Abstract
Objective
To assess the cost‐effectiveness of implementing a patient navigation (PN) program with capitated payment for Medicare beneficiaries diagnosed with lung cancer.
Data Sources/Study Setting
Cost‐effectiveness analysis.
Study Design
A Markov model to capture the disease progression of lung cancer and characterize clinical benefits of PN services as timeliness of treatment and care coordination. Taking a payer's perspective, we estimated the lifetime costs, life years (LYs), and quality‐adjusted life years (QALYs) and addressed uncertainties in one‐way and probabilistic sensitivity analyses.
Data Collection/Extraction Methods
Model inputs were extracted from the literature, supplemented with data from a Centers for Medicare and Medicaid Services demonstration project.
Principal Findings
Compared to usual care, PN services incurred higher costs but also yielded better outcomes. The incremental cost and effectiveness was $9,145 and 0.47 QALYs, respectively, resulting in an incremental cost‐effectiveness ratio of $19,312/QALY. One‐way sensitivity analysis indicated that findings were most sensitive to a parameter capturing PN survival benefit for local‐stage patients. CE‐acceptability curve showed the probability that the PN program was cost‐effective was 0.80 and 0.91 at a societal willingness‐to‐pay of $50,000 and $100,000/QALY, respectively.
Conclusion
Instituting a capitated PN program is cost‐effective for lung cancer patients in Medicare. Future research should evaluate whether the same conclusion holds in other cancers.
Keywords: Cost‐effectiveness analysis, patient navigation, lung cancer
Many national studies have highlighted access barriers and disparities in prevention or treatment encountered by patients from medically underserved populations (IOM 2002; Oluwole et al. 2003). In oncology, it has been shown that many individuals do not receive care consistent with guidelines following an abnormal screening (Haas et al. 2000). Even when these individuals do get initial screening or treatment, there are frequent delays from the time of an abnormal finding to definitive diagnosis and subsequent treatment, which not only can reduce the quality of care and clinical outcomes, but also heighten the level of anxiety in patients (Rimer and Bluman 1997). Individuals least likely to navigate the health care system for cancer diagnosis or treatment are typically those who are poor, less educated, uninsured/underinsured, and belonging to a racial/ethnic minority (Strzelczyk and Dignan 2002).
Patient navigation (PN) is a community‐based, patient‐centered approach that can potentially reduce health disparities by enhancing access to care at an earlier stage of the disease continuum (Dohan and Schrag 2005; Freeman 2006; Wells et al. 2008; Freeman and Rodriguez 2011; Paskett, Harrop, and Wells 2011). The first PN program was pioneered in Harlem, New York City in 1990 to assist breast cancer screening and follow‐up care for low‐income women; effectiveness of the program was demonstrated by a substantial reduction in the proportion of breast cancer diagnosed at late stage (Oluwole et al. 2003). Early success of the pioneer program has attracted tremendous interest in this community‐based approach of reducing cancer disparities, leading to funding of PN programs from federal agencies, such as the National Cancer Institute, Centers for Medicare and Medicaid Services (CMS) and Centers for Disease Control and Prevention, as well as private foundations, such as the American Cancer Society, and Susan G. Komen for the Cure (Hede 2006; Paskett, Harrop, and Wells 2011).
In response to the Cancer Prevention and Treatment Demonstration for Ethnic and Racial Minorities required in Section 122 of the Medicare, Medicaid, and State Children's Health Insurance Program Benefits Improvement and Protection Act of 2000, the CMS funded six 4‐year Patient Navigation Demonstration Projects in 2006 to evaluate the benefit of providing PN services to minorities. These projects randomized study participants into an intervention group with facilitated care through a trained patient navigator (PN arm) versus a control group with current standard of care (usual care [UC] arm). The projects included both screening and treatment cohorts and paid monthly capitated fees for PN services. While capitated payment can potentially become the payment model for financing PN services in the future, a lack of economic information has been voiced as a major barrier to “serious consideration of PN as a policy solution,” especially for the adoption of PN programs in the treatment phase (Institute 2012). Even when economic analysis was attempted, the priority for the CMS has been choosing programs that are cost‐saving or cost‐neutral while achieving equal or better health outcomes. Such prioritization often leads program evaluations to focus on cost analysis, which is considered partial evaluation in the literature of economic evaluation and does not allow policy makers to address allocation efficiency (Drummond et al. 2005). A full evaluation in the form of cost‐effectiveness analysis will provide comprehensive information to help policy makers better understand the harm‐benefit tradeoff of PN programs.
Our study objective was to evaluate the cost‐effectiveness of instituting a capitated PN program for Medicare beneficiaries diagnosed with lung cancer. The choice of lung cancer was driven by two reasons. First, it allows us to isolate the impact of PN services on the treatment phase because patients with lung cancer were only included in the treatment cohort of the demonstration projects. Second, lung cancer is the leading cause of cancer deaths, accounting for approximately 30 percent of all cancer deaths in the United States in 2013 (ACS 2013); therefore, determining the cost‐effectiveness of the PN program for lung cancer has significant public health implications.
Methods
Model Structure
Our cost‐effectiveness analysis employed a payer's perspective in the base case analysis so that findings can be directly applicable to decision makers at the CMS; the cost‐effectiveness from a societal perspective was explored in sensitivity analyses. We designed a decision analytic model to describe the natural history of lung cancer; the model followed a hypothetical cohort of Medicare beneficiaries diagnosed with lung cancer throughout their lifetime, with both costs and outcomes discounted at 3 percent. The model (Figure 1) first captured whether a patient with a confirmed diagnosis of lung cancer would receive treatment because it has been documented that a nontrivial proportion of lung cancer patients did not receive anticancer treatments (Vinod et al. 2010). For those who chose not to receive treatment, a Markov model with a low transition probability (<10 percent) from alive to death was included to capture the poor prognosis in this subgroup (Detterbeck and Gibson 2008). Among those who intended to undergo treatment, the model considered the possibility that following the natural history of lung cancer a small proportion of patients may have died before receiving treatment. Among those who were treated, the model further differentiated between patients' cancer stage at diagnosis and that at treatment initiation, followed by Markov subtrees that described patients' disease progression for each cancer stage after patients started treatment (Detterbeck and Gibson 2008; Fischel and Dillman 2009). Each of these Markov subtrees included two states, alive and death, with the cycle length of 1 year. What differentiated these subtrees was that the transition probability (from alive to death) differed by cancer stage.
Figure 1.
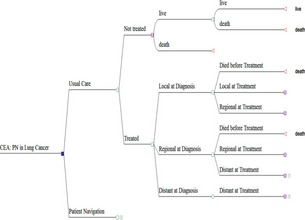
Decision Tree of Clinical Pathway and Natural History of Disease after Lung Cancer Diagnosis
Clinical Benefit of PN Services
Our model was structured to capture two features of PN services that can potentially lead to clinical benefit: timeliness of treatment and care coordination. Because both features were not quantified in our demonstration project, we conducted a literature search to identify relevant publications that linked these features to clinical benefits for patients with a confirmed diagnosis of lung cancer. Specifically, we searched the PubMed database using the following keywords “lung AND ([cancer] OR [oncology] OR [neoplasm]) AND ([nurse navigator] OR [case manager] OR [care coordinator] OR [navigation] OR [patient navigator])” and supplemented information obtained above by hand searching relevant review articles and the references section of original research articles identified from the PubMed search. We summarized our search process in a Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) chart in Appendix SA2 (Moher et al. 2009). As of September, 2014, we identified six studies that provided information for modeling parameters designed to capture clinical benefits of PN services (Murray et al. 2003; Dillman and Chico 2005; Seek and Hogle 2007; Fischel and Dillman 2009; Bjegovich‐Weidman et al. 2010; Alsamarai et al. 2013).
Timeliness of treatment was modeled by taking into consideration the possibility that a patient's disease may have progressed to a more advanced stage from the time of diagnosis to treatment due to delay in initiating treatment, thus placing the patient at a different trajectory of disease progression following his/her natural history of lung cancer. We obtained information on the impact of PN programs with similar features on the duration from diagnosis to treatment initiation from four studies (Murray et al. 2003; Seek and Hogle 2007; Bjegovich‐Weidman et al. 2010; Alsamarai et al. 2013). Murray et al. (2003) pilot tested a two‐stop centralized pathway versus conventional method in a randomized trial among patients suspected of having lung cancer and found significant reductions in the time from presentation to the first treatment, with the average duration decreased from 7 to 3 weeks (Murray et al. 2003). Seek and Hogle (2007) reported that a multidisciplinary program involving patient navigators in a lung cancer clinical trial reduced the average number of days between diagnosis and treatment from 29.3 to 18.76 days, a difference of 10.54 days (Seek and Hogle 2007). Bjegovich‐Weidman et al. (2010) conducted a before–after comparison of a community cancer clinic that established a lung cancer multidisciplinary clinic with a care coordinator and found a reduction in the time from diagnosis to treatment initiation from 24 to 18 days (Bjegovich‐Weidman et al. 2010). Unlike the above three studies, a retrospective cohort analysis by Alsamarai et al. (2013) that evaluated the effectiveness of a cancer care coordination program with a nurse navigator showed no statistically significant difference in the interval from diagnosis to treatment (46 vs. 43 days) (Alsamarai et al. 2013). Therefore, we used the range between 3 days to 4 weeks to capture the benefit of timeliness of treatment associated with PN services and superimposed the impact of this parameter on disease progression based on patients' natural history of lung cancer.
To model the natural history of lung cancer, we obtained information on median survival time (MST) associated with various clinical stages from a systematic review (Detterbeck and Gibson 2008). This article reported that MST for lung cancer patients at local, regional, and distant stage was 11, 5, and 3 months, respectively. To reflect the time intervals of timeliness of care reported in the literature, we modeled the transition probability from one cancer stage to another in 2‐week intervals. Using 2 weeks as the time unit, the MST for local, regional, and distant stage was 23.8, 10.8, and 6.5 units, respectively. On the basis of the MST information, we then used numerical approximation to estimate the survival function for each cancer stage and transition probabilities (see Appendix SA3 for technical details). These transition probabilities captured the natural history of lung cancer. We modeled “timeliness of treatment” by first estimating the probability that a lung cancer patient progressed to a later stage (i.e., local to regional or regional to distant) by the time treatment was initiated for the PN arm. The literature above reported that the time from diagnosis to treatment was in the range of 18.76–43 days for patients in the PN programs with similar features to PN services. We took the average of these two numbers and used 30.88 days (≈ 2 unit) as the duration from diagnosis to treatment for the PN arm to calculate the transition probabilities.1 For the UC arm, the literature suggested that compared to the PN arm, patients in the UC arm experienced an additional delay from diagnosis to treatment in the range of 3 days to 4 weeks. We took the average of these numbers and used 15.5 days (≈ 1 unit) to capture additional delay in the absence of PN services.1
The clinical benefit associated with better coordinated care facilitated by navigators was reflected in improved overall survival. We obtained information on the 5‐year survival for lung cancer patients before and after a program that instituted weekly lung cancer–specific conferences involving nurse navigators to improve care coordination at a community hospital (Dillman and Chico 2005; Fischel and Dillman 2009) and converted the relative survival gain for patients at local, regional, and distant stage to stage‐specific survival probability.
Health Utilities and Costs
We measured the effectiveness of the PN program and UC in terms of life years (LYs) and quality‐adjusted life years (QALYs). To calculate QALYs, we obtained information on health utilities associated with each cancer stage (local, regional, and distant) from published studies that solicited health utilities of lung cancer patients (Ko, Maggard, and Livingston 2003; Sturza 2010). It is possible that PN services, by helping patients better navigate the health care system, can increase the overall health utilities for patients receiving these services. We derived the information on health utility associated with the use of PN services from the treatment cohort in Project FAROS (Facilitated Assistance, Research, and Outreach Services) at MD Anderson Cancer Center. Project FAROS was one of the CMS demonstration sites; it focused on Hispanic beneficiaries in the catchment area of Harris County, Texas. Participants in both the PN and UC groups were asked to complete the Cancer Status Assessment survey at baseline and at exit.
The Cancer Status Assessment included the EQ‐5D questionnaire, which is a standardized instrument for measuring health utilities (Shaw, Johnson, and Coons 2005). The EQ‐5D consists of five dimensions: mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression. Participants' responses to each dimension can be combined into a single health utility index using a published algorithm that transforms the EQ‐5D scores into social preference in the United States (Shaw, Johnson, and Coons 2005). We first converted the EQ‐5D score at baseline and exit interviews to health utilities, next we calculated the change in health utility between exit and baseline interviews as the ratio of mean utility at exit interview divided by that at baseline interview. We then quantified the relative change in health utility as the ratio of the change in utility from baseline to exit for the PN group divided by that for the UC group, and applied the ratio to the PN group to capture the relative utility gain from PN services.
Costs, quantified as Medicare payment, of elderly lung cancer patients at different treatment phases (initial, continuing, and terminal) for each cancer stage were obtained from the literature (Yabroff et al. 2008). For the PN group, we also included the monthly capitated payment of $84 that the CMS reimbursed for each beneficiary randomized to receive PN services. In addition, we added a modeling parameter to capture the possibility that patients utilizing PN services may have more interactions with the health care system, and thus incurring higher costs. All costs were normalized to 2013 US dollars using the Medical Care Consumer Price Index.
Analyses
We conducted both deterministic and probabilistic cost‐effectiveness analyses and applied half‐cycle corrections to all Markov models in our decision tree (Sonnenberg and Beck 1993). In the deterministic analysis, we calculated the incremental cost‐effectiveness ratio (ICER) for PN services versus UC and addressed uncertainties associated with modeling parameters in one‐way sensitivity analyses. One‐way sensitivity analysis investigates the impact of a single parameter on study findings by varying each parameter one at a time while keeping other parameters at their base case values (Drummond et al. 2005). We determined the ranges of values to explore in one‐way sensitivity analysis based on the criteria described below. First, whenever confidence intervals or standard deviations were reported in the literature, we extracted that information directly from the literature. For clinical parameters other than those related to the nature history of lung cancer, we used 50 percent up and down of the base case value as the lower and upper range. For parameters capturing the natural history of lung cancer, we set the range as ±10 percent of the base case values. This is because we felt that there was less uncertainty associated with the natural history of lung cancer as the information came from a systematic review (Detterbeck and Gibson 2008) and a national database (i.e., SEER; Fischel and Dillman 2009). Lastly, for parameters that we are highly uncertain, we chose an even wider range, covering null effect to twice the base case values. Findings from one‐way sensitivity analyses are often depicted as tornado diagrams, which are charts that use horizontal bars to describe the magnitude of effect associated with each parameter. Decision makers can visually inspect a tornado diagram to identify more influential parameters based on the width of each bar in the diagram (Petitti 2000).
For the sensitivity analysis that explored the impact of employing a societal perspective instead of a payor's perspective, we measured societal costs as total health care spending, which included payment from third‐party payers and patients' out‐of‐pocket payment as well as Medicare payment. We obtained information on the ratio of Medicare payment to total health care spending for elderly cancer patients from a study that analyzed Medicare Current Beneficiary Survey linked to Medicare claims to compare out‐of‐pocket expenditure between Medicare beneficiaries with and without cancer (Davidoff et al. 2013). The study found that Medicare payment accounted for approximately 70 percent of all health care spending; therefore, we applied a multiplier (≈ 1.0/0.7) to parameters of Medicare payments in the analysis taking the societal perspective.
Although the above deterministic analysis allowed decision makers to isolate the effect of each parameter and determine which parameters have strong impact on study findings, it does not address overall uncertainty associated with the combined variability in modeling parameters. The latter is achieved by probabilistic analysis (Drummond et al. 2005). In the probabilistic analysis, we followed the recommendation in textbooks of economic evaluation and chose the following distributions for different type of parameters (Table 1): beta distribution for binomial data (e.g., transition probability from local to regional stage), Dirichlet distribution for multinomial data (e.g., proportion of patients at various cancer stages), gamma distribution for costs and utility decrement (i.e., 1 – health utility), and uniform distribution of parameters that were highly speculative in nature and had limited information from the literature (Drummond et al. 2005; Briggs, Claxton, and Sculpher 2007). Results from the probabilistic analysis were presented as cost‐effectiveness acceptability curve (CEAC). The CEAC illustrates the probability that the PN is more cost‐effective than UC at various levels of societal willingness‐to‐pay (WTP). CEAC in our study was calculated by running 10,000 iterations of Monte Carlo simulations that randomly drew value for each modeling parameter from its corresponding distribution shown in Table 1 and propagated these values through the model to capture joint parameter uncertainty.
Table 1.
Parameter Values, Ranges, and Distributions Examined in Sensitivity Analyses, and Data Source
| Parameter Name | Definition | Base Care (Range) | Distributiona | Data Source |
|---|---|---|---|---|
| Clinical parameters | ||||
| TP_LtoRb | Transition probability: local to regional stage | 0.07 (0.035–0.105)c | Beta (0.07, 0.93) | Detterbeck and Gibson (2008) |
| TP_RtoDb | Transition probability: regional to distant stage | 0.22 (0.11–0.33)c | Beta (0.22, 0.78) | |
| B_Delay_PN | Delay (in 2‐week interval) from diagnosis to treatment with PN services | 2 (0–4)d | Uniform (0, 4) | Murray et al. (2003); Seek and Hogle (2007); Bjegovich‐Weidman et al. (2010); Alsamarai et al. (2013) |
| Delay_UC | Additional delay (in 2‐week interval) from diagnosis to treatment in usual care | 1 (0–2)d | Uniform (0, 2) | |
| P_untreat | Proportion of patients with no active treatment | 0.19 (0.1–0.3)c | Beta (0.19, 0.81) | Vinod et al. (2010) |
| P_Loc | Proportion of patients at local stage | 0.17 (0.15–0.19)e | Dirichlet (17, 38, 45) | Detterbeck and Gibson (2008); Fischel and Dillman (2009) |
| P_Reg | Proportion of patients at regional stage | 0.38 (0.34–0.42)e | Dirichlet (17, 38, 45) | |
| Surv_Loc | 1‐year survival for local‐stage lung cancer | 0.87 (0.78–0.96)e | Beta (0.87, 0.13) | |
| Surv_Reg | 1‐year survival for regional‐stage lung cancer | 0.68 (0.61–0.75)e | Beta (0.68, 0.32) | |
| Surv_Dist | 1‐year survival for distant‐stage lung cancer | 0.49 (0.44–0.54)e | Beta (0.49, 0.51) | |
| Surv_Untreat | 1‐year survival for untreated patients | 0.09 (0.08–0.01)e | Beta (0.09, 0.91) | Detterbeck and Gibson (2008) |
| SurvB_PN_Loc | Relative gain in 1‐year survival from PN services for local‐stage lung cancer patients | 5% (0–10%)d | Uniform (1, 1.1) |
Dillman and Chico (2005) Fischel and Dillman (2009) |
| SurvB_PN_Reg | Relative gain in 1‐year survival from PN services for regional‐stage lung cancer patients | 5% (0–10%)d | Uniform (1, 1.1) | |
| SurvB_PN_Dist | Relative gain in 1‐year survival from PN services for distant‐stage lung cancer patients | 0% (0–10%)d | Uniform (1, 1) | |
| E_disc | Discount rate for effectiveness | 0.03 (0–0.06)d | Uniform (0, 0.06) | Assumption |
| Cost parameters | ||||
| C_init_Loc | Cost of initial care phase, local stage | 41,859 (40,336–43,381)f | Gamma (3,023, 0.072) | Yabroff et al. (2008) |
| C_init_Reg | Cost of initial care phase, regional stage | 53,316 (51,653–54,978)f | Gamma (4,114, 0.077) | |
| C_init_Dist | Cost of initial care phase, distant stage | 58,681 (55,891–61,472)f | Gamma (1,769, 0.030) | |
| C_cont | Cost of continuing care phase | 5,335 (4,944–5,727)f | Gamma (744.12, 0.139) | |
| C_term_Loc | Cost of terminal care phase, local stage | 54,023 (52,292–55,754)f | Gamma (3,896, 0.072) | |
| C_term_Reg | Cost of terminal care phase, regional stage | 72,236 (70,828–73,644)f | Gamma (10,523, 0.146) | |
| C_term_Dist | Cost of terminal care phase, distant stage | 91,748 (90,104–93,405)f | Gamma (12,355, 0.135) | |
| C_PN | Annual capitated cost of PN services | 1,000 (500–1,500)c | Uniform (500, 1,500) | Medicare payment |
| C_inc_PN | % increase in costs due to more interactions with providers driven by PN services | 5% (0–10%)d | Uniform (1, 1.1) | Assumption |
| C_disc | Discount rate for cost | 0.03 (0–0.06)d | Uniform (0, 0.06) | Assumption |
| Utility parameters | ||||
| U_init | Utility for lung cancer patients in nonmetastatic initial phase | 0.42 (SD = 0.28)f | 1‐Gamma (4.29, 7.40) | Ko, Maggard, and Livingston (2003) |
| U_int | Utility for lung cancer patients in nonmetastatic interval phase | 0.825 (SD = 0.074)f | 1‐Gamma (5.55, 31.74) | Sturza (2010) |
| U_adv | Utility for lung cancer patients at distant stage or terminal phase | 0.573 (SD = 0.067)f | 1‐Gamma (40.62, 95.12) | |
| U_PN | Relative gain in utility due to PN services | 0.07 (0–0.14)d | Uniform (0, 0.14) | Analysis of FAROS data |
Distributions used in the probabilistic sensitivity analysis.
See supplementary material for details.
±50% of base case value.
Null effect to twice the base case value.
±10% of base case value.
From the literatures or FAROS.
FAROS, Facilitated Assistance, Research, and Outreach Services.
We conducted statistical analysis using STATA 13 and modeling analyses using TreeAge Pro Healthcare 2014. This study was exempt from the Institutional Review Board (IRB).
Results
Analysis of the EQ‐5D data collected from Project FAROS (Table 2) showed that while patients in the PN and UC group had similar health utilities at baseline interview, health utilities at exit interview were higher for patients in the PN group (0.827 vs. 0.771; p < .05). Comparisons across the five EQ‐5D dimensions suggested that the higher utilities observed in the PN group at exit interview were primarily driven by improvement in self‐care (p = .062), usual activities (p = .091), and anxiety/depression (p = .047). None of the five EQ‐5D dimensions showed significant improvement from baseline to exit interview for the UC group.
Table 2.
Comparison of EQ‐5D Dimensions and Utility Score between PN and UC Groups at Baseline and Exit Interviews
| Baseline Interview | Change from Baseline to Exit Interviews | ||||||
|---|---|---|---|---|---|---|---|
| PN | UC | ||||||
| PN (N = 151) | UC (N = 148) | p‐value | Mean Difference | p‐value | Mean Difference | p‐value | |
| Mobility | 68.9% | 64.2% | .391 | 2.80% | .622 | 1.90% | .749 |
| Self‐care | 82.1% | 84.5% | .588 | 8.20% | .062 | 1.60% | .713 |
| Usual activities | 65.6% | 66.2% | .905 | 9.60% | .091 | 0.80% | .9 |
| Pain/discomfort | 56.3% | 45.3% | .057 | −4.10% | .510 | 1.70% | .785 |
| Anxiety/depression | 60.9% | 62.2% | .826 | 11.70% | .048 | −0.50% | .944 |
| EQ‐5D score | 0.793 | 0.794 | .982 | 0.034 | .22 | −0.023 | .41 |
Numbers in the percentages were the proportion of participants reporting “having no problem” in the corresponding EQ‐dimension.
PN, patient navigation; UC, usual care.
Table 3 summarizes the finding from deterministic analyses. The base case analysis showed that instituting a capitated PN program for Medicare beneficiaries diagnosed with lung cancer was associated with an increase of $9,145 lifetime cost per beneficiary and an increase in LY and QALY by 0.43 and 0.47, respectively, resulting in an ICER of $21,383/LY or $19,312/QALY, which was substantially lower than the commonly accepted cost‐effectiveness threshold in the range between $50,000/QALY and $100,000/QALY. Analysis that isolated the effect of “timeliness of treatment” from “care coordination” showed that the majority of clinical benefit from PN services was driven by the navigator's ability in coordinating complicated cancer treatments for patients. The conclusion on the cost‐effectiveness of PN remained robust when changing the study perspective to societal perspective, with the ICER rising to $25,173/QALY. A tornado diagram (Figure 2) shows that the two most influential modeling parameters were the survival benefit of PN for local‐stage patients (SurvB_PN_Loc) and the proportion of increase in Medicare expenditures due to PN services (C_inc_PN). We explored these two parameters in one‐way sensitivity analyses. As shown in Table 3, the conclusion that the capitated PN program is cost‐effective remained robust.
Table 3.
Base Case Cost‐Effectiveness Analysis and One‐Way Sensitivity Analyses
| Mean Cost | Mean LY | Mean QALY | Incremental Costs | Incremental LY | Incremental QALY | ICER ($/LY) | ICER ($/QALY) | |
|---|---|---|---|---|---|---|---|---|
| Base case | ||||||||
| PN | $99,551 | 2.34 | 1.77 | $9,145 | 0.43 | 0.47 | $21,383 | $19,312 |
| Usual care | $90,406 | 1.91 | 1.30 | |||||
| No clinical benefit from “timely treatment” | $8,969 | 0.32 | 0.37 | $28,239 | $24,144 | |||
| No clinical benefit from “care coordination” | $6,857 | 0.08 | 0.17 | $87,175 | $41,435 | |||
| One‐way sensitivity analysis | ||||||||
| Study perspective | ||||||||
| Societal perspective | $11,920 | 0.43 | 0.47 | $27,873 | $25,173 | |||
| Increase in other Medicare spending due to PN services | ||||||||
| 0% | $4,539 | 0.43 | 0.47 | $10,614 | $9,586 | |||
| 5% (base case) | $9,145 | 0.43 | 0.47 | $21,383 | $19,312 | |||
| 10% | $13,750 | 0.43 | 0.47 | $32,152 | $29,038 | |||
| 20% | $22,961 | 0.43 | 0.47 | $53,689 | $48,489 | |||
| Survival benefit of PN for local‐stage patients | ||||||||
| 0% | $7,297 | 0.15 | 0.22 | $50,224 | $32,537 | |||
| 5% (base case) | $9,145 | 0.43 | 0.47 | $21,383 | $19,312 | |||
| 10% | $12,850 | 1.01 | 0.99 | $12,682 | $12,974 | |||
Figure 2.
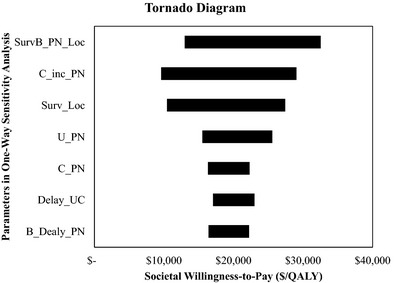
Tornado Diagram of Incremental Cost‐Effectiveness Ratio
Figure 3 presents results from the probabilistic analyses. It shows that the probability that the PN program was cost‐effective (compared to UC) was 0.80 at the societal WTP of $50,000/QALY, and the probability rose to 0.91 at WTP of $100,000/QALY. The probability reduced to 0.76 and 0.88, respectively, if the study employed a societal perspective. If the use of PN services did not lead to an increase in Medicare spending other than PN costs, the probability that the capitated PN program is cost‐effective increased to 0.92 and 0.96 at WTP of $50,000/QALY and $100,000/QALY, respectively (results not shown). However, if the use of PN services was associated with a 20 percent increase in Medicare cost, these probabilities dropped to 0.55 and 0.76 at $50,000/QALY and $100,000/QALY, respectively.
Figure 3.
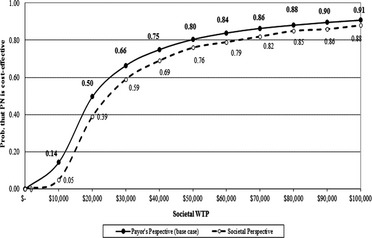
Cost‐Effectiveness Acceptability Curve: PN Versus Usual Care
Discussions
The CMS funded six demonstration projects to explore the impact of a capitated PN program on improving cancer screening and treatment for minority Medicare beneficiaries. The final report evaluating the effectiveness of these demonstration projects was released recently. Participants in the PN group reported considerable satisfaction with the educational materials, and the referral and support services provided by the navigators (Karikari‐Martin et al. 2013). However, the report provided no information regarding the cost‐effectiveness of the PN program. In addition, while the Patient Navigator and Chronic Disease Prevention Act in Section 340a of the Public Health Service Act was sunset in 2010, the Patient Protection and Affordable Care Act of 2010 reauthorized Section 340a for five more years (Institute 2012). With the widespread interests in PN services among public and private funding agencies, information on the cost‐effectiveness of various aspects of PN programs is critically important. Our study offers early evidence on the evaluation of the CMS PN demonstration projects.
Our cost‐effectiveness analysis suggested that paying for PN services for beneficiaries diagnosed with lung cancer is cost‐effective for the Medicare program. The estimated ICER was less than $25,000 per QALY, with the probability of cost‐effectiveness being 0.80 and 0.91 at the societal WTP of $50,000/QALY and $100,000/QALY, respectively. We conducted extensive sensitivity analyses, including the modification of the study perspective from a payor's perspective to a societal perspective, and found that the ICER stayed within favorable range even when the values of key modeling parameters were varied within a wide range. Although our study showed that a capitated PN program is cost‐effective from the Medicare perspective, it also showed that such program could increase overall Medicare spending in the long run. This increase is driven by two factors: (1) medical costs associated with additional LYs gained, and (2) the possibility that better coordinated care would lead to higher utilization as a result of more frequent interaction with the health care system. Current CMS policies that deprioritize programs that are not cost‐saving or cost‐neutral could create a barrier in implementing highly cost‐effective programs, such as the capitated PN program examined in our study.
Besides the policy cost‐effectiveness, our study also provided some insights on the mechanism that PN services improved patients' QALYs. Using the EQ‐5D data collected from Project FAROS, our analysis suggested that the increase in QALY observed between the baseline and exit interviews among patients in the PN group was likely driven by reduction in anxiety and depression, as well as improvement in self‐care and performing usual activities, although most of the observed increases were only marginally significant (i.e., p < .10). The above mechanism of QALY improvement probably reflects the type of navigation services received by patients as well as the qualifications of navigators employed in Project FAROS. A recent review by Paskett and colleagues highlighted the heterogeneity of patient navigators, with a wide variation in their clinical training and preparation (Paskett, Harrop, and Wells 2011). Navigators employed in Project FAROS mostly had a bachelor's degree and received 160 hours of training from a state‐certified agency; none of the navigators had a registered nurse degree. A survey of patients in the treatment cohort showed that navigation services concentrated on three activities: provide information about cancer‐related services/resources, contact patients by phone or mail to remind them about cancer follow‐up, and help patients make additional follow‐up appointments. Thus, the improved EQ‐5D dimensions observed in the PN group likely reflected the fact that navigation activities in Project FAROS largely focused on facilitating patients' contact with the health care system and providing information on cancer knowledge as well as more general health conditions. For the other two EQ‐5D dimensions, mobility and pain/discomfort, navigators in Project FAROS may not be trained to help patients address difficulties associated with these two dimensions.
The demonstration project provides capitated payment for PN services given to beneficiaries in the screening as well as treatment cohorts. Our analysis offers supportive evidence for Medicare to cover PN services for beneficiaries diagnosed with lung cancer. The cost‐effectiveness of this capitated PN program observed in our study could be driven by a number of factors. First, the natural history of lung cancer may differ from other cancers in that once diagnosed, lung cancer tends to be fast‐progressing with substantially worse survival at the advanced stage; therefore, timely treatment is especially important among those patients. Second, treatment of lung cancer is complex and often involves multiple specialties; therefore, the role of navigators in care coordination is highly relevant in diseases requiring multidisciplinary care, such as lung cancer. This point was confirmed in our analysis that separated the effect of timeliness of treatment from care coordination (Table 3) and found a larger benefit associated with care coordination. Lastly, unlike PN services aimed at increasing the rate of screening in the general population, PN services in our study were targeted at patients with an urgent need for medical care; therefore, the impact of PN services on patients' utility and health is immediately detected. Analyses that removed the effect of health utilities and focused only on LY saved also reached the same conclusion, although the ICER was slightly higher. While the extensive sensitivity analyses performed in this study confirmed the robustness of our conclusion, it should be noted that findings from our study do not imply that reimbursing PN services for other cancers will be cost‐effective. Nor do they imply that the use of capitated PN services to increase screening rates in the general Medicare population will be cost‐effective. The cost‐effectiveness of PN services in either scenario needs to be addressed in future research.
Caveats of our study are discussed below. First, given the 4‐year duration of the demonstration project, the survival benefit associated with PN services was not directly observed in our site but was inferred from the literature. In an article discussing the unique challenges for evaluating the cost‐effectiveness of PN programs, Ramsey et al. (2009) acknowledged difficulties in detecting the impact of treatment delay on mortality and recommended addressing this issue using a modeling approach (Ramsey et al. 2009). Following this recommendation, we designed a model that captured two key features of PN services, timeliness of treatment and care coordination, and identified relevant literature that linked these functions to clinical benefits in lung cancer. We then explored the impact of these parameters (i.e., delay_UC, B_Delay_PN, SurvB_PN_loc, and SurvB_PN_Reg) in sensitivity analyses and confirmed from sensitivity analyses that findings from our study are robust within a wide range of parameter values (Figure 2).
Second, our EQ‐5D data were based on all patients in the treatment cohort of Project FAROS, instead of the subset of lung cancer patients. The decision was driven by two concerns: (1) the small number of lung cancer patients in the treatment cohort rendered highly unstable estimates, and (2) the initial randomization of participants both in the intervention (i.e., PN services) and control arm of the treatment cohort was based on all five cancers (breast, cervical, colorectal, lung, and prostate) combined. Thus, while the baseline utilities were similar between patients randomized to the PN and UC group, the same cannot be expected among lung cancer patients in these two groups from the trial design. The use of health utilities data from Project FAROS which focused exclusively on Hispanic Medicare beneficiaries raised a concern regarding the generalizability of our study finding. To address this concern, we included LY as another outcome measure so that our study finding was independent from the source of health utilities data. Nevertheless, we are unaware of any study in the literature that has demonstrated racial disparities in health utility values. We verified this by comparing the EQ‐5D index score between Hispanic and non‐Hispanic Medicare beneficiaries using the 2003 Medical Expenditure Panel Survey. Our analysis showed that the mean score was not significantly different between these two groups (mean = 0.68 for Hispanic beneficiaries and 0.70 for non‐Hispanic beneficiaries; p = .141 in unweighted analysis and 0.326 in weighted analysis), suggesting that our use of health utilities obtained from Hispanic beneficiaries should not bias our estimate of QALY.
Lastly, our base case analysis evaluated the cost‐effectiveness of the PN program from the payer's perspective (i.e., Medicare) and explored findings from the societal perspective in sensitivity analyses. For our analysis taking the societal perspective we did not take into consideration indirect cost in the context of productivity loss by patients and their family caregivers. Given that the study focused on Medicare beneficiaries and many are no longer in the labor force, we think they are unlikely to incur high indirect costs. It is possible that patient navigators could affect the productivity of family caregivers. However, it is not clear whether the use of PN services would increase or decrease the time commitment of family caregivers. On one hand, PN services can reduce family caregivers' productivity loss by assisting patients and their families navigating the health care system. On the other hand, indirect costs may increase if PN services lead to higher utilization of medical services, which could then increase the time caregivers spend accompanying patients to receiving their care. However, the net effect of these two forces is unlikely to drive up the incremental costs substantially that push the ICER over $50,000/QALY.
Supporting information
Appendix SA1: Author Matrix.
Appendix SA2: PRISMA Chart.
Appendix SA3: Programs of Numerical Approximation to Calculate Transition Probabilities.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This study is supported by grant from the National Cancer Institute (RC1CA145799), the University of Chicago Cancer Research Foundation Women's Board, and a grant from the Ministry of Health and Welfare in Taiwan (MOHW104‐TDU‐B‐212‐124‐002). All key individuals involved in the funded study had participated in the preparation of this manuscript and were included as coauthors.
Disclosures: None of the funding agencies affects the independence of the study or causes conflicts of interests. The authors have no other financial and material support to disclose.
Disclaimers: None.
Note
Denote D_Delay_PN as the average duration from diagnosis to treatment for the PN arm, the transition probability from local to regional stage and from regional to distant stage for the PN arm is: exp(−TP_LtoR x (B_delay_PN)) and exp(−TP_RtoD x (B_delay_PN)), respectively. For the UC arm, denote Delay_UC as the additional delay from diagnosis to treatment without PN services, the transition probability from local to regional stage and from regional to distant stage for the UC arm is: exp(−TP_LtoR x (B_Delay_PN + Delay_UC)) and exp(−TP_RtoD x (B_Delay_PN+Delay_UC)), respectively.
References
- ACS . 2013. American Cancer Society. Cancer Facts & Figures 2013. Atlanta, GA: American Cancer Society. [Google Scholar]
- Alsamarai, S. , Yao X., Cain H. C., Chang B. W., Chao H. H., Connery D. M., Deng Y., Garla V. N., Hunnibell L. S., Kim A. W., Obando J. A., Taylor C., Tellides G., and Rose M. G.. 2013. “The Effect of a Lung Cancer Care Coordination Program on Timeliness of Care.” Clinical Lung Cancer 14 (5): 527–534. [DOI] [PubMed] [Google Scholar]
- Bjegovich‐Weidman, M. , Haid M., Kumar S., Huibregtse C., McDonald J., and Krishnan S.. 2010. “Establishing a Community‐Based Lung Cancer Multidisciplinary Clinic as Part of a Large Integrated Health Care System: Aurora Health Care.” Journal of Oncology Practice/American Society of Clinical Oncology 6 (6): e27–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs, A. H. , Claxton K., and Sculpher M.. 2007. Decision Modelling for Health Economic Evaluation. New York: Oxford University Press. [Google Scholar]
- Davidoff, A. J. , Erten M., Shaffer T., Shoemaker J. S., Zuckerman I. H., Pandya N., Tai M. H., Ke X., and Stuart B.. 2013. “Out‐of‐Pocket Health Care Expenditure Burden for Medicare Beneficiaries with Cancer.” Cancer 119 (6): 1257–1265. [DOI] [PubMed] [Google Scholar]
- Detterbeck, F. C. , and Gibson C. J.. 2008. “Turning Gray: The Natural History of Lung Cancer over Time.” Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer 3 (7): 781–792. [DOI] [PubMed] [Google Scholar]
- Dillman, R. O. , and Chico S. D.. 2005. “Cancer Patient Survival Improvement Is Correlated with the Opening of a Community Cancer Center: Comparisons with Intramural and Extramural Benchmarks.” Journal of Oncology Practice/American Society of Clinical Oncology 1 (3): 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohan, D. , and Schrag D.. 2005. “Using Navigators to Improve Care of Underserved Patients: Current Practices and Approaches.” Cancer 104 (4): 848–855. [DOI] [PubMed] [Google Scholar]
- Drummond, M. F. , Sculpher M. J., Torrance G. W., O'Brien B. J., and Stoddart G. L.. 2005. Methods for the Economic Evaluation of Health Care Programmes. New York: Oxford University Press. [Google Scholar]
- Fischel, R. J. , and Dillman R. O.. 2009. “Developing an Effective Lung Cancer Program in a Community Hospital Setting.” Clinical Lung Cancer 10 (4): 239–243. [DOI] [PubMed] [Google Scholar]
- Freeman, H. P. 2006. “Patient Navigation: A Community Centered Approach to Reducing Cancer Mortality.” Journal of Cancer Education: The Official Journal of the American Association for Cancer Education 21 (1): S11–S14. [DOI] [PubMed] [Google Scholar]
- Freeman, H. P. , and Rodriguez R. L.. 2011. “History and Principles of Patient Navigation.” Cancer 117 (15 Suppl): 3539–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas, J. S. , Cook E. F., Puopolo A. L., Burstin H. R., and Brennan T. A.. 2000. “Differences in the Quality of Care for Women with an Abnormal Mammogram or Breast Complaint.” Journal of General Internal Medicine 15 (5): 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hede, K. 2006. “Agencies Look to Patient Navigators to Reduce Cancer Care Disparities.” Journal of the National Cancer Institute 98 (3): 157–159. [DOI] [PubMed] [Google Scholar]
- Institute, G. C. 2012. “The Affordable Care Act and Patient Navigation: Support for Those in Need” [accessed on August 8, 2014]. Available at http://smhs.gwu.edu/gwci/sites/gwci/files/PatientNavigationFullReport.pdf
- IOM . 2002. Institute of Medicine Report: Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Karikari‐Martin, P. , Mitchell J. B., Bir A., Hoover S., and Subramanian S.. 2013. Evaluation of the Cancer Prevention and Treatment Demonstration for Ethnic and Racial Minorities. Final Report to Congress. Waltham, MA: RTI International. [Google Scholar]
- Ko, C. Y. , Maggard M., and Livingston E. H.. 2003. “Evaluating Health Utility in Patients with Melanoma, Breast Cancer, Colon Cancer, and Lung Cancer: A Nationwide, Population‐Based Assessment.” Journal of Surgical Research 114 (1): 1–5. [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati A., Tetzlaff J., and Altman D. G.. 2009. “Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement.” PLoS Medicine 6 (7): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, P. V. , O'Brien M. E., Sayer R., Cooke N., Knowles G., Miller A. C., Varney V., Rowell N. P., Padhani A. R., MacVicar D., Norton A., Ashley S., and Smith I. E.. 2003. “The Pathway Study: Results of a Pilot Feasibility Study in Patients Suspected of Having Lung Carcinoma Investigated in a Conventional Chest Clinic Setting Compared to a Centralised Two‐Stop Pathway.” Lung Cancer 42 (3): 283–290. [DOI] [PubMed] [Google Scholar]
- Oluwole, S. F. , Ali A. O., Adu A., Blane B. P., Barlow B., Oropeza R., and Freeman H. P.. 2003. “Impact of a Cancer Screening Program on Breast Cancer Stage at Diagnosis in a Medically Underserved Urban Community.” Journal of the American College of Surgeons 196 (2): 180–188. [DOI] [PubMed] [Google Scholar]
- Paskett, E. D. , Harrop J. P., and Wells K. J.. 2011. “Patient Navigation: An Update on the State of the Science.” CA: A Cancer Journal for Clinicians 61 (4): 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitti, D. B. 2000. “Sensitivity Analysis” In Meta‐Analysis, Decision Analysis, and Cost‐Effectiveness Analysis: Methods for Quantitative Synthesis in Medicine, edited by Petitti D. B., pp. 229–239. New York, NY: Oxford University Press. [Google Scholar]
- Ramsey, S. , Whitley E., Mears V. W., McKoy J. M., Everhart R. M., Caswell R. J., Fiscella K., Hurd T. C., Battaglia T., and Mandelblatt J.. 2009. “Evaluating the Cost‐Effectiveness of Cancer Patient Navigation Programs: Conceptual and Practical Issues.” Cancer 115 (23): 5394–5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimer, B. K. , and Bluman L. G.. 1997. “The Psychosocial Consequences of Mammography.” Journal of the National Cancer Institute Monographs 1997 (22): 131–138. [DOI] [PubMed] [Google Scholar]
- Seek, A. , and Hogle W. P.. 2007. “Modeling a Better way: Navigating the Healthcare System for Patients with Lung Cancer.” Clinical Journal of Oncology Nursing 11 (1): 81–85. [DOI] [PubMed] [Google Scholar]
- Shaw, J. W. , Johnson J. A., and Coons S. J.. 2005. “US Valuation of the EQ‐5D Health States: Development and Testing of the D1 Valuation Model.” Medical Care 43 (3): 203–220. [DOI] [PubMed] [Google Scholar]
- Sonnenberg, F. A. , and Beck J. R.. 1993. “Markov Models in Medical Decision Making: A Practical Guide.” Medical Decision Making 13 (4): 322–338. [DOI] [PubMed] [Google Scholar]
- Strzelczyk, J. J. , and Dignan M. B.. 2002. “Disparities in Adherence to Recommended Followup on Screening Mammography: Interaction of Sociodemographic Factors.” Ethnicity & Disease 12 (1): 77–86. [PubMed] [Google Scholar]
- Sturza, J. 2010. “A Review and Meta‐Analysis of Utility Values for Lung Cancer.” Medical Decision Making: An International Journal of the Society for Medical Decision Making 30 (6): 685–693. [DOI] [PubMed] [Google Scholar]
- Vinod, S. K. , Sidhom M. A., Gabriel G. S., Lee M. T., and Delaney G. P.. 2010. “Why do Some Lung Cancer Patients Receive no Anticancer Treatment?” Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer 5 (7): 1025–1032. [DOI] [PubMed] [Google Scholar]
- Wells, K. J. , Battaglia T. A., Dudley D. J., Garcia R., Greene A., Calhoun E., Mandelblatt J. S., Paskett E. D., and Raich P. C.. 2008. “Patient Navigation: State of the Art or Is It Science?” Cancer 113 (8): 1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabroff, K. R. , Lamont E. B., Mariotto A., Warren J. L., Topor M., Meekins A., and Brown M. L.. 2008. “Cost of Care for Elderly Cancer Patients in the United States.” Journal of the National Cancer Institute 100 (9): 630–641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.
Appendix SA2: PRISMA Chart.
Appendix SA3: Programs of Numerical Approximation to Calculate Transition Probabilities.


