free article] [PubMed] [Google Scholar]
- Lajud N, Torner L. Early life stress and hippocampal neurogenesis in the neonate: sexual dimorphism, long term consequences and possible mediators. Frontiers in Molecular Neuroscience. 2015;8:3. doi: 10.3389/fnmol.2015.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Rabe A. Infantile handling eliminates reversal learning deficit in rats prenatally exposed to alcohol. Alcohol. 1999;18:49–53. doi: 10.1016/s0741-8329(98)00067-6. [DOI] [PubMed] [Google Scholar]
- Lipska BK, al-Amin HA, Weinberger DR. Excitotoxic lesions of the rat medial prefrontal cortex. Effects on abnormal behaviors associated with neonatal hippocampal damage. Neuropsychopharmacology. 1998;19:451–464. doi: 10.1016/S0893-133X(98)00045-1. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behavioral Neuroscience. 1997;111:104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Lugo JN, Jr, Marino MD, Cronise K, Kelly SJ. Effects of alcohol exposure during development on social behavior in rats. Physiology & Behavior. 2003;78:185–194. doi: 10.1016/s0031-9384(02)00971-x. [DOI] [PubMed] [Google Scholar]
- MacKay-Lyons M. Central pattern generation of locomotion: a review of the evidence. Physical Therapy. 2002;82:69–83. doi: 10.1093/ptj/82.1.69. [DOI] [PubMed] [Google Scholar]
- March SM, Abate P, Spear NE, Molina JC. Fetal exposure to moderate ethanol doses: heightened operant responsiveness elicited by ethanol-related reinforcers. Alcoholism: Clinical and Experimental Research. 2009;33:1981–1993. doi: 10.1111/j.1530-0277.2009.01037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MD, Cronise K, Lugo JN, Jr, Kelly SJ. Ultrasonic vocalizations and maternal-infant interactions in a rat model of fetal alcohol syndrome. Developmental Psychobiology. 2002;41:341–351. doi: 10.1002/dev.10077. [DOI] [PubMed] [Google Scholar]
- Marquadt K, Sigdel R, Caldwell KK, Brigman JL. Prenatal alcohol exposure impairs executive function in mice into adulthood. Alcoholism: Clinical and Experimental Research. 2014;38:2962–2968. doi: 10.1111/acer.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DC, Martin JC, Streissguth AP, Lund CA. Sucking frequency and amplitude in newborns as a function of maternal drinking and smoking. Currents in Alcoholism. 1979;5:359–366. [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 1999;23:1808–1815. [PubMed] [Google Scholar]
- Means LW, Russ RD, Medlin CW, Gray SL. Prenatal ethanol exposure in rats does not alter maze exploration or impair visual discrimination with or without distracting stimuli. Neurobehavioral Toxicology and Teratology. 1986;8:1–5. [PubMed] [Google Scholar]
- Meyer LS, Riley EP. Social play in juvenile rats prenatally exposed to alcohol. Teratology. 1986;34:1–7. doi: 10.1002/tera.1420340102. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Varlinskaya EI, Mooney SM. Molecular substrates of social avoidance seen following prenatal ethanol exposure and its reversal by social enrichment. Developmental Neuroscience. 2012;34:115–128. doi: 10.1159/000337858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalick SM, Crandall JE, Langlois JC, Krienke JD, Dube WV. Prenatal ethanol exposure, generalized learning impairment, and medial prefrontal cortical deficits in rats. Neurotoxicology and Teratology. 2001;23:453–462. doi: 10.1016/s0892-0362(01)00168-4. [DOI] [PubMed] [Google Scholar]
- Mooney SM, Varlinskaya EI. Acute prenatal exposure to ethanol and social behavior: effects of age, sex, and timing of exposure. Behavioural Brain Research. 2011;216:358–364. doi: 10.1016/j.bbr.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Ness JW, Franchina JJ. Effects of prenatal alcohol exposure on rat pups’ ability to elicit retrieval behavior from dams. Developmental Psychobiology. 1990;23:85–99. doi: 10.1002/dev.420230109. [DOI] [PubMed] [Google Scholar]
- Ohta K, Sakata-Haga H, Fukui Y. Prenatal ethanol exposure impairs passive avoidance acquisition and enhances unconditioned freezing in rat offspring. Behavioural Brain Research. 2012;234:255–258. doi: 10.1016/j.bbr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- O’Leary-Moore SK, McMechan AP, Mathison SN, Berman RF, Hannigan JH. Reversal learning after prenatal or early postnatal alcohol exposure in juvenile and adult rats. Alcohol. 2006;38:99–110. doi: 10.1016/j.alcohol.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Osborn JA, Kim CK, Steiger J, Weinberg J. Prenatal ethanol exposure differentially alters behavior in males and females on the elevated plus maze. Alcoholism: Clinical and Experimental Research. 1998;22:685–696. [PubMed] [Google Scholar]
- Ouellette EM, Rosett HL, Rosman NP, Weiner L. Adverse effects on offspring of maternal alcohol abuse during pregnancy. The New England Journal of Medicine. 1977;297:528–530. doi: 10.1056/NEJM197709082971003. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Normansell L, Cox JF, Siviy SM. Effects of neonatal decortication on the social play of juvenile rats. Physiology & Behavior. 1994;56:429–443. doi: 10.1016/0031-9384(94)90285-2. [DOI] [PubMed] [Google Scholar]
- Patten AR, Fontaine CJ, Christie BR. A comparison of the different animal models of fetal alcohol spectrum disorders and their use in studying complex behaviors. Frontiers in Pediatrics. 2014;2:93. doi: 10.3389/fped.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Peadon E, Elliott EJ. Distinguishing between attention-deficit hyperactivity and fetal alcohol spectrum disorders in children: clinical guidelines. Neuropsychiatric Disease and Treatment. 2010;6:509–515. doi: 10.2147/ndt.s7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plonsky M, Riley EP. Head-dipping behaviors in rats exposed to alcohol prenatally as a function of age at testing. Neurobehavioral Toxicology and Teratology. 1983;5:309–314. [PubMed] [Google Scholar]
- Popoola DO, Borrow AP, Sanders JE, Nizhnikov ME, Cameron NM. Can low-level ethanol exposure during pregnancy influence maternal care? An investigation using two strains of rat across two generations. Physiology & Behavior. 2015;148:111–121. doi: 10.1016/j.physbeh.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova NK, Morozova MV, Amstislavskaya TG. Prenatal stress and ethanol exposure produces inversion of sexual partner preference in mice. Neuroscience Letters. 2011;489:48–52. doi: 10.1016/j.neulet.2010.11.064. [DOI] [PubMed] [Google Scholar]
- Popova NK, Morozova MV, Naumenko VS. Ameliorative effect of BDNF on prenatal ethanol and stress exposure-induced behavioral disorders. Neurscience Letters. 2011;505:82–86. doi: 10.1016/j.neulet.2011.09.066. [DOI] [PubMed] [Google Scholar]
- Popović M, Caballero-Bleda M, Guerri C. Adult rat’s offspring of alcoholic mothers are impaired on spatial learning and object recognition in the Can test. Behavioural Brain Research. 2006;174:101–111. doi: 10.1016/j.bbr.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Poremba A, Gabriel M. Amygdalar lesions block discriminative avoidance learning and cingulothalamic training-induced neuronal plasticity in rabbits. The Journal of Neuroscience. 1997;17:5237–5244. doi: 10.1523/JNEUROSCI.17-13-05237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes E, Wolfe J, Savage DD. The effects of prenatal alcohol exposure on radial arm maze performance in adult rats. Physiology & Behavior. 1989;46:45–48. doi: 10.1016/0031-9384(89)90319-3. [DOI] [PubMed] [Google Scholar]
- Riley EP, Barron S, Melcer T, Gonzalez D. Alterations in activity following alcohol administration during the third trimester equivalent in P and NP rats. Alcoholism: Clinical and Experimental Research. 1993;17:1240–1246. doi: 10.1111/j.1530-0277.1993.tb05236.x. [DOI] [PubMed] [Google Scholar]
- Riley EP, Lochry EA, Shapiro NR, Baldwin J. Response perseveration in rats exposed to alcohol prenatally. Pharmacology, Biochemisty, and Behavior. 1979;10:255–259. doi: 10.1016/0091-3057(79)90097-2. [DOI] [PubMed] [Google Scholar]
- Royalty J. Effects of prenatal ethanol exposure on juvenile play-fighting and postpubertal aggression in rats. Psychological Reports. 1990;66:551–560. doi: 10.2466/pr0.1990.66.2.551. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nature Neuroscience. 2013;16:1140–1145. doi: 10.1038/nn.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sams-Dodd F, Lipska BK, Weinberger DR. Neonatal lesions of the rat ventral hippocampus result in hyperlocomotion and deficits in social behaviour in adulthood. Psychopharmacology. 1997;132:303–310. doi: 10.1007/s002130050349. [DOI] [PubMed] [Google Scholar]
- Sanchez Vega MC, Chong S, Burne TH. Early gestational exposure to moderate concentrations of ethanol alters adult behaviour in C57BL/6J mice. Behavioural Brain Research. 2013;252:326–333. doi: 10.1016/j.bbr.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Savage DD, Becher M, de la Torre AJ, Sutherland RJ. Dose-dependent effects of prenatal ethanol exposure on synaptic plasticity and learning in mature offspring. Alcoholism: Clinical and Experimental Research. 2002;26:1752–1758. doi: 10.1097/01.ALC.0000038265.52107.20. [DOI] [PubMed] [Google Scholar]
- Savage DD, Rosenberg MJ, Wolff CR, Akers KG, El-Emawy A, Staples MC, et al. Effects of a novel cognition-enhancing agent on fetal ethanol-induced learning deficits. Alcoholism: Clinical and Experimental Research. 2010;34:1793–1802. doi: 10.1111/j.1530-0277.2010.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training. The Journal of Neuroscience. 2000;20:5179–5189. doi: 10.1523/JNEUROSCI.20-13-05179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaka T, Hashimoto E, Ukai W, Yoshinaga T, Ishii T, Tateno M, et al. Stem cell therapy: social recognition recovery in a FASD model. Translational Psychiatry. 2012;2:e188. doi: 10.1038/tp.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluyter F, Jamot L, Bertholet JY, Crusio WE. Prenatal exposure to alcohol does not affect radial maze learning and hippocampal mossy fiber sizes in three inbred strains of mouse. Behavioral and Brain Functions. 2005;1:5. doi: 10.1186/1744-9081-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples MC, Rosenberg MJ, Allen NA, Porch MW, Savage DD. Impact of combined prenatal ethanol and prenatal stress exposure on anxiety and hippocampal-sensitive learning in adult offspring. Alcoholism: Clinical and Experimental Research. 2013;37:2039–2047. doi: 10.1111/acer.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. The American Journal of Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF. Fetal alcohol syndrome in adolescents and adults. JAMA. 1991;265:1961–1967. [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. Journal of Developmental and Behavioral Pediatrics. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, McDonald RJ, Savage DD. Prenatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal synaptic plasticity in adult offspring. Hippocampus. 1997;7:232–238. doi: 10.1002/(SICI)1098-1063(1997)7:2<232::AID-HIPO9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Tan SE, Abel EL, Berman RF. Brain MAP-2 phosphorylation is decreased following prenatal alcohol exposure in rats. Alcohol. 1993;10:391–396. doi: 10.1016/0741-8329(93)90026-k. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Garrison M, O’Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicology and Teratology. 2004;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Kelly SJ, Mattson SN, Riley EP. Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcoholism: Clinical and Experimental Research. 1998;22:528–533. [PubMed] [Google Scholar]
- Tran TD, Stanton ME, Goodlett CR. Binge-like ethanol exposure during the early postnatal period impairs eyeblink conditioning at short and long CS-US intervals in rats. Developmental Psychobiology. 2007;49:589–605. doi: 10.1002/dev.20226. [DOI] [PubMed] [Google Scholar]
- Vaglenova J, Petkov VV. Fetal alcohol effects in rats exposed pre- and postnatally to a low dose of ethanol. Alcoholism: Clinical and Experimental Research. 1998;22:697–703. [PubMed] [Google Scholar]
- Valenzuela CF, Morton RA, Diaz MR, Topper L. Does moderate drinking harm the fetal brain? Insights from animal models. Trends in Neurosciences. 2012;35:284–292. doi: 10.1016/j.tins.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AF, Hunt PS. Impaired trace fear conditioning following neonatal ethanol: reversal by choline. Behavioral Neuroscience. 2006;120:482–487. doi: 10.1037/0735-7044.120.2.482. [DOI] [PubMed] [Google Scholar]
- Wagner JL, Zhou FC, Goodlett CR. Effects of one- and three-day binge alcohol exposure in neonatal C57BL/6 mice on spatial learning and memory in adolescence and adulthood. Alcohol. 2014;48:99–111. doi: 10.1016/j.alcohol.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright PE, Ward GR, Winfield D, Huang YS, Mills DE, Ward RP, et al. Effects of prenatal ethanol and long-chain n-3 fatty acid supplementation on development in mice. 1. Body and brain growth, sensorimotor development, and water T-maze reversal learning. Alcoholism: Clinical and Experimental Research. 1990;14:405–412. doi: 10.1111/j.1530-0277.1990.tb00495.x. [DOI] [PubMed] [Google Scholar]
- Wan RQ, Giovanni A, Kafka SH, Corbett R. Neonatal hippocampal lesions induced hyperresponsiveness to amphetamine: behavioral and in vivo microdialysis studies. Behavioural Brain Research. 1996;78:211–223. doi: 10.1016/0166-4328(95)00251-0. [DOI] [PubMed] [Google Scholar]
- Wellmann KA, George F, Brnouti F, Mooney SM. Docosahexaenoic acid partially ameliorates deficits in social behavior and ultrasonic vocalizations caused by prenatal ethanol exposure. Behavioural Brain Research. 2015;286:201–211. doi: 10.1016/j.bbr.2015.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JR. Fetal alcohol-induced brain damage and the problem of determining temporal vulnerability: a review. Alcohol and Drug Research. 1987;7:423–441. [PubMed] [Google Scholar]
- Westergren S, Rydenhag B, Bassen M, Archer T, Conradi NG. Effects of prenatal alcohol exposure on activity and learning in Sprague-Dawley rats. Pharmacology, Biochemistry, and Behavior. 1996;55:515–520. doi: 10.1016/s0091-3057(96)00277-8. [DOI] [PubMed] [Google Scholar]
- White JJ, Sillitoe RV. Development of the cerebellum: from gene expression patterns to circuit maps. Wiley Interdisciplinary Reviews Developmental Biology. 2013;2:149–164. doi: 10.1002/wdev.65. [DOI] [PubMed] [Google Scholar]
- Wilcoxon JS, Kuo AG, Disterhoft JF, Redei EE. Behavioral deficits associated with fetal alcohol exposure are reversed by prenatal thyroid hormone treatment: a role for maternal thyroid hormone deficiency in FAE. Molecular Psychiatry. 2005;10:961–971. doi: 10.1038/sj.mp.4001694. [DOI] [PubMed] [Google Scholar]
- Williams JF, Smith VC Committee On Substance Abuse. Fetal Alcohol Spectrum Disorders. Pediatrics. 2015;136:e1395–1406. doi: 10.1542/peds.2015-3113. [DOI] [PubMed] [Google Scholar]
- Workman JL, Raineki C, Weinberg J, Galea LA. Alcohol and pregnancy: Effects on maternal care, HPA axis function, and hippocampal neurogenesis in adult females. Psychoneuroendocrinology. 2015;57:37–50. doi: 10.1016/j.psyneuen.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak DF, Hartman RE, Boyle MP, Vogt SK, Brooks AR, Tenkova T, et al. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiology of Disease. 2004;17:403–414. doi: 10.1016/j.nbd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nature Neuroscience. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg B, Mattson S, Riley EP. Impaired alternation test performance in adult rats following prenatal alcohol exposure. Pharmacology, Biochemistry, and Behavior. 1989;32:293–299. doi: 10.1016/0091-3057(89)90246-3. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Sukel HL, Stekler JD. Spatial learning of adult rats with fetal alcohol exposure: deficits are sex-dependent. Behavioural Brain Research. 1991;42:49–56. doi: 10.1016/s0166-4328(05)80039-7. [DOI] [PubMed] [Google Scholar]
- Zink M, Ferbert T, Frank ST, Seufert P, Gebicke-Haerter PJ, Spanagel R. Perinatal exposure to alcohol disturbs spatial learning and glutamate transmission-related gene expression in the adult hippocampus. The European Journal of Neuroscience. 2011;34:457–468. doi: 10.1111/j.1460-9568.2011.07776.x. [DOI] [PubMed] [Google Scholar]