Abstract
Key points
The concept of symmorphosis predicts that the capacity of each step of the oxygen cascade is attuned to the task demanded of it during aerobic exercise at maximal rates of oxygen consumption () such that no single process is limiting or in excess at .
The present study challenges the applicability of this concept to humans by revealing clear, albeit very different, limitations and excesses in oxygen supply and consumption among untrained and endurance‐trained humans.
Among untrained individuals, is limited by the capacity of the mitochondria to consume oxygen, despite an excess of oxygen supply, whereas, among trained individuals, is limited by the supply of oxygen to the mitochondria, despite an excess of mitochondrial respiratory capacity.
Abstract
The concept of symmorphosis postulates a matching of structural capacity to functional demand within a defined physiological system, regardless of endurance exercise training status. Whether this concept applies to oxygen (O2) supply and demand during maximal skeletal muscle O2 consumption () in humans is unclear. Therefore, in vitro skeletal muscle mitochondrial (Mito , mitochondrial respiration of fibres biopsied from vastus lateralis) was compared with in vivo skeletal muscle during single leg knee extensor exercise (KE , direct Fick by femoral arterial and venous blood samples and Doppler ultrasound blood flow measurements) and whole‐body during cycling (Body , indirect calorimetry) in 10 endurance exercise‐trained and 10 untrained young males. In untrained subjects, during KE exercise, maximal O2 supply (KE O2max) exceeded (462 ± 37 ml kg−1 min−1, P < 0.05) and KE matched (340 ± 22 ml kg−1 min−1, P > 0.05) Mito (364 ± 16 ml kg−1 min−1). Conversely, in trained subjects, both KE O2max (557 ± 35 ml kg−1 min−1) and KE (458 ± 24 ml kg−1 min−1) fell far short of Mito (743 ± 35 ml kg−1 min−1, P < 0.05). Although Mito was related to KE (r = 0.69, P < 0.05) and Body (r = 0.91, P < 0.05) in untrained subjects, these variables were entirely unrelated in trained subjects. Therefore, in untrained subjects, is limited by mitochondrial O2 demand, with evidence of adequate O2 supply, whereas, in trained subjects, an exercise training‐induced mitochondrial reserve results in skeletal muscle being markedly limited by O2 supply. Taken together, these in vivo and in vitro measures reveal clearly differing limitations and excesses at in untrained and trained humans and challenge the concept of symmorphosis as it applies to O2 supply and demand in humans.
Key points
The concept of symmorphosis predicts that the capacity of each step of the oxygen cascade is attuned to the task demanded of it during aerobic exercise at maximal rates of oxygen consumption () such that no single process is limiting or in excess at .
The present study challenges the applicability of this concept to humans by revealing clear, albeit very different, limitations and excesses in oxygen supply and consumption among untrained and endurance‐trained humans.
Among untrained individuals, is limited by the capacity of the mitochondria to consume oxygen, despite an excess of oxygen supply, whereas, among trained individuals, is limited by the supply of oxygen to the mitochondria, despite an excess of mitochondrial respiratory capacity.
Abbreviations
- BIOPS
biopsy preservation fluid
- Body
whole‐body
- KE
knee extensor
- Mito
mitochondrial
- O2max
maximum specific oxygen uptake rate
maximal oxygen uptake
- WRmax
maximum work rate
Introduction
Despite decades of research, it remains unclear which step, if any, along the O2 cascade limits maximal oxygen uptake () (Levine, 2008; Wagner, 2008). Based on the Fick principle, is the product of O2 supply (O2) and mitochondrial O2 extraction (Richardson, 2003), and thus the steps of the O2 cascade that potentially determine can be generalized into two components: O2 supply to the skeletal muscle mitochondria by the cardiopulmonary system and O2 demand by the skeletal muscle mitochondria. Proponents of the theory of symmorphosis have suggested that the capacity of each step of the O2 cascade, with the exception of the lungs, is built in strict proportion to the task demanded of it at , regardless of species (i.e. adaptive variation) or variation in body size (i.e. allometric variation), or training status (i.e. induced variation) (Weibel et al. 1991; Hoppeler & Weibel, 1998). Thus, neither the capacity for O2 supply, nor the capacity for O2 demand would be in excess or limiting at . Although this concept applies well when considering variation in across many species (Hoppeler & Weibel, 1998), how well symmorphosis pertains to humans is unclear because studies in humans often indicate that O2 supply or demand are either limiting or in excess at (Hoppeler & Weibel, 1998; Levine, 2008; Wagner, 2008).
Although many agree that a rate‐limiting step probably exists along the O2 cascade in humans, there is considerable disagreement regarding where the bottleneck lies because of the variety of prior findings. For example, several studies have provided convincing evidence that O2 supply limits by documenting that the capacity of mitochondria to consume O2 exceeds the ability of the cardiopulmonary system to supply O2 (Margaria et al. 1972; Knight et al. 1993; Boushel et al. 2011). However, other studies have also made an equally convincing case for the concept that mitochondrial O2 demand can limit by clearly illustrating that increasing O2 supply with hyperoxia does not always augment (Roca et al. 1992; Cardús et al. 1998).
Although the source of disagreement, in terms of what limits , remains unclear, it has been suggested that physical activity/exercise training may alter the source of limitation (Wagner, 2008). The possibility that the limitation to may shift with endurance training directly conflicts with the concept of symmorphosis (Weibel et al. 1991; Hoppeler & Weibel, 1998). Nevertheless, it has been well‐documented that, when humans undergo endurance training, the resulting gain in mitochondrial capacity far exceeds the accompanying increase in (Gollnick et al. 1973; Holloszy & Coyle, 1984). Thus, in contrast to the concept of symmorphosis, it is possible that exercise training may result in a mitochondrial reserve capacity that shifts the bottleneck at away from the mitochondria toward processes related to O2 supply.
In the present study, we utilized a unique combination of in vitro and in vivo measures of oxygen supply and demand, in both untrained and endurance exercise‐trained humans, aiming to determine whether O2 supply and demand are consistently well‐matched to . Based on previous literature (Roca et al. 1992; Cardús et al. 1998), we hypothesized that, although O2 supply and demand would be relatively well matched in untrained subjects, they would ultimately be limited by mitochondrial O2 demand. Conversely, considering the disproportionate rise in mitochondrial capacity associated with endurance exercise training (Gollnick et al. 1973; Holloszy & Coyle, 1984), we further hypothesized that, in trained individuals, mitochondrial O2 demand would far exceed O2 supply at , causing the fit individuals to be limited by O2 supply. If correct, these hypotheses will challenge the applicability of the concept of symmorphosis to the O2 cascade by revealing clear and very different, limitations and excesses at in untrained and endurance‐trained humans.
Methods
Subjects
Following approval by the Institutional Review Board at the University of Utah and the Salt Lake City Veterans’ Hospital, and in accordance with the Declaration of Helsinki, 10 young, healthy untrained males and 10 young, healthy, endurance‐trained male subjects were recruited for the present study and provided their informed, written consent. The untrained subjects were selected based upon a self‐reported lack of moderate‐to‐vigorous physical activity over the past 2 years, whereas trained subjects reported regularly participating in moderate‐to‐vigorous physical activity and endurance training (e.g. cycling, running, cross‐country skiing) over the past 2 years.
Muscle O2 supply and muscle in vivo
Familiarization sessions and whole‐body (Body ) protocol
Prior to the main experiment, subjects underwent multiple familiarization visits to become accustomed to performing knee extensor (KE) exercise on a custom built knee extension ergometer and cycling exercise on a commercially available cycle ergometer (Lode, the Netherlands). Once familiar with the exercise modalities, the maximum work rate (WRmax) that a subject could sustain for 1 min at the end of maximal graded cycle and KE exercise test was assessed. Specifically, for both cycling and KE exercise, subjects were given a 2–3 min warm‐up, which was followed by an increment in work rate (KE: 5 W, cycling: 25 W) every 1 min until task failure (i.e. inability to maintain 60 rpm during KE exercise or falling from a self‐selected cadence of > 60 rpm to < 50 rpm during cycling). To ensure a test duration of 8–12 min, the starting workload for both exercise modalities was tailored to each subject's ability. Additionally, Body was assessed during the cycling WRmax test utilizing a gas‐calibrated indirect calorimetry system (Parvomedics Inc., Sandy, UT, USA), and was defined as the highest rate of O2 consumption averaged over 30 s prior to cessation of exercise, whereas the respiratory exchange ratio exceeded 1.1 (Garten et al. 2014). Note that, during preliminary visits, KE and cycling tests were separated by at least 48 h, such that subjects were well rested.
KE protocol
After resting from exercise for at least 24 h, and experiment‐related activity for at least 48 h, subjects performed KE exercise at 25%, 50%, 75%, 90% and 100% of WRmax for 2–3 min each (Richardson et al. 1995), during which muscle VO2 of the leg was assessed by the direct Fick method. Specifically, muscle VO2 was calculated as the product of femoral blood flow and the arterial‐venous O2 difference ( = femoral blood flow × arterial‐venous O2 difference). Femoral blood flow was assessed via Doppler ultrasound of the common femoral artery, proximal to the exercising quadriceps femoris, whereas arterial and venous O2 content were assessed by a blood gas analyser (GEM 4000; Instrumentation Laboratories, Bedford, MA, USA) on blood drawn through catheters placed directly in the common femoral artery and vein, as described below. Subsequently, O2 to the muscle was calculated as the product of femoral blood flow and arterial O2 content.
Doppler ultrasound assessments
Femoral artery blood flow was measured with ultrasound Doppler and averaged during the last minute of each workload. Specifically, measurements of common femoral artery blood velocity and artery diameter, 2–3 cm proximal to the superficial/deep bifurcation, were taken using a Logiq 7 ultrasound Doppler system in duplex mode (General Electric Medical Systems, Milwaukee, WI, USA) equipped with a linear array transducer function at an imaging frequency of 14 MHz. Artery diameter, measured as internal diameter, was assessed during the peak of the electrocardiogram R‐wave (average of four or five measurements) for each workload. Femoral artery blood velocity was assessed with the same probe with a Doppler frequency of 5 MHz operated in the high‐pulsed repetition frequency mode (2–25mHz), as described previously (Barrett‐O'Keefe et al. 2013). Blood velocity was assessed with an insonation angle of no more than 60° (Rizzo et al. 1990), whereas the sample size was maximized and centred according to vessel size and position in real time. All ultrasound data were calculated using Logiq 7 software. Ultimately, femoral blood flow (ml·min−1) was calculated using the equation:
where mean blood velocity is expressed in cm s–1 and radius is expressed in cm.
Blood analysis
Three milliliters of blood were drawn at rest and during the last minute of each exercise stage through catheters placed in the common femoral artery and vein. Next, ∼1 ml of blood was analysed for arterial and venous total haemoglobin (tHb) content, haemoglobin saturation (HbO2) and using a GEM 4000 blood gas analyser and co‐oximeter (Instrumentation Laboratories, Bedford, MA, USA). Arterial and venous blood O2 content (ml dl−1) were calculated using the equation:
where total Hb (tHb) is expressed in g dL–1, HbO2 saturation is expressed as a percentage and is expressed in mmHg. The remainder of blood drawn was taken and preserved for further analysis.
Determination of leg muscle mass
Muscle volume of the lower limb was assessed by multiple measurements of thigh and leg circumference and skin fold thickness as described by Layec et al. (2014), which strongly correlates with volume measured by magnetic resonance imaging across a broad spectrum of subjects (r 2 = 0.89–0.98). Muscle mass of the thigh and leg were then determined by assuming a muscle density of 1.049 kg·L−1 (Kemp et al. 2015). Finally, the mass of the quadriceps femoris was calculated as described by Jones & Pearson (1969), at the same time as applying the correction factor recommended by (Râdegran et al. 1999).
Determination of mitochondrial in vitro
Muscle biopsy
Subjects reported to the laboratory for a muscle biopsy (∼1 week before or after the exercise/catheter portion of the study) in a fasted state and having refrained from vigorous exercise for 24 h. Muscle samples of the vastus lateralis were obtained by a percutaneous needle biopsy, 15 cm proximal to the knee at a depth of ∼3.5 cm, under sterile conditions (Richardson et al. 2004). Immediately after the muscle sample (∼150 mg) was biopsied, ∼20% of the sample was immersed in ice‐cold biopsy preservation fluid (BIOPS) for respiratory analysis (Pesta & Gnaiger, 2012), whereas the other portion was snap frozen and stored at –80°C for histological and biochemical analyses.
Mitochondrial respiration
Muscle samples were prepared and permeabilized as described by Pesta & Gnaiger (2012). Briefly, BIOPS‐immersed fibres were carefully separated with fine‐tip forceps and subsequently bathed in a BIOPS‐based saponin solution comprising 50 μg of saponin (ml BIOPS)−1, for 30 min. Following saponin treatment, muscle fibres were rinsed twice in ice‐cold mitochondrial respiration fluid (MIR05) for 10 min each rinse. The wet weight of each muscle sample (∼3–4 mg) was then assessed on a calibrated scale after excess fluid had been removed from the sample by dabbing the muscle several times with a paper towel.
Muscle fibres were then placed in the temperature‐controlled respiration chamber (Oxytherm, Hansatech Instruments, Norfolk, UK) in 2 ml of MIR05 solution and warmed to 37°C. After allowing the muscle 10 min to equilibrate, mitochondrial (Mito ) was assessed by sequentially adding malate (2 mm) + glutamate (10 mm), ADP (5 mm) and succinate (10 mm) to the respiration chamber. Finally, cytochrome C (10 μM) was added to the chamber to verify membrane integrity as recommended by Pesta & Gnaiger (2012). Each respiration state or step lasted as long as required to produce a steady‐state respiration rate, which was ∼3 min in most cases. Note that, prior to each experiment, the respirometer was calibrated, in accordance with the manufacturer's instructions, with two known levels of O2 concentration: MIR05 equilibrated with room air at a known barometric pressure and MIR05 with the O2 in the chamber removed by the introduction of sodium dithionite. Subsequently, background respiration inherent to the experimental set‐up was measured and taken into account when assessing overall mitochondrial respiration. Because the respirometer offered the greatest temperature stability at 37°C but exercise generally results in an increase in muscle temperature from 37°C to 38°C or more (Saltin et al. 1968; Kenny et al. 2003), respiration rates were obtained with the muscle sample at 37°C and then mathematically adjusted, based on a Q 10 of 2 (multiplication factor for O2 consumption at a 10°C difference), to yield predicted values at 38°C (Rasmussen et al. 2001; Boushel et al. 2011). The respiration data for each of the two separate muscle fibre samples were then averaged.
Statistical analysis
Differences in O2 supply, O2 consumption and O2 extraction during KE exercise were assessed with two‐way, repeated measures ANOVA, which, when significant, was followed by Tukey's post hoc test (SPSS, version 17; SPSS Inc., Chicago, IL, USA). Differences in variables between groups, such as subject characteristics and maximum values, were tested with an independent samples t test. Correlations between variables were assessed with Pearson product‐moment correlations. Alpha was set at 0.05 a priori. Data are reported as the mean ± SE.
Results
Subject characteristics
Ten healthy, untrained males and ten healthy, endurance‐trained males completed the present study. As shown in Table 1, subjects in both groups were well matched, with the exception that, by experimental design, there was a clear difference in exercise capacity. Specifically, Body was in the range 31–44 ml kg−1 min−1 in untrained subjects and 54–66 ml kg−1 min−1 in trained subjects. This difference in Body was also apparent in absolute terms (i.e. Body in L·min−1) and was reflected in both cycle and KE exercise WRmax. Thus, although untrained subjects weighed more than trained subjects, absolute was also lower in the untrained group, which, when combined with the fact that body mass index was not different between groups, indicates that the difference in Body was not merely a result of differences in body mass but, instead, was a consequence of real differences in aerobic capacity.
Table 1.
Subject characteristics
| Untrained | Trained | P | |
|---|---|---|---|
| Subjects (n) | 10 | 10 | |
| Age (years) | 25 ± 4 | 24 ± 2 | 0.77 |
| Height (cm) | 178 ± 2 | 174 ± 2 | 0.23 |
| Body mass (kg) | 78 ± 4 | 70 ± 2* | 0.04 |
| Body mass index (kg m–2) | 25 ± 1 | 23 ± 1 | 0.20 |
| Body (l min–1) | 2.9 ± 0.2 | 4.1 ± 0.2* | <0.001 |
| Body (ml kg−1 min−1) | 38 ± 2 | 59 ± 1* | <0.001 |
| Cycling WRmax (W) | 243 ± 38 | 359 ± 63* | <0.001 |
| KE exercise WRmax (W) | 45 ± 7 | 70 ± 5* | <0.001 |
| Total leg muscle mass (kg) | 6.35 ± 0.3 | 6.46 ± 0.3 | 0.45 |
| Quadriceps muscle mass (kg) | 1.94 ± 0.1 | 2.0 ± 0.1 | 0.64 |
*Significantly different compared to untrained subjects.
Cardiovascular and metabolic responses to KE exercise
As shown in Fig. 1 A, muscle mass‐specific during KE exercise increased with each increase in WR (P < 0.05) until WRmax, except for the change in from 90% to 100% of KE exercise WRmax, which did not achieve significance in either untrained or trained subjects. As expected, trained subjects achieved significantly greater rates of muscle O2 consumption compared to untrained subjects during KE exercise, both in absolute (untrained: 641 ± 41 ml·min−1, trained: 858 ± 55 ml·min−1) or relative terms (Fig. 1 A). Femoral artery blood flow (Fig. 1 B) followed a very similar pattern to , with clear increments at each WR, except from 90% to 100% of KE exercise WRmax in trained subjects, with blood flow at WRmax ultimately being significantly greater in trained subjects compared to untrained subjects. The arterial‐venous O2 difference (Fig. 1 C) did not exhibit an increase with each KE exercise WR. In untrained subjects, extraction failed to increase beyond that achieved at 50% of WRmax. Interestingly, although also failing to increase O2 extraction with each stepwise increase in workload, trained subjects exhibited a greater propensity for extracting O2 by increasing the arterial‐venous O2 difference at WRmax beyond that achieved at 75%WRmax. Ultimately, the arterial‐venous O2 difference was significantly greater at KE exercise WRmax in trained subjects compared to untrained subjects (17.5 and 15.9 ml·dl−1 respectively, P < 0.05).
Figure 1. Cardiovascular and metabolic responses during KE exercise in untrained and endurance‐trained males .
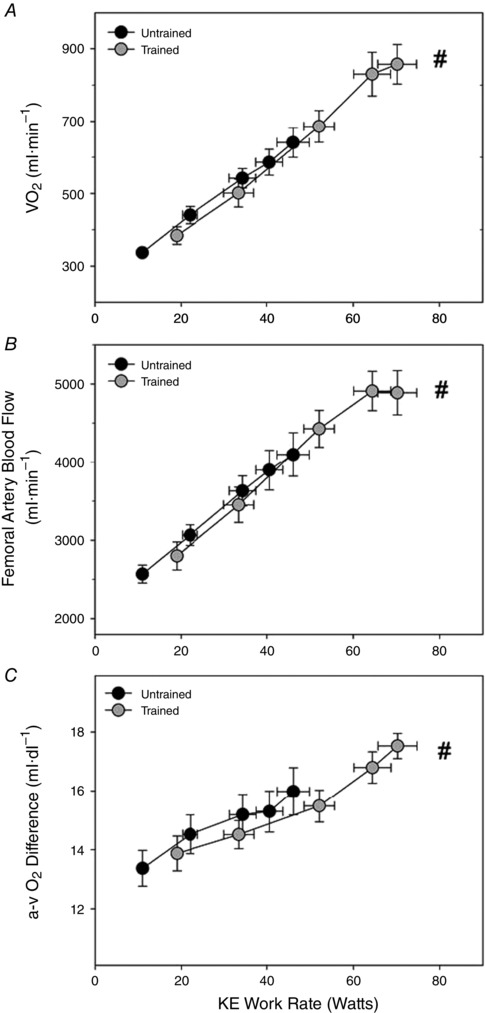
A, muscle O2 consumption () assessed as the product of (B) femoral artery blood flow and (C) arterial‐venous O2 difference across the exercising limb. Exercise was performed at 25%, 50%, 75%, 90% and 100% of WRmax but plotted as absolute WR to allow the appropriate comparisons. #Significantly different from untrained subjects at maximal KE exercise.
O2 supply and mitochondrial O2 demand during KE and cycling exercise
As is commonly observed with endurance training, muscle samples from endurance‐trained subjects exhibited a significantly greater Mito than untrained subjects (743 ± 35 and 364 ± 16 ml kg−1 min−1, respectively, P < 0.05) (Fig. 2 A). As shown in Fig. 2, in untrained subjects, KE (340 ± 21 ml kg−1 min−1) was not significantly different from (P > 0.05), whereas maximum O2 supply during KE exercise (KE O2max, 462 ± 37 ml·kg−1·min1) exceeded Mito (P < 0.05). By contrast, in trained subjects, KE O2max (557 ± 35 ml kg−1 min−1) and subsequently KE (458 ± 24 ml kg−1 min−1) were both significantly lower than Mito (P < 0.05). When considered as a percentage of Mito , KE of trained subjects reached only 63 ± 4 % of Mito , whereas the KE of untrained subjects reached 94 ± 4 % Mito (Fig. 2 B). Of note, an increase in Mito was associated with a proportionate increase in KE among untrained subjects (r = 0.69, P < 0.05) but not in trained subjects (r = 0.01, P = 0.74) (Fig. 3). Interestingly, as shown in Fig. 4, untrained subjects exhibited a strong positive relationship between Mito and Body , whereas, again in contrast to untrained subjects, trained subjects exhibited no relationship between Mito and Body (r = 0.12, P = 0.74).
Figure 2. Utilization of mitochondrial respiratory capacity during maximal KE exercise .
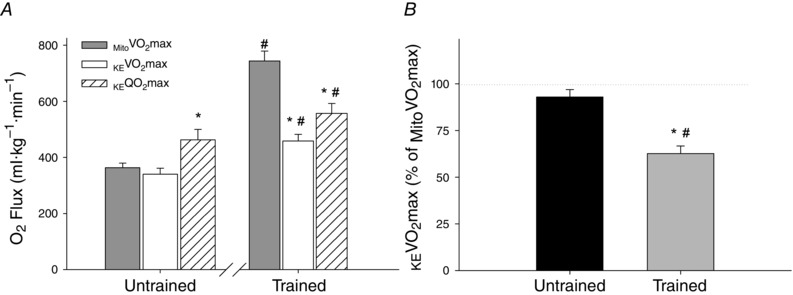
A, maximal oxygen consumption (KE ) and supply (KE O2max) during maximal KE exercise in trained and untrained subjects compared to the maximal mitochondrial oxygen consumption (mito ) of permeabilized fibres from the vastus lateralis in the same subjects. B, percentage utilization of mitochondrial capacity (% mito ) during maximal KE exercise in untrained and endurance‐trained subjects. Mito is normalized by the mass of the sample of muscle, whereas KE and KE O2max are normalized by the muscle mass of the quadriceps femoris. The dotted line in (B) represents the theoretical point where the full respiratory capacity of the mitochondria is utilized during KE exercise (i.e. 100% Mito ). *Significantly different from mito . #Significantly different from untrained subjects.
Figure 3.
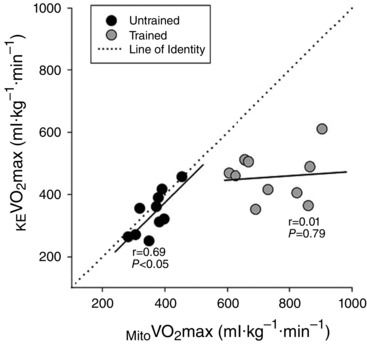
Mito is normalized by the mass of the sample of muscle, whereas KE is normalized by the muscle mass of the quadriceps femoris. The dotted line represents the line of identity (i.e. perfect 1:1 relationship between Mito and KE ).
Figure 4.
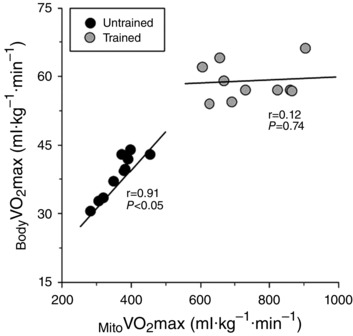
Mito is normalized by the mass of the sample of muscle, whereas Body , which was assessed by indirect calorimetry during cycle exercise, is normalized by the entire body mass.
Discussion
The unique combination of in vitro and in vivo measures of O2 supply and demand in the present study yielded two related sets of novel findings. First, in contrast to the concept of symmorphosis, the present study reveals that KE is limited by mitochondrial O2 demand in untrained and O2 supply in endurance exercise‐trained individuals. Second, again contrary to the concept of symmorphosis, the present study documents that the relationship between mitochondrial capacity and varies with exercise training status. Taken together, these findings clarify the determinants of in untrained and endurance exercise‐trained humans and challenge the invariant matching of structural capacity to functional demand proposed to exist by the concept of symmorphosis.
O2 supply and mitochondrial O2 demand limitations during maximal exercise
In theory, if O2 supply during maximal exercise is sufficient mitochondria should be able to respire at maximal rate (i.e. Mito ), whereas, if it is O2 supply that is insufficient, mitochondria would respire at a submaximal rate during maximal exercise (Wagner, 2008). Given the submaximal strain that KE exercise places on central factors (Richardson & Saltin, 1998), we anticipated that, if subjects were to have sufficient O2 supply to reach Mito during some form of exercise, it would probably be during KE exercise, when pulmonary O2 diffusion and cardiac output are probably not limiting (Richardson & Saltin, 1998). As shown in Fig. 1, KE exercise yielded high maximal rates of O2 consumption that were greater in trained compared to untrained subjects. As hypothesized, during maximal KE exercise, untrained subjects appeared to be limited by Mito , whereas KE O2max was apparently in excess (Fig. 2 B). Conversely, although trained subjects achieved significantly greater rates of O2 consumption during maximal KE exercise than untrained subjects, the increases in KE and KE O2max were not proportionate to the large increase in Mito , which was almost double that of untrained subjects. Thus, it appears that an insufficient O2 supply limited trained subjects to only utilizing 63 ± 4% of their far‐greater Mito during maximal KE exercise (Fig. 2 B).
In theory, if mitochondrial respiratory capacity is important in determining KE in untrained and not in trained subjects, a strong relationship would be expected between Mito and KE among untrained subjects but not trained subjects. Indeed, in the present study, mitochondrial capacity was well‐matched to KE in untrained subjects, whereas it was entirely unrelated to KE among trained subjects (Fig. 3). Interestingly, despite the fact that cycling has a greater probability of eliciting limitations in O2 supply than KE exercise, (Richardson & Saltin, 1998), this pattern persisted when comparing Body and Mito . Specifically, among untrained subjects, a greater Mito was mirrored by a proportionally greater Body but, in contrast, among trained subjects, a greater Mito did not translate into a greater Body . Indeed, among trained subjects, Body was entirely unrelated to Mito , suggesting that something else, probably O2 supply, plays a major role in determining Body (Fig. 4). Thus, it appears that, during both small and large muscle mass exercise, mitochondria are limiting in untrained subjects, and in excess in trained subjects.
These differing constraints to in trained and untrained humans during maximal exercise may help to explain the varied effects of hyperoxia on (Knight et al. 1993; Cardús et al. 1998; Pedersen et al. 1999; Richardson et al. 1999). For example, several studies utilizing untrained subjects have found that augmenting the O2 supply with hyperoxia has no effect on KE (Pedersen et al. 1999) or cycling (Roca et al. 1992; Cardús et al. 1998). In light of the current data, these previous findings make sense because untrained subjects already appear to be operating at Mito under normoxic conditions. By contrast, several other studies examining the effect of hyperoxia on in endurance‐trained individuals have reported that increasing O2 supply with hyperoxia can significantly augment KE by ∼17% (Richardson et al. 1999) and cycling by ∼8% (Margaria et al. 1972; Knight et al. 1993). This finding is also in agreement with the current data, which indicate that trained subjects, who exhibit a significant mitochondrial reserve capacity under normoxic conditions, have the additional mitochondria in place to consume the extra O2 made available by hyperoxia. Importantly, the divergence in the determination of in untrained and trained subjects observed in the present study has also been observed in longitudinal studies of subjects before and after endurance training, supporting the idea that training is at the root of the differing constraints to (Roca et al. 1992).
Although the present study is unique in terms of how the roles of O2 supply and demand in untrained and trained subjects were compared, it is certainly not the only one to compare indices of mitochondrial O2 demand with in vivo. For example, Rasmussen et al. (2001) and Blomstrand et al. (1997) compared multiple measures of muscle oxidative capacity with KE , whereas Boushel et al. (2011) made a similar comparison with cycling . As was the case for untrained subjects in the present study, both Rasmussen (2001) and Blomstrand (1997) reported that in vitro measures of skeletal muscle mitochondrial capacity closely paralleled KE . However, somewhat unexpectedly, Rasmussen et al. (2001) also found that Mito , assessed in isolated mitochondria, was lower than KE . As acknowledged in this prior work, this observation, which disagrees with the findings of the present study, is physiologically improbable and is potentially the result of altered mitochondrial function, inherent to the isolated mitochondria technique (Picard et al. 2011).
More recently, utilizing a similar design to the present study, Boushel et al (2011) reported that, in sedentary subjects, the of the lower limb during cycling exercise did not reach the level of Mito , suggesting that they were O2 supply limited. This is in disagreement with the present study, which suggests that Mito was probably limiting in untrained subjects during both KE and cycling exercise. The discrepancy between the present study and that of Boushel et al. (2011) could be a consequence of differences in the aerobic fitness between untrained subjects in these two studies (i.e. Body ), which was not reported by Boushel et al. (2011), or may be related to an overestimation of the amount of muscle mass of the lower limb engaged during cycling exercise, which Boushel et al. (2011) assumed to be 100%. Importantly, it should be restated that the observation that hyperoxia augments in trained (Knight et al. 1993) but not untrained individuals (Roca et al. 1992; Cardús et al. 1998) supports the findings of the present study. Also in support of our findings, Jacobs et al. (2013) recently reported that the exercise training‐induced increase in of previously sedentary subjects was related to augmented mitochondrial respiratory capacity and not improved O2 supply. Thus, there is considerable evidence to support the contention that untrained individuals are limited mitochondrial O2 demand, whereas trained individuals are limited by O2 supply.
Symmorphosis and determinants of in untrained and trained subjects
Supported by comparative studies that report a constant relationship between the capacity of many steps of the O2 cascade (e.g.) and , the concept of symmorphosis postulates that the capacity of each step of the O2 cascade is well matched to the demand placed on it at . Therefore, an invariant ratio, regardless of body size or training status, should exist between the capacity of each step of the O2 cascade and (Weibel et al. 1991; Hoppeler & Weibel, 1998). For the most part, the current data from untrained subjects appear to support the concept of symmorphosis, with Mito being well matched and proportional to KE and Body (Figs 3 and 4). Nevertheless, KE O2max, which somewhat exceeded Mito , does appear to challenge the matching concept set forth by symmorphosis. However, this somewhat moderate excess may be necessary to maintain a diffusional gradient from blood to muscle cell (Wagner, 2015) and may also serve as a safety factor (Diamond, 1992). By contrast, the data from trained subjects deviate substantially from the concept of symmorphosis. First, as shown in Fig. 2, the capacity for O2 supply clearly limits KE , leaving ∼40% of the respiratory capacity of the mitochondria in excess. Thus, the capacity of each step of the O2 cascade does not appear to be well matched to in trained subjects. Second, as shown in Figs 3 and 4, the strong relationships that exist between mitochondrial respiratory capacity and KE and Body in untrained subjects are no longer evident in trained subjects. Thus, in contrast to the concept of symmorphosis (Weibel et al. 1991; Hoppeler & Weibel, 1998), training status or ‘induced variation’ does appear to affect the relationship between and the steps of the O2 cascade.
The reason that the concept of symmorphosis inadequately applies to the O2 cascade in humans appears to be related to distinct processes (i.e. mitochondrial O2 demand in untrained subjects and cardiopulmonary O2 supply in trained subjects) acting as bottlenecks along the cascade. Although the reasons surrounding the O2 demand limitation of untrained subjects were not specifically examined in the present study, recent evidence suggests that this low Mito may be attributable to differences in the mitochondrial quality, as well as a lower mitochondrial density (Jacobs & Lundby, 2013). Regarding the O2 supply limitation exhibited by trained subjects, it is recognized that the term ‘O2 supply’ covers multiple steps of the O2 cascade, all of which may contribute to the O2 supply limitation. Although central limitations, such as pulmonary diffusion and cardiac output, have been reported to limit O2 supply during maximal exercise (Dempsey et al. 2008; Levine, 2008), the capacity of these factors probably was not exhausted during KE exercise, a small muscle mass model (Richardson & Saltin, 1998). Therefore, it appears that peripheral factors such as capillary‐muscle diffusion (Richardson et al. 1995) or the ability of the vascular system to conduct O2‐rich blood to the exercising muscle may contribute to the O2 supply limitation in trained subjects.
Implications of a mitochondrial reserve capacity
Although mitochondrial respiratory capacity, at maximal KE exercise, in trained subjects appears to be in great excess of metabolic demand, it is doubtful that this reserve capacity serves no purpose. Indeed, Gollnick & Saltin (1982) proposed that increasing mitochondrial enzyme capacity, even without increasing , would greatly increase endurance performance by increasing fatty acid utilization, thus delaying the onset of blood lactate accumulation and the subsequent sparing of glycogen. Thus, as supported by the recent work of Jacobs et al. (2011), the elevated mitochondrial capacity associated with training may be more related to endurance performance (e.g. time trial) than or maximal aerobic power.
Additionally, mitochondrial reserve capacity may benefit the cell by allowing the mitochondria to buffer the additional stresses encountered during times of high metabolic demand (Sansbury et al. 2011). Studies examining mitochondrial reserve capacity in animal models and cultured cells have reported that cells with a greater mitochondrial reserve are more resistant to oxidative stress (Sansbury et al. 2011), defects as a result of mitochondrial toxins (Fern, 2003) and cell death (Nickens et al. 2013) than cells with little‐to‐no reserve capacity. Thus, when confronted with a metabolic challenge, untrained subjects without a mitochondrial reserve capacity may have a greater probablity of showing outward manifestations of this stress (e.g. mitochondrial dysfunction, exercise intolerance, etc.) than exercise‐trained subjects, who may be able to buffer such an insult with their mitochondrial reserve.
Experimental considerations
In the present study, Mito was assessed in permeabilized fibres with saturating levels of ADP and substrates for complex I and complex II of the electron transport chain. Although this general protocol reportedly yields the highest rates of respiration at the same time as maintaining physiological conditions (Gnaiger, 2009; Pesta & Gnaiger, 2012), and has previously been used to represent Mito (Boushel et al. 2011), it is possible that this paradigm and measures do not represent the true maximum capacity of the mitochondria. Nevertheless, in the improbable event that the Mito assessed in the present study was shown not to be maximal, the patterns exhibited in Figs 3 and 4 would still suggest a strong relationship between mitochondrial respiration and in untrained but not trained individuals under similar conditions.
Conclusions
The unique combination of in vivo and in vitro measures utilized in the present study clarifies the determinants of and identifies two very different sources of limitation to in untrained and endurance exercise‐trained individuals. Among untrained individuals, appears to be limited and therefore determined by mitochondrial O2 demand, despite evidence of adequate O2 supply. By contrast, among trained individuals, O2 supply appears to limit , despite the presence of a large mitochondrial respiratory reserve capacity. Given this evidence of reserve capacities at in both untrained and trained individuals, the results of the present study call into question the invariant matching of structural capacity to functional demand that is proposed to exist at by the premise of symmorphosis.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
JG, RG, MR and RR conceived and designed the experiments. JG, RG, AN, JD, GL MW, JW, TM, CH, CE, JJ, AB, DM, DW, MR and RR collected, assembled, analysed and interpretated data. JG, RG, AN, JD, GL MW, JW, TM, CH, CE, JJ, AB, DM, DW and RR drafted or revised the article. All data were collected in the Utah Vascular Research Laboratory at the University of Utah. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This study was funded in part by grants from the NIH National Heart, Lung and Blood Institute (PO1 H1091830) and VA Merit grant E6910R.
References
- Barrett‐O'Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS & Wray DW (2013). Taming the ‘sleeping giant’: the role of endothelin‐1 in the regulation of skeletal muscle blood flow and arterial blood pressure during exercise. Am J Physiol Heart Circ Physiol 304, H162–H169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomstrand E, Rådegran G & Saltin B (1997). Maximum rate of oxygen uptake by human skeletal muscle in relation to maximal activities of enzymes in the Krebs cycle. J Physiol 501, 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boushel R, Gnaiger E, Calbet JAL, Gonzalez‐Alonso J, Wright‐Paradis C, Sondergaard H, Ara I, Helge JW & Saltin B (2011). Muscle mitochondrial capacity exceeds maximal oxygen delivery in humans. Mitochondrion 11, 303–307. [DOI] [PubMed] [Google Scholar]
- Cardús J, Marrades RM, Roca J, Barberà JA, Diaz O, Masclans JR, Rodriguez‐Roisin R & Wagner PD (1998). Effects of FIO2 on leg VO2 during cycle ergometry in sedentary subjects. Med Sci Sports Exerc 30, 697–703. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, McKenzie DC, Haverkamp HC & Eldridge MW (2008). Update in the understanding of respiratory limitations to exercise performance in fit, active adults. Chest 134, 613–622. [DOI] [PubMed] [Google Scholar]
- Diamond JM (1992). Evolutionary physiology. The red flag of optimality. Nature 355, 204–206. [DOI] [PubMed] [Google Scholar]
- Fern R (2003). Variations in spare electron transport chain capacity: the answer to an old riddle? J Neurosci Res 71, 759–762. [DOI] [PubMed] [Google Scholar]
- Garten RS, Groot HJ, Rossman MJ, Gifford JR & Richardson RS (2014). The role of muscle mass in exercise‐induced hyperemia. J Appl Physiol 116, 1204–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnaiger E (2009). Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology. Int J Biochem Cell Biol 41, 1837–1845. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Armstrong RB, Saltin B, Saubert CW, Sembrowich WL & Shepherd RE (1973). Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol 34, 107–111. [DOI] [PubMed] [Google Scholar]
- Gollnick PD & Saltin B (1982). Significance of skeletal muscle oxidative enzyme enhancement with endurance training. Clin Physiol 2, 1–12. [DOI] [PubMed] [Google Scholar]
- Holloszy JO & Coyle EF (1984). Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 56, 831–838. [DOI] [PubMed] [Google Scholar]
- Hoppeler H & Weibel ER (1998). Limits for oxygen and substrate transport in mammals. J Exp Biol 201, 1051–1064. [DOI] [PubMed] [Google Scholar]
- Jacobs RA, Rasmussen P, Siebenmann C, Diaz V, Gassmann M, Pesta D, Gnaiger E, Nordsborg NB, Robach P & Lundby C (2011). Determinants of time trial performance and maximal incremental exercise in highly trained endurance athletes. J Appl Physiol 111, 1422–1430. [DOI] [PubMed] [Google Scholar]
- Jacobs RA, Flück D, Bonne TC, Bürgi S, Christensen PM, Toigo M & Lundby C (2013). Improvements in exercise performance with high‐intensity interval training coincide with an increase in skeletal muscle mitochondrial content and function. J Appl Physiol 115, 785–793. [DOI] [PubMed] [Google Scholar]
- Jacobs RA & Lundby C (2013). Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. J Appl Physiol 114, 344–350. [DOI] [PubMed] [Google Scholar]
- Jones P & Pearson J (1969). Anthropometric determination of leg fat and muscle plus bone volumes in young males and female adults. J Physiol 204, 63P–66P. [PubMed] [Google Scholar]
- Kemp GJ, Ahmad RE, Nicolay K & Prompers JJ (2015). Quantification of skeletal muscle mitochondrial function by (31) P magnetic resonance spectroscopy techniques: a quantitative review. Acta Physiol (Oxf) 213, 107–144. [DOI] [PubMed] [Google Scholar]
- Kenny GP, Reardon FD, Zaleski W, Reardon ML, Haman F & Ducharme MB (2003). Muscle temperature transients before, during, and after exercise measured using an intramuscular multisensor probe. J Appl Physiol 94, 2350–2357. [DOI] [PubMed] [Google Scholar]
- Knight DR, Schaffartzik W, Poole DC, Hogan MC, Bebout DE & Wagner PD (1993). Effects of hyperoxia on maximal leg O2 supply and utilization in men. J Appl Physiol 75, 2586–2594. [DOI] [PubMed] [Google Scholar]
- Layec G, Venturelli M, Jeong E & Richardson RS (2014). The validity of anthropometric leg muscle volume estimation across a wide spectrum: from able‐bodied adults to individuals with a spinal cord injury. J Appl Physiol 116, 1142–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BD (2008). VO2max: what do we know, and what do we still need to know? J Physiol 586, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaria R, Camporesi E, Aghemo P & Sassi G (1972). The effect of O 2 breathing on maximal aerobic power. Pflügers Arch 336, 225–235. [DOI] [PubMed] [Google Scholar]
- Nickens KP, Wikstrom JD, Shirihai OS, Patierno SR & Ceryak S (2013). A bioenergetic profile of non‐transformed fibroblasts uncovers a link between death‐resistance and enhanced spare respiratory capacity. Mitochondrion 13, 662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen PK, Kiens B & Saltin B (1999). Hyperoxia does not increase peak muscle oxygen uptake in small muscle group exercise. Acta Physiol Scand 166, 309–318. [DOI] [PubMed] [Google Scholar]
- Pesta D & Gnaiger E (2012). High‐resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810, 25–58. [DOI] [PubMed] [Google Scholar]
- Picard M, Taivassalo T, Ritchie D, Wright KJ, Thomas MM, Romestaing C & Hepple RT (2011). Mitochondrial structure and function are disrupted by standard isolation methods. PLoS ONE 6, e18317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Râdegran G, Blomstrand E & Saltin B (1999). Peak muscle perfusion and oxygen uptake in humans: importance of precise estimates of muscle mass. J Appl Physiol 87, 2375–2380. [DOI] [PubMed] [Google Scholar]
- Rasmussen UF, Rasmussen HN, Krustrup P, Quistorff B, Saltin B & Bangsbo J (2001). Aerobic metabolism of human quadriceps muscle: in vivo data parallel measurements on isolated mitochondria. Am J Physiol Endocrinol Metab 280, E301–E307. [DOI] [PubMed] [Google Scholar]
- Richardson RS (2003). Oxygen transport and utilization: an integration of the muscle systems. Adv Physiol Educ 27, 183–191. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Grassi B, Gavin TP, Haseler LJ, Tagore K, Roca J & Wagner PD (1999). Evidence of O2 supply‐dependent VO2max in the exercise‐trained human quadriceps. J Appl Physiol 86, 1048–1053. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Knight DR, Poole DC, Kurdak SS, Hogan MC, Grassi B & Wagner PD (1995). Determinants of maximal exercise VO2 during single leg knee‐extensor exercise in humans. Am J Physiol Heart Circ 268, H1453–H1461. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Leek BT, Gavin TP, Haseler LJ, Mudaliar SRD, Henry R, Mathieu‐Costello O & Wagner PD (2004). Reduced mechanical efficiency in chronic obstructive pulmonary disease but normal peak Vo(2) with small muscle mass exercise. Am J Respir Crit Care Med 169, 89–96. [DOI] [PubMed] [Google Scholar]
- Richardson RS & Saltin B (1998). Human muscle blood flow and metabolism studied in the isolated quadriceps muscles. Med Sci Sports Exerc 30, 28–33. [DOI] [PubMed] [Google Scholar]
- Rizzo RJ, Sandager G, Astleford P, Payne K, Peterson‐Kennedy L, Flinn WR & Yao JST (1990). Mesenteric flow velocity variations as a function of angle of insonation. J Vasc Surg 11, 688–694. [DOI] [PubMed] [Google Scholar]
- Roca J, Agusti a G, Alonso A, Poole DC, Viegas C, a Barbera J, Rodriguez‐Roisin R, Ferrer A & Wagner PD (1992). Effects of training on muscle O2 transport at VO2max . J Appl Physiol 73, 1067–1076. [DOI] [PubMed] [Google Scholar]
- Saltin B, Gagge AP & Stolwijk JA (1968). Muscle temperature during submaximal exercise in man. J Appl Physiol 25, 679–688. [DOI] [PubMed] [Google Scholar]
- Sansbury BE, Jones SP, Riggs DW, Darley‐Usmar VM & Hill BG (2011). Bioenergetic function in cardiovascular cells: the importance of the reserve capacity and its biological regulation. Chem Biol Interact 191, 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner PD (2008). Systemic oxygen transport and utilization. J Breath Res 2, 1–12. [DOI] [PubMed] [Google Scholar]
- Wagner PD (2015). CrossTalk proposal: diffusion limitation of O2 from microvessels into muscle does contribute to the limitation of V̇O2 max. J Physiol 593, 3757–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel ER, Taylor CR & Hoppeler H (1991). The concept of symmorphosis: a testable hypothesis of structure‐function relationship. Proc Natl Acad Sci U S A 88, 10357–10361. [DOI] [PMC free article] [PubMed] [Google Scholar]


