Abstract
Aim
Clinical use of glucagon‐like peptide‐1 receptor agonists (GLP‐1RA) is consistently associated with heart rate (HR) acceleration in type 2 diabetes patients. We explored the mechanisms underlying this potential safety concern.
Methods
Ten healthy overweight males (aged 20–27 years) were examined in an open label, crossover study. Automated oscillometric blood pressure measurements and finger photoplethysmography were performed throughout intravenous administration of placebo (saline 0.9%), exenatide (targeting therapeutic concentrations) and a combination of exenatide and the nitric oxide synthase inhibitor L‐NG‐monomethyl arginine (L‐NMMA). Sympathetic nervous system (SNS) activity was measured by heart rate variability and rate‐pressure product.
Results
Exenatide increased HR by a mean maximum of 6.8 (95% CI 1.7, 11.9) beats min–1 (P < 0.05), systolic blood pressure (SBP) by 9.8 (95% CI 3.5, 16.1) mmHg (P < 0.01) and markers of SNS activity (P < 0.05). No changes in total peripheral resistance were observed. Increases in HR, SBP and sympathetic activity were preserved during concomitant L‐NMMA infusion.
Conclusions
Our data argue against exenatide‐induced reflex tachycardia as a response to vasodilation and rather suggest the involvement of SNS activation in humans.
Keywords: GLP‐1 receptor agonist, haemodynamics, heart rate, sympathetic nervous system activity
What is Already Known About This Subject
Glucagon‐like peptide‐1 receptor agonists (GLP‐1RA) consistently increase heart rate in clinical practice. Animal and limited clinical data suggest vascular and autonomic nervous system involvement, yet the exact underlying mechanisms remain speculative.
What This Study Adds
Acute administration of the GLP‐1 receptor agonist exenatide increases heart rate and sympathetic nervous activity, while not affecting vascular resistance in healthy overweight males. These results improve our understanding of the mechanisms involved in the potential safety concerns associated with GLP‐1 receptor agonist treatment in type 2 diabetes.
Introduction
Over the last decade, glucagon‐like peptide‐1 receptor agonists (GLP‐1RA) have emerged as a therapeutic option for type 2 diabetes management. These insulinotropic agents improve glycaemic control, with the added clinical benefit of reducing bodyweight and blood pressure after long term treatment 1. However, in both acute and long term human studies, a persistent GLP‐1RA‐induced increase in heart rate (HR) of approximately 2 beats min–1 was reported in a meta‐analysis that included available data from randomized controlled trials 1. Since elevated HR is independently associated with cardiovascular morbidity and mortality in the general population 2, the long term safety of these antihyperglycaemic drugs in type 2 diabetes patients remains to be established. However, understanding the underlying mechanisms may already lead to more insight into its clinical significance.
Suggested mechanisms that underlie GLP‐1RA‐associated HR acceleration in humans remain speculative. First, GLP‐1 may directly augment sympathetic or reduce parasympathetic nervous system activity 3. Alternatively, GLP‐1‐induced vasodilation may initially reduce total peripheral resistance (TPR), leading to reflex tachycardia 4.
In the current study, we aimed to untangle these opposing theories in healthy overweight males. As the baroreceptor reflex augments HR after TPR lowering by modulating the cardiac autonomic nervous system (ANS), and GLP‐1RAs potentially reduce TPR in a nitric oxide (NO)‐dependent manner 5, we examined the effects of the GLP‐1RA exenatide on systemic haemodynamics and the ANS, with and without concomitant blockage of NO synthase.
Methods
This was a single centre, open label, crossover study with intravenous administration of placebo (saline 0.9%), exenatide, the NO synthase blocker L‐NG‐monomethyl‐arginine‐citrate (L‐NMMA) or a combination of exenatide/L‐NMMA, as described previously 6.
Participants
Ten healthy, overweight (body mass index range 26–31 kg m−2), Caucasian males, aged 20–27 years, were recruited via poster advertisements. Subjects with a recent history of any type of medication or substance abuse (alcohol >3 units per day), nightshift work or abnormalities on physical examination, fasting blood and urine samples, were excluded. A 2 h 75 mg oral glucose tolerance test was performed to ascertain normal glucose metabolism. The study was approved by the Ethics Review Board of the VU University Medical Center and complied with the principles of the Declaration of Helsinki and International Conference on Harmonization on Good Clinical Practice. All subjects provided written informed consent before participation.
Study protocol
Measurements were performed on 2 separate study visits at least 1 week apart. On day 1, the effects of intravenous placebo (saline 0.9%) and subsequently exenatide (AstraZeneca, London, UK; loading dose of 50 ng min−1 for 30 min, followed by a continuous infusion of 25 ng min−1) were assessed. On day 2, the effects of L‐NMMA (Bachem GmbH, Weil am Rhein, Germany; loading dose of 5 mg kg−1, administered over 5 min, followed by continuous infusion of 50 μg kg−1 min−1) and a combination of L‐NMMA and exenatide were assessed 6, 27.
Before testing, subjects adhered to an average salt and protein diet for 2 days, and abstained from heavy physical exercise, alcohol and caffeine. Prior to each measurement, subjects were acclimatized for more than 5 min. All measurements were performed in the fasting state, with the non‐dominant arm comfortably placed at heart level, in a semi‐recumbent position in a temperature‐controlled room (23.0 ± 1.0 °C). Appropriate cuff sizes were used where applicable. Measurements were performed at repeated intervals (see online Supplementary Figure 1). While systemic haemodynamic parameters were measured during all conditions, heart rate variability (HRV) could only be assessed during exenatide infusion (and the combination of exenatide/L‐NMMA), for logistic reasons.
Systolic and diastolic blood pressure (SBP and DBP, respectively), mean arterial pressure (MAP) and HR were measured using an automatic oscillometric device (Dinamap©, GE Healthcare, Little Chalfont, UK). Measurements were performed in triplicate at 1–2 min intervals and the mean of the last two measurements was taken as the final value for each time point. Stroke volume (SV), cardiac output (CO) and TPR were assessed using a beat‐to‐beat finger blood pressure device (Nexfin©, BMEYE, Amsterdam, The Netherlands). A 30 s average was derived using dedicated software (Nexfin@PC version 2, BMEYE, Amsterdam, the Netherlands). The rate‐pressure product (RPP), a marker reflecting sympathetic nervous system (SNS) activity, was calculated as HR × SBP 7.
Cardiac ANS balance was assessed by resting HRV. Measurements were performed with an ECG‐equipped Nexfin© device for a 5 min period during which participants breathed spontaneously (range 10–18 breaths per minute), and refrained from speaking or sleeping. ECG strips were visually inspected and artefacts were manually corrected (linear interpolation). Fast Fourier spectral analyses were performed using Kubios HRV Analysis Software 2.1 (University of of Eastern Finland, Kuopio, Finland) after further automated artefact correction and removal of trend components. The derived spectrum comprises very low frequency (VLF, 0.01 to 0.04 Hz), low frequency (LF, 0.04 to 0.15 Hz) and high frequency (HF, 0.15 to 0.5 Hz), where VLF, LF and HF were expressed in ms2. The LF : HF ratio, a regularly used marker for ANS balance, was calculated as LF (ms2)/HF (ms2) and used for the current analysis 8.
Blood samples were drawn repeatedly from an intravenously placed catheter. Venous plasma glucose was assessed with a YSI 2300 STAT Glucose analyzer (YSI Life Sciences, Yellow Springs, Ohio, USA). Insulin was determined from heparin plasma using an immunometric assay (Advia Centaur XP Immunoassay System, Siemens Healthcare, Erlangen, Germany). Homeostatic model assessment‐insulin resistance 2 (HOMA2‐IR) was calculated using the online available tool (www.dtu.ox.ac.uk).
Statistics
No formal a priori power calculation was performed as this was a mechanistic pilot study designed to assess appreciable effects of exenatide, with or without concomitant NO blockade, on systemic haemodynamics and cardiac ANS balance in healthy overweight subjects.
Subject characteristics are presented as mean ± standard deviation (SD). The study endpoint measurements are presented as mean with 95% confidence interval. The effects of exenatide are given as maximal change compared with placebo, while placebo values are given as time‐averaged mean. Parameters demonstrating a non‐normal distribution were log‐transformed before analysis, which was only needed for LF : HF ratio and insulin, and median [interquartile range] are shown. Analyses were performed using linear mixed models (LMM) using SPSS version 20.0 for Windows (IBM SPSS Inc., Chicago, IL, USA), thereby accounting for paired and repeated data. Intervention and time were included in the model as fixed factors, with a random slope, and the interaction term (condition × time) was the parameter of interest. LMM tests were performed to assess differences in systemic haemodynamic variables between exenatide and placebo (day 1), and between exenatide/L‐NMMA and exenatide (day 2). For HRV parameters, where placebo measurements were not available, within group changes were tested using LMM. Linear correlation analysis was performed between the area under the curve (AUC) for glucose, HR and RPP, using Pearson's correlation coefficient. A two‐sided P value of <0.05 was considered statistically significant.
Results
Ten healthy overweight males were included in this analysis. Full baseline characteristics of the subjects can be found in Table 1.
Table 1.
Subject characteristics (mean ± SD)
| Parameter | Value |
|---|---|
| Age (years) | 22.7 ± 1.8 |
| BMI (kg m −2 ) | 29.4 ± 1.7 |
| Waist circumference (cm) | 101.5 ± 5.9 |
| Systolic blood pressure (mmHg) | 115.9 ± 10.7 |
| Diastolic blood pressure (mmHg) | 64.9 ± 4.9 |
| Fasting plasma glucose (mmol l −1 ) | 4.9 ± 0.3 |
| HOMA2‐IR | 1.0 ± 0.6 |
| Triglycerides (mmol l −1 ) | 1.3 ± 0.7 |
| Total cholesterol (mmol l −1 ) | 4.3 ± 0.9 |
| HDL cholesterol (mmol l −1 ) | 1.2 ± 0.2 |
| LDL cholesterol (mmol l −1 ) | 2.5 ± 0.8 |
| eGFR (ml min −1 1.73 m −2 ) | >60 in all subjects |
BMI, body mass index; eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein; HOMA‐2IR, homeostatic model assessment insulin resistance; LDL, low density lipoprotein
Exenatide infusion acutely elevated HR, with a maximum mean increase of 6.8 (95% CI 1.7, 11.9) beats min–1 (P < 0.05), compared with a time‐averaged mean of 58.3 (95% CI 55.4, 61.2) beats min–1 during placebo (Figure 1 and Table 2). Exenatide increased SBP with a maximum of 9.8 (95% CI 3.5, 16.1) mmHg (P < 0.01), compared with a time‐averaged mean of 114.3 (95% CI 109.4, 119.2) mmHg during placebo. Additionally, exenatide increased CO with a maximum of 1.3 (95% CI 0.2, 2.5) l min−1 (P < 0.05), compared with a time‐averaged mean of 6.9 (95% CI 6.6, 7.2) l min−1 during placebo. Exenatide administration had no statistically significant effect on DBP, MAP or SV, compared with placebo infusion. Moreover, exenatide had no effect on TPR (maximum decrease −50.1 (95% CI −137.0, 237.2) dyn s cm−5) compared with placebo (time‐averaged mean 978.0 (95% CI 896.4, 1085.0) dyn s cm−5) (P = 0.692). Co‐infusion of exenatide/L‐NMMA showed no differences in HR, SBP, DBP or MAP compared with exenatide infusion alone, suggesting an NO independent effect. No differences in TPR were observed between exenatide/L‐NMMA and exenatide infusion alone, although TPR tended to decrease during combined infusion after 90 min (−139 (95% CI −45.8, 294.9) dyn s cm−5, P = 0.079).
Figure 1.
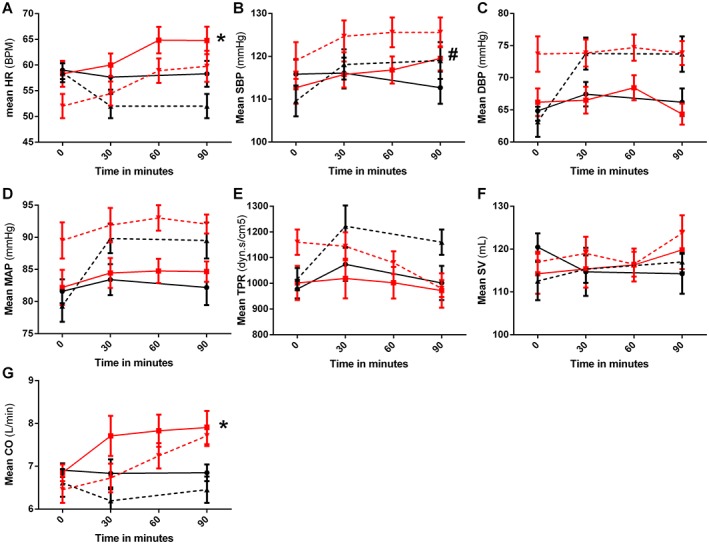
Exenatide‐induced changes in systemic haemodynamics. Changes in systemic haemodynamic parameters after administration of placebo (black lines, circles), exenatide (red lines, squares), L‐NMMA (black dashed lines, triangle) or exenatide/L‐NMMA (red dashed lines, downward triangles). Data points represent mean with 95% CI. A) heart rate (HR), B) systolic blood pressure (SBP), C) diastolic blood pressure (DBP), D) mean arterial pressure (MAP), E) total peripheral resistance (TPR), F) stroke volume (SV) and G) cardiac output (CO). Statistically significant mean differences of exenatide compared with placebo are indicated as * (P < 0.05) or # (P < 0.01)
Table 2.
Effects of interventions after 90 min infusion (mean (95% CI) or median [IQR])
| Parameter | Placebo | Exenatide | L‐NMMA | Exenatide/L‐NMMA |
|---|---|---|---|---|
| HR (beats min −1 ) | 58.3 (52.6–63.9) | 63.6 (57.5–69.6) | 53.4 (48.8–57.9) | 59.6 (52.9–66.6) |
| SBP (mmHg) | 112.7 (104.2–121.2) | 118.7 (112.7–124.7) | 119.0 (109.2–128.8) | 125.2 (119.0–131.4) |
| DBP (mmHg) | 66.2 (61.3–71.1) | 63.6 (59.9–67.3) | 73.7 (67.5–79.9) | 73.9 (69.6–78.1) |
| MAP (mmHg) | 82.2 (75.9–88.4) | 83.8 (80.0–87.6) | 89.5 (83.1–95.9) | 92.1 (88.7–95.4) |
| SV (ml) | 114.2 (103.4–125.0) | 119.8 (109.5–130.1) | 115.7 (110.4–120.9) | 121.9 (112.2–131.5) |
| CO (l min −1 ) | 6.8 (6.4–7.3) | 7.9 (7.0–8.8) | 6.5 (5.7–7.3) | 7.8 (7.2–8.4) |
| TPR (dyns·s cm −5 ) | 1001.5 (847.4–1155.6) | 971.8 (817.4–1126.2) | 1162.8 (1035.6–1289.9) | 983.2 (898.4–1067.9) |
| RPP (mmHg * beats min −1 ) | 6632.5 (5611.9–7653.1) | 7576.2 (6638.7–8513.6) | 6392.1 (5515.6–7268.6) | 7502.7 (6500.3–8505.1) |
| LF : HF ratio * | N/A | 1.86 [0.65–4.79] | N/A | 1.56 [0.85–3.21] |
| Glucose (mmol l −1 ) | 4.24 (4.14–4.35) | 3.68 (3.54–3.82) | 4.40 (4.07–4.73) | 3.80 (3.69–3.92) |
| Insulin (pmol l −1 ) * | 28.8 [21.8–52.8] | 30.6 [20.5–49.2] | 24.5 [17.6–34.2] | 27.8 [18.9–42.7] |
All values represent the variables after 90 min infusion of placebo, exenatide, L‐NMMA and exenatide/L‐NMMA.
non‐Gaussian distribution, thus median [IQR] are shown. CO, cardiac output; DBP, diastolic blood pressure; HR, heart rate; LF : HF ratio, low frequency to high frequency ratio; L‐NMMA, L‐NG‐monomethyl‐arginine‐citrate; MAP, mean arterial pressure; N/A, not available; RPP, rate‐pressure product; SBP, systolic blood pressure; SV, stroke volume; TPR, total peripheral resistance.
Exenatide increased RPP, with a maximum mean increase of 1214.4 (95% CI 423.0, 2205.9) (P < 0.01) mmHg*beats min−1, compared with a time‐averaged mean of 6709.9 (95% CI 6173.3, 7246.8) mmHg*beats min−1 during placebo (Figure 2 and Table 2). Moreover, exenatide increased the LF : HF ratio by a median [IQR] of 0.6 [−0.3–3.2](P < 0.05) compared with baseline values (Figure 2). Exenatide/L‐NMMA showed no statistically different effect on RPP or LF : HF ratio compared with exenatide alone.
Figure 2.
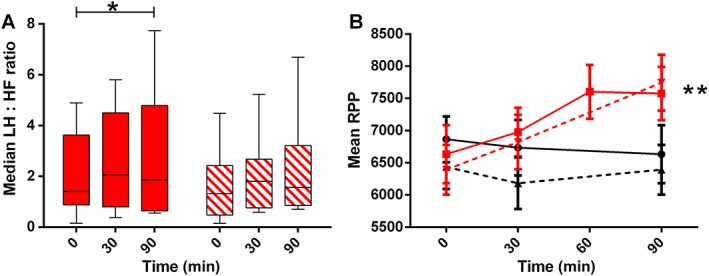
Exenatide‐induced changes in autonomic nervous system. Changes in autonomic nervous system parameters after administration of placebo (black lines, circles), exenatide (red lines, squares), L‐NMMA (black dashed lines, triangle) or exenatide/L‐NMMA (red dashed lines, downward triangles). A) LF : HF ratio, in median with IQR and range and B) rate pressure product (RPP), in mean with 95% CI; Statistically significant mean differences of exenatide compared with placebo are indicated as * (P < 0.05) or ** (P < 0.01). Please note for LF : HF ratio (panel A) no placebo measurements were available and statistics were performed compared with baseline values
Infusion of exenatide decreased glucose levels, with the nadir reaching 3.47 (95% CI 3.21, 3.74) mmol l−1 after 60 min, from a time‐averaged mean of 4.32 (95% CI 4.26, 4.38) mmol l−1 during placebo (P < 0.001). Similar observations were made during concomitant L‐NMMA infusion, during which the lowest plasma glucose concentration was 3.41 (95% CI 3.26, 3.57) mmol l−1 after 60 min of exenatide infusion. Exenatide had no significant effect on insulin levels (P = 0.839) (Figure 3). No significant association between AUC glucose and AUC HR (r = − 0.040) or AUC RPP (r = 0.118) was found during exenatide infusion (online Supplementary Figure 2).
Figure 3.
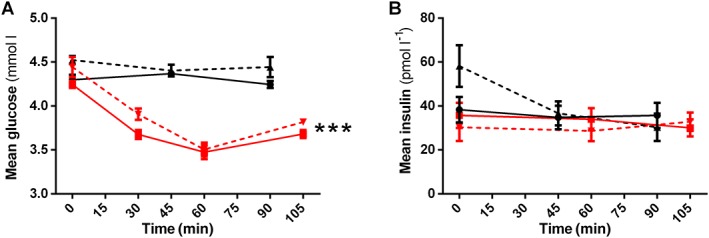
Exenatide‐induced changes in metabolic parameters. Changes in metabolic parameters after administration of placebo (black lines, circles), exenatide (red lines, squares), L‐NMMA (black dashed lines, triangle) or exenatide/L‐NMMA (red dashed lines, downward triangles). Data points represent mean with 95% CI. A) glucose and B) insulin. Statistically significant mean differences of exenatide compared with placebo are indicated as *** (P < 0.001)
Discussion
In the current mechanistic intervention study, we confirm that acute GLP‐1RA exenatide administration induces a modest but consistent heart rate acceleration 1, and increases SBP and markers of sympathetic activity in healthy overweight males. We observed no significant exenatide‐induced changes in TPR, DBP or SV compared with placebo.
The observed HR acceleration of 4.3 beats min−1 is consistent with previous acute GLP‐1(RA) infusion studies, with effects ranging from no effect 3, 9, to increases of up to 8.2 beats min–1 4, 10. With long term treatment, a mean HR acceleration of 2 beats min–1 is commonly observed in type 2 diabetes patients, but can be as high as ~10 beats min–1 1, 11. Importantly, large‐sized epidemiological studies have demonstrated a 17% increase in mortality risk with every 5 beats min–1 HR increase 12. At this point it is unknown whether pharmacologically induced HR acceleration also increases mortality in humans. The recent cardiovascular safety trial ELIXA demonstrated no increased mortality with the use of GLP‐1RA lixisenatide in type 2 diabetes patients with high cardiovascular risk 13. However, since lixisenatide induces minor HR acceleration compared with other GLP‐1RA 11, 14, results of more safety trials are eagerly awaited. Meanwhile, finding the mechanism underlying GLP‐1RA‐induced HR acceleration might help understand the clinical consequences.
To date, several hypotheses have been raised to explain GLP‐1RA‐induced HR acceleration: 1) modulation of cardiac ANS balance, 2) reflex tachycardia in response to vasodilation and 3) direct stimulation of the GLP‐1 receptor on sino‐atrial cells 15. As exenatide increased HR and SBP without effects on TPR, our data suggest that exenatide acts on these variables through SNS activation. This premise was underlined by concomitant increases in RPP and LF : HF ratio 7. Furthermore, as exenatide left TPR and DBP unaltered in the present study, the reflex tachycardia hypothesis seems to be refutable, and moreover suggest that SNS activation does not result from baroreceptor reflex activity. A HR accelerating effect of GLP‐1RA through direct sino‐atrial node stimulation also seems less likely, as we did not observe a reduction in diastolic filling time and subsequent SV.
The observed increase in markers of SNS activity is consistent with previous human studies, in which muscle SNS activity and plasma epinephrine levels increased following GLP‐1 peptide administration 3, 10. In animals, peripheral and central exenatide administration increased c‐fos expression in sympathetic neurons and the adrenal medulla in rodents, also suggesting increased SNS activity 16. Whether exenatide directly stimulates SNS activity or through indirect mechanisms remains hitherto unknown.
In order to prevent the suggested NO‐dependent vasodilation and assess the effects of exenatide on cardiac ANS balance without intervenience of the baroreceptor reflex 4, 5, we assessed exenatide‐induced effects during co‐infusion with L‐NMMA. As expected, during L‐NMMA infusion, systemic vasoconstriction led to increased blood pressure and reduced HR (Figure 1). Interestingly, even in this challenged system, exenatide infusion increased HR, SBP, RPP and LF : HF ratio.
In a previously reported placebo‐controlled trial in healthy males, a single subcutaneous injection of exenatide 10 μg reduced TPR and increased HR by 8.2 beats min–1 within 2 h after dosing 4. We were unable to reproduce the exenatide‐induced effects on TPR, which may be explained by shorter measurement duration in the current study. Moreover, in the study by Mendis et al., HR increased even prior to TPR lowering, suggesting the presence of different HR accelerating mechanisms. Interestingly, we observed a non‐significant decrease in TPR when exenatide was co‐infused with L‐NMMA. Although exenatide may induce NO‐independent vasodilation 5, 6, it is unclear why this only occurred during L‐NMMA infusion in our study, and not also during exenatide infusion alone. Potentially, the vasodilator effects of exenatide can only be seen during a state of vasoconstriction in healthy subjects, as achieved by L‐NMMA infusion. Since vascular resistance in patients with type 2 diabetes is generally higher compared with healthy controls 17, the effects of exenatide on TPR could be different in this patient group. Alternatively, we could speculate that the effects of L‐NMMA on TPR were transient, and consequently the reduction in TPR during co‐infusion of exenatide/L‐NMMA was merely based on a less vasoconstrictive effect of L‐NMMA or compensating vasodilator factors. However, L‐NMMA infusion up until 5 h had a continuous effect on TPR 18, 19, making this hypothesis less likely.
There was an expected modest reduction in glucose after exenatide infusion in our healthy subjects, during both placebo or L‐NMMA co‐infusion, reaching levels of 3.47 mmol l−1, though no subjects experienced symptoms of hypoglycaemia. This exenatide‐induced glucose lowering could theoretically contribute to the activation of the SNS, thereby leading to increased HR. However, we did not observe a significant correlation between exenatide‐induced changes in glucose and HR, or glucose and RPP. Notably, in other studies using exogenous GLP‐1 peptide vs. placebo, HR increased during clamped hyperglycaemia (10 mmol l−1) 9. Moreover, after meal ingestion, HR increased with GLP‐1 infusion despite increases in blood glucose 20. Thus, while exenatide reduced glucose levels, it appears that GLP‐1RA administration is capable of inducing HR‐acceleration independent of glycaemia.
Notably, while we confirm that GLP‐1RA administration increases SBP in the acute setting 10, long term treatment is known to reduce SBP by ~2 mmHg in patients with type 2 diabetes 1. An explanation for this differential effect is currently lacking. However, GLP‐1RA therapy not only affects cardiac ANS balance, it also affects other factors that control blood pressure in type 2 diabetes. As such, GLP‐1RAs are believed to improve endothelial function, increase natriuresis/diuresis, reduce bodyweight, and inhibit renin‐angiotensin‐aldosterone system activity 21, 22, 23. It could be hypothesized that GLP‐1 affects these factors differently during acute and prolonged intervention. We hypothesize that acute administration acutely increases CO and subsequent SBP, while long term effects on bodyweight and natriuresis collectively reduce SBP over time. More studies are needed to understand these functional differences.
Limitations of the current study include its open label design. However, the haemodynamic and ANS analyses were performed using automated and standardized software techniques, thereby minimizing the risk of investigator‐induced bias. Furthermore and unfortunately, we were unable to assess HRV during the placebo phase. Our systemic haemodynamic measurements are modelled from pulse pressure waveforms, and are validated and accurate techniques 24, 25. Moreover, this was an intervention study in healthy overweight males. Thus our data cannot be directly extrapolated to effects in patients with type 2 diabetes. This study population was chosen since GLP‐1RAs are mainly prescribed to overweight subjects. Thus, a study in normal weight individuals would not yield informative data. No patients with type 2 diabetes were included because of the rather high dose of L‐NMMA infusion, which could induce adverse effects in patients with an increased risk of cardiovascular disease. However, as GLP‐1RA treatment (liraglutide 3.0 mg) has now been approved for weight management in obese healthy subjects 26, our results may have clinical relevance for this indication. Lastly, we used intravenous exenatide administration, compared with the clinically used subcutaneous route. However, differences in route of administration have no effect on time to reach therapeutic plasma exenatide levels 27, 28.
In conclusion, as TPR did not change following acute GLP‐1RA administration in healthy overweight males, exenatide‐induced HR acceleration may not be a compensatory mechanism for systemic vasodilation. Our data rather suggest involvement of increased SNS activity as an underlying mechanism for this potential safety issue.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare MD had grant support from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Medtronic, Merck Sharp & Dohme, Novo Nordisk and Sanofi for the submitted work. MHHK had grant support from Boehringer Ingelheim, Novo Nordisk and Sanofi. MD was consultant for Abbott, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, GI Dynamics, Merck Sharp & Dohme, Novo Nordisk, Poxel Pharma and Sanofi in the previous 3 years, MD was speaker for AstraZeneca, Bristol‐Myers Squibb, Eli Lilly, Novo Nordisk and Sanofi. There are no other relationships or activities that could appear to have influenced the submitted work. The other authors declare no competing interests.
The authors are grateful to the study participants whose time and effort was critical to the success of our research protocol. Furthermore, we would like to thank Jeannette Boerop for her invaluable help during the test visits.
The research leading to these results has received funding from the European Community's Seventh Framework Programme (FP7/2007–2013) under grant agreement n° 282 521 ‐ the SAFEGUARD project.
Contributors
M.M. Smits developed the study protocol, performed the measurements and analyses and wrote the manuscript. M.H.A. Muskiet performed measurements, and contributed to the discussion and manuscript writing. M. Diamant developed the study protocol and was involved in the discussion. T. Hoekstra performed statistical analyses and contributed to editing of the manuscript. L. Tonneijck, M.H.H. Kramer and D.H. van Raalte contributed to the discussion and edited the manuscript. All authors have approved the final version of this manuscript.
Supporting information
Figure S1 Study layout and testing day schedule. Tests were performed during 2 study days. On the first day placebo and exenatide were administered, on the second day L‐NMMA and a combination of exenatide and L‐NMMA
Figure S2 Correlation between the time‐averaged area under the curve (AUC) for glucose and heart rate (HR) and rate pressure product (RPP). A correlation line with Pearson's correlation coefficient is given
Supporting info item
Supporting info item
Smits, M. M. , Muskiet, M. H. A. , Tonneijck, L. , Hoekstra, T. , Kramer, M. H. H. , Diamant, M. , and van Raalte, D. H. (2016) Exenatide acutely increases heart rate in parallel with augmented sympathetic nervous system activation in healthy overweight males. Br J Clin Pharmacol, 81: 613–620. doi: 10.1111/bcp.12843.
References
- 1. Robinson LE, Holt TA, Rees K, Randeva HS, O'Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: Systematic review and meta‐analysis. BMJ Open 2013; 3: e001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jensen MT, Marott JL, Allin KH, Nordestgaard BG, Jensen GB. Resting heart rate is associated with cardiovascular and all‐cause mortality after adjusting for inflammatory markers: the Copenhagen City Heart Study. Eur J Prev Cardiol 2012; 19: 102–8. [DOI] [PubMed] [Google Scholar]
- 3. Bharucha AE, Charkoudian N, Andrews CN, Camilleri M, Sletten D, Zinsmeister AR, Low PA. Effects of glucagon‐like peptide‐1, yohimbine, and nitrergic modulation on sympathetic and parasympathetic activity in humans. Am J Physiol Regul Integr Comp Physiol 2008; 295: R874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mendis B, Simpson E, MacDonald I, Mansell P. Investigation of the haemodynamic effects of exenatide in healthy male subjects. Br J Clin Pharmacol 2012; 74: 437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gardiner SM, March JE, Kemp PA, Bennett T. Effects of nitric oxide synthase inhibition with or without cyclooxygenase‐2 inhibition on resting haemodynamics and responses to exendin‐4. Br J Pharmacol 2006; 149: 802–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smits MM, Muskiet MHA, Tonneijck L, Kramer MHH, Diamant M, van Raalte DH, Serné EH. GLP‐1 receptor agonist exenatide increases capillary perfusion independent of nitric oxide in healthy overweight men. Arterioscler Thromb Vasc Biol 2015; 35: 1538–43. [DOI] [PubMed] [Google Scholar]
- 7. Valensi P, Chiheb S, Fysekidis M. Insulin‐ and glucagon‐like peptide‐1‐induced changes in heart rate and vagosympathetic activity: why they matter. Diabetologia 2013; 56: 1196–200. [DOI] [PubMed] [Google Scholar]
- 8. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996; 93: 1043–65. [PubMed] [Google Scholar]
- 9. Berkelaar M, Eekhoff EMW, Simonis‐Bik AMC, Boomsma DI, Diamant M, Ijzerman RG, Dekker JM, ’t Hart LM, de Geus EJC. Effects of induced hyperinsulinaemia with and without hyperglycaemia on measures of cardiac vagal control. Diabetologia 2013; 56: 1436–43. [DOI] [PubMed] [Google Scholar]
- 10. Asmar A, Simonsen L, Asmar M, Madsbad S, Holst JJ, Frandsen E, Moro C, Jonassen T, Bülow J. Renal extraction and acute effects of glucagon‐like peptide‐1 on central and renal hemodynamics in healthy men. Am J Physiol Endocrinol Metab 2015; 308: E641–9. [DOI] [PubMed] [Google Scholar]
- 11. Meier JJ, Rosenstock J, Hincelin‐Méry A, Roy‐Duval C, Delfolie A, Coester H‐V, Menge BA, Forst T, Kapitza C. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open‐label trial. Diabetes Care 2015; 38: 1263–73. [DOI] [PubMed] [Google Scholar]
- 12. Hozawa A, Ohkubo T, Kikuya M, Ugajin T, Yamaguchi J, Asayama K, Metoki H, Ohmori K, Hoshi H, Hashimoto J, Satoh H, Tsuji I, Imai Y. Prognostic value of home heart rate for cardiovascular mortality in the general population: the ohasama study. Am J Hypertens 2004; 17: 1005–10. [DOI] [PubMed] [Google Scholar]
- 13. Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, Lawson FC, Ping L, Wei X, Lewis EF, Maggioni AP, McMurray JJ, Probstfield JL, Riddle MC, Solomon SD, Tardif JC. ELIXA Investigators. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med 2015; 373: 2247–57. [DOI] [PubMed] [Google Scholar]
- 14. Meier JJ. GLP‐1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012; 8: 728–42. [DOI] [PubMed] [Google Scholar]
- 15. Pyke C, Heller RS, Kirk RK, Orskov C, Reedtz‐Runge S, Kaastrup P, Hvelplund A, Bardram L, Calatayud D, Knudsen LB. GLP‐1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 2014; 155: 1280–90. [DOI] [PubMed] [Google Scholar]
- 16. Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK. Glucagon‐like peptide‐1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest 2002; 110: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suzuki E, Yoshimura T, Omura Y, Sakaguchi M, Nishio Y, Maegawa H, Hisatomi A, Fujimoto K, Takeda J, Kashiwagi A. Higher arterial stiffness, greater peripheral vascular resistance and lower blood flow in lower‐leg arteries are associated with long‐term hyperglycaemia in type 2 diabetic patients with normal ankle‐brachial index. Diabetes Metab Res Rev 2009; 25: 363–9. [DOI] [PubMed] [Google Scholar]
- 18. Dijkhorst‐Oei LT, Boer P, Rabelink TJ, Koomans HA. Nitric oxide synthesis inhibition does not impair water immersion‐induced renal vasodilation in humans. J Am Soc Nephrol 2000; 11: 1293–302. [DOI] [PubMed] [Google Scholar]
- 19. Sprangers F, Jellema WT, Lopuhaä CE, Endert E, Ackermans MT, Van Lieshout JJ, Van Der Zee JS, Romijn JA, Sauerwein HP. Partial inhibition of nitric oxide synthesis in vivo does not inhibit glucose production in man. Metabolism 2002; 51: 57–64. [DOI] [PubMed] [Google Scholar]
- 20. Trahair LG, Horowitz M, Hausken T, Feinle‐Bisset C, Rayner CK, Jones KL. Effects of exogenous glucagon‐like peptide‐1 on the blood pressure, heart rate, mesenteric blood flow and glycemic responses to intraduodenal glucose in healthy older subjects. J Clin Endocrinol Metab 2014; 99: E2628–34 [DOI] [PubMed] [Google Scholar]
- 21. Sheikh A. Direct cardiovascular effects of glucagon like peptide‐1. Diabetol Metab Syndr 2013; 5: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tonneijck L, Muskiet MHA, Smits MM, van Raalte DH, Diamant M. Combining incretin‐based drugs and RAAS inhibitors: more cons than pros? Lancet Diabetes Endocrinol 2014; 2: 684–5. [DOI] [PubMed] [Google Scholar]
- 23. Muskiet MHA, Smits MM, Morsink LM, Diamant M. The gut‐renal axis: do incretin‐based agents confer renoprotection in diabetes? Nat Rev Nephrol 2014; 10: 88–103. [DOI] [PubMed] [Google Scholar]
- 24. Bogert LWJ, Wesseling KH, Schraa O, Van Lieshout EJ, de Mol BAJM, van Goudoever J, Westerhof BE, van Lieshout JJ. Pulse contour cardiac output derived from non‐invasive arterial pressure in cardiovascular disease. Anaesthesia 2010; 65: 1119–25. [DOI] [PubMed] [Google Scholar]
- 25. Eeftinck Schattenkerk DW, van Lieshout JJ, van den Meiracker AH, Wesseling KR, Blanc S, Wieling W, van Montfrans GA, Settels JJ, Wesseling KH, Westerhof BE. Nexfin noninvasive continuous blood pressure validated against Riva‐Rocci/Korotkoff. Am J Hypertens 2009; 22: 378–83. [DOI] [PubMed] [Google Scholar]
- 26. Pi‐Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DCW, le Roux CW, Violante Ortiz R, Jensen CB, Wilding JPH. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015; 373: 11–22. [DOI] [PubMed] [Google Scholar]
- 27. Fehse F, Trautmann M, Holst JJ, Halseth AE, Nanayakkara N, Nielsen LL, Fineman MS, Kim DD, Nauck MA. Exenatide augments first‐ and second‐phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2005; 90: 5991–7. [DOI] [PubMed] [Google Scholar]
- 28. Calara F, Taylor K, Han J, Zabala E, Carr EM, Wintle M, Fineman M. A randomized, open‐label, crossover study examining the effect of injection site on bioavailability of exenatide (synthetic exendin‐4). Clin Ther 2005; 27: 210–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Study layout and testing day schedule. Tests were performed during 2 study days. On the first day placebo and exenatide were administered, on the second day L‐NMMA and a combination of exenatide and L‐NMMA
Figure S2 Correlation between the time‐averaged area under the curve (AUC) for glucose and heart rate (HR) and rate pressure product (RPP). A correlation line with Pearson's correlation coefficient is given
Supporting info item
Supporting info item


