Summary
BALB/c mice develop a patent state [release of microfilariae (Mf), the transmission life‐stage, into the periphery] when exposed to the rodent filariae Litomosoides sigmodontis. Interestingly, only a portion of the infected mice become patent, which reflects the situation in human individuals infected with Wuchereria bancrofti. Since those individuals had differing filarial‐specific profiles, this study compared differences in immune responses between Mf+ and Mf– infected BALB/c mice. We demonstrate that cultures of total spleen or mediastinal lymph node cells from Mf+ mice produce significantly more interleukin‐5 (IL‐5) to filarial antigens but equal levels of IL‐10 when compared with Mf– mice. However, isolated CD4+ T cells from Mf+ mice produced significantly higher amounts of all measured cytokines, including IL‐10, when compared with CD4+ T‐cell responses from Mf– mice. Since adaptive immune responses are influenced by triggering the innate immune system we further studied the immune profiles and parasitology in infected Toll‐like receptor‐2‐deficient (TLR2−/−) and TLR4−/− BALB/c mice. Ninety‐three per cent of L. sigmodontis‐exposed TLR4−/− BALB/c mice became patent (Mf+) although worm numbers remained comparable to those in Mf+ wild‐type controls. Lack of TLR2 had no influence on patency outcome or worm burden but infected Mf+ mice had significantly lower numbers of Foxp3+ regulatory T cells and dampened peripheral immune responses. Interestingly, in vitro culturing of CD4+ T cells from infected wild‐type mice with granulocyte–macrophage colony‐stimulating factor‐derived TLR2−/− dendritic cells resulted in an overall diminished cytokine profile to filarial antigens. Hence, triggering TLR4 or TLR2 during chronic filarial infection has a significant impact on patency and efficient CD4+ T‐cell responses, respectively.
Keywords: cytokines, immune regulation, Litomosoides sigmodontis, microfilariae, Toll‐like receptor 2, Toll‐like receptor 4
Abbreviations
- APC
antigen‐presenting cell
- DC
dendritic cell
- FCS
fetal calf serum
- GM‐CSF
granulocyte–macrophage colony‐stimulating factor
- IL‐5
interleukin‐5
- LsAg
L. sigmodontis worm antigen preparation
- medLN
mediastinal lymph nodes
- Mf
microfilariae
- PBMC
peripheral blood mononuclear cells
- p.i.
post infection
- TC
thoracic cavity
- Th2
T helper type 2
- TLR2
Toll‐like receptor 2
- Treg
regulatory T
- WT
wild‐type
Introduction
Filarial infections in man can persist for years due to the strategies developed by the nematodes to ensure their survival.1, 2, 3 As with other chronic helminth infections, they strive for a homeostatic milieu and strong regulatory responses by the host.3, 4, 5, 6, 7 To understand the principle immune players research has focused on observing changes in parasitology and host pathology upon removal of innate or adaptive systems in vivo. For studies on filarial nematodes this can be achieved by exposing mice to the rodent‐specific species, Litomosoides sigmodontis, which is the only filarial species that develops patent [release of microfilariae (Mf) into the periphery] infections in BALB/c mice but not other strains such as C3H or C57BL/6 mice.8, 9, 10, 11 T helper type 2 (Th2) responses in infected BALB/c mice are critical for controlling L. sigmodontis development because their absence results in higher worm burden and extended microfilaraemia.12 Moreover, Th2 responses were shown to be dominant in Mf+ BALB/c mice and interleukin‐5 (IL‐5) was further implicated in the destruction of adult worms through neutrophils.13 Indeed, the absence of IL‐5 extends and enhances Mf levels, indicating that this Th2 cytokine is critical for mediating the host–helminth balance.13, 14 Nevertheless, the factors responsible for initiating these filarial‐specific responses remain unclear. It is well accepted that Toll‐like‐receptors (TLR) and other pathogen recognition receptors play a critical role in initiating innate immune responses, which in turn create appropriate adaptive immune reactions.15, 16 Helminths comprise of a multitude of (glyco)‐proteins and (glyco)‐lipids and components have been shown to trigger pathogen recognition receptors or even alter TLR‐mediated responses.17, 18, 19, 20, 21, 22 To fully understand filarial infections, one must also take into account the role of Wolbachia, an endosymbiotic third partner, which is essential for worm fecundity and survival.23 In vitro studies have shown that filarial‐mediated macrophage tolerance was dependent on MyD88 and promoted by Wolbachia.24 Further studies have also shown that Wolbachia triggers TLR‐associated innate pathways24, 25, 26, 27 but few studies have addressed the role of TLR during chronic L. sigmodontis infections, especially in the development of filarial‐specific CD4+ T‐cell responses.
Immune responses to Mf are of considerable importance because transmission of this life‐stage to blood‐feeding vectors is paramount to their survival. Interestingly, only 50% of asymptomatic Wuchereria bancrofti‐infected individuals become Mf+ 28 and as the Mf– group showed no overt signs of pathology this cohort remained largely undetected for many years. Although the reasons behind this phenomenon remain unclear the immunological profiles of these two cohorts are distinct.29 For example, when compared with Mf– individuals, peripheral blood mononuclear cells (PBMC) isolated from Mf+ individuals displayed decreased tumour necrosis factor‐α (TNF‐α), IL‐10 and IL‐5 upon filarial‐specific re‐stimulation.29 However, in man, it is difficult to differentiate a true amicrofilaraemic status from pre‐patent infections (adult worms are not yet fecund), or situations where only single sex infections exist or when worms are too old to produce further offspring or where Mf levels are simply low due to recent ivermectin treatment.30 Interestingly, the partial development of patent infections also occurs in L. sigmodontis‐infected BALB/c mice.8, 11 Therefore, using this susceptible murine model we first addressed whether different systemic and local immune responses occurred in Mf+ and Mf– infected BALB/c mice and second, whether lack of either TLR2 or TLR4 during infection altered these adaptive responses and in addition effected parasitology. With regards to the first, all IL‐5 responses to worm antigen or live Mf were higher in Mf+ mice when compared with Mf– mice. On the other hand, IL‐10 levels remained comparable and systemic TNF‐α responses to L. sigmodontis worm antigen preparation (LsAg) were higher in Mf– mice. Furthermore, isolated CD4+ T cells from Mf+ mice showed significantly elevated TNF‐α, IL‐5, IL‐13 and IL‐10 responses upon re‐stimulation with worm antigen. These responses were significantly diminished however when using TLR2−/− antigen‐presenting cells (APC); a phenomenon not observed with TLR4‐deficient APC. Interestingly, although infected TLR2−/− mice had no changes in parasitology per se, lack of TLR4 during infection enhanced transmissibility as > 93% of infected mice became patent. Hence, TLR4 and TLR2 play a crucial role during L. sigmodontis infection by facilitating patency and influencing filarial‐specific CD4+ T‐cell responses, respectively.
Material and methods
Ethics statement
This animal study was conducted in accordance with an application to perform in vivo experiments (license number 8.87‐50.10.31.09.096) and was approved by the local government authorities: Landesamt für Natur, Umwelt und Verbraucherschutz NRW, Germany. Mice were kept under specific pathogen‐free conditions at the IMMIP, University Hospital of Bonn, and husbandry was in accordance with the German Tierschutzgesetz (German animal protection laws) and the EU guidelines 2010/63/E4.
Animal maintenance and infections with L. sigmodontis
Wild‐type (WT) BALB/c mice were bred in house alongside TLR2‐ and TLR4‐deficient strains, also on a BALB/c background. Deficient strains were obtained from CNRS Orléans, France. The life‐cycle was maintained in house using infected cotton rats and recovered adult worms were used as the source of LsAg preparation. In brief, adult worms were rinsed with ice‐cold sterile endotoxin‐free PBS (PAA, Linz, Austria) before being mechanically minced. Insoluble material was removed by centrifugation at 300 g for 10 min at 4°C. Protein concentrations of antigenic extracts were determined using the Advanced Protein Assay (Cytoskeleton, ORT, USA). Aliquots of sterile LsAg were frozen at − 80°C until required. Natural mouse infections were performed as previously described.31
Parasite recovery and purification of peripheral blood Mf
Worms were recovered from the thoracic cavity (TC) of individually infected female mice on days 35, 49 and 72 post infection (p.i.). In brief, the TC was flushed with sterile PBS and expulsed worms were retained by passing through a gauze before storing in 4% formalin. Worm number and gender were determined microscopically.31 To determine the presence of Mf, 50 µl EDTA‐treated peripheral blood was stained using Hinkelmann's solution and examined for the number of Mf.12, 31 The Mf for in vitro experiments were isolated from the peripheral blood of L. sigmodontis‐infected cotton rats.32 In brief, blood was diluted 1 : 2 with PBS and carefully loaded onto a 30%–25% Percoll–sucrose gradient (Sigma‐Aldrich GmbH, Munich, Germany). After centrifugation (300 g, 30 min, room temperature, without brake), the recovered Mf were washed twice with non‐supplemented RPMI‐1640 medium (PAA), counted and frozen at − 80°C in medium containing 6% DMSO and 15% fetal calf serum (FCS) (all PAA).33 For each experiment, fresh aliquots of frozen Mf were thawed and controlled for Mf viability microscopically after 30 min of pre‐incubation at 37°C in RPMI‐1640 medium containing 10% FCS. Only aliquots with more than 95% Mf viability were used.
Dendritic cell preparation
Bone‐marrow‐derived dendritic cells (DC) were generated as previously described.18 In brief, recovered bone marrow was washed, depleted of erythrocytes and then differentiated over 7 days in Iscove's modified Dulbecco's medium supplemented with 10% FCS, 1% penicillin/streptomycin, 1% l‐glutamine, 1% non‐essential amino acids, 1% sodium pyruvate, 1% sodium bicarbonate (all from PAA) and 10 µg/ml granulocyte–macrophage colony‐stimulating factor (GM‐CSF; Peprotech, Rocky Hill, NJ).
Cytokine determination
Cytokine concentrations in the culture supernatants of re‐stimulation assays were determined by ELISA according to the manufacturer's instructions (TNF‐α and IL‐13, R&D Systems, Wiesbaden‐Nordenstadt, Germany; IL‐5 and IL‐10, BD Biosciences, Heidelberg, Germany). ELISA plates were read and analysed at 450 and 570 nm using a Spectra Max 340pc384 photometer and softmax pro 3.0 software (from Molecular Devices, Sunnyvale, CA). Cytokine concentrations in the TC fluid were measured using a FlowCytomixPro Fluorescent Bead Immunoassay kit (eBioscience, San Diego, CA) in accordance with the manufacturer's instructions. Samples were analysed using the FACS Canto II (BD Biosciences). Data were analysed with flowcytomix pro 3.0 software (eBioscience).
In vitro filarial‐specific T helper immune responses
Bulk spleen and mediastinal lymph node re‐stimulation assays
Using 96‐well round‐bottomed plates, erythrocyte‐depleted mediastinal lymph nodes (medLN) or spleen cells from individual mice were plated (5·0 × 105) in RPMI‐1640 medium containing 10% FCS, 1% penicillin/streptomycin, 1% l‐glutamine, 0·1% gentamycin (all PAA). Cells were left either unstimulated (medium control) or were stimulated with αCD3/αCD28 (5 µg/1·25 µg/ml) (eBioscience), LsAg (50 µg/ml) or 5000 or 25 000 live Mf for 72 hr at 37°C.
CD4+ T‐cell co‐cultures
The GM‐CSF‐derived bone‐marrow‐derived DC from naive WT, TLR2−/− or TLR4−/− mice were plated at 1·5 × 104 cells/well and left to settle for 2 hr. As previously described,34 CD4+ splenic T cells from infected mice were isolated using the FACS DIVA cell sorter (BD Biosciences) after staining cells with anti‐CD4 allophycocyanin‐conjugated antibodies (eBioscience). Then, 4·0 × 105 CD4+ T cells/well were co‐cultured with DC and left either un‐stimulated or activated with LsAg (50 µg/ml) or αCD3/αCD28 (5 µg/1·25 µg/ml). After 72 hr, all cell culture supernatants were removed and analysed for levels of cytokines.
Flow cytometry staining of Foxp3+ regulatory T cells
Absolute cell counts from the spleen and medLN of individual mice were determined using the CASY® Cell Counter and Analyser System Model TT (Roche Innovatis AG, Reutlingen, Germany). Staining of regulatory T (Treg) cells was performed on medLN and spleen cells as previously described.34 After blocking (anti‐CD16/CD32), cells were stained with phycoerythrin‐conjugated anti‐CD25 and allophycocyanin‐conjugated anti‐CD4 mAb. Intracellular Foxp3 levels were detected using the PE‐Cy7 conjugated anti‐Foxp3 mAb staining kit (eBioscience). Expression levels were determined using the FACS Canto flow cytometer and FACS DIVA software (BD Biosciences).34
Statistical analysis
Statistical differences were determined using graphpad prism 5 software (San Diego, CA). Parametrically distributed data were analysed using unpaired t‐tests or one‐way analysis of variance whereas non‐parametrically distributed data were calculated using Kruskal–Wallis tests. If significant, this was followed by a Mann–Whitney U‐test for a further comparison of the groups.
Results
Differing immune profiles in Mf+ and Mf– L. sigmodontis‐infected BALB/c mice
To assess whether the presence of circulating Mf effected filarial‐specific recall, BALB/c mice were infected with L. sigmodontis, because unlike C57BL/6 and C3H mice, this strain develops patent infections.8 In our cycle, the peak of peripheral Mf output is approximately day 72 p.i. (Fig. 1a). Confirming previous studies,8 only a portion of the infected BALB/c mice become patent (49% Mf+ mice in six independent infection studies). Expanding on these findings, further groups of Mf+ and Mf– mice were screened for changes in parasitology and immunological aspects. A significant decrease in worm burden was observed in amicrofilaraemic mice (Fig. 1b). When compared with naive mice, Mf+ groups had significantly higher cell counts in the spleen (Fig. 1c) and medLN (Fig. 1d). Mf– groups, however, presented only higher cell counts in the medLN (Fig. 1d). To observe differences in immune profiles, lymphocyte preparations from the spleens of individual Mf+ or Mf– mice were re‐stimulated with filarial‐derived worm antigen (LsAg) or live Mf (Fig. 2). After 72 hr, cell culture supernatants were analysed for the presence of TNF‐α (Fig. 2a,b), IL‐5 (Fig. 2c,d) and IL‐10 (Fig. 2e,f). In Mf– mice, TNF‐α responses to LsAg were significantly higher (Fig. 2a) but IL‐5 levels were significantly lower (Fig. 2c) when compared with Mf+ groups. Production of IL‐10 in response to LsAg remained comparable between the groups (Fig. 2e). When cultured with live microfilariae, levels of secreted TNF‐α (Fig. 2b), IL‐5 (Fig. 2d) and IL‐10 (Fig. 2f) from splenic lymphocytes of Mf+ mice were significantly higher than in Mf– mice. These responses were further accentuated with increasing numbers of Mf.
Figure 1.
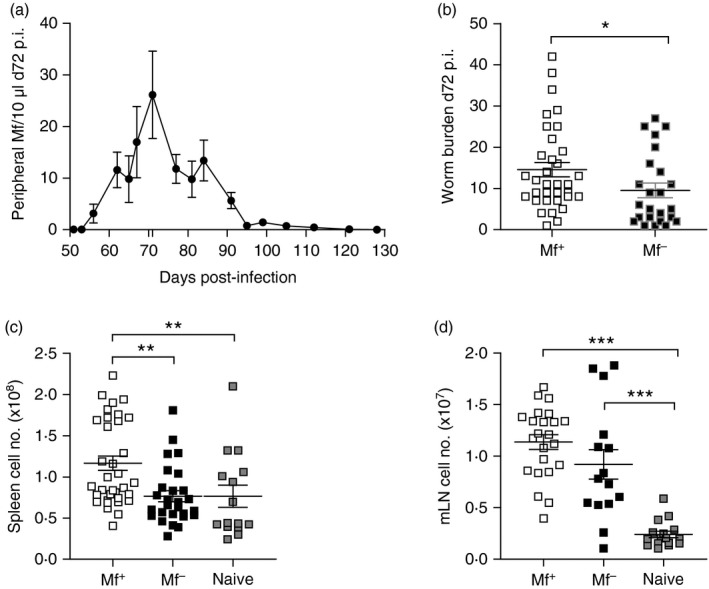
Higher worm burden in Mf+ Litomosoides sigmodontis‐infected BALB/c mice corresponds with an increased lymphocyte number both systemically and in local draining lymph nodes. (a) Wild‐type BALB/c female mice were naturally infected with L. sigmodontis. From day 45 to 120 post infection (p.i.) individual mice were screened for the number of Mf in blood. Symbols represent mean ± SEM of infected mice from six independent infection experiments (n = 20 Mf+ mice). On day 72, L. sigmodontis‐infected BALB/c mice were screened for the presence of peripheral Mf and assessed for changes in worm burden (b), absolute lymphocyte numbers in the spleen (c) and mediastinal lymph nodes (medLN), the draining lymph nodes of the thoracic cavity (d). Symbols represent individual Mf+, Mf– and naive mice from four independent infection experiments (n = 28 Mf+, n = 19 Mf– and n = 15 naive BALB/c mice). Asterisks indicate significant differences (analysis of variance or t‐test) between the groups indicated by the brackets (*P < 0·01, **P < 0·05, ***P < 0·001).
Figure 2.
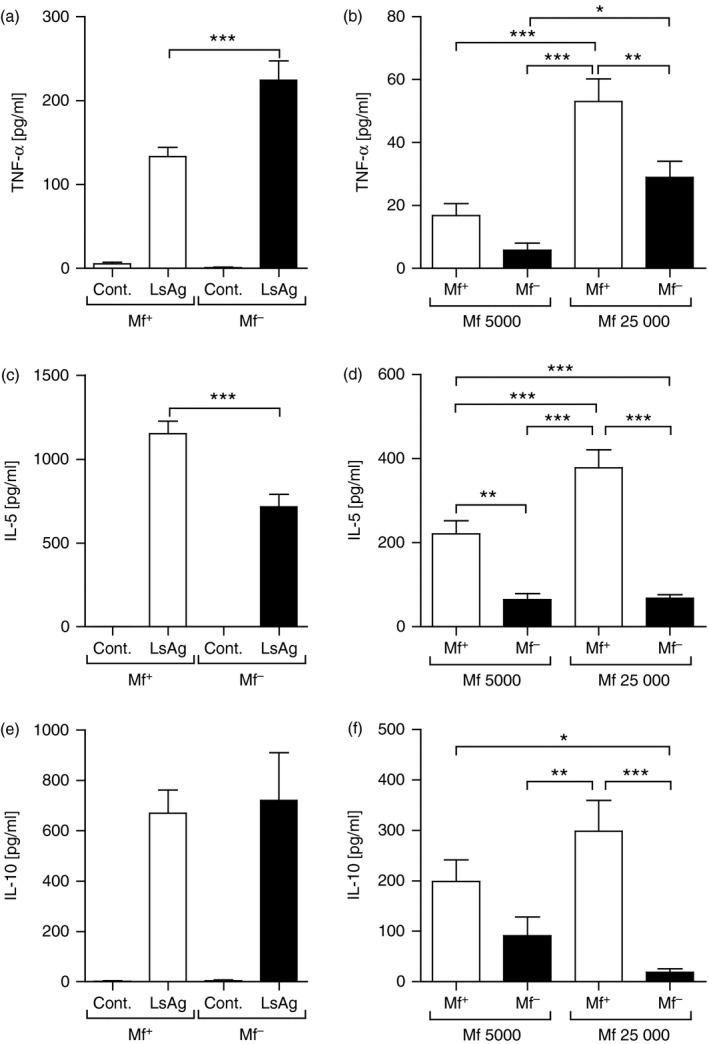
Circulating microfilariae (Mf) in Litomosoides sigmodontis‐infected BALB/c mice alter systemic filarial‐specific immune responses. Wild‐type female BALB/c mice were naturally infected with L. sigmodontis. On day 72, mice were screened for the presence of peripheral Mf and isolated lymphocytes from the spleen were re‐stimulated ex vivo with 50 μg/ml worm antigen preparation (LsAg) or 5000 or 25 000 live Mf/well for 72 hr. Thereafter, levels of TNF‐α (a,b), IL‐5 (c,d) and IL‐10 (e,f) were determined in the culture supernatant by ELISA. Graphs show mean ± SEM of individually assessed mice from four independent infection experiments (n = 28 Mf+ mice and n = 19 Mf– mice). Asterisks indicate significant differences (analysis of variance or Student's t‐test) between the groups indicated by the brackets (*P < 0·01, **P < 0·05, ***P < 0·001).
As the site of infection is the TC we further investigated whether responses from medLN cells in Mf+ and Mf– mice reflected their systemic immune profiles. Here, no differences in TNF‐α responses to LsAg could be observed between the groups (Fig. 3a). Cells from Mf+ mice did make more TNF‐α when co‐cultured with 25 000 Mf (Fig. 3b). The profiles of IL‐5 reflected those seen in the systemic responses with significantly higher levels secreted from cells of Mf+ mice following re‐stimulation with either LsAg or live microfilariae (Fig. 3c and d, respectively). No differences in the production of IL‐10 were observed between the two groups upon LsAg stimulation (Fig. 3e) although significantly more IL‐10 was released by cells from Mf+ mice in the presence of 25 000 live Mf (Fig. 3f). These data reveal that upon Mf release into the periphery, Mf‐specific immune responses are triggered and systemic responses increase with increasing amounts of Mf. To observe whether such profiles also occurred upon T‐cell receptor activation, cell populations were activated using αCD3/αCD28 (see Supplementary material, Fig. S1a–c). Overall, similar profiles were observed: elevated systemic TNF‐α responses by cells from Mf– mice with an overall reduced release by medLN cells from both groups (see Supplementary material, Fig. S1a) and higher IL‐5 levels by cells from Mf+ mice (see Supplementary material, Fig. S1b). With regards to IL‐10, systemic responses remained comparable between both infected groups but in contrast to filarial‐specific responses, medLN cells from Mf– mice produced more of this regulatory cytokine (see Supplementary material, Fig. S1c).
Figure 3.
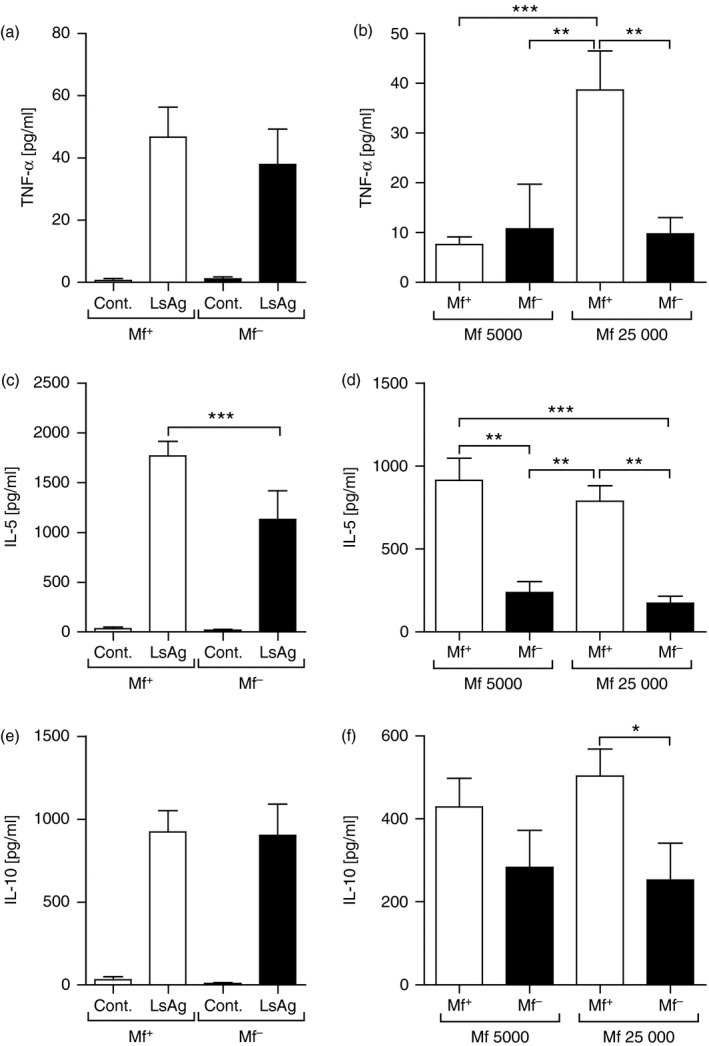
medLN cells from Mf+ Litomosoides sigmodontis‐infected mice present dominant IL‐5 responses upon filarial‐specific activation. On day 72, L. sigmodontis‐infected BALB/c mice were screened for the presence of peripheral Mf and isolated lymphocytes from the draining medLN were re‐stimulated ex vivo with 50 μg/ml L. sigmodontis antigen (LsAg) or 5000 or 25 000 live Mf/well for 72 hr. Thereafter, levels of TNF‐α (a,b), IL‐5 (c,d) and IL‐10 (e,f) were determined in the culture supernatant by ELISA. Graphs show mean ± SEM of individually assessed mice from four independent infection experiments (n = 28 Mf+ mice and n = 19 Mf– mice). Asterisks indicate significant differences (analysis of variance or Student's t‐test) between the groups indicated by the brackets (*P < 0·01, **P < 0·05, ***P < 0·001).
Lack of TLR4 but not TLR2 facilitates patency in L. sigmodontis‐infected mice and deficiency in either TLR impacts filarial‐specific immune responses
As mentioned above, adult L. sigmodontis worms in BALB/c mice begin to produce Mf after approximately 50 days post infection. Moreover, only a portion (40–60%) of these mice become Mf+ (see ref. 8 and Fig. 1a). To study whether lack of TLR2 or TLR4 had an influence on Mf release we infected groups of WT, TLR2−/− and TLR4−/− mice on a BALB/c background and followed Mf release from day 45. As shown in Fig. 4(a), the percentage of WT mice that became Mf+ was within the expected margin and this was also observed for infected TLR2−/− mice. However, 93% of infected TLR4−/− mice became Mf+ (Fig. 4a) and this was not associated with Mf numbers because a kinetic comparison between WT, TLR2−/− and TLR4−/− infected groups revealed comparable amounts throughout infection (Fig. 4b). The increased patency in TLR4−/− mice was not due to increased worm numbers either, as no differences were found between Mf+ and TLR4−/− groups on day 72 p.i. (Fig. 4c). No significant differences between individual Mf levels on day 72 p.i. were observed either (Fig. 4d). To exclude that an increase in patency in infected TLR4−/− mice was not simple the result of more female worms, or a lack of male worms, we differentiated gender as well. As shown in the Supplementary material (Fig. S2), there were no significant differences between the number of female worms from WT, TLR4−/− and TLR2−/− infected mice. This was also the case for male worms. Significant differences were found between male and female worm numbers in TLR4−/− and TLR2−/− mice and this trend was also observable in WT mice, hence, the increased patency was not the result of elevated numbers of female worms and hence more Mf.
Figure 4.
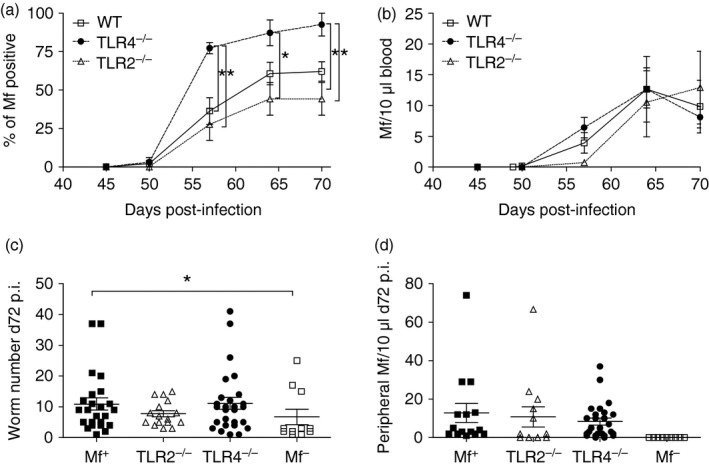
Lack of TLR4 during Litomosoides sigmodontis infection increases the percentage of Mf+ mice. WT, TLR2−/− and TLR4−/− BALB/c mice were naturally infected with L. sigmodontis and from day 45, mice were surveyed for the presence of peripheral Mf. (a) Symbols represent the mean percentage ± SEM of mice that were Mf+ within the different strains. (b) Symbols show the mean percentage ± SEM of Mf number per 10 μl blood in individual mice. On day 72, (c) worm burden and (d) peripheral Mf numbers were determined in individual mice. (a) Data from four independent infection experiments (n = 45 WT, n = 25 TLR2−/− and n = 32 TLR4−/−). (b–d) Values show data from three independent infection studies (n = 20 WT Mf+, n = 11 WT Mf–, n = 12 TLR2−/− Mf+ mice and n = 22 TLR4−/− Mf+ mice) and correlate to the data described in Figs 5, 6, 7. Asterisks indicate significant differences (analysis of variance or Student's t‐test) between the groups indicated by the brackets (*P < 0·01).
To observe whether lack of TLR2 or TLR4 altered adaptive immune profiles, spleen (Fig. 5a–d) and medLN (Fig. 5e–h) cells were isolated on day 72 p.i. and co‐cultured with LsAg in vitro. As 93% of infected TLR4−/− mice became Mf+ (Fig. 4a), only three (n = 32) infected mice were Mf– and therefore for immunological comparisons we studied the following groups: WT Mf+, TLR2−/− Mf+, TLR4−/− Mf+ and WT Mf–. In these further infection studies, the differences in immune profiles of WT Mf+ and Mf– mice were confirmed because responses reflected those seen in Figs. 2 and 3. Cells from TLR2−/− Mf+ mice produced very little TNF‐α both systemically and locally (Fig. 5a, e). With regards to Th2 responses, spleen cells from Mf+ WT mice produced significantly more IL‐5 and IL‐13 when compared with all other groups (Fig. 5b, c). Interestingly, IL‐10 production by spleen cells from TLR2−/− mice was significantly lower than in all other groups, despite them having circulating Mf (Fig. 5d). In contrast, when compared with all other groups, medLN cells from infected TLR2−/− mice produced significantly higher levels of both IL‐5 and IL‐10 (Fig. 5f and h, respectively). Secretion of IL‐13 from medLN was comparably high in all groups (Fig. 5g). In addition, we also measured cytokine levels in TC fluid (the site of infection). No significant differences could be observed between levels of TNF‐α between the groups (Fig. 5i). Levels of IL‐5 and IL‐13 were significantly lower in Mf– mice when compared with Mf+ mice (Fig. 5i and k) confirming the immune profiles seen in Figs. 2 and 3. Levels of IL‐10 within the TC were not significantly regulated between the groups (Fig. 5l). Hence, despite having circulating Mf, systemic Th2 responses in TLR4−/− mice were significantly dampened and were comparable with the responses in Mf– WT mice. Infected TLR2−/− mice on the other hand showed an overall suppressed systemic immune profile (Fig. 5a–d) but accentuated IL‐5 and IL‐10 filarial‐specific responses in the medLN (Fig. 5f, h).
Figure 5.
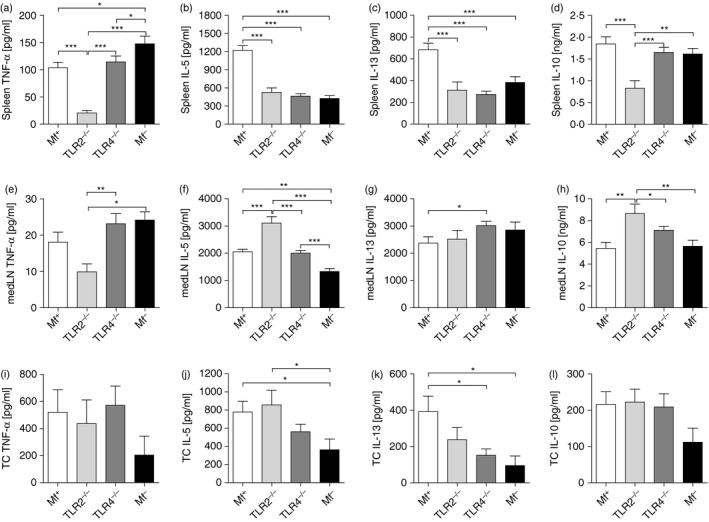
Lack of TLR2 during patent infections of Litomosoides sigmodontis dampens systemic but not local filarial‐specific IL‐5 and IL‐13 responses. WT, TLR2−/− and TLR4−/− BALB/c mice were naturally infected with L. sigmodontis. On day 72, mice were screened for the presence of peripheral microfilariae and lymphocytes were isolated from the spleen (a–d) or medLN (e–h). Cells were stimulated with 50 μg/ml L. sigmodontis antigen (LsAg) for 72 hr. Thereafter, levels of TNF‐α (a,e), IL‐5 (b,f), IL‐13 (c,g) and IL‐10 (d,h) were determined in the culture supernatant by ELISA. Cytokine levels within the thoracic cavity (TC) (i–l) were measured using cytometric bead assays and analysed using a BD FACS Canto and flowcytomix pro 3·0 software. Graphs show mean ± SEM of individually assessed mice from three independent infection experiments (n = 20 WT Mf+, n = 11 WT Mf–, n = 12 TLR2−/− Mf+ mice and n = 22 TLR4−/− Mf+ mice). Asterisks indicate significant differences (analysis of variance or Student's t‐test) between the groups indicated by the brackets (*P < 0·01, **P < 0·05, ***P < 0·001).
Circulating Mf elevates filarial‐specific CD4+ T‐cell responses in the periphery
When comparing the filarial‐specific responses of spleen cells from Mf+ WT mice and TLR2−/− or TLR4−/− mice, that were also Mf+, we observed dampened IL‐5 and IL‐13 responses. Indeed, the secretion levels of these cytokines from cells of TLR‐deficient mice resembled responses from Mf– WT mice (Fig. 5b, c). To determine whether this profile was the result of skewed T helper cell responses we analysed the frequency of regulatory T cells (Fig. 6) and measured filarial‐specific cytokine production by CD4+ T cells (Fig. 7). During infection, no significant differences could be observed in the frequency of CD25hiFoxp3+ Treg cells within the CD4+ T‐cell compartment in the spleen (Fig. 6a). At the draining lymph node however, significantly fewer Treg cells were observed in TLR2−/− mice on days 49 and 72 p.i. (Fig. 6b) even though absolute cell counts were comparable between the groups (Table 1). Although Treg cell numbers were also reduced in TLR4−/− mice at the pre‐patent stage (day 49), levels were comparable to those in WT infected mice on day 72 p.i. (Fig. 6b). To measure filarial‐specific cytokine responses, CD4+ T cells were isolated by flow cytometry from the spleens of infected mice and co‐cultured with GM‐CSF‐derived DC from naive WT BALB/c mice and LsAg (Fig. 7). With cells from Mf– mice, all CD4+ T‐cell responses to LsAg were significantly lower than all other groups and this result was observed for all measured cytokines: TNF‐α (Fig. 7a), IL‐5 (Fig. 7b), IL‐13 (Fig. 7c) and IL‐10 (Fig. 7d). Surprisingly, whereas bulk spleen cell responses from TLR2−/− mice had been poor (Fig. 5a–d), isolated CD4+ T cells from these same mice now presented significantly higher levels of TNF‐α, IL‐5 and IL‐13 when compared with the other groups (Fig. 7a–c). Overall, cytokine secretion by CD4+ T cells to worm antigen was comparable (no significant differences) between WT Mf+ and TLR4−/− Mf+ groups (compare white and dark grey bars).
Figure 6.
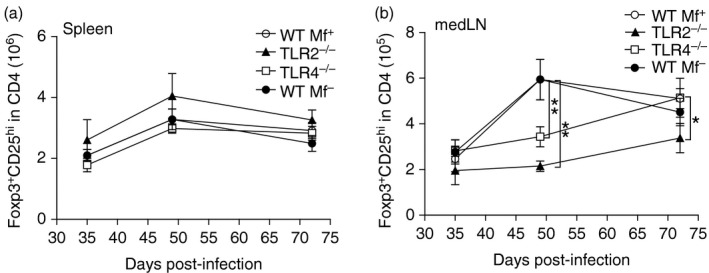
Reduced frequency of Foxp3+ CD25hi regulatory T (Treg) cells in infected TLR2−/− mice during filarial infection. Groups of WT, TLR2−/− and TLR4−/− BALB/c mice were infected with Litomosoides sigmodontis. On days 35, 49 and 72 post infection (p.i.), spleen (a) and medLN (b) cells from individual mice were analysed by flow cytometry for the number of Foxp3+ CD25hi Treg cells within the CD4+ T‐cell compartment. Symbols show mean ± SEM of two independent infection studies. Day 35: WT n = 8, TLR2−/− n = 6 and TLR4 n = 13. Day 49: WT n = 9, TLR2−/− n = 6 and TLR4−/− n = 19. Day 72: WT Mf+ n = 16, WT Mf– n = 11, TLR2 Mf+ n = 9 and TLR4 Mf+ n = 9. Asterisks indicate significant differences (analysis of variance or Student's t‐test) between the groups indicated by the brackets (*P < 0·01, **P < 0·05).
Figure 7.
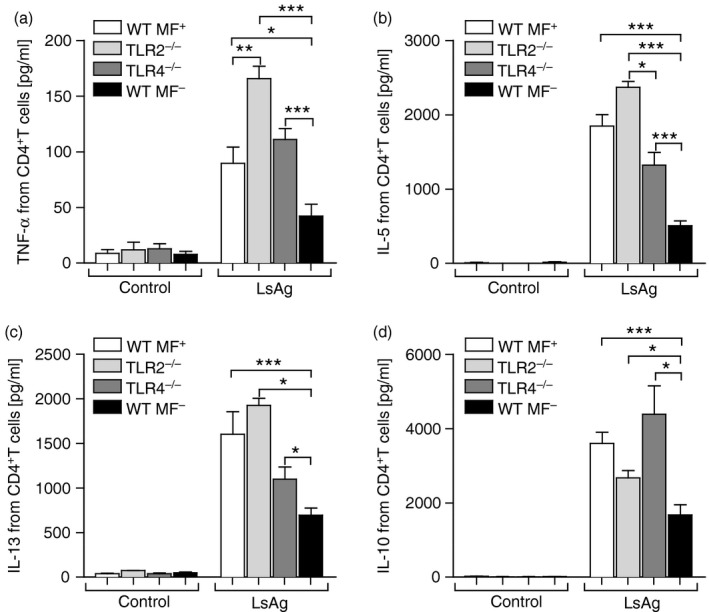
Lack of TLR2 or TLR4 on CD4+ T cells does not hinder filarial‐specific responses. DC from WT BALB/c mice were co‐cultured with Litomosoides sigmodontis antigen (LsAg; 50 μg/ml) and CD4+ T cells were isolated by flow cytometry from WT Mf+; white bars, TLR2−/− Mf+ (light grey bars), TLR4−/− Mf+ (dark grey bars) and WT Mf– (black bars) mice on day 72 post infection (p.i.). After 72 hr, the resulting supernatant was screened for the levels of TNF‐α (a), IL‐5 (b), IL‐13 (c) and IL‐10 (d). From three independent infection studies, graphs show mean ± SD of five independent T‐cell assays using pooled cells from three or four mice per group. Asterisks indicate significant differences (analysis of variance or Student's t‐test) between the groups indicated by the brackets (*P < 0·01, **P < 0·05, ***P < 0·001).
Table 1.
Spleen and medLN cells counts in Litomosoides sigmodontis‐infected WT, TLR2−/− and TLR4−/− BALB/c mice
| Infected BALB/c WT Mf+ | Infected BALB/c WT Mf– | Infected BALB/c TLR2−/− Mf+ | Infected BALB/c TLR4−/− Mf+ | |
|---|---|---|---|---|
| Spleen cell counts (108) (mean ± SD) | 1·61 ± 0·38 | 1·07 ± 0·44 | 1·11 ± 0·39 | 0·91 ± 0·26 |
| medLN cell counts (107) (mean ± SD) | 1·02 ± 0·59 | 1·01 ± 0·32 | 0·89 ± 0·36 | 0·96 ± 0·38 |
Abbreviations: medLN, mediastinal lymph nodes; Mf, microfilariae; TLR2, Toll‐like receptor 2; WT, wild‐type.
Groups of WT, TLR2−/− and TLR4−/− BALB/c mice were infected with L. sigmodontis for 72 days? Thereafter, absolute cell counts in the spleen and medLN were determined using the Casy Cell Counter. Values show data from three independent infection studies (n = 20 WT Mf+, n = 11 WT Mf–, n = 12 TLR2−/− Mf+ mice and n = 22 TLR4−/− Mf+ mice) and correlate with the data described in Figs 4, 5, 6.
TLR2 signalling on DC is crucial for effective filarial‐specific CD4+ T‐cell responses
Above, we demonstrated that filarial‐specific responses by bulk spleen cells from L. sigmodontis‐infected TLR2−/− mice were low (Fig. 5a–d) but co‐cultures of isolated splenic CD4+ T cells with WT APC produced significantly higher amounts of the measured cytokines when compared with T cells isolated from the other infected groups (Fig. 7a–d). These data implied that the reduced cytokine production by bulk cells was not the result of reduced filarial‐specific T cells in TLR2−/− mice but perhaps resided in insufficient antigen presentation. Hence, CD4+ T cells from the spleens of WT Mf+ and WT Mf– mice were isolated by flow cytometry and co‐cultured with LsAg and GM‐CSF‐derived DC from WT, TLR2−/− (Fig. 8) or TLR4−/− (see Supplementary material, Fig. S3) mice. In line with the previous experiment (Fig. 7), CD4+ T cells from Mf– mice produced significantly less TNF‐α (Fig. 8a), IL‐5 (Fig. 8b), IL‐13 (Fig. 8c) and IL‐10 (Fig. 8d) when co‐cultured with DC from WT mice. However, when co‐cultured with DC deficient in TLR2, CD4+ T cells from either Mf+ or Mf– mice produced significantly lower amounts of cytokines (Fig. 8a–d). This finding was not observed when TLR4‐deficient DC were used as APC (see Supplementary material, Fig. S3a–d). To observe whether reduced CD4+ T‐cell activation was a general occurrence with TLR2‐deficient APC, T cells from infected mice were also activated with αCD3/αCD28. Here, no deficit in the production of IL‐5 or IL‐13 was detected (see Supplementary material, Fig. S4a and b, respectively). Co‐cultures of WT CD4+ T cells with TLR4−/− DC did make more IL‐5 and IL‐3 upon αCD3/αCD28 stimulation. These experiments show that in the presence of TLR2−/− APC, but not TLR4−/− APC, there is an overall dampening of filarial‐specific CD4+ T‐cell responses.
Figure 8.
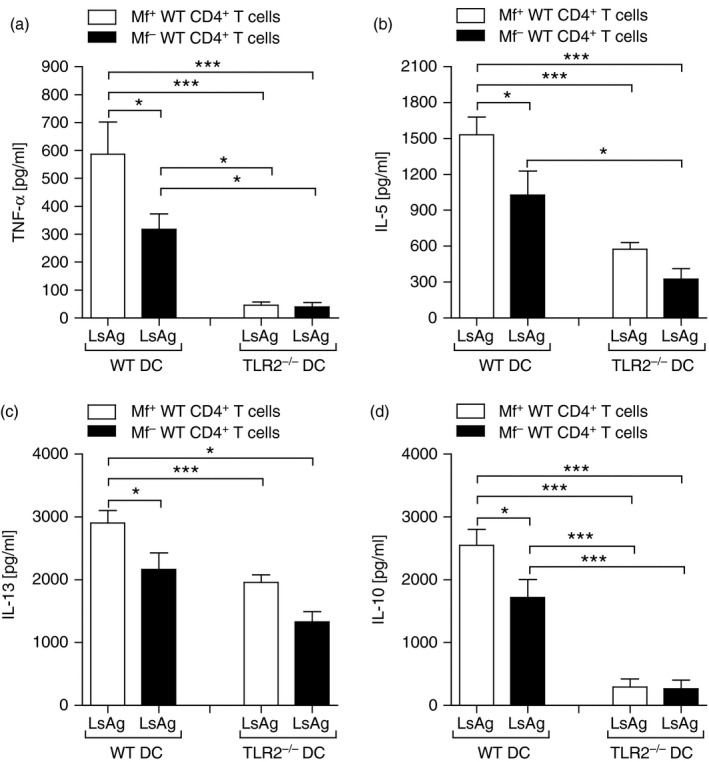
Lack of TLR2 on APC reduces filarial‐specific CD4+ T‐cell responses from WT infected mice. On day 72 post infection (p.i.), CD4+ T cells from Mf+; white bars or Mf– (black bars) infected BALB/c mice were isolated by flow cytometry and co‐cultured with DC from WT or TLR2−/− BALB/c mice. Cell cultures were then stimulated with 50 μg/ml Litomosoides sigmodontis antigen (LsAg) for 72 hr and thereafter, levels of TNF‐α (a), IL‐5 (b), IL‐13 (c) and IL‐10 (d) were determined in the culture supernatant by ELISA. From three independent infection studies, bars show mean ± SD of three co‐culture assays using isolated T cells pooled from three or four mice per group. Asterisks indicated significant differences (analysis of variance or Student's t‐test) between the groups indicated by the brackets (*P < 0·01, ***P < 0·001).
Discussion
The release of Mf into the periphery signals the final stage of filarial nematode life cycles. Studies in individuals presenting asymptomatic W. bancrofti infections revealed that Mf+ individuals had dampened immune responses following filarial‐specific activation when compared with Mf– cohorts.29 Infections in BALB/c mice with L. sigmodontis also results in Mf+ and Mf– animals and this study shows that immune responses between these groups are different. In accordance with the above‐mentioned human study,29 TNF‐α responses to LsAg in spleen cultures from Mf– mice were greater than in those from Mf+ mice. However, in contrast to the studies with W. bancrofti‐infected individuals, IL‐10 responses to filarial antigens were comparable in both infection groups and IL‐5 responses were significantly higher in cultures from Mf+ mice to both LsAg and live Mf. Indeed, medLN IL‐5 responses were twofold to fourfold higher than spleen responses although increasing Mf numbers in splenic cultures correspondingly increased IL‐5 levels as well. Cell cultures from Mf– mice produced little IL‐5 in response to live Mf, which did not change with increasing Mf numbers. The overall low recall response by cells from Mf– mice to live Mf is perhaps somewhat anticipated because Mf‐derived antigens have not been previously seen by these mice although further experiments are required to confirm this hypothesis. In correlation, CD4+ T cells isolated from infected Mf– groups also secreted much lower levels of IL‐5 and IL‐13 upon LsAg re‐stimulation when compared with T‐cell cultures from Mf+ mice. These lower responses may be a consequence of reduced antigen or worm load (as Mf– mice had slightly fewer worms) but since LsAg‐provoked IL‐10 levels remained comparable between the groups (in bulk cultures), further studies are required to resolve this issue. Hence, with the exception of TNF‐α, these findings contrast with immune profiles in W. bancrofti‐infected patients because there, PBMC from Mf– individuals made significantly more IL‐5 and IL‐10 upon worm antigen re‐stimulation.29 Reasons for these differences may lie in the longer infection period in humans compared with mice, the potential impact of previous infections on immune responses in humans and differences between PBMC versus spleen and LN responses.
Th2 responses during filarial infection are considered to control filarial parasite burden.31, 35, 36 The importance of CD4+ T‐cell responses during filarial infection was demonstrated upon administration of anti‐CD4 antibodies in BALB/c during exposure to L. sigmodontis. Such treatment resulted in higher worm burden and increased Mf number in peripheral blood, reduced Th2 and IL‐10 responses by cells from the TC in the pre‐patent period.12 Other in vivo infection studies have demonstrated that lack of IL‐5 increases both worm and Mf numbers and was linked to reduced neutrophil recruitment and impaired phagocytosis in the TC.13, 14 In correlation, using the semi‐resistant CBA/Ca strain, it was demonstrated that over‐expression of IL‐5 resulted in decreased worm burden.37 Moreover, in RAG2−/−IL‐2Rγ −/− C57BL/6 mice, lack of T cells, B cells and natural killer cells results in increased worm burden and 100% patency.31 When compared with Mf+ BALB/c mice, both worm burden and spleen cell counts were reduced in the Mf– group studied here. However, this could not be attributed to increased filarial‐specific IL‐5 levels as these responses were significantly lower in Mf– mice. Hence, other immune signals must be required to develop a patent state and we reveal here that lack of TLR4 signalling creates an immune environment that facilitates Mf release into the periphery because nearly all infected TLR4‐deficient mice became patent (> 93%). These findings correlate with early studies using the lipopolysaccharide non‐responsive C3H/HeJ strain since L. sigmodontis infections in those mice led to Mf release within the TC but not the periphery.38 Using the more susceptible BALB/c strain we now expand on those earlier findings and show that the observed elevated transmissibility was restricted to infected TLR4−/− BALB/c mice because the percentage of Mf+ TLR2−/− mice was similar to that in WT controls. Of note, Mf counts in infected TLR4−/− mice were comparable to TLR2‐deficient and WT Mf+ groups throughout infection, demonstrating that the increased frequency of patency did not correspond with higher Mf counts (Fig. 4b and d) nor elevated worm burden (Fig. 4c) nor increased females (see Supplementary material, Fig. S2). TLR4 has been shown to have an array of functions depending on the immune situation. For example, lipopolysaccharide triggering of TLR4 is associated with the development of Th1 responses via TNF‐α and IL‐12.15, 16, 39 Signalling via TLR4 during schistosome infections is also dependent on the immune setting and applied life stage. For example, its presence on DC was shown to be essential for Th2 responses to a glycan derivative from Schistosoma mansoni.40 Tegument‐derived antigens also activate DC via TLR4 to produce IL‐12p40 and TNF‐α 41 and cercarial antigen extracts from schistosomes, devoid of endotoxins, trigger macrophages in a TLR4‐dependent manner.42 Other studies however have demonstrated that TLR4 and TLR2 are not required for inducing Th2 responses when using DC pulsed with schistosomal egg antigen.43 Indeed, components within schistosome eggs are more likely to modulate pathogen‐activated DC responses via TLRs.17, 18, 44 With regards to other helminth infections, TLR4 is crucial for the correct development of Trichuris muris and its absence elevates IL‐13 responses.45 In correlation, we observed that cultures of medLN from infected TLR4−/− mice produced significantly higher LsAg‐specific IL‐13 responses when compared with Mf+ WT mice. Moreover, CD4+ T cells from WT infected mice produced higher levels of IL‐13 upon αCD3/αCD28 activation when cultured with TLR4−/− DC, indicating that TLR4 ligation may influence adaptive immune responses depending on the stimulus. In infections of C3H/HeJ mice with Strongyloides stercoralis, TLR4 was required for killing worms in the adaptive phase but as Th2 responses were not altered it was considered that TLR4‐mutant mice failed to recruit effector cells to the microenvironment.46
Activation of TLR2 and TLR4 first became associated with filarial infections through Wolbachia activity, the essential endosymbiont bacteria required for worm fecundity and survival.2, 23 Wolbachia‐mediated TLR4 responses are considered to play a role in the immunopathogenesis of Onchocerca volvulus‐induced keratitis47 and adverse reactions in patients following microfilaricidal therapy is associated with increased Wolbachia DNA in the blood and strong pro‐inflammatory responses.48 The specific ligands, however, remain unclear although potential candidates include the Wolbachia surface protein, which induces TLR activity in vitro and can elicit TNF release in PBMC from healthy individuals in a dose‐dependent manner.26 Moreover, PBMC from O. volvulus‐infected individuals produce pro‐inflammatory cytokines when cultured with Wolbachia surface protein,26 and recently, a recombinant peptidoglycan‐associated lipoprotein (wPAL) elicited higher TNF responses in microfilaridermic individuals compared with amicrofilaridermic cohorts: following regression analysis this was shown to be dependent on the presence of Mf.49 In vitro experiments have shown that macrophages respond to Brugia malayi antigen or Wolbachia in a TLR2/TLR6‐dependent manner25 and ES62, a phosphorylcholine‐containing glycoprotein derived from the non‐Wolbachia containing filarial nematode Acanthocheilonema viteae also signals via TLR4, indicating that the filariae contain TLR‐specific components themselves.50 In vivo a study that focused on determining the role of TLR4 and the development of protective immunity noted that C3H/HeJ mice failed to develop immune‐mediated larval killing, and spleen cells from those mice produced significantly higher levels of IL‐5 upon antigen re‐stimulation.51 Systemic IL‐5 responses were not pronounced in cell cultures from the L. sigmodontis‐infected TLR4−/− mice studied here, although responses by medLN or CD4+ T cells, isolated from TLR4−/− mice, were comparable to those of Mf+ mice. Further studies are required to resolve how TLR4−/− mice dampen systemic IL‐5 responses, which may in turn aid Mf release and survival.3, 14 Preliminary evidence does indicate that L. sigmodontis‐infected TLR4−/− mice have decreased numbers of eosinophils in the TC during patent infection (TLR4−/−: 4·53 × 106 ± 1·4 × 106 (n = 12) WT: 9·95 × 106 ± 1·5 × 106 (n = 12) P < 0·0073) which may facilitate Mf survival.
In humans, exposure of monocyte‐derived DC to Brugia malayi Mf down‐regulated the mRNA expression of several TLRs and protein expression of TLR4 was markedly diminished.52 Filarial‐infected individuals also present decreased TLR expression53 and culturing Mf with APCs diminished their capacity to stimulate CD4+ T cells.54, 55 T cells express TLR themselves and in the presence of B cells and monocytes, T cells from lymphatic filariasis patients express lower levels of TLR1, ‐2 and ‐4 than from non‐infected groups, suggesting that filarial parasites can affect T‐cell activity.56 With regards to regulatory cell types, numbers of Foxp3+ Treg cells in the spleen and medLN remained comparable in WT Mf+ and Mf– mice throughout infection. Foxp3+ Treg cell numbers in TLR2−/− mice were however significantly lower in the medLN and this phenotype was observed in both pre‐patent and patent periods, which could also be connected to the inefficient presentation of filarial antigen. Reduced Treg cell numbers in TLR2−/− mice have been observed in other parasitic infections including Schistosoma mansoni.57 There it was shown that TLR2 deficiency led to decreased Treg, which in turn elicited uncontrolled schistosome‐specific responses and aggravated pathology.57 During L. sigmodontis here, lack of TLR2 had no effect on worm burden or on levels of Mf as levels were comparable to WT Mf+ infected groups. However, local and systemic LsAg‐specific responses in infected TLR2−/− mice were lower or even absent, especially TNF‐α and IL‐10. Spleen cell cultures from infected TLR2−/− mice also failed to release cytokines upon LsAg stimulation but upon isolation, CD4+ T cells produced significantly higher amounts of TNF‐α, IL‐5 and IL‐13 when cultured with wild‐type DC. Overall, our data indicate that TLR2 does not influence immune responses per se but only its absence on DC leads to lower responses. This contrasts with the study on schistosome‐infected mice, since there CD4+ T cells from TLR2−/− infected mice produced less IL‐10 and IL‐13 upon culturing with WT DC and schistosome antigen.57 To confirm our hypothesis that lack of TLR2 on APC dampened filarial antigen presentation we cultured WT CD4+ T cells from infected mice with TLR‐deficient mice. All filarial‐specific cytokine responses from CD4+ T cells were markedly reduced using TLR2−/− but not TLR4−/− DC. In conclusion, our data show that triggering TLR2 or TLR4 during chronic filarial infection has a significant impact on efficient T‐cell responses and patency, respectively. This interplay between the different TLR during an ongoing L. sigmodontis infection emphasizes the complexity of host–parasite interactions and the ensuing adaptive responses.
Disclosures
The authors of this work have no financial, personal or professional interests that could have been construed to influence this manuscript.
Funding
This study and SS were supported through a grant from the German Research Council (SFB 704). RT is supported by a German Academic Exchange Service (DAAD) fellowship. AH is a member of the Excellence Cluster Immunosensation (DFG, EXC 1023) and of the German Centre of Infectious Disease (DZIF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supporting information
Figure S1 Circulating microfilariae in Litomosoides sigmodontis BALB/c mice skews adaptive immune responses.
Figure S2 Worm gender differentiation in wild‐type, Toll‐like receptor‐4‐deficient (TLR4−/−) and TLR2−/− infected BALB/c mice.
Figure S3 Lack of Toll‐like receptor 4 on antigen‐presenting cells does not alter filarial‐specific CD4+ T‐cell responses.
Figure S4 Lack of Toll‐like receptor 2 (TLR2) or TLR4 on dendritic cells does not alter T‐cell receptor‐activated CD4+ T‐cell responses from Litomosoides sigmodontis wild‐type mice.
Acknowledgements
Special thanks to Ö. Mutluer and A. Wiszniewsky (IMMIP) and to A. Dolf and P. Wurst (IMMEI) for excellent technical assistance. Thanks to M. P. Hübner for critically reading the manuscript.
References
- 1. Babu S, Nutman TB. Immunopathogenesis of lymphatic filarial disease. Semin Immunopathol 2012; 34:847–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taylor MJ, Hoerauf A, Bockarie M. Lymphatic filariasis and onchocerciasis. Lancet 2010; 376:1175–85. [DOI] [PubMed] [Google Scholar]
- 3. Hoerauf A, Satoguina J, Saeftel M, Specht S. Immunomodulation by filarial nematodes. Parasite Immunol 2005; 27:417–29. [DOI] [PubMed] [Google Scholar]
- 4. Metenou S, Nutman TB. Regulatory T cell subsets in filarial infection and their function. Front Immunol 2013; 4:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McSorley HJ, Maizels RM. Helminth infections and host immune regulation. Clin Microbiol Rev 2012; 25:585–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Adjobimey T, Hoerauf A. Induction of immunoglobulin G4 in human filariasis: an indicator of immunoregulation. Ann Trop Med Parasitol 2010; 104:455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Riet E, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology 2007; 212:475–90. [DOI] [PubMed] [Google Scholar]
- 8. Petit G, Diagne M, Marechal P, Owen D, Taylor D, Bain O. Maturation of the filaria Litomosoides sigmodontis in BALB/c mice; comparative susceptibility of nine other inbred strains. Ann Parasitol Hum Comp 1992; 67:144–50. [DOI] [PubMed] [Google Scholar]
- 9. Le Goff L, Lamb TJ, Graham AL, Harcus Y, Allen JE. IL‐4 is required to prevent filarial nematode development in resistant but not susceptible strains of mice. Int J Parasitol 2002; 32:1277–84. [DOI] [PubMed] [Google Scholar]
- 10. Hoffmann WH, Pfaff AW, Schulz‐Key H, Soboslay PT. Determinants for resistance and susceptibility to microfilaraemia in Litomosoides sigmodontis filariasis. Parasitology 2001; 122:641–9. [DOI] [PubMed] [Google Scholar]
- 11. Graham AL, Taylor MD, Le Goff L, Lamb TJ, Magennis M, Allen JE. Quantitative appraisal of murine filariasis confirms host strain differences but reveals that BALB/c females are more susceptible than males to Litomosoides sigmodontis . Microbes Infect 2005; 7:612–18. [DOI] [PubMed] [Google Scholar]
- 12. Al‐Qaoud KM, Taubert A, Zahner H, Fleischer B, Hoerauf A. Infection of BALB/c mice with the filarial nematode Litomosoides sigmodontis: role of CD4+ T cells in controlling larval development. Infect Immun 1997; 65:2457–6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al‐Qaoud KM, Pearlman E, Hartung T, Klukowski J, Fleischer B, Hoerauf A. A new mechanism for IL‐5‐dependent helminth control: neutrophil accumulation and neutrophil‐mediated worm encapsulation in murine filariasis are abolished in the absence of IL‐5. Int Immunol 2000; 12:899–908. [DOI] [PubMed] [Google Scholar]
- 14. Saeftel M, Arndt M, Specht S, Volkmann L, Hoerauf A. Synergism of γ interferon and interleukin‐5 in the control of murine filariasis. Infect Immun 2003; 71:6978–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schenten D, Medzhitov R. The control of adaptive immune responses by the innate immune system. Adv Immunol 2011; 109:87–124. [DOI] [PubMed] [Google Scholar]
- 16. Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science 2010; 327:291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klaver EJ, Kuijk LM, Laan LC, Kringel H, van Vliet SJ, Bouma G et al Trichuris suis‐induced modulation of human dendritic cell function is glycan‐mediated. Int J Parasitol 2013; 43:191–200. [DOI] [PubMed] [Google Scholar]
- 18. Ritter M, Gross O, Kays S, Ruland J, Nimmerjahn F, Saijo S et al Schistosoma mansoni triggers Dectin‐2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc Natl Acad Sci USA 2010; 107:20459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ludwig‐Portugall I, Layland LE. TLRs, Treg, and B cells, an interplay of regulation during helminth infection. Front Immunol 2012; 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hübner MP, Layland LE, Hoerauf A. Helminths and their implication in sepsis – a new branch of their immunomodulatory behaviour? Pathog Dis 2013; 69:127–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goodridge HS, Marshall FA, Else KJ, Houston KM, Egan C, Al‐Riyami L et al Immunomodulation via novel use of TLR4 by the filarial nematode phosphorylcholine‐containing secreted product, ES‐62. J Immunol 2005; 174:284–93. [DOI] [PubMed] [Google Scholar]
- 22. White RR, Artavanis‐Tsakonas K. How helminths use excretory secretory fractions to modulate dendritic cells. Virulence 2012; 3:668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tamarozzi F, Halliday A, Gentil K, Hoerauf A, Pearlman E, Taylor MJ. Onchocerciasis: the role of Wolbachia bacterial endosymbionts in parasite biology, disease pathogenesis, and treatment. Clin Microbiol Rev 2011; 24:459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Turner JD, Langley RS, Johnston KL, Egerton G, Wanji S, Taylor MJ. Wolbachia endosymbiotic bacteria of Brugia malayi mediate macrophage tolerance to TLR‐ and CD40‐specific stimuli in a MyD88/TLR2‐dependent manner. J Immunol 2006; 177:1240–9. [DOI] [PubMed] [Google Scholar]
- 25. Hise AG, Daehnel K, Gillette‐Ferguson I, Cho E, McGarry HF, Taylor MJ et al Innate immune responses to endosymbiotic Wolbachia bacteria in Brugia malayi and Onchocerca volvulus are dependent on TLR2, TLR6, MyD88, and Mal, but not TLR4, TRIF, or TRAM. J Immunol 2007; 178:1068–76. [DOI] [PubMed] [Google Scholar]
- 26. Brattig NW, Bazzocchi C, Kirschning CJ, Reiling N, Büttner DW, Ceciliani F et al The major surface protein of Wolbachia endosymbionts in filarial nematodes elicits immune responses through TLR2 and TLR4. J Immunol 2004; 173:437–45. [DOI] [PubMed] [Google Scholar]
- 27. Turner JD, Langley RS, Johnston KL, Gentil K, Ford L, Wu B et al Wolbachia lipoprotein stimulates innate and adaptive immunity through Toll‐like receptors 2 and 6 to induce disease manifestations of filariasis. J Biol Chem 2009; 284:22364–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Turner P, Copeman B, Gerisi D, Speare R. A comparison of the Og4C3 antigen capture ELISA, the Knott test, an IgG4 assay and clinical signs, in the diagnosis of Bancroftian filariasis. Trop Med Parasitol 1993; 44:45–8. [PubMed] [Google Scholar]
- 29. Arndts K, Deininger S, Specht S, Klarmann U, Mand S, Adjobimey T et al Elevated adaptive immune responses are associated with latent infections of Wuchereria bancrofti . PLoS Negl Trop Dis 2012; 6:e1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dunyo SK, Nkrumah FK, Simonsen PE. Single‐dose treatment of Wuchereria bancrofti infections with ivermectin and albendazole alone or in combination: evaluation of the potential for control at 12 months after treatment. Trans R Soc Trop Med Hyg 2000; 4:437–43. [DOI] [PubMed] [Google Scholar]
- 31. Layland LE, Ajendra J, Ritter M, Wiszniewsky A, Hoerauf A, Hübner MP. Development of patent Litomosoides sigmodontis infections in semi‐susceptible C57BL/6 mice in the absence of adaptive immune responses. Parasit Vectors 2015; 2:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chandrashekar R, Rao UR, Rajasekariah GR, Subrahmanyam D. Separation of viable microfilariae free of blood cells on Percoll gradients. J Helminthol 1984; 58:69–70. [DOI] [PubMed] [Google Scholar]
- 33. Wang SH, Zheng HJ. Survival and infectivity of Brugia malayi microfilariae after cryopreservation. Southeast Asian J Trop Med Public Health 1991; 22:165–7. [PubMed] [Google Scholar]
- 34. Layland LE, Mages J, Loddenkemper C, Hoerauf A, Wagner H, Lang R et al Pronounced phenotype in activated regulatory T cells during a chronic helminth infection. J Immunol 2010; 184:713–24. [DOI] [PubMed] [Google Scholar]
- 35. Hoerauf A, Fleischer B. Immune responses to filarial infection in laboratory mice. Med Microbiol Immunol 1997; 185:207–15. [DOI] [PubMed] [Google Scholar]
- 36. Lawrence RA. Lymphatic filariasis: what mice can tell us. Parasitol 1996; 12:267–71. [DOI] [PubMed] [Google Scholar]
- 37. Martin C, Le Goff L, Ungeheuer MN, Vuong PN, Bain O. Drastic reduction of a filarial infection in eosinophilic interleukin‐5 transgenic mice. Infect Immun 2000; 68:3651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pfarr KM, Fischer K, Hoerauf A. Involvement of Toll‐like receptor 4 in the embryogenesis of the rodent filaria Litomosoides sigmodontis . Med Microbiol Immunol 2003; 192:53–6. [DOI] [PubMed] [Google Scholar]
- 39. Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll‐like receptors control activation of adaptive immune responses. Nat Immunol 2001; 2:947–50. [DOI] [PubMed] [Google Scholar]
- 40. Thomas PG, Carter MR, Atochina O, Da'Dara AA, Piskorska D, McGuire E et al Maturation of dendritic cell 2 phenotype by a helminth glycan uses a Toll‐like receptor 4‐dependent mechanism. J Immunol 2003; 171:5837–41. [DOI] [PubMed] [Google Scholar]
- 41. Durães FV, Carvalho NB, Melo TT, Oliveira SC, Fonseca CT. IL‐12 and TNF‐α production by dendritic cells stimulated with Schistosoma mansoni schistosomula tegument is TLR4‐ and MyD88‐dependent. Immunol Lett 2009; 125:72–7. [DOI] [PubMed] [Google Scholar]
- 42. Jenkins SJ, Hewitson JP, Ferret‐Bernard S, Mountford AP. Schistosome larvae stimulate macrophage cytokine production through TLR4‐dependent and ‐independent pathways. Int Immunol 2005; 17:1409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kane CM, Jung E, Pearce EJ. Schistosoma mansoni egg antigen‐mediated modulation of Toll‐like receptor (TLR)‐induced activation occurs independently of TLR2, TLR4, and MyD88. Infect Immun 2008; 76:5754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kane CM, Cervi L, Sun J, McKee AS, Masek KS, Shapira S et al Helminth antigens modulate TLR‐initiated dendritic cell activation. J Immunol 2004; 173:7454–61. [DOI] [PubMed] [Google Scholar]
- 45. Helmby H, Grencis RK. Essential role for TLR4 and MyD88 in the development of chronic intestinal nematode infection. Eur J Immunol 2003; 33:2974–9. [DOI] [PubMed] [Google Scholar]
- 46. Kerepesi LA, Hess JA, Leon O, Nolan TJ, Schad GA, Abraham D. Toll‐like receptor 4 (TLR4) is required for protective immunity to larval Strongyloides stercoralis in mice. Microbes Infect 2007; 9:28–34. [DOI] [PubMed] [Google Scholar]
- 47. Hise AG, Gillette‐Ferguson I, Pearlman E. Immunopathogenesis of Onchocerca volvulus keratitis (river blindness): a novel role for TLR4 and endosymbiotic Wolbachia bacteria . J Endotoxin Res 2003; 9:390–4. [DOI] [PubMed] [Google Scholar]
- 48. Cross HF, Haarbrink M, Egerton G, Yazdanbakhsh M, Taylor MJ. Severe reactions to filarial chemotherapy and release of Wolbachia endosymbionts into blood. Lancet 2010; 358:1873–5. [DOI] [PubMed] [Google Scholar]
- 49. Arndts K, Specht S, Debrah AY, Tamarozzi F, Klarmann Schulz U, Mand S et al Immunoepidemiological profiling of onchocerciasis patients reveals associations with microfilaria loads and ivermectin intake on both individual and community levels. PLoS Negl Trop Dis 2014; 8:e2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Harnett W, Goodridge HS, Allen JM, Harnett M. Receptor usage by the Acanthocheilonema viteae‐derived immunomodulator, ES‐62. Exp Parasitol 2012; 132:97–102. [DOI] [PubMed] [Google Scholar]
- 51. Kerepesi LA, Leon O, Lustigman S, Abraham D. Protective immunity to the larval stages of Onchocerca volvulus is dependent on Toll‐like receptor 4. Infect Immun 2005; 73:8291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Semnani RT, Venugopal PG, Leifer CA, Mostböck S, Sabzevari H, Nutman TB. Inhibition of TLR3 and TLR4 function and expression in human dendritic cells by helminth parasites. Blood 2008; 112:1290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Diminished expression and function of TLR in lymphatic filariasis: a novel mechanism of immune dysregulation. J Immunol 2005; 175:1170–6. [DOI] [PubMed] [Google Scholar]
- 54. Semnani RT, Sabzevari H, Iyer R, Nutman TB. Filarial antigens impair the function of human dendritic cells during differentiation. Infect Immun 2001; 69:5813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Semnani RT, Liu AY, Sabzevari H, Kubofcik J, Zhou J, Gilden JK et al Brugia malayi microfilariae induce cell death in human dendritic cells, inhibit their ability to make IL‐12 and IL‐10, and reduce their capacity to activate CD4+ T cells. J Immunol 2003; 171:1950–60. [DOI] [PubMed] [Google Scholar]
- 56. Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Cutting edge: diminished T cell TLR expression and function modulates the immune response in human filarial infection. J Immunol 2006; 176:3885–9. [DOI] [PubMed] [Google Scholar]
- 57. Layland LE, Rad R, Wagner H, da Costa CU. Immunopathology in schistosomiasis is controlled by antigen‐specific regulatory T cells primed in the presence of TLR2. Eur J Immunol 2007; 37:2174–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Circulating microfilariae in Litomosoides sigmodontis BALB/c mice skews adaptive immune responses.
Figure S2 Worm gender differentiation in wild‐type, Toll‐like receptor‐4‐deficient (TLR4−/−) and TLR2−/− infected BALB/c mice.
Figure S3 Lack of Toll‐like receptor 4 on antigen‐presenting cells does not alter filarial‐specific CD4+ T‐cell responses.
Figure S4 Lack of Toll‐like receptor 2 (TLR2) or TLR4 on dendritic cells does not alter T‐cell receptor‐activated CD4+ T‐cell responses from Litomosoides sigmodontis wild‐type mice.


