Abstract
Aims
The aim of this study was to perform an up‐to‐date meta‐analysis on the risk of cardiac malformations associated with gestational exposure to paroxetine, taking into account indication, study design and reference category.
Method
A systematic review of studies published between 1966 and November 2015 was conducted using embase and MEDLINE. Studies reporting major malformations with first trimester exposure to paroxetine were included. Potentially relevant articles were assessed and relevant data extracted to calculate risk estimates. Outcomes included any major malformations and major cardiac malformations. Pooled odds ratios and 95% confidence intervals were calculated using random‐effects models.
Results
Twenty‐three studies were included. Compared with non‐exposure to paroxetine, first trimester use of paroxetine was associated with an increased risk of any major congenital malformations combined (pooled OR 1.23, 95% CI 1.10, 1.38; n = 15 studies), major cardiac malformations (pooled OR 1.28, 95% CI 1.11, 1.47; n = 18 studies), specifically bulbus cordis anomalies and anomalies of cardiac septal closure (pooled OR 1.42, 95% CI 1.07, 1.89; n = 8 studies), atrial septal defects (pooled OR 2.38, 95% CI 1.14, 4.97; n = 4 studies) and right ventricular outflow track defect (pooled OR 2.29, 95% CI 1.06, 4.93; n = 4 studies). Although the estimates varied depending on the comparator group, study design and malformation detection period, a trend towards increased risk was observed.
Conclusions
Paroxetine use during the first trimester of pregnancy is associated with an increased risk of any major congenital malformations and cardiac malformations. The increase in risk is not dependent on the study method or population.
Keywords: cardiac malformations, major malformations, meta‐analysis, paroxetine, pregnancy
Introduction
Up to one‐fifth of women of childbearing age experience moderate to severe depressive symptoms 1. Pregnancy may be a time of risk for both new onset and reoccurrence of depression, with prevalence rates of depression ranging from 7% to 20% 2, 3, 4, 5. Depression during pregnancy is associated with poor maternal nutrition, inadequate weight gain, smoking, alcohol and other substance intake, and increased risk of post‐partum depression 6, 7, 8, 9, 10. Antidepressant prescribing during pregnancy has increased up to four‐fold between 1992 and 2006 with a total of 4.8% of women receiving a prescription in the months prior to pregnancy in the UK 11. Prevalence rates are estimated to be 4.5% (2009) in Canada and up to 13% (2007) in the US 12, 13. The most frequently used treatment for depression in pregnant women is selective serotonin re‐uptake inhibitors (SSRIs) 14, 15, accounting for approximately 80% of prescribed antidepressants during pregnancy 11, 12, 15, 16, 17, 18. The widespread use of antidepressants during pregnancy makes it essential to understand the safety and the risk of adverse outcomes in the fetus.
Up until 2005 paroxetine was considered to be safe for use during pregnancy 19, 20, 21, 22, 23. However, following results from a small unpublished study conducted by the manufacturer, there were suggestions of an increase in the risk of cardiac malformations in infants with in utero exposure to paroxetine, compared with those unexposed to paroxetine. This resulted in a modification of the product label to include warnings of the risk of cardiac malformations with antenatal exposure to paroxetine 24. At the same time, the US FDA changed the classification of paroxetine from pregnancy category C (human data lacking: animal studies positive or not done) to category D (human data show risk, but benefit may outweigh). Subsequent to these changes, numerous studies using various study designs with the use of different populations across Europe and North America have been published. Some supported statistically significant association of the risk of congenital malformations with first trimester exposure of paroxetine 25, 26, 27, 28, 29, 30, 31. Conversely, findings from other studies showed conflicting results in terms of statistical significance, although a trend remained towards an increased risk.
Paroxetine and other SSRIs are known to cross the maternal placental barrier 32, 33 and significant concentrations of antidepressants have been found in the amniotic fluid 34. It is thought that SSRIs may affect fetal cardiovascular and central nervous system development through interference with the serotonin 5‐HT2B receptor 35. In addition, physiological systems such as the sleep‐wake cycle, circadian rhythms and hypothalamic‐pituitary‐adrenal axis are affected by increased serotonergic neurotransmission 36.
As more studies have been conducted overtime, a number of meta‐analyses have been performed in order to find more conclusive answers to the question of teratogenicity associated with paroxetine and/or other SSRIs. Earlier meta‐analyses, that reported an increased risk of cardiac defects associated with paroxetine had methodological limitations such as using studies that did not adjust for all potential risk factors for malformations (confounders) 37, 38. Wurst et al. 38 have performed a meta‐analysis showing that paroxetine use during pregnancy was increasing the risk of cardiac defects. However, some studies were not considered and only studies up to 2009 were used. In an attempt to categorize studies in terms of their quality (by using quality tools), Girgordiadis et al. 39 conducted a meta‐analysis using 19 studies on SSRIs as a class, with a sub‐analysis of individual SSRIs. They found an increased risk of congenital cardiac malformations, but major malformations as a whole were not associated with paroxetine exposure. Myles et al. 40 conducted another meta‐analysis of 16 studies also investigating SSRIs as a whole but excluded studies with any antidepressant medications in the comparator group. Paroxetine was associated with an increase in the risk of major malformations and cardiac malformations. However, some relevant studies were not included in Myles et al. 40, and the majority of studies used did not distinguish between the potential effect of depression (the underlying condition) and the drug (paroxetine) on the risk of major and cardiac malformations.
There is a strong recommendation for studies to include untreated patients with depression and/or other psychiatric diagnoses 41. Recent studies have attempted to overcome this by including a comparison group of untreated depressed patients 16, 42, 43, 44, some of which were not considered in previous meta‐analyses. In light of new publications and recent recommendations, we aimed to conduct a meta‐analysis incorporating more recent findings, stratifying on types of comparison groups to update current understanding of paroxetine and major congenital malformations, with a particular focus on cardiac defects. The impact of study designs, methodologies and comparator groups (reference category) on the quantification of the effect were also studied.
Method
Electronic and hand searches
A systematic electronic literature search of English and French language publications, indexed in MEDLINE and embase databases between 1966 to the 10 November 2015, was conducted by three individual reviewers independently (AB, NI, SC) using a broad combination of search terms. The search strategy was written in Ovid and run in each database (Supplementary file S1). Strategies were based on the subject headings specific to the individual databases searched, combined with appropriate keywords and phrases. After exclusion of duplicates, full text of potentially relevant studies was retrieved and examined. The reference list of manuscripts included in the analysis, were manually searched for additional relevant publications. Corresponding authors of studies that contained information on SSRIs, but not specifically on paroxetine, as an individual drug, were contacted for additional information. Information available in previous meta‐analyses that were obtained by investigators from the corresponding authors was also used 38, 40.
Inclusion and exclusion criteria
Studies were included if 1) they investigated paroxetine use during the first trimester of pregnancy (if the study investigated SSRIs as a class, it was only included if individual data or a sub‐analysis for paroxetine use alone was available), 2) they had a comparator group, 3) they reported an effect measure such as odds ratio (OR), risk ratio (RR) or there were enough information to calculate an unadjusted OR and 4) the outcomes investigated included any major congenital malformations, and/or major cardiac malformations and/or sub‐categories of cardiac defects. Studies were excluded if 1) exposure consisted of SSRIs and/or other antidepressants combined or 2) exposure did not occur during the first trimester of pregnancy. Additionally, if the data source of two or more studies overlapped with each other (time period, population, inclusion and exclusion criteria), the most recent study was included (Supplementary file S2).
Data extraction
Data were extracted from each study independently by two reviewers using a standardized extraction form. Disagreements of data collected were resolved by re‐examining the data by a third reviewer.
Meta‐analysis
Since the prevalence of major congenital malformations is less than 10%, we have assumed that the OR is equivalent to the RR and will refer to the effect measure as OR throughout 45. If the effect size was not reported in the study, we calculated the unadjusted OR and their 95% confidence intervals (95% CI) from the raw data. We used the adjusted ORs in preference to unadjusted ORs, and estimates related to all infants in preference to estimates from subgroups only. Due to the differences in methods between study populations, a random effects model was used to calculate a pooled OR for each outcome in our meta‐analysis 46. In order to evaluate publication bias in our main meta‐analysis, we inspected a funnel plot (eye ball test). However, given the inherent subjectivity of a graphical assessment, the Egger test and trim and fill methods were used to adjust for a potential publication bias 47. Also, abstract data were combined with the included studies in a sensitivity analysis. The I‐squared (I2) test was used to assess the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance).
In an attempt to explain possible heterogeneity between studies, we conducted several sub‐group analyses by study design (cohort/case control), continent (North America, Europe, Australia), type of comparator groups, time to defect ascertainment (less than 1 year/up to 1 year and more), definitions of first trimester exposure, type of data source (administrative claims data/prescription databases linked to registries, clinical data linked to registries and teratogen information services) and timing of the exposure. We also restricted the main analysis to the use of only adjusted OR estimates (no unadjusted values), studies that specified excluding chromosomal and/or genetic defects and other teratogenic substances, studies that adjusted for depression or consisted of a depressed cohort and studies that excluded or adjusted analyses for individuals with epilepsy or hypertension. In addition, sensitivity analyses using fixed effects were performed to ensure the robustness of our results. All pooled ORs were calculated using STATA software (version 11).
Results
After screening abstracts and titles, 53 articles were identified as potentially relevant studies and full texts were obtained (Figure 1). In addition, nine articles were identified through hand searching references. Twenty‐three studies in total were included in the meta‐analysis, 16 with a result on major malformations 16, 19, 20, 23, 24, 26, 27, 28, 30, 41, 47, 48, 50, 51, 52, 54 and 19 with a result on cardiac malformations 16, 19, 23, 24, 26, 27, 28, 29, 30, 41, 43, 47, 48, 49, 50, 51, 52, 54, 55. Thirty of the potentially relevant articles were excluded in the analysis because they did not investigate the outcome of interest (major congenital malformations, major cardiovascular malformations) (n = 5), exposure time window was not in the first trimester (n = 4), exposure group of interest contained other SSRIs or antidepressants (n = 8), overlapped with a previous study/and or combined previously published results/updated later (n = 3), contained a control group from a different population (n = 1), were abstracts (n = 7) or a commentary (n = 1) (Supplementary file S2). Relevant results available from abstracts were not included in the main analysis. However four were added to the sub‐analyses 56, 57, 58, 59.
Figure 1.
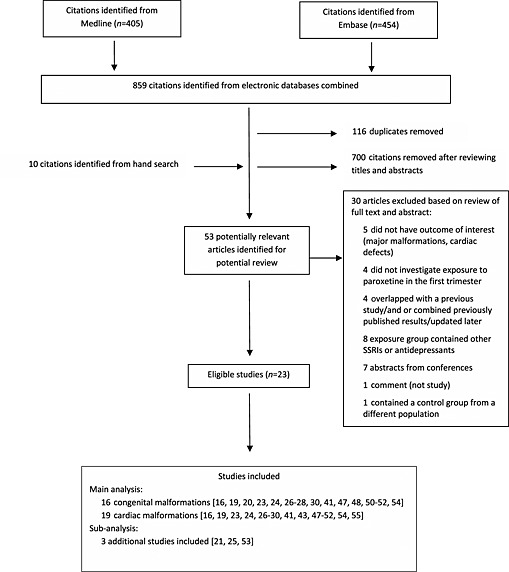
Flow diagram of study selection in the systematic review and meta‐analysis of the effect of paroxetine use during pregnancy on the risk of major malformations and cardiac malformations. [Indexed from 1966 to November 10th, 2015].
Three authors were contacted for additional information 44, 61, 62. Two authors could not give specific information for paroxetine and the studies were not included in the analysis 60, 61. One author provided additional information and the study was included 44. Further information on studies published in previous meta‐analyses that were obtained through author contact was also included in our meta‐analysis 19, 20, 38, 40.
Characteristics of studies included
Included studies contained data collected from Australia, Canada, Denmark, Israel, Italy, The Netherlands, Sweden, United Kingdom and United States. Four studies used a case–control design 27, 29, 30, 51 and the remaining 15 used cohort study designs. Study populations ranged from 534 subjects to 949 504 in size. The most common data sources were prescription databases linked to birth registries. Other sources included claims and administrative databases, active surveillance cohorts and teratogen information service data (Table 1).
Table 1.
Characteristics of included studies
| Study | Study period | Country and data source | Study design | Exposure (first trimester)/sample size | Comparison group | Relevant congenital malformations | Relevant findings (paroxetine use only) |
|---|---|---|---|---|---|---|---|
| Furu et al. 23 | 1996–2010 | Nordic countries (Denmark, Finland, Iceland, Norway,and Sweden) Nationwide health registers | Cohort | SSRIs as a class and individually
Total n = 2 303 647 Paroxetine: 2879 |
Unexposed to any antidepressant | Major congenital malformations | Adjusted OR 1.16, 95% CI 0.95, 1.41 |
| Cardiovascular defects
Atrial and ventricular septal defects Right ventricular outflow tract obstructions Conotruncal and major arch defects |
Adjusted OR 1.30, 95% CI 0.96, 1.75
Adjusted OR 1.37, 95% CI 0.96, 1.95 Adjusted OR 2.54, 95% CI 1.31, 4.90 Adjusted OR 2.27, 95% CI 1.01, 5.07 |
||||||
| Ban et al. 42 | 1990–2009 | UK The Health Improvement Network | Cohort | SSRIs as a class and individually
Total n = 349 127 Paroxetine: 1200 |
1) Depressed untreated 2) No depression (unexposed to any antidepressant) | Major congenital defects | Adjusted OR 1.08, 95% CI 0.77, 1.50 |
| Cardiovascular defects | Adjusted OR 1.79; 95% CI 1.09, 2.89 | ||||||
| Huybrechts et al. 50 | 2000–2007 | US Medicaid Analytic eXtract | Cohort | All antidepressants
Total n = 949 504 Paroxetine: 11 126 |
Unexposed to antidepressant (depressed or not depressed) | Any cardiac malformation | Adjusted OR 0.94, 95% CI 0.73, 1.21 |
| Knudsen et al. 44, †† | 1995–2008 | Denmark Medical Birth Registry; National Hospital Register; National Prescription Registry, Statistic Denmark | Cohort | SSRIs as a class and individually
Total n = 72 280 Paroxetine: 131* |
Unexposed to SSRI (not treated with SSRI from 1 year before to 1 year after pregnancy) | Congenital heart defects* | Adjusted OR (total congenital heart defects) 1.01, 95% CI 0.14, 7.22 |
| Jimenez‐Solem et al. 16 | 1997–2009 | Denmark (Nationwide) Danish Medical Birth Registry, Danish Hospital register, Register of Medicinal Product Statistics | Cohort | SSRIs as a class and individually
Total n = 848 786 Paroxetine: 568 Paused exposure (women with exposure to an SSRI 3 to 12 months before conception and 1 to 12 months after giving birth) |
Unexposed to SSRI | Major congenital malformations | Adjusted OR 1.25, 95% CI 0.84, 1.85 |
| Cardiovascular defects | Adjusted OR 1.54, 95% CI 0.77, 3.10 | ||||||
| Bulbus cordis anomalies and anomalies of cardiac septal closure | Adjusted OR 1.89, 95% CI 0.85, 4.23 | ||||||
| Nordeng et al. 52 | 1999–2005 | Norway Norwegian Mother Child Cohort (information from hospital lists and questionnaires) | Cohort | SSRIs as a class and individually
Total n = 63 395 Paroxetine: 76 |
Non‐exposed to any antidepressants (6 months before pregnancy) | Major congenital malformations | Unadjusted OR 1.70, 95% CI 0.55, 5.63 |
| Colvin et al. 48 | 2002–2005 | Australia Western Australia Data Linkage System; hospital mortality data; national pharmaceutical claims dataset | Cohort | SSRIs as a class and individually
Total n = 96 968 Paroxetine: 572 |
Non‐exposed to SSRI (can be exposed to other antidepressants) | Major congenital malformations | Adjusted OR 0.99, 95% CI 0.65, 1.51 |
| Bulbus cordis anomalies and anomalies of cardiac septal closure | Unadjusted OR 1.76, 95% CI 0.83, 3.72 | ||||||
| Malm et al. 31 | 1996–2006 | Finland Drugs and pregnancy project database (Registries for Medical Birth, Congenital Malformations, Drug reimbursement) | Cohort | SSRIs as a class and Individually
Total n = 635 583 Paroxetine: 968 |
Unexposed to SSRI in analysis of all SSRIs; For paroxetine SSRIs: Unexposed to paroxetine (includes other SSRIs) | Major congenital malformations | Adjusted OR 1.22, 95% CI 0.91, 1.64 |
| Cardiovascular defects | Adjusted OR 1.09, 95% CI 0.66, 1.79 | ||||||
| Bakker et al. 30 | 1997–2006 | Netherlands Eurocat Northern Netherlands database | Case–control | Paroxetine Total n = 1293 (678 cases, 615 controls) Paroxetine: 16 | Unexposed to any SSRIs | Congenital heart defects | Adjusted OR 1.5, 95% CI 0.5, 4.0 |
| Bulbus cordis anomalies and anomalies of cardiac septal closure | Adjusted OR 1.6, 95% CI 0.4, 5.6 | ||||||
| Reis et al. 25 | 1995–2007 | Sweden (birth register) | Cohort | SSRIs as a class and paroxetine individually Total n = 15 017 infants Paroxetine: 1208 | Unexposed to an antidepressant | ‘Relatively severe’ malformation | Adjusted OR 1.20, 95% CI 0.90, 1.61 |
| Cardiovascular defects | Adjusted OR 1.67, 95% CI 1.09, 2.53 | ||||||
| Pedersen et al. 54, † | 1996–2003 | Denmark Birth registry, fertility database, hospital register, medicinal product statistic | Cohort (population based) | SSRIs as a class and Individually
Total n = 493 113 Paroxetine: 299 |
Unexposed to an antidepressants | Any malformations | Adjusted OR 1.41, 95% CI 0.79, 2.51 |
| Diav‐Citrin et al. 55 | 1994–2002 | Israel, Italy, Germany Teratology information service | Prospective cohort | Paroxetine and fluoxetine individually Total n = 2191 Paroxetine: 463 | Unexposed to SSRIs or known teratogens (n = 1467) | Any malformations‡ | Adjusted OR 2.14, 95% CI 1.19, 3.82 |
| Cardiovascular defects | Adjusted OR 2.66, 95% CI 0.80, 8.90 | ||||||
| Einarson et al. 56 | Not specified | Italy; Switzerland; Australia; Germany; Israel; Finland Teratology information services | Case–control | Paroxetine
1174 exposure to Paroxetine identified from the TIS 2061 exposures from published studies, not used in analysis |
Unexposed to known teratogens with similar clinic characteristics to women with case infants | Cardiovascular defects | Adjusted OR 1.11, 95% CI 0.36, 2.78 |
| Oberlander et al. 53 | 1998–2001 | Canada Administrative databases (British Columbia registry of births, hospital separation records, PharmCare registry, Medical Services Plan, PharmNet | Cohort (population based) | SRIs monotherapy SRIs and benzodiazepinesTotal n = 119 547 Paroxetine (monotherapy): 993 | Unexposed to SRIs and benzodiazepines | Major congenital malformations‡ | Unadjusted OR 0.93, 95% CI 0.64, 1.35 |
| Cardiovascular malformations‡ | Unadjusted OR 1.48, 95% CI 0.70, 3.10 | ||||||
| Alwan et al. 27 | 1997–2002 | US NBDPS surveillance study | Retrospective Case–control (population bases) | SSRIs Total n = 13 714 (9622 cases, 4092 controls) Paroxetine: 88 | Controls: Infants with no birth defects Comparison: Unexposed to SSRIs | Major congenital malformations | Adjusted OR 1.60, 95% CI 0.90, 2.77 |
| Cardiovascular defects | Adjusted OR 1.70, 95% CI 0.92, 3.16 | ||||||
| Bulbus cordis anomalies and anomalies of cardiac septal closure | Adjusted OR 1.70, 95% CI 0.8, 3.5 | ||||||
| Berard et al. 29 | 1997–2003 | Canada Québec databases (Quebec Pregnancy Cohort) | Nested case–control (population based) | Paroxetine Total n = 1403 (101 cases, 1302 controls) Paroxetine: 542 | Exposure to non‐SSRI antidepressants | Major congenital malformations | Adjusted OR 1.32, 95% CI 0.79, 2.20 |
| major cardiac malformations | Adjusted OR 1.38, 95% CI 0.49, 3.94 | ||||||
| Bulbus cordis anomalies and anomalies of cardiac septal closure | Unadjusted OR 1.14, 95% CI 0.44, 2.99 | ||||||
| Cole et al. 28 | 1995–2004 | US United Healthcare Claims database | Cohort | Paroxetine
Total n = 5752 Paroxetine: 815 |
Exposed to other antidepressants as monotherapy or polytherapy. | Any congenital malformation | Adjusted OR 2.03, 95% CI 1.28, 3.25 |
| Cardiovascular malformations | Adjusted OR 1.46, 95% CI 0.74, 2.89 | ||||||
| Davis et al. 49 | 1996–2000 | US HMO research network program administrative databases) | Cohort (population based) | SSRIs as a class and individually
Total n = 805 Paroxetine: 182 |
Unexposed to SSRIs | Congenital anomalies | Unadjusted OR 1.03, 95% CI 0.73, 1.48 |
| Cardiac defects‡ | Unadjusted OR 1.03, 95% CI 0.45, 2.32 | ||||||
| Kallen et al. 26, * | 1995–2004 | Sweden Swedish medical birth register, birth defects register and hospital discharge, register | Cohort | SSRIs as a class and individuallyTotal n = 860 215 Paroxetine:959 | Unexposed to any antidepressant | Major congenital malformations | Adjusted OR 1.03, 95% CI 0.76, 1.8 |
| Cardiac malformation | Adjusted OR 1.63, 95% CI 1.05, 2.53 | ||||||
| Bulbus cordis anomalies and anomalies of cardiac septal closure | Unadjusted OR 1.81, 95% CI 0.96, 3.09 | ||||||
| Louik et al. 51 | 1993–2004 | US The birth defects study (population based) | Retrospective Case–control | SSRIs Total n = 15 709 (9849 cases, 5860 controls) Paroxetine: 97 | Controls: Infants with no birth defects Comparison: Unexposed to antidepressants | Major congenital malformations 38 | Adjusted OR 1.38, 95% CI 0.85, 2.27 |
| Any cardiac defect | Adjusted OR 1.40, 95% CI 0.79, 2.46 | ||||||
| Bulbus cordis anomalies and anomalies of cardiac septal closure | Adjusted OR 0.8, 95% CI 0.3, 2.2 | ||||||
| Malm et al. 21, § | 1996–2001 | Finland Drugs and pregnancy project database (Registries for Medical Birth, Congenital Malformations, Drug reimbursement) | Cohort (Population based) | SSRIs Total n = 1920 Paroxetine: 149 | Women with no drug purchases | Major congenital malformations | Adjusted OR 0.4, 95% CI 0.1, 3.3 |
| Cardiac malformations | Adjusted OR 0.66, 95% CI 0.09, 4.95 | ||||||
| Simon et al. 20 | 1986–1998 | US
Group Health Cooperative (claims database) |
Retrospective Cohort | SSRIs as a group TCAsTotal n = 787 Paroxetine:38 | Unexposed to SSRIs | Major and minor malformations¶ | Unadjusted OR 1.00, 95% CI 0.06, 16.78 |
| Kulin et al. 19 | Not specified | Canada, US Teratogen information service (Motherisk Program) | Controlled cohort | SSRIs as a group Total n = 534 Paroxetine: 97 | Unexposed to known teratogenic agents | Major congenital malformations¶ | Unadjusted OR 1.77, 95%CI 0.57, 5.47 |
| Cardiac malformations¶ | Unadjusted OR 0.77, 95% CI 0.09, 6.89 |
Information obtained from authors.
Potential overlap of data with Jimenez‐Solem et al. 16. However overlap is minimal and therefore considered as an independent study.
Unadjusted OR (calculated via a 2 by 2 table).
This study overlaps with Malm et al. 31; used when restricting comparison group to: unexposed to all antidepressants.
Unadjusted OR (obtained via pervious meta‐analysis).
Overlaps with Reis et al. 25; used in sub‐analysis.
In order to include prescriptions received immediately before pregnancy and potentially used during pregnancy (duration overlaps with the start of pregnancy), the majority of studies defined the exposure time window as 30 days prior to conception date until the end of the first trimester (14 weeks of gestation; nine studies). Comparison groups consisted of unexposed to any antidepressants (non‐medicated depressed and non‐depressed women), those unexposed to SSRIs (non‐SSRI treated patients or other antidepressant treated patients, non‐medicated depressed patients, non‐depressed women) and unexposed to paroxetine (can be exposed to other SSRIs or non‐SSRI antidepressants). Two studies used non‐medicated controls with a diagnosis of depression 42, 50 and the control groups in two studies consisted of all subjects treated with other antidepressants 28, 29. Oberlander et al. 53 and Nordeng et al. 52 adjusted for depression in their analyses. Knudsen et al. 44 and Jiminez‐Solem et al. 16 included an exposure group of women who stopped SSRIs treatment 3 to 12 months before the last menstrual period, did not use their SSRIs during pregnancy but restarted taking the same SSRIs after pregnancy. Most studies adjusted for maternal age. Other confounding factors that were adjusted for included smoking, alcohol use, folic acid intake, year of birth, parity, presence of chronic diseases, body mass index, education, other medications, income and maternal depression (Table 1).
Major congenital malformations
Based on 15 studies using women unexposed to paroxetine as reference category (women could be using other antidepressants including other SSRIs), the use of paroxetine was associated with a statistically significant 23% increased risk of major congenital malformations (pooled OR 1.23, 95% CI 1.10, 1.38) 16, 19, 20, 25, 27, 28, 29, 31, 42, 48, 49, 51, 52, 53, 55 (Figure 2A). After restricting the analysis to studies that compared women using paroxetine with women unexposed to SSRIs, the ORs remained similar (Table 2 , Figure 2). The risk estimates were slightly higher in subgroups using different data sources such as clinic surveillance linked to registries (pooled OR 1.49, 95% CI 1.05, 2.12; n = 3 studies) (Table 2).
Figure 2.
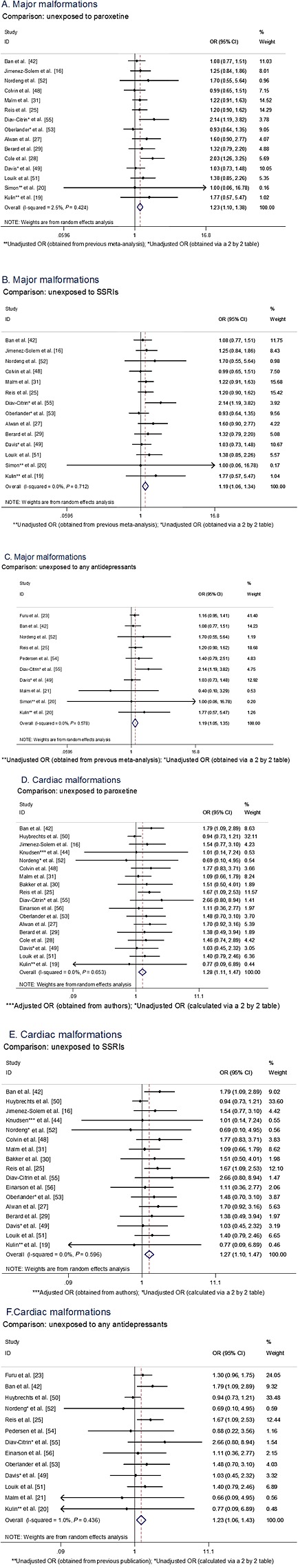
Forest plots and pooled measures of risks for overall malformation and cardiac malformation according to the comparator group. (A) Major malformations, comparison: unexposed to paroxetine, (B) major malformations, comparison: unexposed to SSRIs, (C) major malformations, comparison: unexposed to any antidepressants, (D) cardiac malformations, Comparison: unexposed to paroxetine, (E) cardiac malformations, comparison: unexposed to SSRIs, (F) cardiac malformations, comparison: unexposed to any antidepressants.
Table 2.
Sub‐analyses of major malformations and cardiac malformations
| Major malformations (all/aggregate) | Cardiac malformations (all/aggregate) | |||||
|---|---|---|---|---|---|---|
| Number of studies | Effect size Pooled OR (95% CI) | Between‐sample heterogeneity I 2 (P value) | Number of studies |
Effect size
Pooled
OR (95% CI) |
Between‐sample heterogeneity I 2 (P value) | |
| Comparison group | ||||||
| Unexposed to paroxetine (unadjusted or adjusted ORs combined) | 15 | 1.23 (1.10, 1.38) | 1.8% (0.431) | 18 | 1.28 (1.11, 1.47) | 0.0% (0.653) |
| Unexposed to paroxetine (adjusted ORs only) | 10 | 1.26 (1.11, 1.43) | 0.0% (0.615) | 13 | 1.35 (1.23, 1.62) | 8.5% (0.361) |
| Unexposed to SSRIs (unadjusted or adjusted ORs combined) | 14 | 1.19 (1.06, 1.34) | 0.0% (0.722) | 17 | 1.27 (1.10, 1.47) | 0.0% (0.596) |
| Unexposed to SSRIs (adjusted ORs only) | 9 | 1.21 (1.06, 1.38) | 0.0% (0.936) | 11 | 1.36 (1.09, 1.56) | 21.2% (0.241) |
| Unexposed to any antidepressants (unadjusted or adjusted ORs combined) | 10 | 1.19 (1.05, 1.35) | 0.0% (0.578) | 13 | 1.23 (1.06, 1.43) | 1.0% (0.436) |
| Unexposed to any antidepressants (adjusted ORs only) | 6 | 1.21 (1.03, 1.43) | 17.5% (0.300) | 8 | 1.27 (1.03, 1.56) | 27.1% (0.212) |
| Exposed to antidepressants other than paroxetine | 2 | 1.66 (1.09, 2.53) | 30.9% (0.229) | 2 | 1.44 (0.81, 2.54) | 0.0% (0.925) |
| Diagnosed with depression and/or anxiety (exposed or unexposed to an antidepressant other than paroxetine) | 3 | 1.28 (0.74, 2.22) | 81.4% (0.005) | 4 | 1.33 (0.87, 1.71) | 38.4% (0.181) |
| Diagnosed with depression and/or anxiety but not exposed to any antidepressant | 1 | ‐ | ‐ | 2 | 1.20 (0.69, 2.08) | 73.1% (0.051) |
| Addition of other data | ||||||
| Addition of data from abstracts and unpublished studies | 18 | 1.26 (1.11, 1.42) | 16.2% (0.260) | 21 | 1.27 (1.10, 1.46) | 0.0% (0.585) |
| Study design | ||||||
| Case–control | 3 | 1.42 (1.05, 1.91) | 0.0% (0.879) | 4 | 1.51 (1.01, 2.17) | 0.0% (0.972) |
| Cohort | 13 | 1.21 (1.06, 1.38) | 15.2% (0.295) | 15 | 1.23 (1.01, 1.45) | 0.0% (0.449) |
| Where study took place | ||||||
| North America | 8 | 1.28 (1.04, 1.58) | 21.1% (0.262) | 9 | 1.23 (1.01, 1.5) | 8.0% (0.369) |
| Europe | 6 | 1.19 (1.01, 1.41) | 0.0% (0.946) | 7 | 1.40 (1.06, 1.84) | 0.0% (0.808) |
| Australia | 1 | ‐ | ‐ | 1 | ‐ | ‐ |
| More than one continent | 1 | ‐ | ‐ | 2 | 1.6 (0.69, 3.79) | 16.2% (0.275) |
| Time to malformation ascertainment | ||||||
| Less than 1 year of age | 4 | 1.69 (1.22, 2.3) | 0.0% (0.532) | 4 | 1.04 (0.84, 1.29) | 0.0% (0.438) |
| Up to 1 year or beyond | 5 | 1.27 (1,01, 1.61) | 10.5% (0.346) | 6 | 1.10 (0.66, 1.86) | 50.7% (0.07) |
| Not specified | 7 | 1.41 (0.99, 1.31) | 0.0% (0.800) | 9 | 1.51 (1.22, 1.87) | 0.0% (0.895) |
| Data source | ||||||
| Administrative/claims database | 4 | 1.39 (0.78, 2.49) | 61.1% (0.077) | 4 | 1.00 (0.79, 1.25) | 0.0% (0.491) |
| Prescription database and/or linked to birth registry | 6 | 1.21 (0.96, 1.30) | 0.0% (0.804) | 7 | 1.46 (1.13, 1.89) | 0.0% (0.884) |
| Clinic/surveillance and/or link to registries | 3 | 1.49 (1.05, 2.12) | 0.0% (0.901) | 4 | 1.48 (1.02, 2.17) | 0.0% (0.846) |
| Teratogen information services | 3 | 1.50 (0.99, 2.26) | 37.9% (0.200) | 4 | 1.62 (1.21, 2.32) | 0.0% (0.646) |
| Inclusions and exclusions | ||||||
| Studies excluded chromosomal and/or genetic defects | 5 | 1.28 (1.05, 1.55) | 21.5% (0.277) | 6 | 1.25 (0.93,1.68) | 43.8% (0.113) |
| Studies excluded or adjusted for the use of teratogenic drugs | 6 | 1.23 (1.01, 1.52) | 20.0% (0.283) | 8 | 1.18 (0.94,1.48) | 14.0% (0.320) |
| Covariates adjustments | ||||||
| Adjusted for maternal chronic diseases | 7 | 1.25 (1.01, 1.54) | 32.9% (0.189) | 10 | 1.16 (0.97,1.38) | 0.0% (0.476) |
| Adjusted for depression or used a cohort of depressed pregnant women | 5 | 1.26 (0.95, 1.69) | 45.3% (0.120) | 6 | 1.25 (0.93, 1.67) | 27.5% (0.229) |
| Definition of first trimester exposure | ||||||
| At least 30 days before to end of first trimester | 8 | 1.15 (1.00, 1.33) | 0.0% (0.681) | 10 | 1.49 (1.20, 1.85) | 0.0% (0.958) |
| Not specified | 5 | 1.27 (0.98, 1.63) | 17.7% (0.302) | 5 | 1.50 (1.08, 2.09) | 0.0% (0.623) |
OR odds ratio; 95% CI 95% confidence interval.
The estimate was higher (pooled OR 1.66, 95% CI 1.09, 2.53; n = 2 studies) when the comparison group was restricted to women prescribed a non‐paroxetine antidepressant. However, a higher inter‐study heterogeneity was observed in this latter subgroup (I2 = 30.9%) (Table 2). It remained nevertheless that the inter‐study heterogeneity was null or very low in the majority of combinations.
Cardiac malformations
An aggregated outcome for all cardiac malformations was reported in 19 studies with a total of 20251 women exposed to paroxetine (Figure 2). The estimate for all major cardiac malformations in women exposed to paroxetine compared with non‐paroxetine exposure (can be exposed to other antidepressants) was pooled OR 1.28 (95% CI 1.11, 1.47; n = 19 studies) (Table 2 , Figure 2D). This estimate was similar when the comparison group was restricted to women with no antidepressant use (Table 2 , Figure 2F). The risk estimate increased slightly when the analysis was restricted to studies with adjusted estimates (pooled OR 1.35, 95% CI 1.23, 1.62; n = 13 studies) (Table 2). Estimates varied amongst different data sources; i.e. lower pooled risk was seen in studies conducted with data from administrative databases (pooled OR 1.00, 95% CI 0.79, 1.25; n = 4 studies). A higher risk was seen in studies conducted with data obtained in Europe (pooled OR 1.40, 95% CI 1.06, 1.84; n = 7 studies) (Table 2).
Specific cardiac malformations
Table 3 presents the specific cardiac malformations reported. Compared with women not exposed to paroxetine, the use of paroxetine was associated with an increased risk of bulbus cordis anomalies and anomalies of cardiac septal closure (pooled OR 1.42, 95% CI 1.07, 1.89; n = 8 studies), atrial septal defects (pooled OR 2.38, 95% CI 1.14, 4.97; n = 4 studies) and right ventricular outflow track obstruction defects (pooled OR 2.29, 95% CI 1.06, 4.93; n = 4 studies). One study reported the risk of pulmonary valve defect (unadjusted OR 1.84, 95% CI 0.75, 4.54) 48, left‐sided defects (adjusted OR 2.1, 95% CI 0.5, 8.7) 30 and the risk of other anomalies of the peripheral vascular system (unadjusted OR 2.91, 95% CI 1.82, 4.65) 48. There was higher heterogeneity between studies with the reporting of specific individual cardiac outcomes.
Table 3.
Meta‐analysis of specific cardiac malformations
| Number of studies | Effect size Pooled OR (95% CI) | I 2 (P value) | |
|---|---|---|---|
| Specific cardiac defects | |||
| Bulbus cordis anomalies and anomalies of cardiac septal closure | 8 | 1.42 (1.07, 1.89) | 0.0% (0.756) |
| Ventricular septal defect | 5 | 1.26 (0.69, 2.32) | 59.1% (0.044) |
| Atrial septal defect | 4 | 2.38 (1.14, 4.97) | 65.8% (0.032) |
| Ventricular and atrial septal defects combined | 1 | 1.37 (0.96, 1.95) | ‐ |
| Other cardiac defects/other congenital anomalies of heart | 4 | 1.28 (0.96, 1.69) | 0.0% (0.254) |
| Left ventricular outflow tract obstruction | 2 | 1.00 (0.38, 2.61) | 0.0% (0.387) |
| Right ventricular outflow tract obstruction | 4 | 2.29 (1.06, 4.93) | 81.0% (0.001) |
| Conotruncal heart defects including tetralogy of Fallot, interrupted aortic arch, ventricular septal defect and persistent truncus arteriosus | 3 | 1.77 (0.96, 3.26) | 0.0% (0.875) |
OR, odds ratio; 95% CI, 95% confidence interval.
Impact of study design, methodology and adjustment for indication bias
Study designs, inclusion and exclusion criteria, exposure time window definitions, duration/time period of malformation ascertainment, adjustment for confounders including the indication and whether abstracts without full length paper data were considered had minimal impact on the pooled estimates (Table 2 ). Paroxetine use during the first trimester of pregnancy was always increasing the risk of malformations and cardiac malformations specifically.
Publication bias
A publication bias was present in the meta‐analysis on the risk of major congenital malformations (n = 16 studies) (Supplementary file S3) and cardiac malformations (n = 19 studies). We performed an adjustment for publication bias using the trim and filled method 47 which imputed four theoretical missing estimates for major congenital malformations and three for cardiac malformations. The revised pooled, estimates taking into account publication bias, were pooled ORadjusted for publication bias 1.16 (95% CI 1.01, 1.33) for major malformations and pooled ORadjusted for publication bias 1.20 (95% CI 1.05, 2.69) for cardiac malformations, compared with the pooled ORs of 1.23 (major malformations) and 1.28 (cardiac malformations) before adjustment. Publication bias of the eight studies on bulbus cordis anomalies and anomalies of cardiac septal closure was not present (Egger >0.05). The fit and trim method did not impute any hypothetical ‘missing’ studies for analyses on this specific defect.
Discussion
Main findings
Our systematic review and meta‐analysis showed a 23% increased risk of any major congenital malformations and a 28% increased risk of major cardiac malformations associated with paroxetine exposure during the first trimester of pregnancy. This risk of major congenital malformations increased to 42% when only case–control studies were considered, to 69% in studies that followed up infants for ascertaining outcomes less than 1 year after birth and to 49% when clinic or surveillance data were linked to registry. The risk of major cardiac malformations increased to 51% when only case–control studies were considered and to 62% when teratogen information services were used as a data source. The use of paroxetine during the first trimester of pregnancy was associated with a two‐fold increased risk of atrial septal defects and right ventricular outflow tract obstruction compared with non‐use of SSRIs during pregnancy. Although there was a clear overall increase in the risk of major and cardiac malformations overall, these findings highlight the influence of different aspects of the study design, the data source used, and the exposure and outcome time window of ascertainment when studying the use of antidepressant drugs during pregnancy and the risk of major congenital malformations.
The risk of any major malformations or cardiac malformations differs according to the comparison group used. In our meta‐analysis, the highest risk estimates were obtained when the comparator included women exposed to a non‐paroxetine antidepressant, hence women who might be treated with other SSRIs or other antidepressants. These studies are therefore adjusting for the indication per design. The most used comparison group included women unexposed to any antidepressant. Although choice of comparator group varied the risk estimate, it remains that there was a general trend towards increase in risk.
Comparison with existing reviews
The increases in the risk of major and cardiac malformations in this meta‐analysis coincide with earlier meta‐analyses. Indeed, initial meta‐analyses conducted in 2007 and 2010 38, 63, reported pooled risk estimates for cardiac malformations of 1.72 (95% CI, 1.22, 2.42) 63 and 1.46 (95% CI 1.17, 1.82) 38. The most recent meta‐analysis conducted by Myles et al. 40 estimated the risk of cardiac malformations in association with paroxetine use during pregnancy to be 1.44 (95% CI 1.12, 1.86). Our meta‐analysis included subsequently published studies with significantly larger population sizes, with data originating from different countries such as the UK and Australia 16, 42, 48, 50. The 28% increased risk of major cardiac malformations in our meta‐analysis is concordant with the 44% increased risk of major cardiac malformations following paroxetine use obtained from a previous meta‐analysis 40. The adjustment for publication bias did not change our conclusion on the risk of any major congenital malformations and major cardiac malformations.
Effect of methodological parameters on risk estimates
Restricting findings according to different data sources showed higher risk estimates in studies using teratogen information services, and lower estimates in studies using administrative and claims data sources, which is important in interpreting findings from studies. Potential bias from information services may exist, as mothers who feel the need to call and enquire may be more likely to be at higher risk than those who have no concerns. In addition there is a risk of recall bias. Many of the claims databases estimated the start of the first trimester and subsequent exposure by using algorithms based on the delivery date to backdate to time of conception, potentially leading to exposure misclassification. Indeed, this is not as accurate as using ultrasound and the date of the last menstrual period, which is used in sources linked to registries and hospital records 28. In addition, the risk was lower in the pooled estimate of studies that reported time to ascertainment of defect to be less than 1 year. This factor is particularly important in defects such as atrial septal defects, which can remain undetected until later on in life.
Previous literature emphasizes the need to correct for confounding by indication (i.e. to separate the effects of depression from the potential effect of paroxetine). In order to address the potential confounding by indication (effect of depression on the risk estimates), we restricted our analyses to include studies that used women diagnosed with depression and/or anxiety in their comparison group. This was further limited to include studies with depressed women unexposed to any antidepressant in the comparison group. Although this had an impact on the estimates, it did not change the overall finding of an increased risk. This can be explained by the fact that the majority of studies already adjust for maternal depression in their multivariate analyses.
Clinical implications
In practice, the main question that physicians are faced with when treating depressed women during pregnancy is whether the risk to the fetus after continuing antidepressant therapy, such as paroxetine, is lower or greater than the risk associated with the depression itself or to the risk from other similar treatments (other SSRIs or antidepressants). Due to changes in metabolism during pregnancy, SSRIs are often cleared from the body at a faster rate and hence the concentration of paroxetine in the blood may be reduced to below the therapeutic optimum 64, 65. In addition, given that pregnant women usually decrease or maintain pre‐pregnancy dosage during gestation, it is hypothesized that antidepressants, and paroxetine specifically, put mothers and unborn children at greater risks, which is highlighted in this meta‐analysis.
Strengthens and limitations
We carried out several sub‐analyses in order to consider different aspects of study designs and data sources that may influence study results. Although variations in estimates were seen depending on the populations, study designs and comparator groups, it remained that there was a constant trend in showing an increasing risk of major malformation and cardiac malformations. We also performed a sub‐analysis, which used only adjusted estimates, as malformations have several risk factors. However, some prevalence of specific defects are small and thus are more difficult to study, partly explaining why they are less likely to be reported in individual studies.
Conclusions
Paroxetine is associated with a significantly increased risk of major malformations, and cardiac malformations specifically cardiac septal and atrial septal defects, and right ventricular outflow tract obstruction. Studies consisting of a depressed cohort or comparing paroxetine exposure to clinically depressed unexposed pregnant women showed similar risk estimates. Few studies controlled for indication by using depressed non‐treated women in the comparison group but the majority took into account maternal depression in the multivariate analyses. Our meta‐analysis is novel because it includes up to date findings, and mostly because it has studied the effect of methodological choices on the reported outcome and adjusted pooled estimates for publication bias. Given the increased metabolism during gestation and thus the decrease of benefit at comparable dosage, it is believed that paroxetine bears more risks than benefits when used during organogenesis. The baseline risk of major malformations is 3% and of cardiac malformations is 1%. However, given that the benefit of using these medications during pregnancy is debatable, any increase in risk is significant. Hence, regardless of the size of the risk, it is essential to disseminate these findings given that they should be used to change practice and impact appropriate antidepressant use during pregnancy.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare AB is a consultant for plaintiffs in the litigation involving antidepressants and birth defects. All other authors report no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
This study was funded by the Fonds de la Recherche du Québec ‐ Santé (FRQ‐S) and the Réseau Québécois de recherche sur les médicaments (RQRM). AB is the holder of a research chair on Medications and Pregnancy from the FRQ‐S. Jin‐Ping Zhao is the recipient of a Quebec‐China post‐doctoral fellowship from the Canadian Institutes of Health Research. The funding body had no involvement in the data collection or analysis, the preparation of the manuscript or the decision to submit the paper for publication.
Author Contributions
Anick Bérard had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: AB, NI, SC, FTM, BT, JPZ
Acquisition of data: AB, NI, SC, FTM, BT, JPZ
Analysis and interpretation of data: AB, NI, SC, FTM, BT, JPZ
Drafting the manuscript: AB, NI, JPZ
Critical revision of the manuscript and important intellectual content: AB, NI, SC, FTM, BT, JPZ
Obtained funding: AB
Study supervision : AB
Supporting information
S1 Example of the electronic search strategy in Embase with the predefined key words
S2 Articles excluded from the analyses
S3 Assessment of publication bias
Supporting info item
Bérard, A. , Iessa, N. , Chaabane, S. , Muanda, F. T. , Boukhris, T. , and Zhao, J.‐P. (2016) The risk of major cardiac malformations associated with paroxetine use during the first trimester of pregnancy: a systematic review and meta‐analysis. Br J Clin Pharmacol, 81: 589–604. doi: 10.1111/bcp.12849.
References
- 1. Marcus SM. Depression during pregnancy: rates, risks and consequences–motherisk update 2008. Can J Clin Pharmacol 2009; 16: e15–22. [PubMed] [Google Scholar]
- 2. Grigoriadis S, Robinson GE. Gender issues in depression. Ann Clin Psychiatry 2007; 19: 247–55. [DOI] [PubMed] [Google Scholar]
- 3. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry 2005; 62: 593–602. [DOI] [PubMed] [Google Scholar]
- 4. Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol 2004; 103: 698–709. [DOI] [PubMed] [Google Scholar]
- 5. Silva R, Jansen K, Souza L, Quevedo L, Barbosa L, Moraes I, Horta B, Pinheiro R. Sociodemographic risk factors of perinatal depression: a cohort study in the public health care system. Rev Bras Psiquiatr 2012; 34: 143–8. [DOI] [PubMed] [Google Scholar]
- 6. Ahluwalia IB, Mack KA, Mokdad A. Mental and physical distress and high‐risk behaviors among reproductive‐age women. Obstet Gynecol 2004; 104: 477–83. [DOI] [PubMed] [Google Scholar]
- 7. Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta‐analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry 2010; 67: 1012–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Keane V, Marsh MS. Depression during pregnancy. BMJ 2007; 334: 1003–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chung TK, Lau TK, Yip AS, Chiu HF, Lee DT. Antepartum depressive symptomatology is associated with adverse obstetric and neonatal outcomes. Psychosom Med 2001; 63: 830–4. [DOI] [PubMed] [Google Scholar]
- 10. Henrichs J, Schenk JJ, Roza SJ, van den Berg MP, Schmidt HG, Steegers EA, Hofman A, Jaddoe VW, Verhulst FC, Tiemeier H. Maternal psychological distress and fetal growth trajectories: the generation R study. Psychol Med 2010; 40: 633–43. [DOI] [PubMed] [Google Scholar]
- 11. Petersen I, Gilbert RE, Evans SJ, Man SL, Nazareth I. Pregnancy as a major determinant for discontinuation of antidepressants: an analysis of data from the health improvement network. J Clin Psychiatry 2011; 72: 979–85. [DOI] [PubMed] [Google Scholar]
- 12. Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol 2007; 196: 544 e1–5. [DOI] [PubMed] [Google Scholar]
- 13. Berard A, Sheehy O. The Quebec pregnancy cohort–prevalence of medication use during gestation and pregnancy outcomes. PLoS One 2014; 9: e93870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ververs T, Kaasenbrood H, Visser G, Schobben F, de Jong‐van den Berg L, Egberts T. Prevalence and patterns of antidepressant drug use during pregnancy. Eur J Clin Pharmacol 2006; 62: 863–70. [DOI] [PubMed] [Google Scholar]
- 15. Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, Rolnick SJ, Roblin D, Smith DH, Willy ME, Staffa JA, Platt R. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol 2008; 198: 194 e1–5. [DOI] [PubMed] [Google Scholar]
- 16. Jimenez‐Solem E, Andersen JT, Petersen M, Broedbaek K, Jensen JK, Afzal S, Gislason GH, Torp‐Pedersen C, Poulsen HE. Exposure to selective serotonin reuptake inhibitors and the risk of congenital malformations: a nationwide cohort study. BMJ Open 2012; 2: doi: 10.1136/bmjopen-2012-001148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bakker MK, Kolling P, van den Berg PB, de Walle HE, de Jong van den Berg LT. Increase in use of selective serotonin re‐uptake inhibitors in pregnancy during the last decade, a population‐based cohort study from The Netherlands . Br J Clin Pharmacol 2008; 65: 600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wichman CL, Fothergill A, Moore KM, Lang TR, Heise RH Jr, Watson WJ. Recent trends in selective serotonin reuptake inhibitor use in pregnancy. J Clin Psychopharmacol 2008; 28: 714–6. [DOI] [PubMed] [Google Scholar]
- 19. Kulin NA, Pastuszak A, Sage SR, Schick‐Boschetto B, Spivey G, Feldkamp M, Ormond K, Matsui D, Stein‐Schechman AK, Cook L, Brochu J, Rieder M, Koren G. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors: a prospective controlled multicenter study. JAMA 1998; 279: 609–10. [DOI] [PubMed] [Google Scholar]
- 20. Simon GE, Cunningham ML, Davis RL. Outcomes of prenatal antidepressant exposure. Am J Psychiatry 2002; 159: 2055–61. [DOI] [PubMed] [Google Scholar]
- 21. Malm H, Klaukka T, Neuvonen PJ. Risks associated with selective serotonin reuptake inhibitors in pregnancy. Obstet Gynecol 2005; 106: 1289–96. [DOI] [PubMed] [Google Scholar]
- 22. Hendrick V, Smith LM, Suri R, Hwang S, Haynes D, Altshuler L. Birth outcomes after prenatal exposure to antidepressant medication. Am J Obstet Gynecol 2003; 188: 812–5. [DOI] [PubMed] [Google Scholar]
- 23. Furu K, Kieler H, Haglund B, Engeland A, Selmer R, Stephansson O, Valdimarsdottir UA, Zoega H, Artama M, Gissler M, Malm H, Norgaard M. Selective serotonin reuptake inhibitors and venlafaxine in early pregnancy and risk of birth defects: population based cohort study and sibling design. BMJ 2015; 350: h1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Williams M, Wooltorton E. Paroxetine (Paxil) and congenital malformations. CMAJ 2005; 173: 1320–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reis M, Kallen B. Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychol Med 2010; 40: 1723–33. [DOI] [PubMed] [Google Scholar]
- 26. Kallen BA, Otterblad OP. Maternal use of selective serotonin re‐uptake inhibitors in early pregnancy and infant congenital malformations. Birth Defects Res A Clin Mol Teratol 2007; 79: 301–8. [DOI] [PubMed] [Google Scholar]
- 27. Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM, National Birth Defects Prevention S. Use of selective serotonin‐reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med 2007; 356: 2684–92. [DOI] [PubMed] [Google Scholar]
- 28. Cole JA, Ephross SA, Cosmatos IS, Walker AM. Paroxetine in the first trimester and the prevalence of congenital malformations. Pharmacoepidemiol Drug Saf 2007; 16: 1075–85. [DOI] [PubMed] [Google Scholar]
- 29. Berard A, Ramos E, Rey E, Blais L, St‐Andre M, Oraichi D. First trimester exposure to paroxetine and risk of cardiac malformations in infants: the importance of dosage. Birth Defects Res B Dev Reprod Toxicol 2007; 80: 18–27. [DOI] [PubMed] [Google Scholar]
- 30. Bakker MK, Kerstjens‐Frederikse WS, Buys CH, de Walle HE, de Jong‐van den Berg LT. First‐trimester use of paroxetine and congenital heart defects: a population‐based case–control study. Birth Defects Res A Clin Mol Teratol 2010; 88: 94–100. [DOI] [PubMed] [Google Scholar]
- 31. Malm H, Artama M, Gissler M, Ritvanen A. Selective serotonin reuptake inhibitors and risk for major congenital anomalies. Obstet Gynecol 2011; 118: 111–20. [DOI] [PubMed] [Google Scholar]
- 32. Hendrick V, Stowe ZN, Altshuler LL, Hwang S, Lee E, Haynes D. Placental passage of antidepressant medications. Am J Psychiatry 2003; 160: 993–6. [DOI] [PubMed] [Google Scholar]
- 33. Oberlander TF, Misri S, Fitzgerald CE, Kostaras X, Rurak D, Riggs W. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry 2004; 65: 230–7. [DOI] [PubMed] [Google Scholar]
- 34. Loughhead AM, Fisher AD, Newport DJ, Ritchie JC, Owens MJ, DeVane CL, Stowe ZN. Antidepressants in amniotic fluid: another route of fetal exposure. Am J Psychiatry 2006; 163: 145–7. [DOI] [PubMed] [Google Scholar]
- 35. Noorlander CW, Ververs FF, Nikkels PG, van Echteld CJ, Visser GH, Smidt MP. Modulation of serotonin transporter function during fetal development causes dilated heart cardiomyopathy and lifelong behavioral abnormalities. PLoS One 2008; 3: e2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morrison JL, Riggs KW, Rurak DW. Fluoxetine during pregnancy: impact on fetal development. Reprod Fertil Dev 2005; 17: 641–50. [DOI] [PubMed] [Google Scholar]
- 37. Gentile S. Selective serotonin reuptake inhibitor exposure during early pregnancy and the risk of birth defects. Acta Psychiatr Scand 2011; 123: 266–75. [DOI] [PubMed] [Google Scholar]
- 38. Wurst KE, Poole C, Ephross SA, Olshan AF. First trimester paroxetine use and the prevalence of congenital, specifically cardiac, defects: a meta‐analysis of epidemiological studies. Birth Defects Res A Clin Mol Teratol 2010; 88: 159–70. [DOI] [PubMed] [Google Scholar]
- 39. Grigoriadis S, VonderPorten EH, Mamisashvili L, Roerecke M, Rehm J, Dennis CL, Koren G, Steiner M, Mousmanis P, Cheung A, Ross LE. Antidepressant exposure during pregnancy and congenital malformations: is there an association? A systematic review and meta‐analysis of the best evidence. J Clin Psychiatry 2013; 74: e293–308. [DOI] [PubMed] [Google Scholar]
- 40. Myles N, Newall H, Ward H, Large M. Systematic meta‐analysis of individual selective serotonin reuptake inhibitor medications and congenital malformations. Aust N Z J Psychiatry 2013; 47: 1002–12. [DOI] [PubMed] [Google Scholar]
- 41. Gentile S, Bellantuono C. Selective serotonin reuptake inhibitor exposure during early pregnancy and the risk of fetal major malformations: focus on paroxetine. J Clin Psychiatry 2009; 70: 414–22. [DOI] [PubMed] [Google Scholar]
- 42. Ban L, Gibson JE, West J, Fiaschi L, Sokal R, Smeeth L, Doyle P, Hubbard RB, Tata LJ. Maternal depression, antidepressant prescriptions, and congenital anomaly risk in offspring: a population‐based cohort study. BJOG 2014; 121: 1471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huybrechts KF, Palmsten K, Mogun H, Kowal M, Avorn J, Setoguchi‐Iwata S, Hernandez‐Diaz S. National trends in antidepressant medication treatment among publicly insured pregnant women. Gen Hosp Psychiatry 2013; 35: 265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Knudsen TM, Hansen AV, Garne E, Andersen AM. Increased risk of severe congenital heart defects in offspring exposed to selective serotonin‐reuptake inhibitors in early pregnancy–an epidemiological study using validated EUROCAT data. BMC Pregnancy Childbirth 2014; 14: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Viera AJ. Odds ratios and risk ratios: what's the difference and why does it matter? South Med J 2008; 101: 730–4. [DOI] [PubMed] [Google Scholar]
- 46. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986; 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 47. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000; 56: 455–63. [DOI] [PubMed] [Google Scholar]
- 48. Colvin L, Slack‐Smith L, Stanley FJ, Bower C. Dispensing patterns and pregnancy outcomes for women dispensed selective serotonin reuptake inhibitors in pregnancy. Birth Defects Res A Clin Mol Teratol 2011; 91: 142–52. [DOI] [PubMed] [Google Scholar]
- 49. Davis RL, Rubanowice D, McPhillips H, Raebel MA, Andrade SE, Smith D, Yood MU, Platt R, Hmo Research Network Center for Education RiT . Risks of congenital malformations and perinatal events among infants exposed to antidepressant medications during pregnancy. Pharmacoepidemiol Drug Saf 2007;16:1086‐94. [DOI] [PubMed] [Google Scholar]
- 50. Huybrechts KF, Hernandez‐Diaz S, Avorn J. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med 2014; 371: 1168–9. [DOI] [PubMed] [Google Scholar]
- 51. Louik C, Lin AE, Werler MM, Hernandez‐Diaz S, Mitchell AA. First‐trimester use of selective serotonin‐reuptake inhibitors and the risk of birth defects. N Engl J Med 2007; 356: 2675–83. [DOI] [PubMed] [Google Scholar]
- 52. Nordeng H, van Gelder MM, Spigset O, Koren G, Einarson A, Eberhard‐Gran M. Pregnancy outcome after exposure to antidepressants and the role of maternal depression: results from the Norwegian mother and child cohort study. J Clin Psychopharmacol 2012; 32: 186–94. [DOI] [PubMed] [Google Scholar]
- 53. Oberlander TF, Warburton W, Misri S, Riggs W, Aghajanian J, Hertzman C. Major congenital malformations following prenatal exposure to serotonin reuptake inhibitors and benzodiazepines using population‐based health data. Birth Defects Res B Dev Reprod Toxicol 2008; 83: 68–76. [DOI] [PubMed] [Google Scholar]
- 54. Pedersen LH, Henriksen TB, Vestergaard M, Olsen J, Bech BH. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ 2009; 339: b3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Diav‐Citrin O, Shechtman S, Weinbaum D, Wajnberg R, Avgil M, di Gianantonio E, Clementi M, Weber‐Schoendorfer C, Schaefer C, Ornoy A. Paroxetine and fluoxetine in pregnancy: a prospective, multicentre, controlled, observational study. Br J Clin Pharmacol 2008; 66: 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Einarson A, Pistelli A, DeSantis M, Malm H, Paulus WD, Panchaud A, Kennedy D, Einarson TR, Koren G. Evaluation of the risk of congenital cardiovascular defects associated with use of paroxetine during pregnancy. Am J Psychiatry 2008; 165: 749–52. [DOI] [PubMed] [Google Scholar]
- 57. Chambers C. Birth outcomes among pregnant women taking paroxetine (PAXIL). 2007.
- 58. Nash CM, O'Connell CM, Howlett AA. Neonatal outcomes associated with maternal antidepressant use in a population cohort of Nova Scotian pregnancies between 1993 and 2004. Pediatr Child Health 2007; 12: 42. [Google Scholar]
- 59. Vial T, Cournot MP, Bernard N, Carlier P, Jonville‐Bera P, Jean‐Pastor MJ, Bajhoux C, Robert E, Elefant E, Descotes J. Paroxetine and congenital malformations: a prospective comparative study. Drug Saf 2006; 29: 911–1010. [Google Scholar]
- 60. Schloemp S, Einarson TR, Sterzik K, Stox F. Congenital malformations after antidepressant medication asociated with paroxetine in early pregnancy? Hum REprod 2006; 21 (Sup 1). [Google Scholar]
- 61. Vasilakis‐Scaramozza C, Aschengrau A, Cabral H, Jick SS. Antidepressant use during early pregnancy and the risk of congenital anomalies. Pharmacotherapy 2013; 33: 693–700. [DOI] [PubMed] [Google Scholar]
- 62. Margulis AV, Abou‐Ali A, Strazzeri MM, Ding Y, Kuyateh F, Frimpong EY, Levenson MS, Hammad TA. Use of selective serotonin reuptake inhibitors in pregnancy and cardiac malformations: a propensity‐score matched cohort in CPRD. Pharmacoepidemiol Drug Saf 2013; 22: 942–51. [DOI] [PubMed] [Google Scholar]
- 63. Bar‐Oz B, Einarson T, Einarson A, Boskovic R, O'Brien L, Malm H, Berard A, Koren G. Paroxetine and congenital malformations: meta‐analysis and consideration of potential confounding factors. Clin Ther 2007; 29: 918–26. [DOI] [PubMed] [Google Scholar]
- 64. Heikkinen T, Ekblad U, Kero P, Ekblad S, Laine K. Citalopram in pregnancy and lactation. Clin Pharmacol Ther 2002; 72: 184–91. [DOI] [PubMed] [Google Scholar]
- 65. Tracy TS, Venkataramanan R, Glover DD, Caritis SN, National Institute for Child H , Human Development Network of Maternal‐Fetal‐Medicine U . Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A activity) during pregnancy. Am J Obstet Gynecol 2005;192:633‐9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1 Example of the electronic search strategy in Embase with the predefined key words
S2 Articles excluded from the analyses
S3 Assessment of publication bias
Supporting info item


