Abstract
Aim
The haemodynamic effects of intravenous paracetamol have not been systematically investigated. We compared the physiological effects of intravenous mannitol‐containing paracetamol, and an equivalent dosage of mannitol, and normal saline 0.9% in healthy volunteers.
Methods
We performed a blinded, triple crossover, randomized trial of 24 adult healthy volunteers. Participants received i.v. paracetamol (1 g paracetamol +3.91 g mannitol 100 ml–1), i.v. mannitol (3.91 g mannitol 100 ml–1) and i.v. normal saline (100 ml). Composite primary end points were changes in mean arterial pressure (MAP), systolic blood pressure (SBP) and diastolic blood pressure (DBP) measured pre‐infusion, during a 15 min infusion period and over a 45 min observation period. Systemic vascular resistance index (SVRI) and cardiac index were measured at the same time points.
Results
Infusion of paracetamol induced a transient yet significant decrease in blood pressures from pre‐infusion values (MAP –1.85 mmHg, 95% CI –2.6, –1.1, SBP –0.54 mmHg, 95% CI –1.7, 0.6 and DBP −1.92 mmHg, 95% CI –2.6, –1.2, P < 0.0001), associated with a transient reduction in SVRI and an increase in cardiac index. Changes were observed, but to a lesser extent with normal saline (MAP –0.15 mmHg, SBP +1.44 mmHg, DBP −–0.73 mmHg, P < 0.0001), but not with mannitol (MAP +1.47 mmHg, SBP +4.03 mmHg, DBP +0.48 mmHg, P < 0.0001).
Conclusions
I.v. paracetamol caused a transient decrease in blood pressure immediately after infusion. These effects were not seen with mannitol or normal saline. The physiological mechanism was consistent with vasodilatation. This study provides plausible physiological data in a healthy volunteer setting, supporting transient changes in haemodynamic variables with i.v. paracetamol and justifies controlled studies in the peri‐operative and critical care setting.
Keywords: acetaminophen, adverse event, blood pressure, haemodynamic, intravenous, paracetamol
What is Already Known About This Subject
I.v. paracetamol is ubiquitous in critical care and peri‐operative medicine. I.v. paracetamol formulations that are manufactured by major pharmaceutical companies contain as much as 3.91 g of mannitol per dose of 1 g 100 ml–1 i.v. paracetamol. It is unknown if the i.v. formulations are associated with transient hypotension.
What this Study Adds
In a healthy volunteer model, i.v. paracetamol caused a transient decrease in blood pressure immediately after infusion. The physiological mechanism was consistent with vasodilatation. These haemodynamic effects were transient and of small magnitude. However they may have a different intensity and duration in patients who are critically ill.
Introduction
Paracetamol (also known as acetaminophen) is ubiquitously used in hospitals as an antipyretic and analgesic. It is frequently administered intravenously (i.v.) to patients undergoing major surgery and to critically ill patients, where oral intake and rectal suppositories may not be possible 1. Paracetamol has negligible solubility in aqueous solutions. Mannitol on the other hand, is commonly used in i.v. paracetamol formulations as a stabilizing compound. The inclusion of mannitol in i.v. paracetamol is not widely appreciated. I.v. paracetamol formulations that are manufactured by major pharmaceutical companies contain as much as 3.91 g of mannitol per dose of 1 g 100 ml–1 i.v. paracetamol. Bristol‐Myers Squibb, Cadence Pharmaceuticals and Actavis Pharmaceuticals use mannitol as a stabilizing compound, while Pfizer has produced a non‐mannitol containing formulation.
I.v. paracetamol is widely believed to be safe and free of adverse effects. However, with growing use, concerns have developed in critical care literature, which suggest that i.v. formulations may be associated with transient hypotension 1, 2, 3, 4, 5, 6, 7, 8. Additionally, studies examining the haemodynamic effects of i.v. paracetamol are notably limited by their retrospective design, small patient numbers, and lack of randomization and blinding. No studies have investigated these putative adverse effects in the absence of disease‐related confounders in a healthy volunteer setting or tested whether its main excipient (mannitol) contributes to such effects. We hypothesized that the i.v. paracetamol formulation (3.91 g mannitol +1 g paracetamol 100 ml–1) would lower blood pressure in healthy volunteers when compared with i.v. mannitol (3.91 g 100 ml) or 0.9% normal saline (100 ml) (placebo), and conducted a double‐blinded, randomized, triple crossover study to test our hypothesis.
Methods
The Austin Health Research and Ethics Committee approved this study (number 05 005/2013) and all patients gave written informed consent. We registered the study with the Australian New Zealand Clinical Trials Registry (number 12 615 000 533 594). Between October 2013 and April 2014 we recruited participants by word of mouth from peri‐operative medical personnel at the Austin Hospital, a large University‐affiliated, metropolitan hospital in Melbourne, Australia. Inclusion criteria included normotensive healthy participants, on no medications and between 18 and 60 years of age. Exclusion criteria included requirement for any chronic medications, pregnancy, body mass index greater than 35 kg m−2, intellectual disability, known history of liver and/or renal impairment, previous allergy to the study drugs, consumption of caffeine within 12 h of each experiment and consumption of NSAID or paracetamol containing products within 24 h of each experiment.
The primary composite end points were changes in mean arterial pressure (MAP), systolic blood pressure (SBP) and diastolic blood pressure (DBP) pre‐infusion, during a 15 min infusion period, and then post‐infusion during a 45 min observation period. Other variables collected at the same time points included systemic vascular resistance index (SVRI), cardiac index, stroke volume index (SVI), heart rate (HR), and plasma osmolality. Normal reference values for these end points were MAP 70–105 mmHg, SBP 100–140 mmHg, DBP 60–90 mmHg, SVRI 1970–2395 mmHg l−1 min−1 m−2, cardiac index 2.5–4.0 l min−1 m−2, SVI 33–47 ml m−2/beat, HR 60–90 beats min–1 and plasma osmolality 275–295 mosm kg−1.
Analytical methods
All continuous beat‐to‐beat haemodynamic variables were measured with the fourth generation non‐invasive Edwards Lifesciences Nexfin™ system, which uses the volume clamp method 9 to continuously measure blood pressure (MAP, SBP and DBP) and the physiocal method 10 for initial and continuous calibration. Real‐time reconstruction of the blood pressure waveform allowed computation of stroke volume, cardiac output, systemic vascular resistance and dP/dt using a pulse contour method. Blood pressure measurements with Nexfin™ technology have been reported as being more accurate than using a traditional upper arm blood pressure cuff 11, 12. Nexfin™ technology is approved by the Association for the Advancement of Medical Instrumentation criteria 13 and is validated against intermittent non‐invasive 14 and continuous invasive methods 15.
Currently there is no reliable assay to determine the level of mannitol in plasma or serum, nor any biochemical indicator or metric to measure the potential haemodynamic effects of mannitol in plasma. Being an osmotic diuretic that is filtered at the glomerulus yet not significantly reabsorbed in the proximal tubule, mannitol acutely raises plasma/extracellular osmolarity. In order to quantify any diuretic effects of mannitol on osmolality, plasma osmolality was measured by placing a tourniquet on the arm for blood sampling, 5 ml of blood was taken and discarded, followed by a 3 ml sample for plasma osmolality in a 6 ml lithium‐heparin blood Vacuette® (Greigner bio‐one, Kremsmünster, Austria). Plasma osmolality was measured with an Advanced® Model 3300 Micro‐Osmometer (Advanced Instruments, Inc., Norwood, Massachusetts, USA). This device calculates osmolality via freezing point depression osmometry by measuring the total molar concentration of dissolved solids in any solution.
Standardization of setting
Participants attended on 3 separate trial days with a washout period between trial days of between 24 h and 1 week. All participants were fasted for solid foods for 6 h and 2 h for clear liquids prior to each experiment. On arrival, participants were encouraged to empty their bladders to avoid discomfort during the study. The study was conducted in a dedicated research laboratory with standardized of illumination intensity and noise, and with ambient air temperature set to 21 °C to avoid distractions that may alter haemodynamics during the continuous measurements.
Participants were placed in a supine position on a standard hospital bed with their heads raised at 45º and resting on a pillow for comfort. The finger cuff of the Nexfin™ was placed on the middle phalanx of the index finger of the dominant hand. Stable baseline measurements were observed prior to each study arm in pre‐study monitoring over a period of 10 min. A 22‐guage Introcan Safety® intravenous cannula (Braun, Melsungen, Germany) was placed either in the peripheral veins located on the back of the non‐dominant hand or the cubital fossa of the non‐dominant arm. A Safsite® injection site safety connector was fitted for the i.v. infusion set and blood sampling. After the insertion of the cannula, blood pressure was allowed to stabilize to baseline values over a further 10 min period. At the end of this time period, continuous haemodynamics were recorded for a period of 2 min to capture stable baseline haemodynamic variables. Participants then received i.v. paracetamol (1 g paracetamol +3.91 g mannitol 100 ml–1) (Actavis Australia, The Rocks, NSW, Australia), i.v. mannitol (3.91 g mannitol 100 ml–1) (Baxter Healthcare, Toongabbie, NSW, Australia) or i.v. normal saline (100 ml) as placebo (Baxter Healthcare, Toongabbie, NSW, Australia). The composition of 100 ml Actavis formulation of i.v. paracetamol includes paracetamol 1000 mg, mannitol 3910 mg in the following stabilizers ‐ cysteine hydrochloride monohydrate, dibasic dehydrate sodium phosphate, sodium hydroxide, hydrochloric acid and water for injection. Study drugs were infused at room temperature via a volumetric pump (Alaris GP, Cardinal Health, Seven Hills, NSW, Australia) over 15 min. The cannula and Edwards Lifesciences Nexfin™ finger cuff were removed at the end of the study period. Participants were assigned to receive all three treatments in randomly allocated orders via a computer generated randomization program without blocking, stratification or other restrictions.
Blinding
Participants received their own unique randomization code using a computer generated randomization program (www.randomization.com). Random permutations of treatments for each subject were created using the randomization program second generator application (seed number: 203), and entering ‘Paracetamol’, ‘Mannitol’ and ‘Normal Saline’ as the treatment labels. Participant 1 had the randomization code: PHV1A (PHV denoted paracetamol healthy volunteers, 1 denoted the participant number and A denoted the first trial arm). As there were three trial arms, the second and third trial arms were respectively listed as ‘B’ and ‘C’ in each randomization code. All randomization codes were individually sealed in opaque envelopes. All study investigators were blinded to the trial fluid intervention. Independent hospital pharmacy staff provided the solutions in identical unmarked blinded vials. Treatment allocation was only revealed after data analysis was performed.
Statistical methods
In order to determine a difference in MAP of 5 mmHg between the groups, given a baseline MAP of 90 mmHg, with an adjusted significance level of 0.025, a standard deviation of 3 mmHg and a power of 0.9, 24 participants were recruited. Repeated measures analysis of variance modelling was performed by fitting the main effects for treatment (i.e. paracetamol, mannitol, normal saline) and time and an interaction between treatment and time to determine if the three treatments behaved differently over time. Within each variable, analysis was stratified by period (pre‐infusion, infusion, observation) so there were three sets of analysis for each variable. Because there were three groups, the overall P value for a group effect simply answered the question ‘were these three groups significantly different from each other overall’. It did not inform us specifically where the differences were. For this assessment, we performed post hoc analyses, looking for specific pairwise comparisons (paracetamol vs. mannitol, paracetamol vs. normal saline, mannitol vs. normal saline). To account for multiple comparisons, a reduced two‐sided P value of 0.01 was used to indicate statistical significance for post hoc comparisons. Modelling was performed using the PROC Mixed procedure in SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Twenty‐five participants were screened for eligibility. All participants were deemed eligible to participate in the trial after pre‐study monitoring. One participant withdrew due to the time commitments required for the study. Withdrawal was prior to randomization. Twenty‐four healthy volunteers consented for this study and were randomized to receive three treatment arms in a randomly allocated order. All participants completed all interventions and there were no breaches in the trial protocol. Fifty‐eight‐percent of the participants were male. The median (IQR) age and body mass index were 36.5 years (28–40.3 years) and 23.3 kg m−2 (22.0–25.4 kg m−2), respectively. Baseline haemodynamic values together with the standard error of difference from the baseline (confidence intervals) are summarized in Table 1. There were no statistical differences in baseline demographic variables.
Table 1.
Baseline haemodynamic demographics. Shown as absolute values ± standard error of difference between trial arms
| Baseline variable | Treatment | Mean (SE) |
|---|---|---|
| Systolic blood pressure (mmHg) | Paracetamol | 121.1 (1.7) |
| Saline | 123.6 (1.7) | |
| Mannitol | 122.4 (1.7) | |
| Mean arterial pressure (mmHg) | Paracetamol | 91.6 (1.3) |
| Saline | 94.0 (1.3) | |
| Mannitol | 92.7 (1.3) | |
| Diastolic blood pressure (mmHg) | Paracetamol | 72.2 (0.97) |
| Saline | 74.4 (0.97) | |
| Mannitol | 73.2 (0.97) | |
| Systemic vascular resistance index (mmHg l −1 min −1 m −2 ) | Paracetamol | 2285 (100.5) |
| Saline | 2450 (100.4) | |
| Mannitol | 2342 (100.5) | |
| Cardiac index (l min −1 m −2 ) | Paracetamol | 3.4 (0.17) |
| Saline | 3.3 (0.17) | |
| Mannitol | 3.4 (0.17) | |
| Heart rate (beats min –1) | Paracetamol | 64.4 (2.2) |
| Saline | 69 (2.2) | |
| Mannitol | 65.5 (2.2) | |
| Stroke volume index (ml m −2 ) | Paracetamol | 52.5 (1.1) |
| Saline | 51.3 (1.1) | |
| Mannitol | 52.1 (1.1) |
Primary ends points: MAP, SBP and DBP
The three groups were different when averaged across the infusion period and observation periods (P < 0.001). Changes in mean SBP, MAP and DBP during the infusions of paracetamol, mannitol and normal saline are graphically summarized in Figures 1, 2, 3. There were no decreases from baseline blood pressure with the infusions of mannitol and normal saline, but the infusion of paracetamol induced transient decreases in SBP, MAP, and DBP with a nadir at 15 min (end of infusion). In the paracetamol group compared with baseline values the mean SBP decreased by 0.5 mmHg (95% CI –1.6, 0.6), MAP decreased by 1.85 mmHg (95% CI –2.6, –1.1) and DBP decreased by 1.9 mmHg (95% CI –2.6, –1.2).
Figure 1.
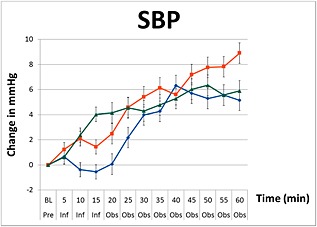
Changes in systolic blood pressure (SBP) including standard error bars from baseline pre‐infusion (Pre), during a 15 min infusion period (Inf), and 60 min post‐infusion (Obs) of paracetamol ( ), mannitol (
), mannitol ( ) and normal saline (
) and normal saline ( ) (repeated measures anova, P < 0.001)
) (repeated measures anova, P < 0.001)
Figure 2.
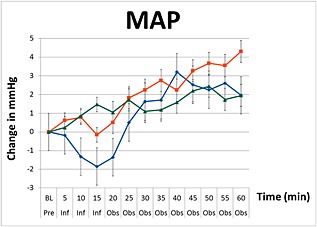
Changes in mean arterial pressure (MAP) including standard error bars from baseline pre‐infusion (Pre), during a 15 min infusion period (Inf), and 60 min post‐infusion (Obs) of paracetamol ( ), mannitol (
), mannitol ( ) and normal saline (
) and normal saline ( ) (repeated measures anova, P < 0.001)
) (repeated measures anova, P < 0.001)
Figure 3.
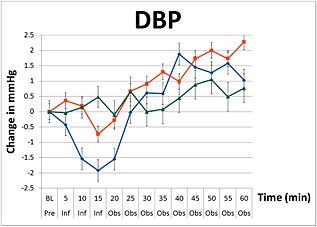
Changes in diastolic blood pressure (DBP) including standard error bars from baseline pre‐infusion (Pre), during a 15 min infusion period (Inf), and 60 min post‐infusion (Obs) of paracetamol ( ), mannitol (
), mannitol ( ) and normal saline (
) and normal saline ( ) (repeated measures anova
P < 0.001
) (repeated measures anova
P < 0.001
In the paracetamol group, the SBP, MAP and DBP blood pressure variables remained significantly lower than the mannitol and saline groups (P < 0.0001) for the first 25 min. At the end of the 60 min observation period, these changes were no longer present. These changes in blood pressure were not observed with mannitol, which showed a consistent increase in blood pressure during and after infusion with a peak effect at 60 min in SBP by 5.9 mmHg (95% CI 4.3, 7.5), MAP by 1.5 mmHg (95% CI 0.8, 3.1) and DBP by 0.8 mmHg (95% CI –0.2, 1.7). Compared with baseline values, infusion of normal saline resulted in negligible changes in blood pressure after 15 min (MAP 0.2 mmHg, 95% CI –0.9, 0.6 and DBP 0.7 mmHg, 95% CI –1.4, 0.0), but produced the greatest increase in blood pressure at the end of the 60 min observation period (MAP +4.3 mmHg, 95% CI 3.2, 5.5).
Secondary ends points: SVRI, cardiac index, SVI, HR
For all the secondary endpoints, there was statistical evidence to suggest that the three groups were different when averaged across the infusion and observation periods (P < 0.001). In the paracetamol group there was a transient reduction in SVRI decreasing by 88.3 mmHg l−1 min−1 m−2 (95% CI –147.4, –29.2) immediately after the infusion (P < 0.001), which was similar to normal saline, which decreased by 124.0 mmHg l−1 min−1 m−2 (95% CI –64.9, –183.1) (Figure 4) Figure 5. Approximately 25–30 min post‐infusion, the SVRI in the paracetamol group returned to pre‐infusion values and then remained above baseline values until completion. SVRI for the paracetamol group peaked at 55 min after infusion with a total increase of 83.5 mmHg l−1 min−1 m−2 (95% CI 21.4, 145.5). This post‐infusion effect was significantly different from the mannitol and normal saline groups, with both groups increasing towards baseline 20 min after infusion.
Figure 4.
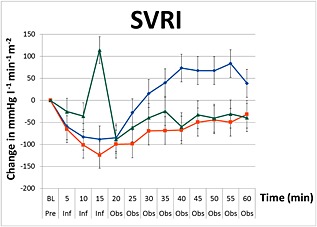
Changes in systemic vascular resistance index (SVRI) including standard error bars from baseline pre‐infusion (Pre), during a 15 min infusion period (Inf), and 60 min post‐infusion (Obs) of paracetamol ( ), mannitol (
), mannitol ( ) and normal saline (
) and normal saline ( ) (repeated measures anova, P < 0.001)
) (repeated measures anova, P < 0.001)
Figure 5.
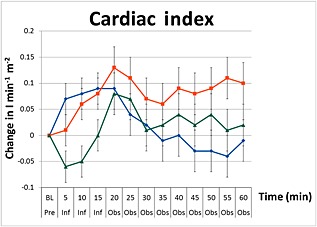
Changes in cardiac index including standard error bars from baseline pre‐infusion (Pre), during a 15 min infusion period (Inf), and 60 min post‐infusion (Obs) of paracetamol ( ), mannitol (
), mannitol ( ) and normal saline (
) and normal saline ( ) (repeated measures anova, P < 0.001)
) (repeated measures anova, P < 0.001)
In the mannitol group, compared with baseline values there was a transient increase in SVRI by 114.3 mmHg l−1 min−1 m−2 (95% CI 55.14, 173.5), a finding not observed with the other two solutions. At the end of the study, SVRI for normal saline and mannitol were comparable (−31.5 mmHg l−1 min−1 m−2 (95% CI –93.5, 30.5) vs. –39.3 mmHg l−1 min−1 m−2 (95% CI –101.3, 22.7). Post hoc analysis of changes in SVRI between the three infusions over the duration of the study were primarily driven by paracetamol, i.e. there was no statistical difference observed between mannitol and normal saline (P = 0.87).
I.v. paracetamol induced a significant increase in cardiac index during the infusion with a peak of increase of 0.09 l min−1 m−2 from baseline (95% CI 0.03, 0.1), (P < 0.001) at 15 min. This effect was maintained until the 20 min time point. Normal saline produced a similar increase in cardiac index by 0.08 l min−1 m−2 (95% CI 0.0, 0.1) during infusion. However cardiac index continued to increase to a peak of 0.13 l min−1 m−2 (95% CI 0.0, 0.2) at the 20 min time point. Mannitol induced a transient decrease in cardiac index during infusion with a nadir of −0.06 l min−1 m−2 (95% CI –0.1, 0.0) at the 5 min infusion time. Thereafter, the cardiac index returned to pre‐infusion values by the end of the observation period. Post hoc analysis of the cardiac index between the three infusions over the duration of the study showed that the changes in cardiac index between the three infusions were primarily driven by paracetamol as evident by lack of a statistical difference observed between mannitol and normal saline (P = 0.13).
During the infusion, the HR decreased significantly (P < 0.0001) in all three groups and remained below baseline during the observation period. The decrease in HR was greatest in the mannitol group with an overall decrease at the end of the study in HR by 2.8 beats min−1 (95% CI –3.9, –1.6) at 30 min compared with paracetamol which decreased HR by 2.5 beats min−1 (95% CI –3.5, –1.2) at 35 min and normal saline which decreased HR by 1.4 beats min−1 (95% CI –2.4, –0.2) at 30 min. During the infusion of all three interventions, there were significant increases in SVI compared with pre‐infusion values. SVI remained increased in all three groups throughout the observation period compared with pre‐infusion values with a study end increase of 1.8 ml m−2 (95% CI 1.2, 2.6) in the paracetamol group, 2.8 ml m−2 (95% CI 2.1, 3.5) in the normal saline group and 2.9 ml m−2 (95% CI 2.1, 3.6) in the mannitol group with paracetamol increasing by 1.9 ml m−2 (95% CI 1.2, 2.6), normal saline increasing by 2.8 ml m−2 (95% CI 201, 3.5) and mannitol increasing by 2.9 ml m−2 (95% CI 2.1, 3.6).
Changes in plasma osmolality
Plasma osmolality levels remained within the normal laboratory reference range (275–295 mosm kg−1) in all groups throughout the study. There were no statistical differences between the groups.
Reported adverse events
One participant in the saline group and three in the mannitol group expressed a need to void urine after completion of the study. The volume of urine voided was not documented.
Discussion
Key study findings
We conducted a double‐blind, randomized, triple crossover study in healthy volunteers to compare intravenous paracetamol infusion (3.91 g mannitol +1 g paracetamol 100 ml–1) with an equivalent administration of its excipient (mannitol) or placebo (normal saline). Our aim was to test the hypothesis of whether paracetamol itself is responsible for lowering arterial blood pressure. This healthy volunteer study provides plausible physiological data that i.v. paracetamol causes a transient decrease in BP immediately after infusion. These changes were not observed with mannitol or normal saline infusions suggesting that the adverse haemodynamic effects of paracetamol appear to be a function of the paracetamol itself, rather than that of its excipient ‐ mannitol. Furthermore, the mechanism of this transient decrease in blood pressure appears to be secondary to a transient reduction in SVRI and not due to a reduction in cardiac index as previously reported.
Relationship to previous studies
Recently, in the context of critical illness, clinical studies have suggested that i.v. paracetamol may cause hypotension 1, 2, 3, 4, 5, 6, 7, 8. Most of these studies did not report on the possible mechanism for the hypotension observed 1, 3, 5, 6, 7. In addition, all studies identified in our literature search were non‐randomized, non‐blinded and uncontrolled, making it difficult to draw firm conclusions about this potential effect of paracetamol. One study reported anecdotal data 1 and others used small patient numbers 5, 6, 8. Moreover, use of vasopressors could have also masked the magnitude of the adverse haemodynamic effects of paracetamol observed 2, 3, 5, 6, 7. Recently, Krajcova et al. performed a non‐blinded, non‐randomized study on six intensive care patients who received 48 paracetamol and 35 control drug administrations 8. These authors reported that i.v. paracetamol decreased cardiac index. In contrast to these findings, we observed a small increase in cardiac index after the infusion of paracetamol. Consistent with this increase in cardiac index, we also observed an increase in SVI. The transient blood pressure changes observed in our study were associated with a reduction in SVRI. In the present study, we also investigated the effects of 3.91 g of mannitol over a 15 min infusion time. Sabharwal et al. studied 11 participants who were administered i.v. mannitol (1 g kg−1) over a 10 min period and reported a decrease in blood pressure post‐infusion 16. In contrast, we observed an increase in blood pressure during and post‐infusion of mannitol, albeit it at a much lower dose (1/20th). From the results our study, it is unlikely that the mannitol component present in the i.v. paracetamol formulation contributes to the haemodynamic effects we observed with the paracetamol infusion.
Study implications
The clinical implication of our findings in this healthy volunteer study is that i.v. paracetamol transiently lowers arterial blood pressure. The effects of paracetamol on haemodynamics could be considered clinically insignificant. However, these haemodynamics derangements may have different intensities and durations in patients who are already haemodynamically compromised. The mechanism of paracetamol‐induced hypotension has previously been reported to be secondary to a reduction in cardiac index in a non‐randomized non‐blinded study. In contrast, we observed that the decrease in blood pressure was due to a reduction in SVRI and not cardiac index. Of particular importance was the increase in SVRI with mannitol observed shortly after its infusion. Taking into consideration the mannitol content of the i.v. paracetamol formulation used in our study, the true value of the observed reduction in SVRI may have been further masked by the effects of mannitol. Further studies may be required to examine these effects in non‐mannitol containing i.v. paracetamol formulations. The observed reduction in SVRI may have clinically important implications when considering the administration of i.v. paracetamol to patients with low SVR states e.g. septic shock or post‐operative inflammatory vasodilatation.
Strengths and limitations
This study has several methodological strengths. It was blinded and randomized, thus minimizing selection and assessment bias and increasing internal validity. It was a triple crossover study, which reduced the influence of confounding covariates. The concealment of the intervention further decreased selection bias. Comprehensive electronic data collection using continuous beat‐to‐beat measurements of haemodynamic variables of the Edwards Lifesciences Nexfin™ enabled detailed quantitative comparisons. Furthermore, SBP, DBP, MAP and HR were all objective variables not amenable to ascertainment bias or manipulation, and our findings were further strengthened by measurements of derived physiological haemodynamic data such as cardiac index and SVRI. Finally, to date this is the first study to compare comprehensively i.v. paracetamol (1 g paracetamol +3.91 g mannitol 100 ml–1), its excipient ‐ mannitol (3.91 g mannitol 100 ml–1) and placebo (normal saline) over a 60 min period.
There are also several limitations to our study. First, the changes in blood pressure observed were small and could be considered clinically insignificant. Similarly, the increase in SVRI seen with mannitol at 15 min should also be interpreted with caution. Given that SVRI has a wide normal range between 1970–2396 mmHg l−1 min−1 m−2, changes observed in SVRI could also be considered to be within normal limits and not of clinical importance. Secondly, the trial was small and only powered to demonstrate composite changes in blood pressure and not changes in SVRI or cardiac index, which are derived variables. Whilst the use of the Lifesciences Nexfin™ invasive haemodynamic is well validated in many clinical studies, and validated against both the sphygmomanometer and arterial line, measurement of central venous pressure via a central venous catheter would have been useful in identifying the effects of the paracetamol on preload and in more accurately calculating SVRI. The use of a central venous catheter and other invasive haemodynamic monitoring tools, e.g. arterial line, pulmonary artery catheter, were considered too invasive in a healthy volunteer population. Although we did not use invasive techniques to measure arterial blood pressure and cardiac output, the accuracy of the Nexfin™ cardiac output technology has been validated against pulmonary thermodilution 17, transpulmonary thermodilution 18, transoesophageal and transthoracic echocardiography 19, 20 and inert gas rebreathing 21. Percentage errors range from 23% to 39%, comparable with more invasive methods 17, 21. Larger errors have been reported, but these are in the setting of critically ill patients where compromised flow to the finger may affect Nexfin™ performance 22. This is clearly not applicable in a healthy volunteer setting making it physiologically plausible that the Nexfin™ technology used was able to track reliably changes in cardiac index, a finding supported by other studies 17, 18, 23. Use of transthoracic echocardiography would have allowed valuable non‐invasive and focused estimations of cardiac function and fluid responsiveness. However, its continuous use over the entire duration of each experiment was not pragmatic and lack of availability of a dedicated TTE machine and a skilful operator for all experiments unfortunately precluded its use. Finally, whilst the carrier solution in i.v. paracetamol is sterile water, we used normal saline as the placebo solution as 100 ml infusion of either solution over a 15 min period would have little, if any effect, on haemodynamic differences.
In summary, in a double‐blind, randomized, triple crossover healthy volunteer study, we found evidence that i.v. paracetamol caused a transient decrease in BP after infusion when compared with its excipient (mannitol) and placebo (normal saline). The reduction in blood pressure was most noticeable at 15 min, returning to baseline values within 30 min. These findings appeared to be a function of the paracetamol itself, rather than that of its excipient, mannitol. The primary physiological mechanism of these haemodynamic changes was transient systemic vasodilatation, rather than a reduction in cardiac index. This study provides plausible physiological data in a healthy volunteer setting, which supports the recently reported adverse haemodynamic effects of i.v. paracetamol in the critical care setting and suggests the need for similar double‐blind, randomized controlled trials in patients.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
This work was supported by a research grant from the Intensive Care Foundation.
Meetings at which this work has been presented
This trial was presented at Euroanesthesia, The European Anesthesiology Congress Conference 2015, 30 May–2 June 2015, Berlin, Germany.
Chiam, E. , Weinberg, L. , Bailey, M. , McNicol, L. , and Bellomo, R. (2016) The haemodynamic effects of intravenous paracetamol (acetaminophen) in healthy volunteers: a double‐blind, randomized, triple crossover trial. Br J Clin Pharmacol, 81: 605–612. doi: 10.1111/bcp.12841.
References
- 1. Duncan C, Seet J, Baker S. Centrally administered parenteral paracetamol: a potentially under‐reported cause of haemodynamic instability within the adult intensive care unit. Aust Crit Care 2012; 25: 131. [Google Scholar]
- 2. Boyle M, Nicholson L, O'Brien M, Flynn G, Collins D, Walsh W, Bihari D. Paracetamol induced skin blood flow and blood pressure changes in febrile intensive care patients: an observational study. Aust Crit Care 2010; 23: 208–14. [DOI] [PubMed] [Google Scholar]
- 3. de Maat M, Tijssen T, Brüggemann R, Ponssen H. Paracetamol for intravenous use in medium‐ and intensive care patients: pharmacokinetics and tolerance. Eur J Clin Pharmacol 2010; 66: 713–9. [DOI] [PubMed] [Google Scholar]
- 4. Needleman S. Safety of rapid intravenous infusion of acetaminophen. Proc (Bayl Univ Med Cent) 2013; 26: 235–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Picetti E, De Angelis A, Villani F, Antonini M, Rossi I, Servadei F, Caspani M. Intravenous paracetamol for fever control in acute brain injury patients: cerebral and hemodynamic effects. Acta Neurochir 2014; 156: 1953–9. [DOI] [PubMed] [Google Scholar]
- 6. Picetti E, Rossi I, Ceccarelli P, Risolo S, Schiavi P, Donelli V, Crocamo A, Antonini M, Caspani M. Intravenous paracetamol for fever control in acute brain‐injured patients: cerebral and hemodynamic effects. Crit Care 2013; 17: 329. [Google Scholar]
- 7. Vera P, Zepata L, Gich I, Mancebo J, Betbesé A. Hemodynamic and antipyretic effects of paracetamol, metamizol and dexketoprofen in critical patients. Med Intensiva 2012; 36: 619–25. [DOI] [PubMed] [Google Scholar]
- 8. Krajčová A, Matoušek V, Duška F. Mechanism of paracetamol‐induced hypotension in critically ill patients: a prospective observational cross‐over study. Aust Crit Care 2013; 26: 136–41. [DOI] [PubMed] [Google Scholar]
- 9. Penaz J. Criteria for set point estimation in the volume clamp method of blood pressure measurement. Physiol Res 1992; 41: 5–10. [PubMed] [Google Scholar]
- 10. Wesseling K, De Wit B, Van der Hoeven G, Van Goudoever J, Settels J. Physiocal, calibrating finger vascular physiology for Finapres. Homeostasis 1995; 36: 67–82. [Google Scholar]
- 11. Kalmar A, Vos J, Weening M, Mooyaart E, Poterman M, Struys M, Scheeren T. Validation of continuous noninvasive arterial blood pressure measurements during general anesthesia, abstract presented at the Anesthesiology Annual Meeting. 2012.
- 12. Sterr J, Scholz S, Habicher M, Krämer M, Treskatsch S, Sander M. Comparison of the continuous noninvasive Nexfin monitoring system with conventional invasive methods to measure arterial blood pressure in high risk hip surgery: 3AP3‐6. Eur J Anaesthesiol 2013; 30: 45–5. [Google Scholar]
- 13. Krenzelok E, Royal M. Confusion: acetaminophen dosing changes based on NO evidence in adults. Drugs R&D 2012; 12: 45–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eeftinck Schattenkerk D, van Lieshout J, van den Meiracker A, Wesseling K, Blanc S, Wieling W, van Montfrans G, Settles J, Wesseling K, Westerhof B. Nexfin noninvasive continuous blood pressure validated against Riva‐Rocci/Korotkoff. Am J Hypertens 2009; 22: 378–83. [DOI] [PubMed] [Google Scholar]
- 15. Martina J, Westerhof B, van Goudever J, Truijen J, Kim Y, Immink R, Jöbsis D, Hollmann M, Lahpor J, de Mol B, van Lieshout J. Noninvasive continuous arterial blood pressure monitoring with Nexfin®. Anesthesiology 2012; 116: 1092–103. [DOI] [PubMed] [Google Scholar]
- 16. Sabharwal N, Rao G, Radhakrishnan M. Hemodynamic changes after administration of mannitol measured by a noninvasive cardiac output monitor. J Neurosurg Anesthesiol 2009; 21: 248–52. [DOI] [PubMed] [Google Scholar]
- 17. Broch O, Renner J, Gruenewald M, Meybohm P, Schöttler J, Caliebe A, Steingath M, Malbrain M, Bein B. A comparison of the Nexfin® and transcardiopulmonary thermodilution to estimate cardiac output during coronary artery surgery. Anaesthesia 2012; 67: 377–83. [DOI] [PubMed] [Google Scholar]
- 18. Chen G, Meng L, Alexander B, Tran N, Kain Z, Cannesson M. Comparison of noninvasive cardiac output measurements using the Nexfin monitoring device and the esophageal Doppler . J Clin Anesth 2012; 24: 275–83. [DOI] [PubMed] [Google Scholar]
- 19. van der Spoel A, Voogel A, Folkers A, Boer C, Bouwman R. Comparison of noninvasive continuous arterial waveform analysis (Nexfin) with transthoracic Doppler echocardiography for monitoring of cardiac output. J Clin Anesth 2012; 24: 304–9. [DOI] [PubMed] [Google Scholar]
- 20. Bartels S, Stok W, Boksem R, van Goudoever J, Cherpanath T, van Lieshout J, Westerhof B, Karemaker J, Ince C. Noninvasive cardiac output monitoring during exercise testing: nexfin pulse contour analysis compared to an inert gas rebreathing method and respired gas analysis. J Clin Monit Comput 2011; 25: 315–21. [DOI] [PubMed] [Google Scholar]
- 21. Alhashemi J, Cecconi M, Hofer C. Cardiac output monitoring: an integrative perspective. Crit Care 2011; 15: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monnet X, Picard F, Lidzborski E, Mesnil M, Duranteau J, Richard C, Teboul J. The estimation of cardiac output by the Nexfin device is of poor reliability for tracking the effects of a fluid challenge. Crit Care 2012; 16: R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bubenek‐Turconi S, Craciun M, Miclea I, Perel A. Noninvasive continuous cardiac output by the Nexfin before and after preload‐modifying maneuvers: a comparison with intermittent thermodilution cardiac output. Anesth Analg 2013; 117: 366–72. [DOI] [PubMed] [Google Scholar]


