Abstract
Aim
The aim of the study was to examine mortality risk associated with use of antidepressants and antipsychotics classified with torsades de pointes (TdP) risk in elderly.
Methods
A matched case–control register study was conducted in people 65 years and older dying outside hospital from 2008–2013 (n = 286 092) and matched controls (n = 1 430 460). The association between prescription of antidepressants and antipsychotics with various TdP risk according to CredibleMeds (www.crediblemeds.org) and all‐cause mortality was studied by multivariate conditional logistic regression adjusted for comorbidity and several other confounders.
Results
Use of antidepressants classified with known or possible TdP risk, was associated with higher adjusted risk for mortality (OR 1.53, 95% CI 1.51, 1.56 and OR 1.63, 95% CI 1.61, 1.67, respectively) compared with antidepressants classified with conditional TdP risk (OR 1.25, 95% CI 1.22, 1.28) or without TdP classification (OR 0.99, 95% CI 0.94, 1.05). Antipsychotics classified with known TdP risk were associated with higher risk (OR 4.57, 95% CI 4.37, 4.78) than antipsychotics with possible risk (OR 2.58, 95% CI 2.52, 2.64) or without TdP classification (OR 2.14, 95% CI 2.03, 2.65). The following risk ranking was observed for commonly used antidepressants: mirtazapine > citalopram > sertraline > amitriptyline and for antipsychotics: haloperidol > risperidone >olanzapine > quetiapine.
Conclusion
The CredibleMeds system predicted drug‐associated risk for mortality in the elderly at the risk class level. Among antipsychotics, haloperidol, and among antidepressants, mirtazapine and citalopram, were associated with the highest risks. The results suggest that the TdP risk with antidepressants and antipsychotics should be taken into consideration when prescribing to the elderly.
Keywords: all‐cause mortality, antidepressants, antipsychotics, elderly, torsades de pointes
What is already known about this subject
Antidepressants and antipsychotic drugs are classified in different risk classes as regards development of life threatening torsades de pointes cardiac arrhythmia (TdP).
Several drugs classified with known or possible TdP risk are extensively used in the elderly, a patient population having increased risk to develop TdP.
Little is known whether use of drugs in various TdP risk classes in the elderly differs in their mortality risk.
What this study adds
Our large nationwide case control study showed an association with increased mortality in users of antidepressants and antipsychotics classified with known and possible TdP risk, compared with users of compounds in the same therapeutic class with no classification or conditional TdP risk.
Mirtazapine and citalopram among antidepressants and haloperidol among antipsychotics, were associated with the highest risks.
The results suggest that TdP risk should be taken into consideration when prescribing to older persons.
Introduction
Antidepressants, antipsychotics, sedatives and hypnotics are extensively used among older people for a wide variety of mental conditions 1. In a previous nationwide study in Sweden, adjusted for several potential confounders, we showed an increased risk for mortality in older people prescribed antipsychotics and antidepressants, in contrast to prescription of hypnotics or sedatives 2. A number of studies have shown an increased mortality risk in people using antipsychotics, especially in older patients with dementia 3, 4, 5. Only one study has evaluated all‐cause mortality in the elderly prescribed antidepressants 6. The results showed that antidepressants were associated with increased risk of death, with a variation in risk according to the individual drug used.
One possible contributing factor to the increased risk of death is the potential of several antipsychotics and antidepressants, in contrast to sedatives and hypnotics, to interfere with cardiac repolarization in a dose‐dependent manner. This is manifested as QT prolongation on ECG and may result in risk for drug‐induced life threatening cardiac ventricular arrhythmia of the type torsades de pointes (TdP) 7. Most published cases of TdP associated with antidepressants and antipsychotics involved additional risk factors for TdP. These risk factors are common in older persons and include heart disease, electrolyte changes and simultaneous use of other drugs prolonging the QT interval or interfering with drug metabolism 7, 8. In addition, the risk for exposure to supra‐therapeutic concentrations is increased due to known effects of ageing on drug metabolism and excretion 9. Studies also suggest an increased risk for seriously prolonged QT interval and increased risk to develop TdP in the elderly 8, 10.
CredibleMeds developed by the Arizona Center for Education on Research on Therapeutics, is a valuable tool to get information on drugs with TdP risk. The Food and Drug Administration (FDA) collaborates with CredibleMeds with the goal to establish an electronic clinical decision support system to assist physicians in avoiding unnecessary prescription of antibiotics with TdP risk. The CredibleMeds process for assigning drugs with TdP risk is strict and based on statistical analyses of case reports of TdP and associated adverse events, such as long QT syndrome, sudden cardiac death and cardiac arrest reported to the FDA's Adverse Event Reporting System (AERS) 11. Relevant information in the scientific literature is analyzed, including case reports of TdP, and whether a suspected drug has a relevant pharmacologic action, e.g. inhibition of IKr or hERG. According to CredibleMeds, all TdP classified drugs are associated with QTc prolongation and TdP in clinical use, but the risks differ when used as directed in the regulatory Summary of Product Characteristics (SPC) labelling (=‘normal use’): 1) known TdP risk, 2) possible TdP risk in normal use and 3) no TdP risk in normal use, but TdP risk exists in certain conditions, e.g. overdose or being the index or interacting agent in a drug–drug interaction 7.
However, the CredibleMeds system only informs about whether there is a TdP risk with the use of a drug. No information is given on the magnitude of TdP risk for individual compounds or on differences between TdP risk categories in a therapeutic class. In this context, it has to be stressed that it is very difficult to estimate the true incidence of TdP related death, since ECG monitoring is necessary in order to set the diagnosis TdP. Ventricular arrhythmia or sudden cardiac death may not be properly identified in health care registers 12, 13. This is problematic when death occurs outside of hospitals as is the case for 65% of the deaths in Sweden, for example if an older woman who recently started treatment with an antipsychotic drug dies at home in connection with gastroenteritis causing electrolyte changes. All‐cause death has therefore been used as an alternative outcome in some recent studies investing risk of mortality in antipsychotic users 13, 14.
Sweden has excellent possibilities for large scale epidemiological studies through the long standing tradition of national registers with almost complete national coverage. The aim of this study was to investigate the prescription of antidepressants and antipsychotics labelled in different TdP classes in relation to death outside hospitals from 2008–2013 in Sweden in a large unselected population of older adults by applying a matched case–control design.
Methods
Study population and registers used
We performed a nationwide matched case–control study based on several Swedish registers through record‐linkage based on the personal identification number in persons 65 years or older. Information about deaths outside hospitals was retrieved from the Swedish Cause of Death register and information about diagnoses was collected from the Swedish Patient Register, which covers all inpatient and specialized outpatient care in the whole of Sweden 15. Diagnoses are registered according to the International Classification of Diseases 10 (ICD 10). From the Register of the Total Population, we selected five controls matched for age and sex by using an incidence density sampling scheme 16, on the case event day (= index date for controls). Hence, for each case, we selected controls from the age and gender specific at‐risk populations. Exposure data were retrieved from the Swedish Prescribed Drug Register, which has detailed individual‐based information about all prescribed dispensed drugs in Sweden 17. Finally, the highest attained level of formal educational level of individuals on December 31st 2013 was collected from the Swedish Education Register, containing information for individuals aged 16 years and older. The highest level of education was categorized into primary school, secondary school and university. The study was performed within the responsibilities of the National Board of Health and Welfare and therefore no ethical approval from outside ethics committees was needed.
The study comprised all persons aged 65 years or over in Sweden who died outside hospitals between January 1 2008 and December 31 2013 (n = 286 092) and their age and gender matched alive controls (n = 1 430 460). The controls were matched using an incidence density sampling scheme. That is, the controls were selected from the age and gender specific at‐risk group. In total 904 246 unique individuals participated. Some individuals were selected as controls for several cases, as customary in the incidence density sampling. The reason for this is that an individual is in the risk set as long as he/she does not become a case, and can thus be selected at every case event date up to that point. Suicides as well as deaths due to an event of undetermined intent (ICD 10 codes X60‐X84 and Y10‐Y14) were excluded.
Exposure to antidepressant, antipsychotics and other drugs
Prescription data for both cases and their matched controls were collected for the time period of 90 days before the outcome mortality. For each drug a person was prescribed, the duration of the treatment was calculated by dividing the amount of dispensed drug with by the prescribed mean daily dose 18. In order to as much as possible assure relevant exposure, only prescriptions lasting until or beyond the case event day (death) or index date (controls) were included in the analysis.
Individual antipsychotics and antidepressants were divided into different TdP risk classes according to CredibleMeds (www.crediblemeds.org, 15 January 2015, Table S1 in the on line version on the web). CredibleMeds label drugs in three TdP risk categories: 1) known TdP risk, 2) possible TdP risk and 3) conditional TdP risk. In addition we introduced a fourth group, 4) antidepressant and antipsychotic drugs lacking TdP labelling (see Table 2 for details).
Table 2.
Prescription of antidepressants in various TdP cardiac arrhythmia risk classes. Total 16 951 832 dispenses by 539 111 individuals aged 65 years and older 2008–2013
| Known TdP risk | Possible TdP risk | Conditional TdP risk | No TdP labeling | ||||
|---|---|---|---|---|---|---|---|
| Citalopram | 7 446 863 | Mirtazapine | 3 759 411 | Desipramine | <5 | Opipramol | <5 |
| Escitalopram | 754 972 | Venlafaxine | 690 360 | Imipramine | 468 | Lofepramine | 202 |
| Clomipramine | 232 910 | Maprotiline | Z20 276 | ||||
| Trimipramine | 3322 | Fluvoxamine | 4090 | ||||
| Amitriptyline | 930 485 | Fenelzine | 533 | ||||
| Nortriptyline | 37 920 | Tranylcypro‐mine | 588 | ||||
| Protriptyline | <5 | Moclobemide | 13 162 | ||||
| Fluoxetine | 192 916 | Tryptophan | 833 | ||||
| Paroxetine | 340 287 | Mianserin | 379 695 | ||||
| Sertraline | 1 788 262 | Nefazodone | 452 | ||||
| Bupropion | 32 785 | ||||||
| Tianeptine | <5 | ||||||
| Reboxetine | 25 839 | ||||||
| Duloxetine | 286 536 | ||||||
| Agomelatine | 8608 | ||||||
| % of all prescriptions: | |||||||
| 48% | 26% | 21% | 5% | ||||
Statistical analysis and adjustments for confounding
Both univariate and multivariate conditional logistic regression analysis were used for investigating the association between use of antidepressant and antipsychotic drugs and mortality. In the univariate model, the cases and controls were matched for age, gender and case event day, but were otherwise unadjusted. The multivariate model was additionally adjusted for, education, number of contacts with primary care, inpatient days, number of other drugs, use of other TdP labelled drugs, number of drugs within the same therapeutic class and comorbidities including cancer, cerebrovascular disease, congestive heart failure, chronic pulmonary disease, dementia, diabetes, metastatic carcinoma, myocardial infarction, mild and moderate or severe liver disease, paraplegia or hemiplegia, peptic ulcer disease, peripheral vascular disease, renal disease and rheumatic disease the year before the death (Table S2 in the online version on the web). The results are expressed as odds ratios (ORs) with 95% confidence intervals (CIs). The ORs from this design, in which controls are selected from the at‐risk group, can be interpreted as incidence rate ratios 19. All analyses were performed in SAS, version 9.2.
Results
Socio‐demographic characteristics in the study population
The mean age was 83.9 years. The cases had a higher number of inpatient days and number of disease diagnoses, such as cancer, metastatic cancer, cerebrovascular disease, congestive heart failure, myocardial infarction, dementia, renal and liver disease (Table 1). Prescription of antidepressants, antipsychotics and other TdP classified drugs were more common in cases than controls. Also the prescribed mean number of drugs was higher in cases (Table 1). All above mentioned variables were controlled for in the analyses.
Table 1.
Socio‐demographic characteristics, comorbidities in cases and controls among persons aged 65 years and older in Sweden 2008–2013 1 year before the date of death and drug use at case date in cases (n = 286 092) and their corresponding controls (n = 1 430 460)
| Socio‐demographic characteristics | Cases | Controls | Total |
|---|---|---|---|
| Years of age, mean | 83.9 | 83.9 | 83.9 |
| Gender: female % | 57 | 57 | 57 |
| Primary and lower secondary school | 58 | 54 | 55 |
| Upper secondary school | 27 | 29 | 29 |
| Universities and university colleges | 9.8 | 13 | 12 |
| No information | 4.6 | 3,3 | 3,5 |
| Mean number of outpatient visits in specialized care | 3,2 | 1,8 | 2,0 |
| Mean number inpatient days | 7,7 | 2,0 | 2,9 |
| Comorbidities | |||
| Cancer % | 22 | 8,1 | 10 |
| Cerebrovascular disease % | 12 | 4,1 | 5,4 |
| Congestive heart failure % | 17 | 5,6 | 7,4 |
| Chronic pulmonary disease % | 8,2 | 2,9 | 3,7 |
| Dementia, % | 18 | 5,5 | 7,5 |
| Diabetes % | 17 | 11,0 | 12 |
| Metastatic carcinoma % | 9,9 | 0,6 | 2,1 |
| Myocardial infarction % | 9,9 | 4,0 | 5,0 |
| Mild liver disease % | 0,7 | 0,1 | 0,2 |
| Moderate or severe liver disease % | 0,3 | 0,0 | 0,1 |
| Paraplegia or hemiplegia % | 1,6 | 0,3 | 0,5 |
| Peptic ulcer disease % | 1,3 | 0,5 | 0,6 |
| Peripheral vascular disease % | 4,3 | 1,6 | 2,0 |
| Renal disease % | 5,3 | 1,6 | 2,2 |
| Rheumatic disease % | 2,7 | 1,6 | 1,8 |
| Drug use at case date | |||
| Mean number of drugs used, n | 5,6 | 3,8 | 4,1 |
| Antidepressant drugs % | 25,0 | 13,0 | 15 |
| No TdP labelling | 0,7 | 0,5 | 0,5 |
| Conditional TdP risk | 4,4 | 2,7 | 3,0 |
| Possible TdP risk | 8,5 | 3,5 | 4,3 |
| Known TdP risk | 14,0 | 7,2 | 8,3 |
| Antipsychotic drug use % | 8,2 | 2,0 | 3,1 |
| No TdP labelling | 1,1 | 0,4 | 0,5 |
| Conditional TdP risk | 5,0 | 1,5 | 2,0 |
| Known TdP risk | 2,5 | 0,3 | 0,7 |
| Any other drug % | 91 | 84 | 85 |
| No TdP labelling | 91 | 83 | 84 |
| Conditional TdP risk | 38 | 21 | 24 |
| Possible TdP risk | 2,9 | 3,7 | 3,6 |
| Known TdP risk | 2,9 | 2,1 | 2,2 |
Extent of prescription with antidepressant and antipsychotic drugs
Around 15% of individuals aged 65 years or older were prescribed antidepressants and 3% antipsychotic drugs each year during 2008–2013. During the study period 539 111 unique individuals had at least one prescription of an antidepressant drug. A total of 16 951 832 prescriptions for antidepressants were issued. The majority of the prescriptions of antidepressants were selective serotonin re‐uptake inhibitors (SSRIs, citalopram escitalopram, sertraline, fluoxetine and paroxetine) and serotonin norepinephrine re‐uptake inhibitors (SNRI, mirtazapine and venlafaxine), while other types of antidepressants, including tricyclic antidepressants (TCAs), were prescribed to a lesser extent (Table 2 and Figure 1). According to TdP risk class, 48% of the prescriptions consisted of antidepressant drugs classified with known risk, 26% with possible TdP risk and 21% with conditional risk, while 5% had no TdP labelling (Table 2). Citalopram classified with known TdP risk was the most commonly prescribed antidepressant (44% of the prescriptions), followed by mirtazapine with possible TdP risk (22%), sertraline (11%) and amitriptyline (5.5%), both with conditional TdP risk. The most prescribed antidepressant without TdP labelling during the study period was mianserin with 2.2% (Figure 1).
Figure 1.
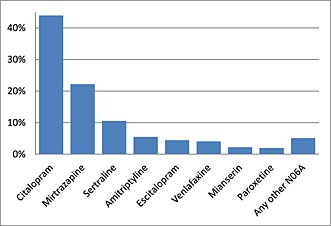
Percentages for the most commonly prescribed antidepressants. In total 16 951 832 anti‐depressant prescriptions were dispensed to individuals aged 65 years or over (n = 539 111) during the study period
For antipsychotics, 133 874 unique individuals aged 65 years or older had at least one prescription of an antipsychotic drug and a total of 4 879 409 prescriptions for antipsychotics were issued. Atypical antipsychotics like risperidine, olanzapine and quetiapine dominated the prescriptions (Table 3 and Figure 2) Eleven percent of the prescriptions consisted of antipsychotic drugs classified with known risk (TdP 1), 70% were classified with possible TdP risk, and 19% were prescriptions of antipsychotics without TdP labelling (Table 3). Risperidone (39%) and olanzapine (12%) classified with possible TdP risk was the most prescribed antipsychotic drugs followed by haloperidol with known TdP risk (11%) The most prescribed antipsychotic drug lacking TdP labelling was levomepromazine with 7.4% of the prescriptions (Figure 2).
Table 3.
Prescription of antipsychotic drugs in various TdP cardiac arrhythmia risk classes. Total 4 879 409 dispensed for 133 874 individuals aged 65 years and older during 2008–2013
| Known TdP risk | Possible TdP risk | No TdP labeling | |||
|---|---|---|---|---|---|
| Chlorpromazine* | 1062 | Sertindole | 79 | Levomepromazine | 362 187 |
| Thioridazine* | 3248 | Ziprasidone | 16 573 | Dixyrazine | 885 |
| Haloperidol | 528 019 | Clozapine | 133 354 | Flufenazine | 3337 |
| Droperidol | 70 | Olanzapine | 599 109 | Perphenazine | 114 292 |
| Pimozide* | 1165 | Quetiapine | 321 907 | Prochlorperazine | 12 904 |
| Sulpiride* | 574 | Lithium | 337 956 | Periciazine | 16 |
| Risperidone | 1 924 156 | Melperone | 129 317 | ||
| Aripiprazole | 77 688 | Flupentixol | 110 195 | ||
| Paliperidone | 3550 | Chlorprothixene | 27 470 | ||
| Zuclopenthixol | 170 296 | ||||
| % of all prescriptions: | |||||
| 11% | 70% | 19% |
Not registered in Sweden. Licence use.
Figure 2.
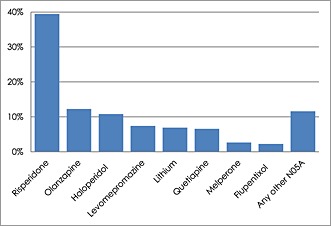
Percentages for commonly prescribed antipsychotic drugs. In total 4 879 409 prescriptions were dispensed to individuals aged 65 years or over (n = 133 874) during the study period
Analyses by TdP class
Table 4 shows the results of the analyses of the prescription of antidepressants and antipsychotic drugs in any class and in different TdP risk classes in relation to all‐cause mortality. We found higher adjusted odds risk for users of antidepressants labelled with known TdP risk (OR 1.53, 95% CI 1.51, 1.56) and possible TdP risk (OR 1.63, 95% CI 1.61, 1.67) compared with antidepressants with conditional risk (OR 1.25, 95% CI 1.22, 1.28). Users of antidepressants without labelling (OR 0.99, 95% CI 0.94, 1.05) had no increase in risk. The risk was the same as in non‐users of antidepressants. Adjusted ORs for antipsychotics with known TdP risk (OR 4.57, 95% CI 4.37, 4.78) were higher than antipsychotics with possible risk (OR 2.58, 95% CI 2.52, 2.64) and antipsychotics lacking TdP classification (OR 2.14, 95% CI 2.03, 2.65).
Table 4.
Mortality odds ratios: antidepressant drugs by TdP class
| Unadjusted risk * | Adjusted risk ** | Numbers (% cases) | |
|---|---|---|---|
| Use of antidepressants, any class | 2.31 (2.29, 2.33) | 1.62 (1.60, 1.63) | 254 072 (28%) |
| No TdP listing | 1.23 (1.17, 1.30) | 0.99 (0.94, 1.05) | 9432 (23%) |
| Conditional TdP risk | 1.64 (1.61, 1.68) | 1.25 (1.22, 1.28) | 51 704 (24%) |
| Possible TdP risk | 2.29 (2.25, 2.32) | 1.63 (1.61, 1.67) | 73 452 (32%) |
| Known TdP risk | 2.06 (2.04, 2.09) | 1.53 (1.51, 1.56) | 142 798 (28%) |
Table 5.
Mortality odds ratios: antipsychotic drugs by TdP class
| Unadjusted risk * | Adjusted risk ** | Numbers (% cases) | |
|---|---|---|---|
| Use of antipsychotics, any class | 4.30 (4.23, 4.38) | 2.98 (2.92, 3.04) | 52 834 (45%) |
| No TdP listing | 2.52 (2.40, 2.64) | 2.14 (2.03, 2.65) | 8166 (38%) |
| Possible TdP risk | 3.46 (3.39, 3.54) | 2.58 (2.52, 2.64) | 35 142 (41%) |
| Known TdP risk | 8.35 (8.04, 8.68) | 4.57 (4.37, 4.78) | 11 377 (63%) |
Conditional logistical model on age and gender.
Reference: No use of Table 4A: antidepressants Table 5B: antipsychotics.
Conditional logistical model on age and gender. Controlled for educational level, number of other drugs at case date, indicator of use of another TdP classed drug at case date, out‐patient visits in specialized care, in‐patient days and comorbidites 365 days prior to case date
Analyses of individual drugs
Figure 3 shows the adjusted ORs for mortality for the eight most prescribed individual antidepressants (with 95% CI). For the sake of brevity, unadjusted ORs are not presented for individual antidepressants and antipsychotic drugs. The adjusted ORs were 1.56 for citalopram and 1.15 for escitalopram classified with known TdP risk, and 1.67 for mirtazapine and 1.15 for venlafaxine with possible TdP risk. Among antidepressants classified with conditional TdP risk, the ORs were 1.10 for amitriptyline, 1.08 for paroxetine and 1.37 for sertraline. For the only drug without TdP labelling among the most antidepressants, mianserin, the adjusted OR was 1.12. For the 20 other antidepressants classified with conditional or lacking TdP labelling (Table 1), the OR was 0.97.
Figure 3.
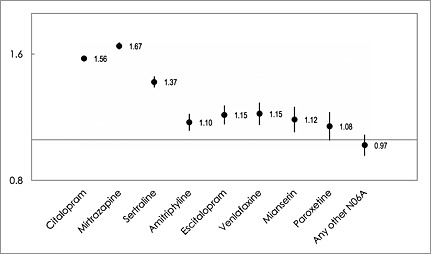
Adjusted odds ratios (ORs) with 95% confidence intervals for mortality in people aged 65 years and older in Sweden 2008–2013 for commonly prescribed antidepressants (n = 539 111)
Figure 4 shows the adjusted ORs for mortality for commonly prescribed antipsychotics. Haloperidol, the only drug labelled with known risk for TdP among the most used antipsychotics, was associated with the highest adjusted OR of 4.63. None of the other commonly prescribed antipsychotic drugs was associated with an adjusted OR > 3,0. The adjusted mortality ORs for users of antipsychotics classified with possible risk were as follows: 2.93 for risperidone, 2.02 for olanzapine, 1.61 for quetiapine and 0.75 for lithium. Adjusted ORs for antipsychotics lacking TdP labelling were: levomepromazine 1.77, melperone 2.46 and flupentixol 1.40. For the other antipsychotics in various TdP labelling classes which in total represented less than 19% of the prescriptions, (Figure 1), the OR was 2.60.
Figure 4.
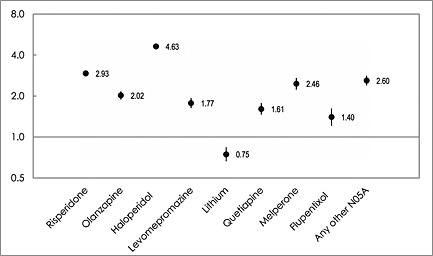
Adjusted odds ratios (ORs) with 95% confidence intervals for mortality in people aged 65 years and older in Sweden 2008–2013 for commonly prescribed antipsychotic drugs in different TdP risk classes(n = 133 874)
Discussion
Use of antidepressants or antipsychotic drugs classified with known or possible TdP cardiac arrhythmia risk was associated with an increased risk for death in older persons, compared with use of compounds in the same therapeutic group without classification or conditional risk. All observed risks in the study remained after adjustment for potential confounders including several comorbidities, such as dementia, cancer, cardiovascular disease and concomitant use of other TdP classified drugs.
Antidepressants
This is the first study showing an association between the TdP rating of different antidepressants and increased mortality risk in older adults. The adjusted risk ratios for death in users of antidepressants without TdP classification were similar to those non‐users, while the risk was increased for users of antidepressants classified with known TdP risk (OR 1.53), possible TdP risk (OR 1.63) and conditional TdP risk (OR 1.25). Mirtazapine and citalopram classified with possible and known TdP risk, respectively, were the most commonly used antidepressants in Sweden and were also the compounds associated with the highest adjusted risk ratio (OR 1.7 and OR 1.6, respectively).
Our results show good agreement with a large register study investigating the association between the most commonly used antidepressants in the UK and all‐cause mortality in patients 65 years or over with depression 6. To our knowledge, the UK study is the only published investigation focused on death in the elderly using antidepressants. As in our study, the SNRI mirtazapine and the SSRI citalopram were associated with higher adjusted hazard ratios for death (1.8 and 1.6, respectively), compared with the SSRI paroxetine (1.2) and the TCA amitriptyline (1.1). The SSRI sertraline was associated with intermediate risk (1.4). As class effects, SSRIs and SNRIs were previously considered to have a more favourable cardiac safety profile compared with TCAs, due to the absence or only slight QT prolongation at therapeutic doses and less cardiac toxicity in overdose 20. However, several studies have shown that cardiac risks exist also for SSRIs and, in accordance with our results, suggest an increased risk for sudden death in users of SSRIs compared with users of TCAs 21. In a large US study, the SNRI mirtazapine and the SSRI citalopram showed a slightly higher risk for sudden death and ventricular arrhythmia than the reference drug paroxetine (SSRI) and the TCA amitriptyline in a mixed population of 30–75 years old 22. A large number of case reports have also associated various SNRIs and SSRIs, especially citalopram, with TdP 8, 23.
Even if block of the hERG K+ channel, resulting in inhibition of the cardiac repolarization IKr current, is a predictor of drug induced TdP, the link between hERG block, QT interval prolongation and TdP arrhythmia is complex. TdP classified antidepressants have different potencies to block hERG channels at clinically relevant concentrations and bind to the channel in various ways, e.g. in open, inactivated or close states 24, 25, 26. Several of them also block the L‐calcium channel, counteracting the effect of the hERG channel block, and TCAs block Na+ channels as well 24, 25. The severe cardiac toxicity in overdose by TCAs seems to be related mainly to the block of Na+ channels at high concentrations, rather than inhibition of IKr 27. Overdose studies give limited information on the risk to develop TdP in normal use. A review of overdose studies with SSRIs and SNRIs shows that only citalopram stands out with pronounced QT prolongation, but no cases of TdP or death were reported 23, 28. Mirtazapine for example, showed no QT prolongation, TdP or mortality in single agent overdose in individuals 26–49 years old 29, despite association with QT prolongation 30, 31, 32 and several cases of TdP (also in monotherapy) 30, 33, 34, ventricular arrhythmia/sudden death 22 and increased mortality in the elderly 6. Altogether, the complexity makes it almost impossible to compare and evaluate TdP risk for individual antidepressants in at‐risk patients seen in clinical practice on the basis of overdose or hERG studies 23.
Finally and most importantly, recent thorough analyses of TdP case reports for antidepressants 23 and other drugs classified with TdP risk 23, 35, 36 show that QT prolongation by itself cannot predict TdP arrhythmia. The use as of QT as surrogate for TdP has also been challenged 37, 38. Instead the presence of one or often two or more additional risk factors, such as old age, female gender, and heart disease, seems to be more important for the development of TdP 23, 35, 36. In our study in older at‐risk individuals, we thoroughly adjusted for comorbidity, concurrent drug use and several other confounders (see Table [Link] in the online version). The mortality in users of antidepressants without TdP classification was higher (OR 1.23) compared with non‐users before adjustment (OR = 1), but was the same as in non‐users (OR 0.99) after adjustment. This result may suggest that we used adequate methods for the adjustment of potential confounders, e.g. in patients who were treated for depression related to physical comorbidity. However, we cannot exclude that residual confounding or unknown factors could have influenced the results.
The Credible Meds system is updated regularly, and citalopram and mirtazapine were not classified with known and possible TdP risk, respectively, until 2012 and 2013. The risk for TdP with citalopram has resulted in regulatory warnings in both the US and EU. Doses above 40 mg day–1 should not be used in adults 39, 40 and not over 20 mg daily in the elderly 40. It has been questioned if these regulatory actions were based on sufficient information to warrant the limitation of prescribing practices for citalopram 41. Our results support these regulatory actions and may suggest that the CredibleMeds TdP risk rating overall is a good predictor of the likelihood of antidepressant‐associated death in elderly. Given the widespread use of antidepressants in the elderly, however, our findings call for confirmation in independent populations.
Antipsychotic drugs
Both typical and atypical antipsychotic drugs have been associated with increased risk of sudden cardiac death and all‐cause mortality 42, 43, 44. In US, the whole therapeutic class carries a box warning referencing death in elderly patients with behavioural problems associated with dementia 45. The results in our study support the idea of an increased risk for the whole therapeutic class and indicate an association with increased risk for death even in a general population of older persons using antipsychotics. Our results also suggest that the potential of many antipsychotics to induce life threatening TdP, which is reflected by their TdP classification, is a contributing factor to explain the increased risk for death. Despite the relatively large number of studies, none has previously compared risks for death of antipsychotics effect in relation to their TdP risk class level. This is important given that the arrhythmogenic potential for antipsychotics differs greatly.
Our results show that antipsychotics classified with known TdP risk were associated with the highest risk for death, followed by agents classified with possible risk and antipsychotics with no TdP classification. However, in contrast to antidepressants, antipsychotics without TdP labelling were also associated with a marked increased risk for death. There are other mechanisms than interference with cardiac repolarization and development of TdP which may be of importance to explain the elevated ‘baseline risk’ for death among users of antipsychotics. These include indirect effects on metabolism and weight gain, other cardiovascular effects such as hypertension or alterations in heart rate variability, increased risk for stroke, as well as increased risk to die in cardiovascular disease by nature of their illness 46, 47, 48, 49, 50. Furthermore, the lack of TdP classification cannot conclude that these medicines confer no risk in relation to development of TdP arrhythmia 7.
The risk ranking of individual antipsychotics in our study is in agreement with results in other studies. In a cohort study in more than 75 000 aged 65 years or older, haloperidol had a two times higher mortality risk than risperidone and olanzapine, while quetiapine had the lowest risk 51. In a retrospective cohort study in 33 000 individuals aged 65 years or older with dementia, starting out‐patient treatment with antipsychotic drugs, the same risk ranking in relation to mortality was observed: haloperidol > risperidone = olanzapine > quetiapine 52. A large registry study, with more than 450 000 incident antipsychotic users aged 30–75 years with sudden cardiac death and all‐cause death as outcomes, also showed similar results. Haloperidol and chlorpromazine were associated with a higher risk than olanzapine and risperidone, and quetiapine the lowest for both the studied outcomes 13.
Limitations
By combined analyses of different national registers with almost complete coverage, our study can circumvent shortcomings of many other studies based on small and selected samples of older individuals. However, it must be emphasized that our study has several limitations and cannot prove causality. One limitation is that confounding by indication cannot be ruled out. The Swedish Prescribed Drug Register does not include information about the underlying indications and diagnoses for prescription of drugs. Therefore, we do not know for which psychiatric symptoms the drugs were prescribed. However, as discussed below antidepressant and antipsychotic drugs are often prescribed without a documented diagnosis to elderly patients. The Swedish Patient Register includes all inpatient and specialized outpatient care in the whole of Sweden, but lacks data from primary care, where the majority of older patients with psychiatric problems are treated 2. Thus, it is difficult to obtain data about mental disorders in older patients and therefore also to adjust adequately for underlying psychiatric diagnoses.
Dementia status was assessed based on data from the Swedish Patient Register and the Swedish Prescribed Drug Register. This underestimates the number of dementia cases, as we lack information about dementia diagnoses in primary care (not included in the Patient Register) as well as undiagnosed dementia. We adjusted for education, number of inpatient days and contacts with open specialized health care, number of drugs and other drugs with TdP liability and a range of potential co‐morbidity confounders (see Table S2 in the on line version for details). However, there may still be residual confounding regarding differences in co‐morbidity levels. Finally, a general limitation related to drug register data, is that they may not fully reflect the patients' actual drug use, if adherence to treatment is low.
In conclusion, this large study found that the great majority of prescribed antidepressants and antipsychotic drugs in the elderly were classified with known or possible TdP risk, and that drugs in these TdP classes were associated with an increased risk of mortality compared with drugs without classification or with conditional risk. The risk varied for individual compounds. Haloperidol among antipsychotics and mirtazapine and citalopram among antidepressants, were associated with higher risks. Prescription of antidepressants and antipsychotics to older persons often occurs without any indication for the treatment. A recent study in Sweden showed that only 18% of the patients aged 65 years or older treated with antipsychotics had indication for treatment. The corresponding figure for antidepressants was 40% 53. Other studies show similar results 54. Our results emphasize that antipsychotics and antidepressants should be used in elderly only when there is an appropriate indication. Moreover, the results may suggest that the TdP risk for individual compounds should be taken into consideration in the risk–benefit assessment, particularly if additional risk factors are present, which is often the case in the elderly. Given the widespread use of especially citalopram and mirtazapine, our findings call for confirmation in independent populations, ideally with knowledge of diagnosis for treatment.
Competing Interests
All authors have completed the ICMJE uniform disclosure form (available on request from the corresponding author) at www.icmje.org/coi_disclosure.pdf and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Contributors
All authors were involved in the study design and interpretation of data. GJB and JC were responsible for statistical analysis. BD wrote the first draft. All authors contributed to further drafts and approved the final manuscript.
We thank Lucas Ryan and Hans Schwartz, both at the Swedish National Board of Health and Welfare and native in the English language, for review and correction of spelling and grammar errors.
Supporting information
Table S1 List of drugs classified with torsades de pointes cardiac ventricular arrhythmia risk according to CredibleMeds (www.crediblemeds.org, date 15 January 2015), including their ATC code and main indication. CredibleMeds divide drugs in three TdP risk classes: 1) known TdP risk or 2) possible TdP risk when used as directed in SPC labelling and 3) conditional TdP risk under certain conditions, e.g. excessive dose or overdose, or being the index or interacting agent in a drug–drug interaction
Table S2 List of comorbidities adjusted for in the multivariate conditional logistic regression analysis, including health‐care registers used and relevant ICD/ATC codes
Supporting info item
Supporting info item
Danielsson, B. , Collin, J. , Jonasdottir Bergman, G. , Borg, N. , Salmi, P. , and Fastbom, J. (2016) Antidepressants and antipsychotics classified with torsades de pointes arrhythmia risk and mortality in older adults ‐ a Swedish nationwide study. Br J Clin Pharmacol, 81: 773–783. doi: 10.1111/bcp.12829.
References
- 1. Volkert J, Schulz H, Harter M, Wlodarczyk O, Andreas S. The prevalence of mental disorders in older people in western countries ‐ a meta‐analysis. Ageing Res Rev 2013; 12: 339–53. [DOI] [PubMed] [Google Scholar]
- 2. Salmi P, Jonasdottir G, Danielsson B. Psykisk ohälsa bland äldre och behandling inom vården. Article number 2013–6‐22. National Board of Health and Welfare publication (in Swedish). www.socialstyrelsen.se
- 3. Kales HC, Valenstein M, Kim HM, McCarthy JF, Ganoczy D, Cunningham F, Blow FC. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry 2007; 164: 1568–76. [DOI] [PubMed] [Google Scholar]
- 4. Gill SS, Bronskill SE, Normand SL, Anderson GM, Sykora K, Lam K, Bell CM, Lee PE, Fischer HD, Herrmann N, Gurwitz JH, Rochon PA. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007; 146: 775–86. [DOI] [PubMed] [Google Scholar]
- 5. Hartikainen S, Rahkonen T, Kautiainen H, Sulkava R. The use of psychotropics and survival in demented elderly individuals. Int Clin Psychopharmacol 2005; 20: 227–31. [DOI] [PubMed] [Google Scholar]
- 6. Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley‐Cox J. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ 2011; 343: d4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arizona Center for Education on Research on Therapeutics . Credible Meds ‐ Available TdP risk categories. https://www.crediblemeds.org/
- 8. Wenzel‐Seifert K, Wittmann M, Haen E. QTc prolongation by psychotropic drugs and the risk of torsade de pointes. Dtsch Arztebl Int 2011; 108: 687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mangoni AA, Jackson SH. Age‐related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol 2004; 57: 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rabkin SW. Aging effects on QT interval: Implications for cardiac safety of antipsychotic drugs. J Geriatr Cardiol 2014; 11: 20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. CredibleMeds process for evaluating evidence and assigning risk. https://www.crediblemeds.org/research‐scientists/why‐lists/
- 12. Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate‐based review in a large U.S. community. J Am Coll Cardiol 2004; 44: 1268–75. [DOI] [PubMed] [Google Scholar]
- 13. Jones ME, Campbell G, Patel D, Brunner E, Shatapathy CC, Murray‐Thomas T, Volkert J, Schulz H, Harter M, Wlodarczyk O, Andreas S. The prevalence of mental disorders in older people in western countries ‐ a meta‐analysis. Ageing Res Rev 2013; 12: 339–53. [DOI] [PubMed] [Google Scholar]
- 14. Leonard CE, Freeman CP, Newcomb CW, Bilker WB, Kimmel SE, Strom BL, Hennessy S. Antipsychotics and the risks of sudden cardiac death and all‐cause death: Cohort studies in Medicaid and dually‐eligible Medicaid‐Medicare beneficiaries of five states. J Clin Exp Cardiol 2013; Suppl 10: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish National Inpatient Register. BMC Public Health 2011; 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lubin JH, Gail MH. Biased selection of controls for case–control analyses of cohort studies. Biometrics 1984; 40: 63–75. [PubMed] [Google Scholar]
- 17. Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, Bergman U, Persson I, Sundström A, Westerholm B, Rosén M. The new Swedish Prescribed Drug Register ‐ opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007; 16: 726–35. [DOI] [PubMed] [Google Scholar]
- 18. Wallerstedt SM, Fastbom J, Johnell K, Sjöberg C, Landahl S, Sundström A. Drug treatment in older people before and after the transition to a multi‐dose drug dispensing system–a longitudinal analysis. PLoS One 2013; 8: e67088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodrigues L, Kirkwood BR. Case–control designs in the study of common diseases: updates on the demise of the rare disease assumption and the choice of sampling scheme for controls. Int J Epidemiol 1990; 19: 205–13. [DOI] [PubMed] [Google Scholar]
- 20. Witchel HJ, Hancox JC, Nutt DJ. Psychotropic drugs, cardiac arrhythmia, and sudden death. J Clin Psychopharmacol 2003; 23: 58–77. [DOI] [PubMed] [Google Scholar]
- 21. Jolly K, Gammage MD, Cheng KK, Bradburn P, Banting MV, Langman MJ. Sudden death in patients receiving drugs tending to prolong the QT interval. Br J Clin Pharmacol 2009; 68: 743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leonard CE, Bilker WB, Newcomb C, Kimmel SE, Hennessy S. Antidepressants and the risk of sudden cardiac death and ventricular arrhythmia. Pharmacoepidemiol Drug Saf 2011; 20: 903–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hasnain M, Vieweg WV. QTc interval prolongation and torsade de pointes associated with second‐generation antipsychotics and antidepressants: a comprehensive review. CNS Drugs 2014; 28: 887–920. [DOI] [PubMed] [Google Scholar]
- 24. Sicouri S, Antzelevitch C. Sudden cardiac death secondary to antidepressant and antipsychotic drugs. Expert Opin Drug Saf 2008; 7: 181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wiśniowska B, Mendyk A, Fijorek K, Glinka A, Polak S. Predictive model for L‐type channel inhibition: multichannel block in QT prolongation risk assessment. J Appl Toxicol 2012; 32: 858–66. [DOI] [PubMed] [Google Scholar]
- 26. Lee SH, Sung MJ, Lee HM, Chu D, Hahn SJ, Jo SH, Choe H, Choi BH. Blockade of HERG human K+ channels by the antidepressant drug paroxetine. Biol Pharm Bull 2014; 37: 1495–504. [DOI] [PubMed] [Google Scholar]
- 27. Thanacoody HK, Thomas SH. Tricyclic antidepressant poisoning: cardiovascular toxicity. Toxicol Rev 2005; 24: 205–14. [DOI] [PubMed] [Google Scholar]
- 28. Kelly CA, Dhaun N, Laing WJ, Strachan FE, Good AM, Bateman DN. Comparative toxicity of citalopram and the newer antidepressants after overdose. J Toxicol Clin Toxicol 2004; 42: 67–71. [DOI] [PubMed] [Google Scholar]
- 29. Berling I, Isbister GK. Mirtazapine overdose is unlikely to cause major toxicity. Clin Toxicol 2014; 52: 20–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goodnick PJ, Jerry J, Parra F. Psychotropic drugs and the ECG: focus on the QTc interval. Expert Opin Pharmacother 2002; 3: 479–98. [DOI] [PubMed] [Google Scholar]
- 31. Castro VM, Clements CC, Murphy SN, Gainer VS, Fava M, Weilburg JB, Erb JL, Churchill SE, Kohane IS, Iosifescu DV, Smoller JW, Perlis RH. QT interval and antidepressant use: a cross sectional study of electronic health records. BMJ 2013; 346: f288. doi:10.1136/bmj.f288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rajpurohit N, Aryal SR, Khan MA, Stys AT, Stys TP. Propafenone associated severe central nervous system and cardiovascular toxicity due to mirtazapine: a case of severe drug interaction. S D Med 2014; 67: 137–9. [PubMed] [Google Scholar]
- 33. Poluzzi E, Raschi E, Moretti U, De Ponti F. Drug‐induced torsades de pointes: data mining of the public version of the FDA adverse event reporting system (AERS). Pharmacoepidemiol Drug Saf 2009; 18: 512–8. [DOI] [PubMed] [Google Scholar]
- 34. Jasiak NM, Bostwick JR. Risk of QT/QTc prolongation among newer non‐SSRI antidepressants. Ann Pharmacother 2014; 48: 1620–8. [DOI] [PubMed] [Google Scholar]
- 35. Vieweg WV, Hancox JC, Hasnain M, Koneru JN, Gysel M, Baranchuk A. Clarithromycin, QTc interval prolongation and torsades de pointes: the need to study case reports. Ther Adv Infect Dis 2013; 1: 121–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gysel M, Vieweg WV, Hasnain M, Hancox JC, Kunanithy V, Baranchuk A. Torsades de pointes following clarithromycin treatment. Expert Rev Cardiovasc Ther 2013; 11: 1485–93. [DOI] [PubMed] [Google Scholar]
- 37. Sala M, Coppa F, Cappucciati C, Brambilla P, d'Allio G, Caverzasi E, Barale F, De Ferrari GM. Antidepressants: their effects on cardiac channels, QT prolongation and torsade de pointes. Curr Opin Investig Drugs 2006; 7: 256–63. [PubMed] [Google Scholar]
- 38. Hondeghem LM. QT prolongation is an unreliable predictor of ventricular arrhythmia. Heart Rhythm 2008; 5: 1210–2. [DOI] [PubMed] [Google Scholar]
- 39. Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. 2012: http://www.fda.gov/Drugs/DrugSafety/ucm297391.htm. [Accessed 15 May 2015].
- 40. European Medicines Agency Homamg, Pharmacovigilance Working Party (PhVWP) October 2011 plenary meeting. EMA/CHMP/PhVWP/845939/2011. http://www.ema.europa.eu/docs/en_GB/document_library/Report/2011/10/WC500117061.pdf. [Accessed 15 May 2015].
- 41. Vieweg WV, Hasnain M, Howland RH, Hettema JM, Kogut C, Wood MA, Pandurangi AK. Citalopram, QTc interval prolongation, and torsade de pointes. How should we apply the recent FDA ruling? Am J Med 2012; 125: 859–68. [DOI] [PubMed] [Google Scholar]
- 42. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009; 360: 225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liperoti R, Onder G, Landi F, Lapane K, Mor V, Bernabei R, Gambassi G. All‐cause mortality associated with atypical and conventional antipsychotics among nursing home residents with dementia: a retrospective cohort study. J Clin Psych 2009; 70: 1340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murray‐Thomas T, Jones ME, Patel D, Brunner E, Shatapathy CC, Motsko S, Van Staa TP. Risk of mortality (including sudden cardiac death) and major cardiovascular events in atypical and typical antipsychotic users: a study with the general practice research database. Cardiovasc Psychiatry Neurol 2013; 2013: 247486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Information for Healthcare Professionals: Conventional Antipsychotics FDA ALERT [6/16/2008]:www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationPatientsandProviders/ucm124830.htm. [Accessed 26 June 2015].
- 46. Woo YS, Kim W, Chae JH, Yoon BH, Bahk WM. Blood pressure changes during clozapine orolanzapine treatment in Korean schizophrenic patients. World J Biol Psychiatry 2009; 10: 420–5. [DOI] [PubMed] [Google Scholar]
- 47. Silke B, Campbell C, King DJ. The potential cardiotoxicity of antipsychotic drugs as assessed by heart rate variability. J Psychopharmacol 2002; 16: 355–60. [DOI] [PubMed] [Google Scholar]
- 48. American Diabetes Association , American Psychiatric Association , American Association of Clinical Endocrinologists , North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004; 27: 596–601. [DOI] [PubMed] [Google Scholar]
- 49. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA 2007; 298: 1794–6. [DOI] [PubMed] [Google Scholar]
- 50. Shin JY, Choi NK, Lee J, Park MJ, Lee SH, Park BJ. A comparison of risperidone and haloperidol for the risk of ischemic stroke in the elderly: a propensity score‐matched cohort analysis. J Psychopharmacol 2015; Mar 31. pii: 0269881115578162. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 51. Huybrechts KF, Gerhard T, Crystal S, Olfson M, Avorn J, Levin R, Lucas JA, Schneeweiss S. Differential risk of death in older residents in nursing homes prescribed specific antipsychotic drugs: population based cohort study. BMJ 2012; 344: e977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kales HC, Kim HM, Zivin K, Valenstein M, Seyfried MS, Chang C, Cunningham F, Schneider LS, Blow FC. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry 2012; 169: 71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Skoogh J . Analysis of factors of importance for drug use. ISBN 978‐91‐7619‐120‐0 Thesis, Lund University 2015 lup.lub.lu.se/record/5275763/file/5275768.
- 54. Akincigil A, Olfson M, Walkup JT, Siegel MJ, Kalay E, Amin S, Zurlo KA, Crystal S. Diagnosis and treatment of depression in older community‐dwelling adults: 1992–2005. J Am Geriatr Soc 2011; 59: 1042–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 List of drugs classified with torsades de pointes cardiac ventricular arrhythmia risk according to CredibleMeds (www.crediblemeds.org, date 15 January 2015), including their ATC code and main indication. CredibleMeds divide drugs in three TdP risk classes: 1) known TdP risk or 2) possible TdP risk when used as directed in SPC labelling and 3) conditional TdP risk under certain conditions, e.g. excessive dose or overdose, or being the index or interacting agent in a drug–drug interaction
Table S2 List of comorbidities adjusted for in the multivariate conditional logistic regression analysis, including health‐care registers used and relevant ICD/ATC codes
Supporting info item
Supporting info item


