The development of effective and well‐tolerated biologic therapies has advanced the management of psoriasis by targeting specific downstream mediators in the inflammatory cascade. In this setting, the interleukin (IL) 12/23 inhibitor ustekinumab is indicated for the treatment of moderate‐to‐severe plaque psoriasis and has recently also received approval for active psoriatic arthritis in adults 1.
Although biologic drugs generally present a reassuring safety profile, there are several reports in the literature regarding skin disorders associated with their use.
A 55‐year‐old man presented to the psoriasis unit of our department with a 1‐week history of multiple asymptomatic, nonscaly, arciform reddish plaques of the right thoracomammary region, together with multiple, often coalescing, papules of the same colour located on his back (Figure 1). Physical examination revealed several skin lesions, two on the thorax and six on the back, ranging from 2 cm to 7 cm in diameter.
Figure 1.
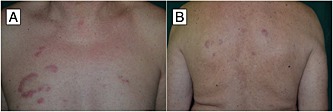
Erythematous arciform plaques in the thoracomammary region (A); similar lesions were also present on the back (B)
The patient had been followed for severe chronic plaque psoriasis and psoriatic arthritis since 2010. He had received acitretin, methotrexate, cyclosporin, etanercept and phototherapy. However, as his symptoms had become refractory to these treatments, he had been switched to ustekinumab. His baseline Psoriasis Area and Severity Index (PASI) score was 11.2, with a body surface area involvement of >10% and a Dermatology Life Quality Index score >10. He was administered ustekinumab 90 mg subcutaneously at weeks 0 and 4, which led to complete remission of the psoriatic skin lesions.
The eruption had arisen about 6 weeks after initiation of the drug, presenting as papules on the upper back, then involving the lower part and the thorax with similar elements, enlarging and clearing in the centre, and arranged in a circinate pattern.
We performed a 6‐mm incisional biopsy of one of the chest lesions. Histological findings were consistent with a diagnosis of lymphocytic infiltration, of the Jessner–Kanof‐type (Figure 2). The direct immunofluorescence and the colloidal iron stain for mucin were negative.
Figure 2.
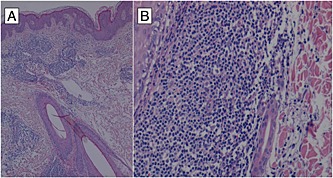
(A) dense lymphocytic infiltrate surrounding dermal vessels with focal involvement of the wall without epidermotropism or basal layer changes. Adjacent ectatic lymphatic vessels were also present. Hematoxylin‐eosin stain; original magnification x 100 and (B) the inflammatory infiltrate is predominantly formed by T lymphocytes (CD3+/CD4+/CD8+), few histiocytes and plasma cells. It involves the full thickness of the dermis with Jessner‐type pattern around vascular plexuses, adnexal structures and nerve endings. Rare extravasated red blood cells were also present. Hematoxylin‐eosin stain; original magnification x 250
All routine blood tests, including differential blood count, erythrocyte sedimentation rate, C‐reactive protein, Borrelia serology, complement levels, and liver and renal function tests, were normal or negative, revealing only hyperglycaemia [with a glucose level of 142 mg dl–1 (normal range 65–110 mg dl–1)] and hypercholesterolaemia [with a total cholesterol level of 241 mg dl–1 (normal range 130–220 mg dl–1)]. A complete autoantibody screening panel revealed positive antinuclear antibodies (ANA) with a titre of 1 : 320 and a speckled pattern (ANA were not assayed prior to undergoing ustekinumab treatment). Extractable nuclear antigen (ENA), antidouble‐stranded DNA autoantibody, antihistone antibody, lupus anticoagulant and anticardiolipin antibody tests were negative. There was neither clinical nor instrumental (chest X‐ray, abdominal and regional lymph node sonography, electrocardiography and echocardiography) evidence of any systemic involvement.
The patient had no prior history of atopic dermatitis, eczema or drug allergy. It was then suggested that he suspend ustekinumab, and lesions resolved within a few weeks, following application of topical hydrocortisone. Reintroduction of the drug 1 month later was followed by a relapse of the condition within 20 days, with the appearance of multiple coin‐like, slightly elevated, reddish papulo‐plaques widely involving the thoracomammary region, the left scapula and arm, and the middle back.
Ustekinumab was then discontinued permanently, and the patient is still in the course of washing out the drug.
Drug‐induced lymphocytic infiltration (Jessner–Kanof type) or chronic cutaneous lupus erythematosus are rarely reported skin conditions following the administration of a wide variety of substances. They are characterized by the eruption of asymptomatic erythematous discoid lesions or, less frequently, oedematous plaques of lupus tumidus, involving the face, central chest and upper back of middle‐aged adults. Central clearing of the lesions may result in an arciform pattern, with the course of the disease switching between remission and relapse, then resolving within a few weeks 2.
In our patient, lupus erythematosus tumidus was ruled out because of the negative results of direct immunofluorescence and the lack of interstitial deposition of mucin. However, most of the authors consider Jessner–Kanof lymphocytic infiltration and lupus erythematosus (tumidus form) to be part of the same spectrum 3, 4.
Apart from its precise nosological assessment, the pathophysiology of this adverse reaction remains subject to debate 2, 5. Some hypothesize the development of new autoantibodies upon drug exposure, and also a drug‐induced photosensitivity reaction which in turn triggers immune activation, as possible causes. Alternatively, drugs may be oxidized to reactive species that bind to carrier proteins and become immunogenic, thus also influencing apoptosis and T‐cell function 2.
An updated search on Pubmed found four similar reports on adverse reactions to ramipril 5, leflunomide 6, glatiramer acetate 7 and, more recently, duloxetine 8. A further case, characterized by histological features similar to those described here, has been described, in the form of an allergic contact dermatitis due to a hydroquinone‐based cream 9.
In our patient, the temporal relationship between ustekinumab administration and the development of the lesions, the complete remission after drug discontinuation and the recurrence of the disease following readministration 1 month later all support the hypothesis that this was a drug‐related event.
The Naranjo probability scale for causality assessment 10 scored 7 (probable). The score was derived as follows: two points for the adverse event occurring after drug intake; one point for improvement in the adverse reaction when ustekinumab administration was stopped; two points for the recurrence of the event after readministration; and two points for the absence of alternative causes.
To the best of our knowledge, there are no previous reports of similar skin disorders in patients treated with biologics.
Competing Interests
All the authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf and declare: 1) no support from any organization for the submitted work; 2) no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; 3) no other relationships or activities that could appear to have influenced the submitted work.
Guarneri, C. , Lentini, M. , Polimeni, G. , Giuffrida, R. , and Cannavò, S. P. (2016) Ustekinumab‐induced drug eruption resembling lymphocytic infiltration (of Jessner–Kanof) and lupus erythematosus tumidus. Br J Clin Pharmacol, 81: 792–794. doi: 10.1111/bcp.12837.
References
- 1. Talamonti M, Galluzzo M, Bianchi L, Boca AN, Costanzo A, Chimenti S. What happened after the clinical trials: long‐term safety and efficacy of ustekinumab in daily clinical practice. Dermatology 2014; 229: 324–32. [DOI] [PubMed] [Google Scholar]
- 2. Ahronovitz I, Fox LP. Severe drug‐induced dermatoses. Semin Cutan Med Surg 2014; 33: 49–58. [DOI] [PubMed] [Google Scholar]
- 3. Lipsker D, Mitschler A, Grosshans E, Cribier B. Could Jessner's lymphocytic infiltrate of the skin be a dermal variant of lupus erythematosus? An analysis of 210 cases. Dermatology 2006; 213: 15–22. [DOI] [PubMed] [Google Scholar]
- 4. Kuhn A, Sonntag M, Ruzicka T, Lehmann P, Megahed M. Histopathologic findings in lupus erythematosus tumidus: review of 80 patients. J Am Acad Dermatol 2003; 48: 901–8. [DOI] [PubMed] [Google Scholar]
- 5. Schepis C, Lentini M, Siragusa M, Batolo D. ACE‐inhibitor‐induced drug eruption resembling lymphocytic infiltration (of Jessner–kanof) and lupus erythematosus tumidus. Dermatology 2004; 208: 354–5. [DOI] [PubMed] [Google Scholar]
- 6. Sparsa L, Afif N, Goetz J, Sordet C, Chatelus E, Lipsker D, Sibilia J. Jessner–Kanof disease induced by leflunomide: a dermal variant of cutaneous lupus? Rheumatol Int 2011; 31: 255–8. [DOI] [PubMed] [Google Scholar]
- 7. Nolden S, Casper C, Kuhn A, Petereit HF. Jessner–Kanof lymphocytic infiltration of the skin associated with glatiramer acetate. Mult Scler 2005; 11: 245–8. [DOI] [PubMed] [Google Scholar]
- 8. Caroli UM, Berner D, Schlegel C, Metzler G, Rochen M, Biedermann T. Lymphocytic infiltration of the skin Jessner–Kanof after treatment with a hydroquinone‐containing bleaching cream. Arch Dermatol 2006; 142: 1655–6. [DOI] [PubMed] [Google Scholar]
- 9. Corazza M, Borghi A, Minghetti S, Mantovani L, Gafà E, Virgili A. Duloxetine‐induced pseudolymphoma with features of lymphocytic infiltration of Jessner–Kanof. Acta Derm Venereol 2014; 94: 605–6. [DOI] [PubMed] [Google Scholar]
- 10. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239–45. [DOI] [PubMed] [Google Scholar]


