Abstract
Background
Chronic inflammatory diseases induce cachexia that increases mortality and morbidity of the illness. Adjuvant‐induced arthritis is an experimental model of rheumatoid arthritis that is associated with body weight loss and muscle wasting. Alpha‐melanocyte stimulating hormone has an anti‐inflammatory effect in arthritic rats and decreases muscle wasting. The aim of this work was to elucidate whether the anti‐cachectic action of alpha‐melanocyte stimulating hormone is mediated by the melanocortin receptor type 3 pathway.
Methods
Arthritis was induced in male Wistar rats by intradermal injection of Freund's adjuvant, and 6 days afterwards, arthritic rats were injected with the selective melanocortin receptor type 3 agonist d‐Trp(8)‐gammaMSH ( d‐Trp(8)‐γMSH) 500 µg/kg subcutaneously. or saline twice a day, for 10 days.
Results
d‐Trp(8)‐γMSH decreased the external signs of inflammation and body weight loss, but it was not able to modify the anorexigenic effect of arthritis or the increase in hypothalamic cyclooxygenase‐2 (COX‐2) expression. In contrast, d‐Trp(8)‐γMSH prevented arthritis‐induced increase in hypothalamic IL‐1β and serum corticosterone levels and the decrease in serum IGF‐I levels. d‐Trp(8)‐γMSH treatment also prevented arthritis‐induced NF‐kB(p65) phosphorylation and tumour necrosis factor‐α mRNA increase in the gastrocnemius. d‐Trp(8)‐γMSH administration to arthritic rats increased gastrocnemius mass, its cross‐sectional area, and mean fast fibre area. Those effects of d‐Trp(8)‐γMSH were associated with a decreased expression of atrogin‐1 and muscle ring‐finger protein‐1 in the gastrocnemius. In rats treated with saline, arthritis increased the expression of autophagy marker genes LC3b, Bnip‐3, and Gabarap1 as well as the conversion of LC3b I to LC3b II by lipidation in the gastrocnemius. d‐Trp(8)‐γMSH decreased gastrocnemius LC3b, Bnip‐3, and Gabarap1 mRNA expression and prevented the increase in LC3b II in arthritic rats.
Conclusion
These data suggest that d‐Trp(8)‐γMSH administration prevents the effect of arthritis on corticosterone and insulin‐like growth factor‐I serum levels and decreases muscle wasting, by down‐regulating atrogenes and autophagy through modifying the NF‐kB(p65)/tumour necrosis factor‐α signalling transduction pathway.
Keywords: Muscle wasting, γMSH, Atrogenes, Autophagy, Corticosterone, IGF‐I, NF‐kB
Introduction
Chronic inflammatory diseases such as cancer, sepsis, and rheumatoid arthritis induce cachexia, which increases morbidity and mortality. Adjuvant‐induced arthritis is an experimental model of rheumatoid arthritis that is associated with body weight loss and skeletal muscle wasting. Although inflammation and arthritis induce anorexia, skeletal muscle wasting in arthritic rats is not secondary to the decrease in food intake.1, 2 Similarly, loss of fat‐free mass in rheumatoid arthritis patients is not attributable to reduced food intake.3 It has recently been reported in other experimental models of arthritis that, although arthritic animals have decreased mobility,4 muscle atrophy is associated with the disease and not with decreased mobility.5
In inflammatory cachexia, there are several factors involved in muscle atrophy such as inflammatory mediators and neuroendocrine modifications. Inflammatory response includes an increased release of cytokines, such as tumor necrosis factor (TNF‐α), and of glucocorticoids, which are well known inducers of muscle wasting.6, 7 In addition, inflammation decreases insulin‐like growth factor I (IGF‐I) serum levels,8, 9 which is an anabolic hormone that increases muscle mass by stimulating protein synthesis and preventing muscle proteolysis.8, 10 Therefore, the increased release of cytokines and glucocorticoids along with the decreased secretion of IGF‐I might play an important role in inflammatory cachexia.
It has been postulated that in inflammatory cachexia muscle wasting is secondary to an increased rate of muscle proteolysis rather than to a decrease in muscle synthesis.11 The ubiquitin‐proteasome system plays an important role in muscle proteolysis in several chronic illnesses that are associated with cachexia.12 In arthritic rats, as in other conditions promoting muscle atrophy, the expression of two muscle‐specific Ub ligases, muscle ring‐finger protein‐1 (MuRF1) and muscle atrophy F‐box (atrogin‐1), are up‐regulated.13 In addition to the ubiquitin‐proteasome system, other proteolytic systems such as autophagy lysosomal proteases can contribute to muscle wasting in inflammatory cachexia.14
Melanocortins are peptides derived from proopiomelanocortin, which have many physiological functions that include immunomodulation and decrease of inflammatory response.15 It is well known that alpha‐melanocyte stimulating hormone (αMSH) is able to decrease inflammation both at central and peripheral levels.16 Blockade of NF‐kB activation is an important mechanism through which αMSH decreases inflammation in different cell types.17, 18 αMSH treatment has been shown to ameliorate several experimental models of inflammatory diseases such as systemic inflammatory response,19 autoimmune encephalomyelitis,20 and systemic lupus erythematosus.21 Similarly, αMSH administration decreases the clinical and histological signs of experimental arthritis.22 We have recently reported that peripheral αMSH administration to arthritic rats has anti‐inflammatory and anti‐cachectic effects, since it ameliorates arthritis‐induced anorexia and muscle proteolysis.23
There are five melanocortin receptors (MCR), MC1‐R to MC5‐R. αMSH released from the pituitary is the main MSH in plasma and is a non‐selective agonist of MCRs MC1, 2, 3, 4, and 5. Of those receptors, MCR type 3 (MC3‐R) has been reported to mediate the inhibitory properties of the melanocortins in experimental models of arthritis.24, 25 Furthermore, MC3‐R agonists have been recently reported to be a novel class of anti‐arthritic treatment, because they are able to ameliorate joint disease without unwanted side effects such as alveolar bone loss.26 Therefore, the aim of this work was to elucidate whether the anti‐cachectic effects of αMSH are mediate through activation of MC3‐R. Accordingly, arthritic rats were treated with d‐Trp(8)‐γMSH, a specific MC3‐R agonist, for 10 days. Our data indicate that d‐Trp(8)‐γMSH has an anti‐cachectic effect decreasing the arthritis‐induced increase in the expression of atrogenes and autophagic markers. Those effects can be secondary to the effects of d‐Trp(8)‐γMSH, preventing inflammation responses such as activation of NF‐kB/TNF‐α signalling, increase in glucocorticoids, and decrease in IGF‐I serum levels, which are responses that play a role in muscle wasting.
Materials and methods
Animals
Arthritic and control male Wistar rats (150 g/6 weeks old) were purchased from Charles River Laboratories (Barcelona, Spain). Arthritis was induced in the rats by an intradermal injection of 4 mg heat‐inactivated Mycobacterium butyricum in the right paw, under isoflurane anaesthesia. Control animals were injected with vehicle (0.1 mL of paraffin oil). After arriving (day 3 after adjuvant injection), rats were housed 3–4, under controlled conditions of temperature (22°C) and light (lights on from 7:30 a.m. to 7:30 p.m.). Food and water were available ad libitum. All procedures on animals were carried out according to the guidelines recommended by the European Union for the care and use of laboratory animals and were approved by the Complutense University Animal Care Committee.
On Day 6 after adjuvant injection, rats were randomly divided into four groups of 10 rats. (i) Control rats injected with 250 μL of saline i.p. twice a day (at 9:00 a.m. and at 5:00 p.m.); (ii) arthritic rats injected with saline; (iii) arthritic rats injected i.p. with 500 µg/kg d‐Trp(8)‐γMSH (American Peptide, Sunnyvale, CA, USA) dissolved in saline twice a day. At this dosage, d‐Trp(8)‐γMSH has been shown to attenuate experimental arthritis in mice.25 (iv) As arthritis decreases food intake, we included a pair‐fed group injected with saline that received the same amount of food (g/100 g body weight) eaten by the arthritic rats treated with saline on the previous day. Body weight, food intake, and arthritis index scores were examined daily. Food intake per cage was calculated by measuring the difference between the initial and the remaining amount of pellets in the feeder.
Evaluation of arthritis severity was performed by measuring the arthritis index of each animal, which was clinically scored by grading each paw from 0 to 4, since inflammation of the paw is associated with radiological and histological alterations of the joints. Grading was determined as follows: 0—no erythema or swelling, 1—slight erythema or swelling of one or more digits, 2—swelling of paw, 3—swelling of entire paw and the ankle, and 4—ankylosis, incapacity to bend the ankle. The severity score was the sum of the clinical scores of the four limbs, the maximum value being 16. After 9 days of d‐Trp(8)‐γMSH treatment and 15 days after adjuvant or vehicle injection, all rats were euthanized by decapitation between 11:30 a.m. and 12:30 p.m., in a separate room, within 30 s after being removed from their cages. Trunk blood was collected in cooled tubes, allowed to clot, centrifuged, and the serum was stored at −20°C until corticosterone and IGF‐I assays were performed. The left hind paw volume, epididymal adipose white tissue, and diaphragm were measured. Immediately after decapitation, left gastrocnemius was removed, dissected, weighed, and frozen in liquid nitrogen and stored at −80°C until RNA or protein extraction. Medial basal hypothalami were dissected as previously described,27 quickly frozen in liquid nitrogen, and stored at −80°C for RNA isolation. Isolation and manipulation of tissues were always performed under sterile conditions.
RNA extraction and real‐time PCR
Hypothalami and gastrocnemius muscles (100 mg) were homogenized, and total RNA was extracted using TriSure (Bioline, London, UK), following the manufacturer's protocol. The final concentration of RNA was determined with a BioPhotometer (Eppendorf, Germany), and the integrity of the RNA was confirmed by agarose gel electrophoresis. First‐strand cDNA synthesis was performed using 1 µg of total RNA with a Quantiscript reverse transcription kit (Qiagen Combh Hilden, Valencia, CA, USA).
Real‐time PCR for quantification of mRNA was performed on a SmartCycler® (Cepheid, Sunnyvale, CA, USA) using a SYBR Green protocol on the fluorescence temperature cycler. Each real‐time PCR reaction consisted of 10 ng total RNA equivalents, 1× Takara SYBR green premix Ex Taq (Takara BIO INC, Otsu, Shiga, Japan) and 300 nM forward and reverse primers in a reaction volume of 25 μL. Primers for real‐time PCR were obtained from Roche (Madrid, Spain) by using the EXIQON universal probe library. The thermal cycling profile consisted of a preincubation step at 95°C for 10 s followed by 40 cycles of 95°C denaturation steps for 15 s, 60°C annealing steps for 30 s, and 72°C extension steps for 30 s. Results were expressed relatively to the control animals treated with saline, where the relative mRNA abundance has been arbitrarily set to 1, using cycle threshold 2(∆∆CT) method with 18S as reference gene. PCR products were separated using agarose gel electrophoresis to confirm the product presence and size.
Western blotting analysis
Gastrocnemius was homogenized in Radio‐Immunoprecipitation Assay (RIPA) buffer (10 μL/mg) with protease inhibitors cocktail, sodium deoxycholate 12.5 mM, phenylmethane sulfonyl fluoride 100 mM, sodium orthovanadate 12.5 mM and with phosphatase inhibitors (Sigma‐Aldrich, Madrid, Spain) for pNF‐kB(p65). The homogenate was later centrifuged at 13 000 rpm at 4°C for 30 min to remove tissue debris. Protein concentration was determined using the Bradford protein assay with bovine serum albumin as standard. The protein extracts were boiled for 5 min in a 1:1 volume of Laemmli loading buffer. Proteins (100 µg) were resolved by electrophoresis on 10–20% polyacrylamide gels under reducing conditions and transferred onto nitrocellulose or polyvinylidene fluoride membranes (Bio‐Rad, Madrid, Spain) that were blocked by incubation in 5% non‐fat dry milk and 0.1% Tween (Sigma‐Aldrich), in Tris‐buffered saline. Ponceau‐S staining was performed to ensure equal transfer prior to blocking. Membranes were probed overnight at 4°C sequentially with antibodies against NF‐kB(p65) (C20), pNF‐kB(p65) ser(536), fast‐type 200 kDa myosin heavy chain (MHC) IIa, atrogin‐1, and MuRF1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), microtubule‐associated protein‐1 light chain 3b (LC3b) (Cell Signaling, Danvers, MA, USA) with stripping of membranes, using stripping buffer (Restore Western Blot Stripping Buffer, Thermo‐scientific Rockford, IL, USA) before each new antibody. Membranes were incubated for 90 min in the appropriate secondary antibody conjugated to horseradish peroxidase [anti‐mouse IgG (Amersham Biosciences, Little Chalfont, UK); anti‐rabbit IgG (GE Healthcare, Madrid, Spain); anti‐goat IgG (Santa Cruz)], and peroxidase activity was detected using enhanced chemiluminescent reagent (Thermo Scientific, Rockford, IL, USA). Band intensities were quantified by densitometry using Gene Tools analysis software.
Gastrocnemius morphology
Left gastrocnemius were dissected and weighed. For immunohistochemical studies, the medial part of the left gastrocnemius was placed on a transparency film, glued at one end to a cork with gum tragacanth (Fibraguar, Fardi, Madrid, Spain), frozen in isopentane, cooled by liquid nitrogen, and stored at −80 C. Ten micrometre cryostat sections were fixed with 100% acetone and stained with haematoxylin–eosin. Parallel sections were kept at −80°C until further processing for immunohistochemical analysis. The haematoxylin–eosin sections were scanned (Epson scanner 4990) with a transparent rule, and the area was measured with Image J software.
Muscle fibre cross‐sectional size was measured as an index of fibre atrophy. The extracellular matrix was detected by wheat germ agglutinin labelled with Texas Red (W849—Invitrogen; 1 µg/mL), and slow muscle fibres were detected with a monoclonal antibody against slow MHC form (Novocastra™ Lyophilized‐MHCs; 1:80, Newcastle upon Tyne, UK) and secondary Alexa Fluor 488 Goat anti‐mouse IgG (A11001—Invitrogen; 1:100, Invitrogen, Madrid, Spain). Sections were mounted with Prolong‐Gold anti‐fade reagent combined with 4',6‐diamidino‐2‐phenylindole (DAPI) (P36931—Invitrogen). Digital images were acquired with a Leica DMI300 microscope. Fibre boundaries were detected from wheat germ agglutinin fluorescent images using difference of Gaussians algorithm by GNU Image Manipulation Program (GIMP) software. According to the distribution of the slow fibres, two zones were differentiated on each section, one zone with only fast fibres and one mixed zone with slow and fast fibres. At least two images of the medial area (mixed area with slow and fast fibres) and two images of the lateral area (only fast fibres) from each section were used to measure the mean slow and fast fibre area with Image J software. The number of fibres measured on each section oscillated between 200 and 700.
Serum corticosterone and insulin‐like growth factor‐I measurement
Serum IGF‐I was measured using the anti‐serum to human IGF‐I (UB2‐495) from Dr Underwood and Dr Van Wik and is distributed by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Hormone Distribution Programme through the National Hormone and Pituitary Programme. Levels of IGF‐I were expressed in terms of rat IGF‐I from Gropep Ltd. (Adelaide, Australia). The intra‐assay coefficient of variation was 8%. All samples from the same experiment were run in the same assay. Serum corticosterone was analysed by a commercial kit from MP Biomedicals, LLC (New York, USA).
Statistical analysis
Statistics were computed using the statistics programme STATGRAPHICS plus for Windows. Data are presented as means ± standard error of the mean and were tested with analysis of variance; post‐hoc comparisons were made using the least significant difference multiple range test. Statistical significance was set at P < 0.05.
Results
Administration of d‐Trp(8)‐γMSH decreased arthritis scores (P < 0.01, Table 1). Similarly, d‐Trp(8)‐γMSH decreased arthritis‐induced increase in left paw volume (P < 0.01, Tabla 1). As expected, arthritis decreased body weight gain (P < 0.01, Figure 1A) compared with both control and pair‐fed rats. Administration of d‐Trp(8)‐γMSH attenuated the decrease in body weight gain, where arthritic rats injected with d‐Trp(8)‐γMSH had a body weight gain similar to pair‐fed rats. This effect was not due to an increase in food intake, since d‐Trp(8)‐γMSH did not modify the inhibitory effect of arthritis on food intake (Figure 1C). Arthritis decreased epididymal white adipose tissue (WAT) (P < 0.01, Table 1) and gastrocnemius weight (P < 0.01, Figure 1B). Pair‐feeding the rats decreased WAT weight P < 0.01, whereas it did not modify gastrocnemius weight. Arthritic rats injected with d‐Trp(8)‐γMSH had higher WAT (P < 0.05) and gastrocnemius (P < 0.01) weights than arthritic rats injected with saline.
Table 1.
Arthritis scores, left paw volume and epididymal white adipose tissue (WAT) weights in control rats, pair‐fed rats, arthritic rats (AA), and arthritic rats injected with d‐Trp(8)‐γMSH (γMSH)
| Arthritis scores | Left paw volume (mL) | Epididymal WAT (mg) | |
|---|---|---|---|
| Control | 1.4 ± 0.03 | 1428 ± 75 | |
| Pair‐fed | 1.5 ± 0.01 | 936 ± 84** | |
| AA | 10.75 ± 0.98 | 2.5 ± 0.14** , ºº | 437 ± 65** , ºº |
| AA‐γMSH | 6.75 ± 0.92++ | 1.9 ± 0.12++ , ºº | 667 ± 80+ , º |
Data represent mean ± standard error of the mean (n = 9–10).
P < 0.05.
P < 0.01 vs. control rats.
P < 0.01 vs. pair‐fed rats.
P < 0.05.
P < 0.01 vs. AA.
Figure 1.
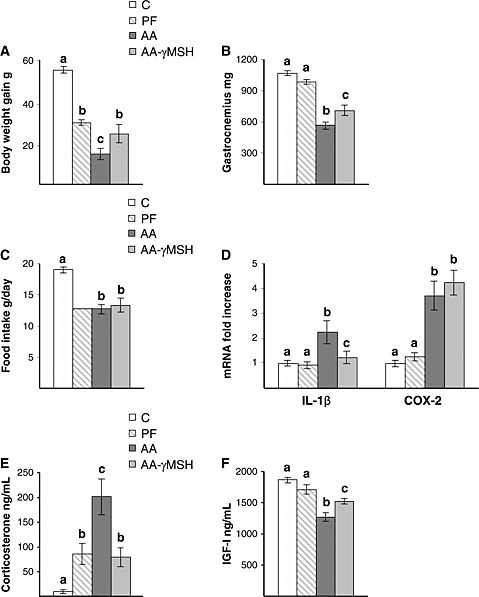
Administration of d‐Trp(8)‐γMSH (γMSH; 500 µg/kg bw. i.p. twice daily) to arthritic rats increased body weight gain (P < 0.05, A) and gastrocnemius weight (P < 0.01, B), whereas it did not modify food intake (C). Hypothalamic interleukin‐1β and cyclooxygenase‐2 expression (D) was increased in arthritic rats (P < 0.01), whereas administration of d‐Trp(8)‐γMSH to arthritic rats decreased interleukin‐1β but not cyclooxygenase‐2 expression in the hypothalamus. Arthritis increased serum concentration of corticosterone (P < 0.01) and d‐Trp(8)‐γMSH treatment prevented the increase in serum corticosterone (E). Serum concentration of insulin‐like growth factor‐I was decreased by arthritis (P < 0.01), and d‐Trp(8)‐γMSH administration increased serum insulin‐like growth factor‐I levels, P < 0.01 (F). C, control rats; AA, arthritic rats; PF, pair‐fed rats. Data represent means ± standard error of the mean (n = 7–10). Values without the same letter are significantly different. Least significant difference multiple comparison test.
Hypothalamic IL‐1β and COX‐2 expression are shown in Figure 1D. Arthritis increased both IL‐1β and COX‐2 mRNA in the hypothalamus (P < 0.01). d‐Trp(8)‐γMSH treatment prevented arthritis‐induced IL‐1β mRNA up‐regulation. In contrast, d‐Trp(8)‐γMSH was not able to modify arthritis‐induced increase in COX‐2 mRNA in the hypothalamus. Pair‐feeding rats did not modify IL‐1β or COX‐2 expression in the hypothalamus.
Serum concentrations of corticosterone were increased in arthritic rats (P < 0.01, Figure 1E). Pair‐fed rats had higher corticosterone levels than control rats (P < 0.05) but lower than arthritic rats (P < 0.01). In arthritic rats, d‐Trp(8)‐γMSH administration decreased serum corticosterone (P < 0.01) to levels similar to those observed in pair‐fed rats. Arthritis decreased serum IGF‐I concentrations (P < 0.01, Figure 1F), whereas d‐Trp(8)‐γMSH administration to arthritic rats increased serum IGF‐I to levels between those of arthritic rats injected with saline and pair‐fed rats.
As shown in Figure 2A and C, the decrease in gastrocnemius weight in arthritic rats was associated with lower cross‐sectional area (P < 0.01). Arthritis decreased fast‐type 200 kDa MHC IIa protein (P < 0.01, Figure 2E) and mean fast and slow fibre area, where the decrease was higher in fast fibre (P < 0.01, Figure 2B and D). In addition, MHC I (slow type) and MHC IIa mRNA were significantly decreased in arthritic rats P < 0.01 (Figure 2F), this decrease was also higher in MCH IIa than in MHC I mRNA (0.24 ± 0.04 vs. 0.5 ± 0.05, P < 0.01). Administration of d‐Trp(8)‐γMSH increased cross‐sectional area, mean fast fibre, MHC IIa protein, and its mRNA in the gastrocnemius muscle. In the arthritic rats treated with d‐Trp(8)‐γMSH, mean slow fibre area values were between those of pair‐fed and the arthritic rats treated with saline, whereas MHC I mRNA levels were between those of control and pair‐fed rats. Pair‐feeding the rats decreased both MHC I (P < 0.05) and IIa mRNA (P < 0.01). In contrast, pair‐feeding the rats did not modify cross‐sectional area, mean fast and slow fibre area, or MHC IIa protein.
Figure 2.
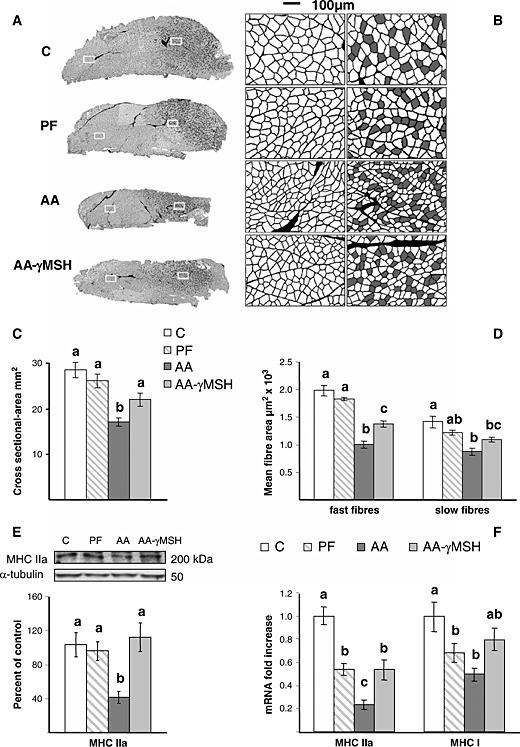
Effect of arthritis and d‐Trp(8)‐γMSH (γMSH; 500 µg/kg bw. i.p. twice daily) on gastrocnemius cross‐sectional area and myofibre size. (A) Representative cross sections of the mid‐belly region of the gastrocnemius with fibre boundaries delineated using difference of Gaussians from the wheat germ agglutinin fluorescent images. On the right side of each section, slow fibres (filled on grey from the slow type I MHC immunofluorescence) are mixed with fast fibres. (B) Amplification of the inserts marked on each cross section: the mixed region on the right and the fast fibre region on the left. (C) Average cross‐sectional areas of gastrocnemius from control, arthritic (AA), or pair‐fed (PF) rats. (D) Mean fast and slow fibre area. (E) MHC IIa protein and (F) MCH IIa and MHC I mRNA. Arthritis decreased gastrocnemius cross‐sectional area and mean fast and slow fibre cross‐sectional area, as well as MCH IIa and MCH I expression (P < 0.01). d‐Trp(8)‐γMSH increased gastrocnemius cross‐sectional area, fast fibre size, and MCH IIa expression in arthritic rats (P < 0.05). Data represent means ± standard error of the mean (n = 5–10). Values without the same letter are significantly different. Least significant difference multiple comparison test.
Arthritis increased phosphoNF‐kB(p65) levels in the gastrocnemius muscle (P < 0.01), whereas it did not modify NF‐kB(p65) levels (Figure 3A). The anti‐inflammatory effect of d‐Trp(8)‐γMSH treatment was also observed in the gastrocnemius muscle, since d‐Trp(8)‐γMSH prevented arthritis‐induced increase in NF‐kB(p65) phosphorylation (P < 0.01). Activation of NF‐kB(p65) was associated with an increase in the expression of TNF‐α in the gastrocnemius muscle (P < 0.01, Figure 3B), and d‐Trp(8)‐γMSH treatment decreased TNF‐α mRNA expression (P < 0.01). Pair‐feeding the rats did not modify NF‐kB(p65) phosphorylation or TNF‐α mRNA levels in the gastrocnemius.
Figure 3.
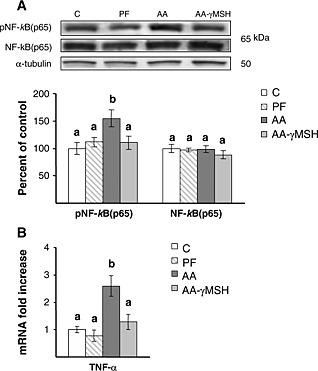
(A) Representative blots and group data for phosphoNF‐kB(p65) and NF‐kB(p65). (B) TNF‐α mRNA in gastrocnemius muscle of C, controls; PF, pair‐fed; and in AA, arthritic rats treated with saline or d‐Trp(8)‐γMSH (γMSH; 500 µg/kg bw. i.p. twice daily). Arthritis increased gastrocnemius phosphoNF‐kB(p65) (P < 0.01) and TNF‐α mRNA (P < 0.01) in rats treated with saline but not in rats treated with d‐Trp(8)‐γMSH. Data represent mean ± standard error of the mean (n = 8–10 rats). Values without the same letter are significantly different. Least significant difference multiple comparison test.
Arthritis increased muscle atrogin‐1 mRNA and protein (P < 0.01, Figure 4B and D), and d‐Trp(8)‐γMSH treatment reduced this increase, but atrogin‐1 levels in arthritic rats treated with d‐Trp(8)‐γMSH were still higher than those observed in control or pair‐fed rats (P < 0.01). MuRF1 mRNA and protein were also increased in the gastrocnemius muscle of arthritic rats (P < 0.01, Figure 4A and C). Administration of d‐Trp(8)‐γMSH to arthritic rats prevented the increase in muscle MuRF1 protein and attenuated that of MuRF1 mRNA.
Figure 4.
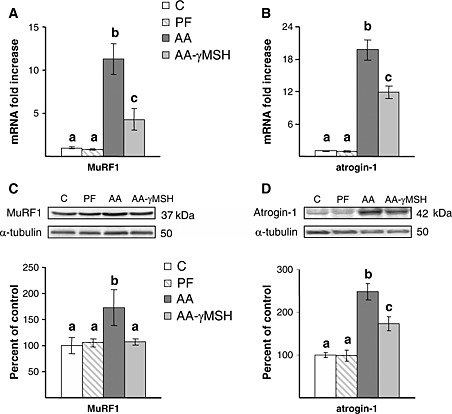
Effect of arthritis and d‐Trp(8)‐γMSH treatment (γMSH; 500 µg/kg bw. i.p. twice daily) on gastrocnemius muscle ring‐finger protein 1 mRNA (A), atrogin‐1 mRNA (B), muscle ring‐finger protein 1 protein (C) and atrogin‐1 protein (D). d‐Trp(8)‐γMSH administration decreased arthritis‐induced increase in muscle ring‐finger protein 1 and atrogin‐1 mRNA, as well as atrogin‐1 protein (P < 0.01). Arthritis increased muscle ring‐finger protein 1 protein in rats treated with saline (P < 0.05) but not in those treated with d‐Trp(8)‐γMSH. C, control rats; AA, arthritic rats; PF, pair‐fed rats. Data represent means ± standard error of the mean (n = 7–9). Values without the same letter are significantly different. Least significant difference multiple comparison test.
Muscle expression of autophagy marker genes: LC3b, BCL2/adenovirus E1B 19 kDa protein‐interacting protein 3 (Bnip‐3), and gamma‐aminobutyric acid receptor‐associated protein (Gabarap1) were increased in arthritic rats (P < 0.01, Figure 5A), whereas pair‐feeding the rats did not modify their expression in the gastrocnemius. d‐Trp(8)‐γMSH administration to arthritic rats prevented the increase in Bnip‐3 mRNA (P < 0.01) and decreased LC3b and Gabarap1 mRNA levels (P < 0.01) to values between those of control and arthritic rats treated with saline. Arthritis also increased both the protein LC3b I and the phospholipid‐associated form LC3b II (P < 0.01, Figure 5B). d‐Trp(8)‐γMSH administration prevented the stimulatory effect of arthritis on LC3b I lipidation, where the arthritic rats treated with d‐Trp(8)‐γMSH had levels of LC3b II similar to those of pair‐fed and control rats.
Figure 5.
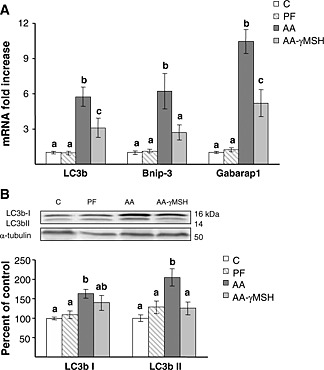
Autophagy is up‐regulated in gastrocnemius muscle of arthritic rats. (A) Autophagy‐related genes LC3b, Bnip‐3, and Gabarap1 mRNA. d‐Trp(8)‐γMSH (γMSH) treatment (500 µg/kg bw. i.p. twice daily) decreased arthritis‐induced increased expression of the autophagy‐related genes (P < 0.01). (B) Representative blots and group data for the autophagic marker LC3b I and LC3b II. Arthritis increased LC3b I and LC3b II levels (P < 0.01), and d‐Trp(8)‐γMSH administration decreased LC3b II (P < 0.05). C, control rats; AA, arthritic rats; PF, pair‐fed rats. Data represent means ± standard error of the mean (n = 7–10). Values without the same letter are significantly different. Least significant difference multiple comparison test.
Discussion
Our data show that d‐Trp(8)‐γMSH administration to arthritic rats decreased inflammation, whereas it attenuated arthritis‐induced decrease in body weight and muscle wasting. The anti‐cachectic effect of d‐Trp(8)‐γMSH was not secondary to an increase in food intake, since d‐Trp(8)‐γMSH was not able to modify the anorexic effect of arthritis. The role of MC3‐R on food intake regulation is not well known. Peripheral d‐Trp(8)‐γMSH administration to normal mice increases food intake, but this stimulation was of short duration (4–6 h), and with repeated daily injections of this compound, the stimulatory effect of food intake was not observed.28 However, it has been proposed that activation of MC3‐R in the hypothalamus has an anorexigenic effect.29
In accordance with our data, an effect of MC3‐R on cancer‐induced cachexia has been reported but not on food intake.30 In tumour‐bearing mice, blockade of MC3‐R increased tumour‐induced cachexia, but it did not modify the anorexigenic effect of cancer.30 Furthermore, deletion of the MC3‐R produces an obesity syndrome with a loss in lean mass and increase in adipose mass, without the behavioural hyperphagia seen in the MC4‐R—null mice.31, 32 All these data suggest that MC3‐R activation in experimental cancer or arthritis ameliorates muscle wasting, although it does not prevent anorexia induced by the illnesses. In contrast, αMSH treatment is able to decrease anorexia induced by experimental arthritis or lipopolysaccharide (LPS) injection.23, 33 Differences in the effects of the two melanocortins, αMSH and d‐Trp(8)‐γMSH, on food intake can be due to their different receptor affinities; d‐Trp(8)‐γMSH is a specific MC3‐R agonist, whereas αMSH is a pan‐agonist of the MCRs.
Both αMSH and d‐Trp(8)‐γMSH have anti‐inflammatory effects in arthritic rats; they decrease the external signs of arthritis and prevent arthritis‐induced increase in the expression of proinflammatory cytokine, IL‐1β in the hypothalamus (present data and those of Gómez‐SanMiguel et al.23). However, d‐Trp(8)‐γMSH, unlike αMSH, was unable to prevent the increased expression of COX‐2 in the hypothalamus of arthritic rats. Taking into account the role of COX‐2 in inflammatory anorexia,34, 35 the lack of effect of d‐Trp(8)‐γMSH treatment on hypothalamic COX‐2 induction can explain the different effects of both melanocortins on inflammatory anorexia.
d‐Trp(8)‐γMSH treatment prevented the effect of chronic arthritis on two hormones that play an important role in the regulation of muscle mass: corticosterone and IGF‐I. This effect can contribute to the anti‐cachectic action of d‐Trp(8)‐γMSH. The relationship between MC3‐R and the adrenal secretion has been previously reported. Administration of γMSH prevents the stimulatory effect of IL‐1β on corticosterone secretion.36 In addition, MC3‐R deficiency was found to produce mild hypercorticosteronemia.30, 32 To our knowledge, present findings demonstrate for the first time that d‐Trp(8)‐γMSH can prevent the inhibitory effect of chronic inflammation on serum IGF‐I levels.
As previously reported, d‐Trp(8)‐γMSH decreased clinical signs of experimental arthritis.25, 26 The anti‐inflammatory effect of d‐Trp(8)‐γMSH treatment was also evident in the gastrocnemius muscle. Administration of d‐Trp(8)‐γMSH was able to prevent arthritis‐induced activation of TNF‐α/NF‐kB pathway in muscle, since it decreased phosphorylation of the transcription factor p65 and TNF‐α expression. As mentioned above, an important mechanism by which αMSH decreases inflammation in different cell types is by blocking NF‐kB activation.17, 18 In this sense, we have recently reported that αMSH prevents endotoxin‐induced NF‐kB(p65) phosphorylation in the gastrocnemius.33 These data and the fact that MC3‐R is expressed in skeletal muscle37 suggest that the inhibitory effect of αMSH on NF‐kB activation is mediated through MC3‐R activation. Furthermore, it has been postulated that MC3‐R is the predominant anti‐inflammatory receptor for melanocortins.24, 25, 26, 38
Our data show that fast fibre was affected more than slow fibre in arthritic rats and are in accordance with the fact that in cachexia induced by inflammatory illness, fast glycolytic muscles are more prone to atrophy than slow oxidative muscles.8, 39 d‐Trp(8)‐γMSH administration attenuated arthritis‐induced decrease in gastrocnemius mass, cross‐sectional area, mean fast fibre area, and MHC IIa levels. The role of the NF‐kB transcription factors in the induction of muscle atrophy under conditions of cachexia, associated with increased expression of the E3 ligases MuRF1 and atrogin‐1, is well known.40, 41 Therefore, the anti‐cachectic effect of d‐Trp(8)‐γMSH treatment can be, in part, secondary to its effect on TNF‐α/NF‐kB pathway, decreasing ubiquitin‐proteasome activity. Similarly, αMSH administration prevents the activation of muscle TNF‐α/NF‐kB pathway and increased expression of atrogenes in rats injected with LPS.33
Arthritis increased the expression of several genes related to autophagy LC3b, Bnip‐3, and Gabarap1. Furthermore, the increased expression of these genes was associated with an increase in both forms of the protein LC3b I and II. When autophagy is acutely induced, levels of LC3b I decrease, because the protein is converted to LC3b II through lipidation. Therefore, the LC3bII/LC3bI ratio increases. However, when autophagy is chronically activated, the novo synthesis of LC3b I can offset its decrease, and both proteins are increased, as has been previously reported by other authors.14 These data suggest that, in addition to the proteasome system, muscle wasting in arthritic rats also seems to be secondary to an increase in autophagy.
d‐Trp(8)‐γMSH treatment to arthritic rats was also able to decrease muscle expression of autophagy genes and LC3b I conversion to LC3b II. The Akt/FoxO3 pathway has been involved in both autophagic and proteasomal degradation pathways in skeletal muscle.42 FoxO3 activation drives the expression of key autophagic genes such as LC3, Bnip‐3, and Gabarap1.43 However, we have previously reported that adjuvant‐induced arthritis does not change Akt or FoxO3 phosphorylation in gastrocnemius,44 suggesting no involvement of this mechanism in activating autophagy or atrogene up‐regulation in gastrocnemius. Autophagy in arthritis can be induced through NF‐kB activation, as has recently reported in hepatocytes after LPS administration.45 Therefore, the effect of d‐Trp(8)‐γMSH on atrogenes and autophagy in arthritic rats could be secondary to its effect in preventing NF‐kB(p65) activation in muscle.
In this study, we report that d‐Trp(8)‐γMSH exerts anti‐inflammatory activities and decreases muscle wasting, down‐regulating atrogene and autophagy in an experimental model of rheumatoid arthritis. Those effects can be due to the fact that d‐Trp(8)‐γMSH decreases the effect of arthritis on IGF‐I and glucocorticoid secretion as well as on muscle NF‐kB(p65)/TNF‐α signalling transduction pathway. Our data support d‐Trp(8)‐γMSH as a novel potential therapeutic agent for clinical use in patients with chronic inflammatory illnesses, such as rheumatoid arthritis, in order to decrease inflammatory responses and to preserve body weight and muscle mass.
Funding
MINECO n° BFU2012‐38468 and Complutense University of Madrid for ABGSM.
Conflict of interest
None declared.
Acknowledgements
The authors are indebted to Christina Bickart for the English correction of the manuscript.
The authors certify that they have complied with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle (von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle 2010;1:7–8).
Gómez‐SanMiguel, A. B. , Martín, A. I. , Nieto‐Bona, M. P. , Fernández‐Galaz, C. , Villanúa, M. Á. , and López‐Calderón, A. (2016) The melanocortin receptor type 3 agonist d‐Trp(8)‐γMSH decreases inflammation and muscle wasting in arthritic rats. Journal of Cachexia, Sarcopenia and Muscle, 7: 79–89. doi: 10.1002/jcsm.12036.
References
- 1. Martín A, Castillero E, Granado M, López‐Menduiña M, Villanúa MA, López‐Calderón A Adipose tissue loss in adjuvant arthritis is associated with a decrease in lipogenesis, but not with an increase in lipolysis. J Endocrinol 2008; 197: 111–119. [DOI] [PubMed] [Google Scholar]
- 2. Castillero E, Martín AI, López‐Menduiña M, Granado M, Villanúa MA, López‐Calderón A IGF‐I system, atrogenes and myogenic regulatory factors in arthritis induced muscle wasting. Mol Cell Endocrinol 2009; 309: 8–16. [DOI] [PubMed] [Google Scholar]
- 3. Binymin K, Herrick A, Carlson G, Hopkins S The effect of disease activity on body composition and resting energy expenditure in patients with rheumatoid arthritis. J Inflamm Res 2011; 4: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Filippin LI, Teixeira VN, Viacava PR, Lora PS, Xavier LL, Xavier RM Temporal development of muscle atrophy in murine model of arthritis is related to disease severity. J Cachexia Sarcopenia Muscle 2013; 4: 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Oliveira Nunes Teixeira V, Filippin LI, Viacava PR, de Oliveira PG, Xavier RM Muscle wasting in collagen‐induced arthritis and disuse atrophy. Exp Biol Med (Maywood) 2013; 238: 1421–1430. [DOI] [PubMed] [Google Scholar]
- 6. Schakman O, Kalista S, Barbé C, Loumaye A, Thissen JP Int J Biochem & Cell Biol 2013; 45: 2163–2172. [DOI] [PubMed] [Google Scholar]
- 7. Bonaldo P, Sandri M Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech 2013; 6: 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. López‐Menduiña M, Martín AI, Castillero E, Villanúa MA, López‐Calderón A Systemic IGF‐I administration attenuates the inhibitory effect of chronic arthritis on gastrocnemius mass and decreases atrogin‐1 and IGFBP‐3. Am J Physiol Regul Integr Comp Physiol 2010; 299: R541–551. [DOI] [PubMed] [Google Scholar]
- 9. Castillero E, López‐Menduiña M, Martín AI, Villanúa MÁ, López‐Calderón A Comparison of the effects of the n‐3 polyunsaturated fatty acid eicosapentaenoic and fenofibrate on the inhibitory effect of arthritis on IGF1. J Endocrinol 2011; 2103: 361–368. [DOI] [PubMed] [Google Scholar]
- 10. Clemmons DR Role of IGF‐I in skeletal muscle mass maintenance. Trends Endocrinol Metab 2009; 20: 349–356. [DOI] [PubMed] [Google Scholar]
- 11. Klaude M, Mori M, Tjäder I, Gustafsson T, Wernerman J, Rooyackers O Protein metabolism and gene expression in skeletal muscle of critically ill patients with sepsis. Clin Sci (Lond) 2012; 122: 133–142. [DOI] [PubMed] [Google Scholar]
- 12. Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 2004; 18: 39–51. [DOI] [PubMed] [Google Scholar]
- 13. Granado M, Priego T, Martín AI, Villanúa MA, López‐Calderón A Ghrelin receptor agonist GHRP‐2 prevents arthritis‐induced increase in E3 ubiquitin‐ligating enzymes MuRF1 and MAFbx gene expression in skeletal muscle. Am J Physiol Endocrinol Metab 2005; 289: E1007–1014. [DOI] [PubMed] [Google Scholar]
- 14. Hosokawa S, Koseki H, Nagashima M, Maeyama Y, Yomogida K, Mehr C, et al. Title efficacy of phosphodiesterase 5 inhibitor on distant burn‐induced muscle autophagy, microcirculation, and survival rate. Am J Physiol Endocrinol Metab 2013; 304: E922–933. [DOI] [PubMed] [Google Scholar]
- 15. Catania A, Lonati C, Sordi A, Carlin A, Leonardi P, Gatti S The melanocortin system in control of inflammation. Sci World J 2010; 10: 1840–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Macaluso A, McCoy D, Ceriani G, Watanabe T, Biltz J, Catania A, et al. Antiinflammatory influences of alpha‐MSH molecules: central neurogenic and peripheral actions. J Neurosci 1994; 14: 2377–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Manna SK, Aggarwal BB Alpha‐melanocyte‐stimulating hormone inhibits the nuclear transcription factor NF‐kappa B activation induced by various inflammatory agents. J Immunol 1998; 161: 2873–2880. [PubMed] [Google Scholar]
- 18. Brzoska T, Luger TA, Maaser C, Abels C, Böhm M Alpha‐melanocyte‐stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune‐mediated inflammatory diseases. Endocr Rev 2008; 29: 581–602. [DOI] [PubMed] [Google Scholar]
- 19. Bitto A, Polito F, Altavilla D, Irrera N, Giuliani D, Ottani A, et al. Melanocortins protect against multiple organ dysfunction syndrome in mice. Br J Pharmacol 2011; 162: 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taylor AW, Kitaichi N The diminishment of experimental autoimmune encephalomyelitis (EAE) by neuropeptide alpha‐melanocyte stimulating hormone (alpha‐MSH) therapy. Brain Behav Immun 2008; 22: 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Botte DA, Noronha IL, Malheiros DM, Peixoto TV, de Mello SB Alpha‐melanocyte stimulating hormone ameliorates disease activity in an induced murine lupus‐like model. Clin Exp Immunol 2014;doi:10.1111/cei.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ceriani G, Diaz J, Murphree S, Catania A, Lipton JM The neuropeptide alpha‐melanocyte‐stimulating hormone inhibits experimental arthritis in rats. Neuroimmunomodulation 1994; 1: 28–32. [DOI] [PubMed] [Google Scholar]
- 23. Gómez‐SanMiguel AB, Martín AI, Nieto‐Bona MP, Fernández‐Galaz C, López‐Menduiña M, Villanúa MÁ, et al. Systemic α‐melanocyte‐stimulating hormone administration decreases arthritis‐induced anorexia and muscle wasting. Am J Physiol Regul Integr Comp Physiol 2013; 304: R877–86. [DOI] [PubMed] [Google Scholar]
- 24. Getting SJ, Christian HC, Flower RJ, Perretti M Activation of melanocortin type 3 receptor as a molecular mechanism for adrenocorticotropic hormone efficacy in gouty arthritis. Arthritis Rheum 2002; 46: 2765–2775. [DOI] [PubMed] [Google Scholar]
- 25. Patel HB, Bombardieri M, Sampaio AL, D'Acquisto F, Gray M, Grieco P, et al. Anti‐inflammatory and antiosteoclastogenesis properties of endogenous melanocortin receptor type 3 in experimental arthritis. FASEB J 2010; 24: 4835–4843. [DOI] [PubMed] [Google Scholar]
- 26. Montero‐Melendez T, Madeira MF, Norling LV, Alsam A, Curtis MA, da Silva TA, et al. Association between periodontal disease and inflammatory arthritis reveals modulatory functions by melanocortin receptor type 3. Am J Pathol 2014; doi:10.1016/j.ajpath.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. López‐Calderón A, Ariznavarreta C, Chen CL Influence of chronic restraint stress on pro‐opiomelanocortin mRNA and beta‐endorphin in the rat hypothalamus. J Mol Endocrinol 1991; 7: 197–204. [DOI] [PubMed] [Google Scholar]
- 28. Marks DL, Hruby V, Brookhart G, Cone RD The regulation of food intake by selective stimulation of the type 3 melanocortin receptor (MC3R). Peptides 2006; 27: 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rowland NE, Schaub JW, Robertson KL, Andreasen A, Haskell‐Luevano C Effect of MTII on food intake and brain c‐Fos in melanocortin‐3, melanocortin‐4, and double MC3 and MC4 receptor knockout mice. Peptides 2010; 31: 2314–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marks DL, Butler AA, Turner R, Brookhart GB, Cone RD Differential role of melanocortin receptor subtypes in cachexia. Endocrinology 2003; 144: 1513–1523. [DOI] [PubMed] [Google Scholar]
- 31. Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, et al. Inactivation of the mouse melanocortin‐3 receptor results in increased fat mass and reduced lean body mass. Nat Genet 2000; 26: 97–102. [DOI] [PubMed] [Google Scholar]
- 32. Renquist BJ, Murphy JG, Larson EA, Olsen D, Klein RF, Ellacott KL, et al. Melanocortin‐3 receptor regulates the normal fasting response. Proc Natl Acad Sci U S A 2012; 109: E1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martín AI, Gómez‐SanMiguel AB, Gómez‐Moreira C, Villanúa MA, López‐Calderón A αMSH blunts endotoxin‐induced MuRF1 and atrogin‐1 upregulation in skeletal muscle by modulating NF‐kB and Akt/FoxO1 pathway. Mediators of inflamm 2014;doi:10.1155/2014/179368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson PM, Vogt SK, Burney MW, Muglia LJ COX‐2 inhibition attenuates anorexia during systemic inflammation without impairing cytokine production. Am J Physiol Endocrinol Metab 2002; 282: E650–656. [DOI] [PubMed] [Google Scholar]
- 35. Granado M, Martín AI, Villanúa MA, López‐Calderón A Experimental arthritis inhibits the insulin‐like growth factor‐I axis and induces muscle wasting through cyclooxygenase‐2 activation. Am J Physiol Endocrinol Metab 2007; 292: E1656–1665. [DOI] [PubMed] [Google Scholar]
- 36. Cragnolini AB, Perelló M, Schiöth HB, Scimonelli TN alpha‐MSH and gamma‐MSH inhibit IL‐1beta induced activation of the hypothalamic‐pituitary‐adrenal axis through central melanocortin receptors. Regul Pept 2004; 122: 185–190. [DOI] [PubMed] [Google Scholar]
- 37. Chhajlani V Distribution of cDNA for melanocortin receptor subtypes in human tissues. Biochem Mol Biol Int 1996; 38: 73–80. [PubMed] [Google Scholar]
- 38. Getting SJ, Christian HC, Lam CW, Gavins FN, Flower RJ, Schiöth HB, et al. Redundancy of a functional melanocortin 1 receptor in the anti‐inflammatory actions of melanocortin peptides: studies in the recessive yellow (e/e) mouse suggest an important role for melanocortin 3 receptor. J Immunol 2003; 170: 3323–3330. [DOI] [PubMed] [Google Scholar]
- 39. Minnaard R, Drost MR, Wagenmakers AJ, van Kranenburg GP, Kuipers H, Hesselink MK Skeletal muscle wasting and contractile performance in septic rats. Muscle Nerve 2005; 31: 339–348. [DOI] [PubMed] [Google Scholar]
- 40. Cai D, Frantz JD, Tawa NE Jr, Melendez PA, Oh BC, Lidov HG, et al. IKKbeta/ NF‐kappaB activation causes severe muscle wasting in mice. Cell 2004; 119: 285–298. [DOI] [PubMed] [Google Scholar]
- 41. Rhoads MG, Kandarian SC, Pacelli F, Doglietto GB, Bossola M Expression of NF‐kappaB and IkappaB proteins in skeletal muscle of gastric cancer patients. Eur J Cancer 2010; 46: 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Neel BA, Lin Y, Pessin JE Skeletal muscle autophagy: a new metabolic regulator. Trends Endocrinol Metab 2013; 24: 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab 2007; 6: 472–483. [DOI] [PubMed] [Google Scholar]
- 44. Castillero E, Nieto‐Bona MP, Fernández‐Galaz C, Martín AI, López‐Menduiña M, Granado M, et al. Fenofibrate, a PPAR alpha agonist, decreases atrogenes and myostatin expression and improves arthritis‐induced skeletal muscle atrophy. Am J Physiol Endocrinol Metab 2011; 300: E790–799. [DOI] [PubMed] [Google Scholar]
- 45. Chen C, Deng M, Sun Q, Loughran P, Billiar TR, Scott MJ Lipopolysaccharide stimulates p62‐dependent autophagy‐like aggregate clearance in hepatocytes. Biomed Res Int 2014; doi:10.1155/2014/267350. [DOI] [PMC free article] [PubMed] [Google Scholar]


