Abstract
Approximately 15% of men with hormone naïve metastatic prostate cancer primarily fail to respond to androgen deprivation treatment (ADT). The reason why the response to ADT differs in this subgroup of men with prostate cancer remains unclear. The aim of this study was to describe the characteristics of these men and to thereby define predictors of early ADT failure in prostate cancer patients with bone metastases. The study was based on 915 men from the prospective randomized multicenter trial (no. 5) conducted by the Scandinavian Prostate Cancer Group comparing parenteral estrogen with total androgen blockade. Early ADT failure was defined as death from metastatic prostate cancer within 12 months after the start of ADT. Multivariate logistic regression models were applied to identify clinical predictors of early ADT failure. Ninety‐four (10.3%) men were primarily nonresponders to ADT. Independent predictors of early ADT failure were poor Eastern Cooperative Oncology Group performance status (PS), analgesic consumption, low hemoglobin, and high Soloway score (extent of disease observed on the scan), in where patients with poor PS and/or high analgesic consumption had a threefold risk of early ADT failure. Not significantly factors related to early ADT failure were age, treatment, cardiovascular comorbidity, T category, grade of malignancy, serum estrogen level, and SHBG at enrolment. We analyzed characteristics of a subgroup of patients who primarily failed to respond to ADT. Four independent clinical predictors of early ADT failure could be defined, and men exhibiting these features should be considered for an alternative treatment.
Keywords: Androgen deprivation treatment, bone metastases, clinical predictors, early failure, prostate cancer
Introduction
Androgen deprivation treatment (ADT) has been the gold standard in treatment of prostate cancer with distant metastases for more than half a century 1, 2, 3. However, the response to ADT varies, and approximately 15% of men with metastatic disease primarily fail to respond to ADT 1, 4, 5, 6, 7, 8, 9, 10, 11, 12. Not only did Huggins and coworkers describe the significant improvement in the clinical condition of patients with metastatic prostate cancer treated with ADT, they also defined a new disease state of early ADT‐refractory prostate cancer, that is, early ADT failure 1. In their series of 21 consecutive patients “a noticeable improvement occurred in the clinical status for all but three patients.”
In the vast majority of cases ADT leads to symptomatic relief, regression of metastases, and a fall in serum Prostate Specific Antigen (PSA). With optimal management, median survival time for men with metastatic disease is now 30–49 months 13 and survival longer than 10 years occasionally occurs 14. With time, however, the disease ceases to respond to hormone manipulation, becomes castration resistant, and the patient eventually dies of cancer progression unless death due to an unrelated cause intervenes. The secondary ADT failure (castration resistance) of metastatic prostate cancer after long‐term ADT has received increasing attention over the last two decades. The reason for this is that new hormonal and chemotherapeutic methods have been introduced and the mechanisms behind the development of castration resistance have been extensively explored 11, 15, 16, 17. As yet there are no predictors of early ADT failure. Results of previous studies on clinical associations between androgen receptors and treatment response in prostate cancer have been questioned 4, 18. Theories of the present time include both androgen receptor dependent and androgen receptor independent mechanisms 19, 20. Biochemical and histochemical assays have failed to predict primary response to ADT 21, 22, 23. Much focus has been placed on serum testosterone and other sex hormone levels and their relation to prostate cancer; but the results have been inconclusive 24, 25, 26. Although certain gene alterations or signatures have prognostic significance in primary prostate cancer, the clinical impact of primary prostate cancer genomic events has yet to be seen 17, 27, 28, 29. Models using molecular profiles of the primary tumor were not superior to models using clinical variables only to predict disease progress 30. The aim of this study was to analyze early ADT failure and to define clinical predictors associated with short survival due to early ADT failure in prostate cancer patients with bone metastases.
The 915 patients in this study were taken from a prospective noninferiority clinical trial (No. 5) conducted by the Scandinavian Prostate Cancer Group.
Methods and Patients
Clinical characteristics
This study was based on a Phase III study on 915 men with hormone‐naive metastatic prostate cancer from 61 centers in Denmark, Finland, Iceland, Norway and Sweden. Patients were included between December 1992 and June 1997 31, 32. To be eligible, patients were required to have skeletal metastases (M1) and an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2; 0 = denotes fully active; 1 = restricted in strenuous activity but ambulatory; 2 = ambulatory and capable of self‐care but unable to work). The primary objective of the trial was to determine whether or not overall‐ and cause‐specific survival following high‐dose parenteral estrogen therapy was inferior to that following Total Androgen Blockade (TAB). The extent of the primary tumor was determined using digital rectal examination according to the TNM classification from 1987 33 and cytological or histological specimens graded according to the World Health Organization (WHO) system 34. PSA, Alkaline Phosphatase (ALP), testosterone and hemoglobin levels were determined by routine laboratory testing before the start of treatment. Serum estrogen and Sex Hormone‐Binding Globulin (SHBG) levels were optional.
Skeletal involvement was assessed using bone scans supplemented by X‐ray when necessary. The extent of skeletal metastases was calculated according to a modified Soloway score: 1 = the total area of hot spots less than three lumbar vertebral bodies; 2 = total area of hot spots larger than of score 1, but <75% of the total scan; and 3 ≥ 75% of the total scan or super scan 35. The presence of nonregional lymph nodes and soft tissue metastases was not investigated by CT or MRT scan.
Treatment
The patients were randomized to TAB or treatment with polyestradiol phosphate (240 mg) given by intramuscular injection every 2 weeks for 8 weeks and monthly thereafter. TAB consisted of orchiectomy or medical castration with the LHRH agonist triptorelin, according to clinician and patient preference, combined with 250 mg flutamide taken orally three times daily. In the TAB group, 298 patients chose orchiectomy, and 159 men received medical castration by monthly injection of the LHRH agonist triptorelin. No significant differences in survival between the two treatment groups were seen 31.
Ethics
The study was performed in accordance with the recommendations of the Helsinki Declaration and approved by the ethics committees of all centers partaking in the study. Patients were given verbal and written information, and gave their informed consent to be included in the study.
Statistical methods
In this study, early ADT failure was defined as a patient dying of prostate cancer within 12 months after start of treatment. The cohort of 915 patients was then divided into two groups: cases that were defined as having an early ADT failure (n = 94) and those in the remaining cohort (n = 821) (Fig. 1).
Figure 1.
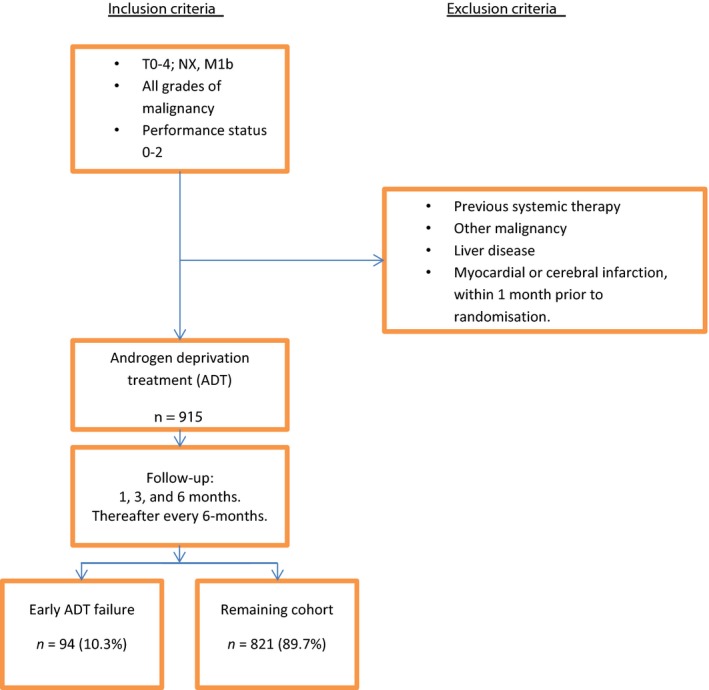
Flowchart showing study group assembly and follow‐up of the Scandinavian Prostate Cancer Group (SPCG) No. 5 Trial on androgen deprivation treatment (ADT) in men with prostate cancer and bone metastases. Early ADT failure was defined as disease‐specific death within 12 months after start of treatment.
Differences in the characteristics of the early ADT failure group and those in the remaining cohort were tested using the chi‐squared test based on age at enrolment, cancer‐related pain, treatment, cardiovascular comorbidity, ECOG PS, analgesic consumption, grade of malignancy, T category, PSA level, Soloway score, hemoglobin, SHBG, estradiol, and testosterone levels. A univariate logistic regression model with odds ratio (OR) and corresponding 95% confidence interval (CI) was used to estimate the likelihood of early ADT failure for each variable. In order to identify independent predictors of early ADT failure, a multivariate logistic regression model was then constructed using all variables that were statistically significant in the univariate analyses. We applied three different criteria for early ADT failure. In a first scenario analysis, we defined primary ADT as death of any cause within 12 months (N = 137). In the second, we applied the same definition, but excluded 44 men who died of other causes them prostate cancer within 12 months from the study group, leaving 871 men for analyses. In the final scenario, we included all men who died of prostate cancer, regardless of time since randomization (N = 661 patients) but excluded those who died of other causes. For each scenario, we repeated all the univariate and multivariate logistic regression analyses. The study group assembly of the first scenario is shown in Figure 1. All P‐values were two‐sided and statistical significance was considered at P < 0.05. All analyses were performed using the R version 3.1.1 (Vienna, Austria).
Results
Baseline demographic and clinical characteristics for the 915 patients enrolled in the study by the early ADT failure group and the remaining cohort are presented in Table 1.
Table 1.
Demographic and clinical characteristics by the primary androgen deprivation treatment ADT failure group and the remaining cohort
| Primary ADT failure | Remaining cohort | P‐value | Total | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| All cases | 94 | 100.0 | 821 | 100.0 | – | 915 | 100.0 |
| Age, years | |||||||
| <65 | 10 | 10.6 | 123 | 15.0 | 133 | 14.5 | |
| 65–74 | 41 | 43.6 | 393 | 47.9 | 434 | 47.4 | |
| ≥75 | 43 | 45.7 | 305 | 37.1 | 0.217 | 348 | 38.0 |
| Initial treatment | |||||||
| Polyestradiol phosphate | 44 | 46.8 | 414 | 50.4 | 458 | 50.1 | |
| TAB | 50 | 53.2 | 407 | 49.6 | 0,579 | 457 | 49.9 |
| Cardiovascular comorbidity | |||||||
| None | 80 | 86.0 | 731 | 90.0 | 811 | 89.6 | |
| Present | 13 | 14.0 | 81 | 10.0 | 0.354 | 94 | 10.4 |
| Missing | 1 | – | 9 | – | 10 | – | |
| Cancer‐related pain | |||||||
| No pain | 21 | 22.3 | 356 | 43.6 | 377 | 41.4 | |
| Pain | 73 | 77.7 | 460 | 56.4 | <0.001 | 533 | 58.6 |
| Missing | – | – | 5 | – | 5 | – | |
| ECOG performance status | |||||||
| 0 | 16 | 17.0 | 389 | 47.7 | 405 | 44.5 | |
| 1 | 50 | 53.2 | 305 | 37.4 | 355 | 39.0 | |
| 2–3 | 28 | 29.8 | 122 | 15.0 | <0.001 | 150 | 16.5 |
| Missing | – | – | 5 | – | 5 | – | |
| Analgesic consumption | |||||||
| Negligible | 21 | 22.3 | 414 | 50.7 | 435 | 47.8 | |
| ≥1 | 73 | 77.7 | 402 | 49.3 | <0.001 | 475 | 52.2 |
| Missing | – | – | 5 | – | 5 | – | |
| Grade of malignancy | |||||||
| WHO 1 | 12 | 13.3 | 124 | 15.5 | 136 | 15.3 | |
| WHO 2 | 34 | 37.8 | 380 | 47.5 | 414 | 46.5 | |
| WHO 3 | 44 | 48.9 | 296 | 37.0 | 0.086 | 340 | 38.2 |
| Missing | 4 | – | 21 | – | 25 | – | |
| T‐category | |||||||
| T0–T2 | 12 | 13.0 | 177 | 21.9 | 189 | 21.0 | |
| T3–T4 | 80 | 87.0 | 633 | 78.1 | 0.067 | 713 | 79.0 |
| Missing | 2 | – | 11 | – | 13 | – | |
| PSA, μg/L | |||||||
| PSA <100 | 24 | 25.5 | 223 | 27.3 | 247 | 27.1 | |
| PSA 100–500 | 39 | 41.5 | 353 | 43.3 | 392 | 43.1 | |
| PSA >500 | 31 | 33.0 | 240 | 29.4 | 0.770 | 271 | 29.8 |
| Missing | – | – | 5 | – | 5 | – | |
| Soloway score | |||||||
| 1 | 14 | 15.1 | 305 | 37.8 | 319 | 35.4 | |
| 2–3 | 79 | 84.9 | 502 | 62.2 | <0.001 | 581 | 64.6 |
| Missing | 1 | – | 14 | – | 15 | – | |
| Hemoglobin, g/L | |||||||
| Median, q1–q3 | 108.5 | 11.3–129.0 | 125.0 | 11.6–141.0 | 0.004 | 124.0 | 11.6–140.0 |
| Missing | 0 | – | 12 | – | 12 | – | |
| SHBG | |||||||
| Median, q1–q3 | 47.5 | 39.8–53.3 | 39.5 | 28.0–57.8 | 0.164 | 42.0 | 29.3–57.0 |
| Missing | 78 | – | 711 | – | 789 | – | |
| Estradiol | |||||||
| Median, q1–q3 | 64.0 | 0.1–82.0 | 65.0 | 0.2–98.0 | 0.366 | 64.5 | 0.1–96.0 |
| Missing | 79 | – | 712 | – | 791 | – | |
| Testosterone | |||||||
| Median, q1–q3 | 12.0 | 8.3–16.6 | 13.2 | 10.0–17.4 | 0.042 | 13.0 | 9.8–17.2 |
| Missing | 11 | – | 79 | – | 90 | – | |
A total of 94 of 915 (10.4%) patients died of progressive metastatic prostate cancer within 12 months after start of ADT and fulfilled the criteria for early ADT failure. The early ADT failure group had a statistically significant higher burden of cancer‐related pain, a poorer PS, analgesic consumption, and a higher Soloway score compared to the remaining cohort. There were also statistically significant differences with regards to level of hemoglobin and testosterone levels at the time of enrolment between the two groups. Not significantly factors related to early ADT failure were age, treatment, cardiovascular comorbidity, T‐category, grade of malignancy, serum estrogen level, and SHBG at enrolment (Table 1).
Factors prognostically associated with early ADT failure were defined using univariate and multivariate logistic regression analyses (Table 2). Factors significantly associated with early ADT failure in the univariate analyses were as follows: cancer‐related pain, a poor ECOG PS, an analgesic consumption, high Soloway score, and a low level of hemoglobin at enrolment. In the multivariate logistic regression analyses, a poor ECOG PS, analgesic consumption, a high Soloway score, and low levels of hemoglobin were all independent predictors of early ADT failure. Patients with an ECOG PS of 1 or higher, for example, had a threefold likelihood of early ADT failure compared to patients with PS of 0 (Table 2). The results of the 3 scenario analyses did not alter from the base case analyses (the 94 patients who died of progressive metastatic prostate cancer within 12 months), where a poor ECOG PS, analgesic consumption, a high Soloway score, and low levels of hemoglobin were all independent predictors of early ADT failure (Data not shown).
Table 2.
Univariate and multivariate logistic regression for primary ADT failure
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR | CI 95% | OR | CI 95% | |
| Age, years | ||||
| <65 | 1.00 | Ref. | – | – |
| 65–74 | 1.28 | 0.65–2.78 | – | – |
| ≥75 | 1.73 | 0.88–3.75 | – | – |
| Initial treatment | ||||
| Polyestradiol phosphate | 1.00 | Ref. | – | – |
| TAB | 1,16 | 0.75–1.78 | – | – |
| Cardiovascular comorbidity | ||||
| None | 1.00 | Ref. | – | – |
| Present | 1,47 | 0.75–2.67 | – | – |
| Cancer‐related pain | ||||
| No pain | 1.00 | Ref. | 1.00 | Ref. |
| Pain | 2.69 | 1.65–4.56 | 0.53 | 0.22–1.27 |
| ECOG Performance status | ||||
| 0 | 1.00 | Ref. | 1.00 | Ref. |
| 1 | 3.99 | 2.28–7.35 | 3.09 | 1.65–6.09 |
| 2–3 | 5.58 | 2.96–10.88 | 3.47 | 1.67–7.44 |
| Analgesic consumption | ||||
| Negligible | 1.00 | Ref. | 1.00 | Ref. |
| ≥1 | 3.58 | 2.20–6.06 | 2.92 | 1.25–7.22 |
| Grade of malignancy | ||||
| WHO 1 | 1.00 | Ref. | – | – |
| WHO 2 | 0.92 | 0.48–1.91 | – | – |
| WHO 3 | 1.54 | 0.81–3.13 | – | – |
| T‐category | ||||
| T0–T2 | 1.00 | Ref. | 1.00 | Ref. |
| T3–T4 | 1.86 | 1.03–3.67 | 1.80 | 0.95–3.73 |
| PSA, μg/L | ||||
| PSA <100 | 1.00 | Ref. | – | – |
| PSA 100–500 | 1.03 | 0.61–1.77 | – | – |
| PSA >500 | 1.20 | 0.69–2.13 | – | – |
| Soloway score | ||||
| 1 | 1.00 | Ref. | 1.00 | Ref. |
| 2–3 | 3.43 | 1.97–6.41 | 2.22 | 1.24–4.25 |
| Hemoglobin, g/L | ||||
| High | 1.00 | Ref. | 1.00 | Ref. |
| Low | 2.11 | 1.35–3.35 | 1.65 | 1.03–2.69 |
| SHBG | ||||
| Low | 1.00 | Ref. | – | – |
| High | 1.43 | 0.50–4.27 | – | – |
| Estradiol | ||||
| Low | 1.00 | Ref. | – | – |
| High | 0.86 | 0.28–2.55 | – | – |
| Testosterone | ||||
| Low | 1.00 | Ref. | – | – |
| High | 0.72 | 0.45–1.13 | – | – |
Discussion
One in ten men with metastatic prostate cancer primarily fails to respond to ADT. In this study we defined a sample of men with prostate cancer and bone metastases as being nonresponders to ADT based on the fact that they died of the disease within 12 months after start of ADT. Nonresponders had a significantly poorer ECOG PS, analgesic consumption, lower hemoglobin, and greater extent of bone metastases. Although it was already realized at the beginning of the ADT‐era that there was a subgroup of prostate cancer patients, who do not primarily responded to ADT, few studies have been undertaken to elucidate this phenomenon even though it continues to be mentioned in the uro‐oncologic literature 1, 4, 5, 6, 7, 10, 11, 36, 37.
A state of castration resistance usually develops after long‐term ADT. Improving the results of metastatic castration‐resistant prostate cancer (mCRPC) treatment is currently regarded as one of the most critical goals in the management of the disease 12, 38, 39, 40. A better understanding of the mechanisms behind secondary resistance following long‐term ADT may eventually result in a better understanding of early ADT failure. However, mCRPC following long‐term ADT is probably a much more heterogeneous condition than metastatic prostate cancer primarily failing to respond to ADT 17.
All men who died of progressive prostate cancer within 12 months of starting ADT were included, though there was a slight bias in the criteria in that any disease‐specific death during the first 12 months was considered to be early ADT failure. There may also have been men who did not respond to ADT that died after 12 months of treatment as well. In the pre‐ADT era, prostate cancer with bone metastases had a very short survival expectation, with a nine‐month mortality rate of 66%. However, long‐term survival was sometimes seen even in the pre‐ADT era 41.
When the idea of ADT evolved, it was suggested that androgen regulation played a central role. When assessing early “failure cases” 42, it was found that those with small testes at time of castration had a poor prognosis. Later, Houghton et al. reported no influence of the pretreatment testosterone concentrations on the outcome of prostate cancer 25. In some studies, however, prostate cancer has been considered to be more aggressive in the presence of low testosterone levels at start of ADT 43, 44.
Medical castration achieved by administration of long‐acting LHRH agonists or estrogens has been demonstrated to be as effective as surgical castration. Although LHRH agonists formulations may differ from estrogens in their suppression of testosterone, there are no data relating these differences to cause‐specific survival. Furthermore, there is no generally accepted limit for serum testosterone after ADT. Recently a value of 0.2 ng/mL or 0.69 nmol/L was suggested, but studies comparing clinical outcome with different castration limits are lacking 45. Uncertainties concerning the role of circulating sex hormones, and response to ADT have prompted the need to measure intraprostatic hormone levels and to compare these with their circulating correlates 26, 46, 47. Residual androgens at levels sufficient to activate the androgen receptor were found in prostate tissue, despite castrate levels of serum testosterone 48.
The previous findings of PSA as prognostic marker for prostate cancer treatment 49 could not be confirmed in this study. The first clinical indication that a patient responds to ADT is that the serum PSA level decreases. In 856 of the originally 915 cases, the serum PSA level had fallen 3 months after start of ADT. The observed PSA decrease in these cases was probably not only due to regression of tumor volume. At the cellular level a fall in PSA levels reflects a decrease in androgen receptor stimulation corresponding to a decreased transcription of the androgen‐responsive PSA gene and secretion of its encoded protein. The reason for the decrease in PSA may, to some extent, be due to the elimination of stimulation of PSA production by circulating androgens 50. We have not assessed the role of PSA kinetics, but focused on patients' variables before treatment start. The reason was the small number of patients in the early ADT failure group. Seven of the 94 patients already died within 3 months and 44 within 9 months. A previous study found that the level of pretreatment PSA or Gleason score at diagnosis could not predict patients likely to be unresponsive to ADT 51. 52, however, demonstrated a correlation between high Gleason score and positivity for chromogranin A in prostate cancer bone metastases and with poor outcome.
Our finding that prostate cancer with more extensive bone metastases is associated with ADT failure agrees with the results of other studies 53. Similarly, poor PS 43, 54, 55, and analgesic consumption 56, have been shown to be predictive for short survival in some studies. Analyses within groups having pain versus no pain at entry revealed poor survival when pain initially was present 5.
In this study all patients had bone metastases, but the presence of distant metastases at other sites was not explored. There is some evidence from clinical trials that treatment results are not related to the site of distant metastases 57. Recently, however, it was shown that the pattern of spread of distant metastases affects prognosis 58. These data, however, require validation.
The negative influence of low hemoglobin at the start of ADT seen in this study is in accordance with previous trials 54, 56.
Conclusion
Using data from a previous randomized clinical trial, we analyzed and compared the clinical characteristics of men with prostate cancer with bone metastases receiving ADT, and who showed early treatment failure. Using multivariate analyses, poor ECOG PS, analgesic consumption, high Solowey score, and a low hemoglobin were found to be predictive of early ADT failure. Men with these characteristics should be considered for alternative treatment.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This study was financially supported by Ferring AB Malmö, Sweden; Ferring Laegemidler A/S Copenhagen, Denmark; Pharmacia AB, Uppsala, Sweden; and Schering‐Plough AB, Stockholm, Sweden.
Cancer Medicine 2016; 5(3): 407–414
References
- 1. Huggins, C. , Stevens R. E., and Hodges C. V.. 1941. Studies on prostatic cancer, II. The effects of castration on advanced carcinoma of the prostate gland. Arch. Surg. 43:209–223. [Google Scholar]
- 2. Heidenreich, A. , Bastian P. J., Bellmunt J., Bolla M., S. Joniau , van der T., et al. 2014. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration‐resistant prostate cancer. Eur. Urol. 65:467–479. [DOI] [PubMed] [Google Scholar]
- 3. Institute NC . 2015. NIH. Available at http: 77 www.cancer.gov/types/prostate (accessed April 6, 2015).
- 4. Gustafsson, J. A. , Ekman P., Snochowski M., Zetterberg A., Pousette A., and Hogberg B.. 1978. Correlation between clinical response to hormone therapy and steroid receptor content in prostatic cancer. Cancer Res. 38(11 Pt. 2):4345–4348. [PubMed] [Google Scholar]
- 5. Murphy, G. P. , Beckley S., Brady M. F., Chu T. M., deKernion J. B., Dhabuwala C., et al. 1983. Treatment of newly diagnosed metastatic prostate cancer patients with chemotherapy agents in combination with hormones versus hormones alone. Cancer 51:1264–1272. [DOI] [PubMed] [Google Scholar]
- 6. Santen, R. J. 1992. Clinical review 37: endocrine treatment of prostate cancer. J. Clin. Endocrinol. Metabol. 75:685–689. [DOI] [PubMed] [Google Scholar]
- 7. Gudziak, M. R. , and Smith A. Y.. 1994. Hormonal therapy for stage D cancer of the prostate. West. J. Med. 160:351–359. [PMC free article] [PubMed] [Google Scholar]
- 8. Presti, J. C. , Tanagho E. A., and McAninch J. W.. 2000. P. 416 in Neoplasms of the prostate gland, Metastatic disease. Initial endocrine treatment Smith's, General Urology. 15th edn. [Google Scholar]
- 9. Nilsson, B. J . 2007. Pp. 3082–3100 in Hormone therapy for prostate cancer Campbell‐Walsh, Urology, 9th edn, vol. 3.. [Google Scholar]
- 10. Klotz, L. , Boccon‐Gibod L., Shore N. D., Andreou C., Persson B. E., Cantor P., et al. 2008. The efficacy and safety of degarelix: a 12‐month, comparative, randomized, open‐label, parallel‐group phase III study in patients with prostate cancer. BJU Int. 102:1531–1538. [DOI] [PubMed] [Google Scholar]
- 11. Harris, W. P. , Mostaghel E. A., Nelson P. S., and Montgomery B.. 2009. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Pract. Urol. 6:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karantanos, T. , Corn P. G., and Thompson T. C.. 2013. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 32:5501–5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tangen, C. M. , Hussain M. H., Higano C. S., Eisenberger M. A., Small E. J., Wilding G., et al. 2012. Improved overall survival trends of men with newly diagnosed M1 prostate cancer: a SWOG phase III trial experience (S8494, S8894 and S9346). J. Urol. 188:1164–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klaff, R. , Berglund A., Varenhorst E., Hedlund P. O., Jonler M., Sandblom G., et al. 2015. Clinical characteristics and quality‐of‐life in patients surviving a decade of prostate cancer with bone metastases. BJU Int. doi: 10.1111/bju.13190. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15. Sharifi, N. 2013. Minireview: androgen metabolism in castration‐resistant prostate cancer. Mol. Endocrinol. 27:708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shafi, A. A. , Yen A. E., and Weigel N. L.. 2013. Androgen receptors in hormone‐dependent and castration‐resistant prostate cancer. Pharmacol. Ther. 140:223–238. [DOI] [PubMed] [Google Scholar]
- 17. Robinson, D. , Van Allen E. M., Wu Y. M., Schultz N., Lonigro R. J., Mosquera J. M., et al. 2015. Integrative clinical genomics of advanced prostate cancer. Cell 161:1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pavone‐Macaluso, M. , Carruba G., and Castagnetta L.. 1994. Steroid receptors in prostate cancer tissues and cells: pathophysiology, problems in methodology, clinical value and controversial questions. Arch. Esp. Urol. 47:189–201. [PubMed] [Google Scholar]
- 19. Feldman, B. J. , and Feldman D.. 2001. The development of androgen‐independent prostate cancer. Nat. Rev. Cancer 1:34–45. [DOI] [PubMed] [Google Scholar]
- 20. Devlin, H. L. , and Mudryj M.. 2009. Progression of prostate cancer: multiple pathways to androgen independence. Cancer Lett. 274:177–186. [DOI] [PubMed] [Google Scholar]
- 21. Abrahamsson, P. A. 1999. Neuroendocrine differentiation in prostatic carcinoma. Prostate 39:135–148. [DOI] [PubMed] [Google Scholar]
- 22. Hirano, D. , Okada Y., Minei S., Takimoto Y., and Nemoto N.. 2004. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur. Urol. 45:586–592; discussion 592. [DOI] [PubMed] [Google Scholar]
- 23. Hansson, J. , and Abrahamsson P. A.. 2003. Neuroendocrine differentiation in prostatic carcinoma. Scand. J. Urol. Nephrol. Suppl. 212:28–36. [DOI] [PubMed] [Google Scholar]
- 24. Robinson, M. R. , and Thomas B. S.. 1971. Effect of hormonal therapy on plasma testosterone levels in prostatic carcinoma. Br. Med. J. 4:391–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Houghton, A. L. , and Jacobs H. S.. 1978. Advanced carcinoma of the prostate‐ does the pre‐treatment Leydig cell function determine the response to orchidectomy? Postgrad. Med. J. 54:261–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Endogenous, H. , Prostate Cancer Collaborative G ; Roddam A. W., Allen N. E., Appleby P., Key T. J.. 2008. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J. Natl. Cancer Inst. 100:170–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Setlur, S. R. , Mertz K. D., Hoshida Y., Demichelis F., Lupien M., Perner S., et al. 2008. Estrogen‐dependent signaling in a molecularly distinct subclass of aggressive prostate cancer. J. Natl. Cancer Inst. 100:815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hieronymus, H. , Schultz N., Gopalan A., Carver B. S., Chang M. T., Xiao Y., et al. 2014. Copy number alteration burden predicts prostate cancer relapse. Proc. Natl. Acad. Sci. USA 111:11139–11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lalonde, E. , Ishkanian A. S., Sykes J., Fraser M., Ross‐Adams H., Erho N., et al. 2014. Tumour genomic and microenvironmental heterogeneity for integrated prediction of 5‐year biochemical recurrence of prostate cancer: a retrospective cohort study. Lancet Oncol. 15:1521–1532. [DOI] [PubMed] [Google Scholar]
- 30. Sboner, A. , Demichelis F., Calza S., Pawitan Y., Setlur S. R., Hoshida Y., et al. 2010. Molecular sampling of prostate cancer: a dilemma for predicting disease progression. BMC Med. Genomics 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hedlund, P. O. , Damber J. E., Hagerman I., S. Haukaas , Henriksson P., Iversen P., et al. 2008. Parenteral estrogen versus combined androgen deprivation in the treatment of metastatic prostatic cancer: part 2. Final evaluation of the Scandinavian Prostatic Cancer Group (SPCG) Study No. 5. Scand. J. Urol. Nephrol. 42:220–229. [DOI] [PubMed] [Google Scholar]
- 32. Hedlund, P. O. , Ala‐Opas M., Brekkan E., Damber J. E., Damber L., Hagerman I., et al. 2002. Parenteral estrogen versus combined androgen deprivation in the treatment of metastatic prostatic cancer – Scandinavian Prostatic Cancer Group (SPCG) Study No. 5. Scand. J. Urol. Nephrol. 36:405–413. [DOI] [PubMed] [Google Scholar]
- 33. Hermanek, P. , Scheibe O., Spiessl B., and Wagner G.. 1987. TNM classification of malignant tumors: new edition 1987. RoFo : Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 146:732–733. [DOI] [PubMed] [Google Scholar]
- 34. Mostofi, F. K . 1980. Pp. 17–21 in Histologic typing of prostate cancer tumors. World Health Organisation, Geneva. [Google Scholar]
- 35. Soloway, M. S. , Hardeman S. W., Hickey D., Raymond J., Todd B., Soloway S., et al. 1988. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer 61:195–202. [DOI] [PubMed] [Google Scholar]
- 36. Resnick, M. I. , and Grayhack J. T.. 1975. Treatment of stage IV carcinoma of the prostate. Urol. Clin. North Am. 2:141–161. [PubMed] [Google Scholar]
- 37. Presti, J. C , Tanagho E. A., and Mc Annich J. W.. 2000. P. 416 in Metastatic disease: initial endocrine treatment Smith's Feneral Urology. [Google Scholar]
- 38. Suzman, D. L. , and Antonarakis E. S.. 2014. Castration‐resistant prostate cancer: latest evidence and therapeutic implications. Ther. Adv. Med. Oncol. 6:167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Frieling, J. S. , Basanta D., and Lynch C. C.. Jan2015. Current and emerging therapies for bone metastatic castration‐resistant prostate cancer. Cancer Control 22:109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hsieh, C. L. , Botta G., Gao S., Li T., Van Allen E. M., Treacy D. J., et al. 2015. PLZF, a tumor suppressor genetically lost in metastatic castration‐resistant prostate cancer, is a mediator of resistance to androgen deprivation therapy. Cancer Res. 75:1944–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bumpus, H. C. 1926. Carcinoma of the prostate: a clinical study of one thousand cases. Surg. Gynecol. Obstet. 43:150–155. [Google Scholar]
- 42. Huggins, C. 1942. Effect of orchiectomy and irradiation on cancer of the prostate. Ann. Surg. 115:1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chodak, G. W. , Vogelzang N. J., Caplan R. J., Soloway M., and Smith J. A.. 1991. Independent prognostic factors in patients with metastatic (stage D2) prostate cancer. The Zoladex Study Group. JAMA 265:618–621. [PubMed] [Google Scholar]
- 44. Schatzl, G. , Madersbacher S., Haitel A., Gsur A., Preyer M., Haidinger G., et al. 2003. Associations of serum testosterone with microvessel density, androgen receptor density and androgen receptor gene polymorphism in prostate cancer. J. Urol. 169:1312–1315. [DOI] [PubMed] [Google Scholar]
- 45. Morote, J. , Orsola A., Planas J., Trilla E., Raventos C. X., Cecchini L., et al. 2007. Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J. Urol. 178(4 Pt. 1):1290–1295. [DOI] [PubMed] [Google Scholar]
- 46. Hsing, A. W. , Chu L. W., and Stanczyk F. Z.. 2008. Androgen and prostate cancer: is the hypothesis dead? Cancer Epidemiol. Biomark. Preven. 17:2525–2530. [DOI] [PubMed] [Google Scholar]
- 47. Kjellman, A. , Akre O., Norming U., Tornblom M., and Gustafsson O.. 2008. Dihydrotestosterone levels and survival in screening‐detected prostate cancer: a 15‐yr follow‐up study. Eur. Urol. 53:106–111. [DOI] [PubMed] [Google Scholar]
- 48. Mohler, J. L. , Gregory C. W., Ford O. H. III, Kim D., Weaver C. M., Petrusz P., et al. 2004. The androgen axis in recurrent prostate cancer. Clin. Cancer Res. 10:440–448. [DOI] [PubMed] [Google Scholar]
- 49. Glass, T. R. , Tangen C. M., Crawford E. D., and Thompson I.. 2003. Metastatic carcinoma of the prostate: identifying prognostic groups using recursive partitioning. J. Urol. 169:164–169. [DOI] [PubMed] [Google Scholar]
- 50. Leo, M. E. , Bilhartz D. L., Bergstralh E. J., and Oesterling J. E.. 1991. Prostate specific antigen in hormonally treated stage D2 prostate cancer: is it always an accurate indicator of disease status? J. Urol. 145:802–806. [DOI] [PubMed] [Google Scholar]
- 51. Figg, W. D. , Franks M. E., Venzon D., Duray P., Cox M. C., Linehan W. M., et al. 2004. Gleason score and pretreatment prostate‐specific antigen in survival among patients with stage D2 prostate cancer. World J. Urol. 22:425–430. [DOI] [PubMed] [Google Scholar]
- 52. Cheville, J. C. , Tindall D., Boelter C., Jenkins R., Lohse C. M., Pankratz V. S., et al. 2002. Metastatic prostate carcinoma to bone: clinical and pathologic features associated with cancer‐specific survival. Cancer 95:1028–1036. [DOI] [PubMed] [Google Scholar]
- 53. Noguchi, M. , Kikuchi H., Ishibashi M., and Noda S.. 2003. Percentage of the positive area of bone metastasis is an independent predictor of disease death in advanced prostate cancer. Br. J. Cancer 88:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mulders, P. F. , Dijkman G. A., Fernandez del Moral P., Theeuwes A. G., Debruyne F. M.. 1990. Analysis of prognostic factors in disseminated prostatic cancer. An update. Dutch Southeastern Urological Cooperative Group. Cancer 65:2758–2761. [DOI] [PubMed] [Google Scholar]
- 55. James, N. D. , Spears M. R., Clarke N. W., Dearnaley D. P., De Bono J. S., Gale J., et al. 2014. Survival with Newly Diagnosed Metastatic Prostate Cancer in the “Docetaxel Era”: data from 917 Patients in the Control Arm of the STAMPEDE Trial (MRC PR08, CRUK/06/019). Eur. Urol. 67:1028–1038. [DOI] [PubMed] [Google Scholar]
- 56. Sylvester, R. J. , Denis L., and de Voogt H.. 1998. The importance of prognostic factors in the interpretation of two EORTC metastatic prostate cancer trials. European Organization for Research and Treatment of Cancer (EORTC) Genito‐Urinary Tract Cancer Cooperative Group. Eur. Urol. 33:134–143. [DOI] [PubMed] [Google Scholar]
- 57. Saeter, G. , Fossa S. D., Ous S., Blom G. P., and Kaalhus O.. 1984. Carcinoma of the prostate with soft tissue or non‐regional lymphatic metastases at the time of diagnosis: a review of 47 cases. Br. J. Urol. 56:385–390. [DOI] [PubMed] [Google Scholar]
- 58. Pond, G. R. , Sonpavde G., de Wit R., Eisenberger M. A., Tannock I. F., and Armstrong A. J.. 2014. The prognostic importance of metastatic site in men with metastatic castration‐resistant prostate cancer. Eur. Urol. 65:3–6. [DOI] [PubMed] [Google Scholar]


