Abstract
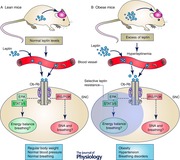
With the global epidemic of obesity, breathing disorders associated with excess body weight have markedly increased. Respiratory dysfunctions caused by obesity were originally attributed to mechanical factors; however, recent studies have suggested a pathophysiological component that involves the central nervous system (CNS) and hormones such as leptin produced by adipocytes as well as other cells. Leptin is suggested to stimulate breathing and leptin deficiency causes an impairment of the chemoreflex, which can be reverted by leptin therapy. This facilitation of the chemoreflex may depend on the action of leptin in the hindbrain areas involved in the respiratory control such as the nucleus of the solitary tract (NTS), a site that receives chemosensory afferents, and the ventral surface of the medulla that includes the retrotrapezoid nucleus (RTN), a central chemosensitive area, and the rostral ventrolateral medulla (RVLM). Although the mechanisms and pathways activated by leptin to facilitate breathing are still not completely clear, evidence suggests that the facilitatory effects of leptin on breathing require the brain melanocortin system, including the POMC–MC4R pathway, a mechanism also activated by leptin to modulate blood pressure. The results of all the studies that have investigated the effect of leptin on breathing suggest that disruption of leptin signalling as caused by obesity‐induced reduction of central leptin function (leptin resistance) is a relevant mechanism that may contribute to respiratory dysfunctions associated with obesity.
Abbreviations
- ARC
arcuate nucleus
- CNS
central nervous system
- LR
leptin receptor
- MC3/4R
melanocortin 3 and 4 receptors
- NTS
nucleus of the solitary tract
- POMC
proopiomelanocortin
- RSNA
renal sympathetic nerve activity
- RTN
retrotrapezoid nucleus
- RVLM
rostral ventrolateral medulla
Introduction
With the global epidemic of obesity, the prevalence of breathing disorders associated with excess body weight has markedly increased (Leung & Bradley, 2001; Gilat et al. 2014). The most common types of breathing disorder exhibited by obese persons are obesity hypoventilation syndrome (OHS) and obstructive sleep apnoea (OSA) (Malhotra & White, 2002; Olson & Zwillich, 2005). These conditions are not mutually exclusive and the majority of the patients with OHS also have severe OSA (Malli et al. 2010). Furthermore, a pathological consequence of OSA is the development of sympathetic overactivity and arterial hypertension (Malli et al. 2010; Mansukhani et al. 2014). The impact of obesity on breathing disorders was originally thought to be due to excessive fat tissue in the upper airways that increases the risk of obstruction during sleep (Malhotra & White, 2002; Olson & Zwillich, 2005). However, recent studies have suggested that an excess of adipose tissue can contribute to the genesis of these syndromes (OHS and OSA) through its exaggerated production of adipokines (Makinodan et al. 2008; Malli et al. 2010; Cundrle et al. 2013).
Leptin, a hormone produced by adipocytes as well as other cells, is a respiratory stimulant which raises the possibility that obese patients may exhibit hypoventilation as a consequence of, at least in part, reduced leptin action in the brain (Morris & Rui, 2009; Malli et al. 2010). In agreement with this hypothesis, experimental studies have demonstrated that leptin‐deficient (ob/ob) mice have an impaired ventilatory response to hypercapnia, a deficiency that can be rescued by systemic or central leptin replacement independently of changes in body weight (O'Donnell et al. 2000; Bassi et al. 2012). Additionally, intravenous infusion of leptin for 60 min elicited a long‐lasting increase in the amplitude of phrenic nerve discharge in rats (peaked at 90 min, 30 min after terminating leptin infusion), supporting the role of leptin as an important facilitator of ventilation (Chang et al. 2013).
Where does leptin act in the CNS to modulate ventilation?
Leptin might act in the nucleus of the solitary tract (NTS) to stimulate breathing and the chemoreflex
Leptin circulates freely in the plasma and crosses the blood–brain barrier via a saturable receptor‐mediated transport system (Morris & Rui, 2009) to enter the central nervous system (CNS) and regulate neural pathways that control appetite (Grill et al. 2002), sympathetic nerve activity (SNA), thermogenesis (Rahmouni & Morgan, 2007; Mark et al. 2009) and potentially ventilatory function (Inyushkina et al. 2010; Bassi et al. 2012, 2014 a).
The hypothalamic arcuate nucleus (ARC) was initially considered the main site of leptin actions in modulating energy balance and sympathetic activity; however, increasing evidence suggests that leptin may also act on a more extensive brain network that includes hindbrain areas such as the NTS and the ventral surface of the medulla (Grill et al. 2002; Ciriello & Moreau, 2013; Barnes & McDougal, 2014; Arnold & Diz, 2014; Ciriello & Caverson, 2014; Bassi et al. 2014 a). The presence of functional leptin receptors (LRs) was demonstrated on cell bodies within the NTS and systemic or focal injections of leptin into the NTS enhanced c‐fos expression in the caudal NTS subnuclei, suggesting that the NTS is an area activated by leptin (Mercer et al. 1998; Elias et al. 2000; Hosoi et al. 2002; Ciriello & Moreau, 2013). Injections of leptin into the NTS of anaesthetized rats acutely increased basal renal sympathetic nerve activity (RSNA) and the pressor response to peripheral chemoreflex activation (Mark et al. 2009; Ciriello & Moreau, 2012, 2013; Ciriello & Caverson, 2014). In addition, leptin in the NTS attenuated the arterial baroreflex response (Arnold et al. 2009; Arnold & Diz, 2014) and enhanced basal respiration activity (Inyushkin et al. 2009; Inyushkina et al. 2010). These data are evidence that the NTS might be a site of leptin action to control chemoreflex responses (Table 1, and Figs 1 and 2).
Table 1.
Ventilatory responses to leptin in different conditions
| Species |
|
Body weight | Leptin therapy and breathing responses | References | |
| ob/ob mice (in vivo) | ↑ or ↔ basal | ↑↑ | Systemic or 4V (3 days) | O'Donnell et al. (2000) | |
| ↓ –CO2 | ↑ basal | Bassi et al. (2012) | |||
| ↑ –CO2 | |||||
| ob/ob mice (in vivo) | ↔ basal | ↑↑ | RTN/pFRG (3 days) | Bassi et al. (2014 a) | |
| ↓ –CO2 | ↑ basal | ||||
| ↑ –CO2 | |||||
| Mice LepR/POMC‐cre | ↓ basal | ↑↑ | — | Bassi et al. (2014 b) | |
| LepR/Nestin‐cre | ↓ –CO2 | ||||
| MC4R−/− | |||||
| Rats (anesthetized) | ↔ | ↔ | NTS (bolus) | Inyushkin et al. (2009) | |
| ↑ basal | Inyushkina et al. (2010) | ||||
| Rats (anesthetized) | ↔ | ↔ | NTS (bolus) | ||
| ↑ AP ↓ bradycardia | Ciriello & Moreau, (2012) | ||||
| ↑ chemoreflexic response | |||||
| Rats (in vitro) | ↔ | ↔ | Brainstem slices | Bassi et al. (2013) | |
| ↔ RTN–CO2 sensitivity | |||||
| Rats (in vivo) | ↔ | ↔ | LV (6 days) | Bassi et al. (2014 b) | |
| ↑ basal | |||||
| ↑ –CO2 | |||||
| Rats (in vivo) | ↔ | ↔ | LV + MC3/4R blockade | Bassi et al. (2014 b) | |
| (6 days) | |||||
| ↔ basal | |||||
| ↔ –CO2 |
, ventilation; RTN/pFRG, retrotrapezoid nuclei/parafacial respiratory group; NTS, nucleus of the solitary tract; AP, arterial pressure; LV, lateral ventricle; 4V, fourth ventricle; MC3/4R, melanocortin 3 and 4 receptors. ↑ increased or elevated; ↑↑ extreme elevated; ↓ reduced; ↔ no change.
Figure 1. Main CNS sites of leptin action to modulate food intake and sympathetic activity .
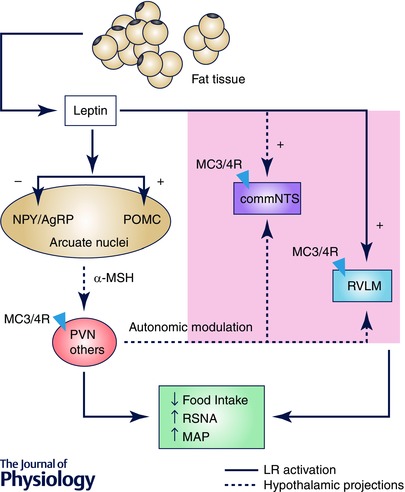
Leptin activates leptin receptors (LRs) in the arcuate nucleus of the hypothalamus causing inhibition of neuropeptide Y/agouti‐related peptide (NPY/AgRP) and stimulation of POMC neurons. Once activated, POMC neurones release α‐MSH that stimulates the MC3/4R (depicted as triangles) mainly in PVN, promoting reduction of food intake and increase of renal sympathetic nerve activity (RSNA) and mean arterial pressure (MAP). Hypothalamic projections to the NTS and RVLM may mediate leptin effects on autonomic function. Moreover, leptin can also act directly in brainstem nuclei such as NTS and RVLM to modulate energy balance and RSNA potentially via activation of MC3/4R. Abbreviations: MC3/4R, melanocortin 3 and 4 receptors; commNTS, commissural nucleus of the solitary tract; POMC, proopiomelanocortin; PVN, paraventricular nucleus; RVLM, rostral ventrolateral medulla.
Figure 2. Acute leptin application had no effect on the activity of chemosensitive RTN neurones or astrocytes .
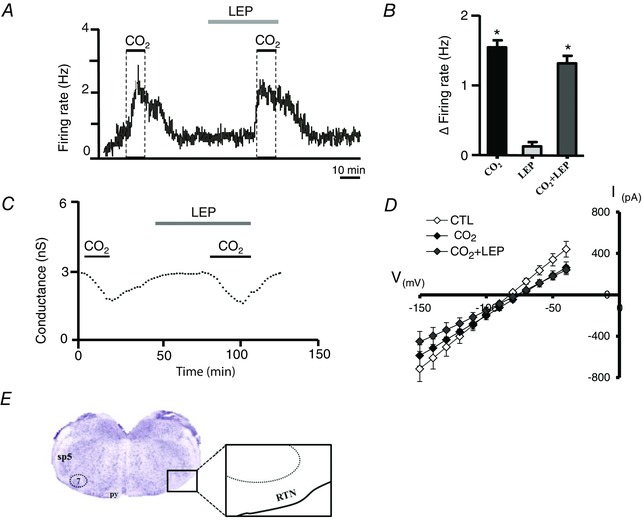
A, firing rate trace showing the response of a chemosensitive RTN neurone to 15% CO2 under control conditions and in the presence of 100 nm leptin (LEP). B, summary data showing the firing rate response of RTN chemoreceptors (n = 10) to 15% CO2, leptin and 15% CO2 plus leptin. C, conductance trace (holding potential = −80 mV, in TTX to block neuronal activity) showing the response of a chemosensitive RTN astrocyte to 15% CO2 under control conditions and after 30 min incubation in 100 nm leptin. D, summary data (n = 9) showing the current–voltage relationship under control conditions, during 15% CO2 and in 15% CO2 plus leptin. *Different from baseline conditions (P ˂ 0.05). After recording, the cells were filled with biocytin for later immunohistochemical detection of leptin receptors. E, coronal brain section showing the location of RTN; py, pyramidal tract; sp5, spinal trigeminal tract.
The effects of LR stimulation are suggested to be mediated by cytokine receptor‐like signals, including the activation of Janus kinases (JAKs) andsignal transducers and activators of transcription (STATs) (Ghilardi & Skoda, 1997). In the CNS, leptin increases the activity of JAK2 to trigger three major intracellular pathways: (1) phosphorylation of the tyrosine (Tyr) residue 1138 of LR, resulting in phosphorylation and nuclear translocation of STAT3; (2) phosphorylation of insulin receptor substrate 2 (IRS2), activating phosphatidylinositol 3‐kinase (PI3K); and (3) phosphorylation of Tyr985 of LR, which recruits the tyrosine phosphatase SHP2 to activate ERK/MAPK protein kinases.
Although the involvement of these intracellular signalling pathways mediating the various actions of leptin on appetite behaviour and regulation of sympathetic activity has been the subject of numerous studies (Dubinion et al. 2013; do Carmo et al. 2013, 2014; Harlan & Rahmouni, 2013), the signalling pathways related to breathing function is poorly understood. Still controversial is the phosphorylation of STAT3 in proopiomelanocortin (POMC) neurons. One study proposed that leptin activates STAT3 in ARC POMC neurons, not in NTS POMC neurons (Huo et al. 2006), whereas another study demonstrated phosphorylation of STAT3 by leptin in NTS neurons of mice, but not in NTS neurons of rats, suggesting an apparent species divergence (Hosoi et al. 2002; Nagaishi et al. 2014; Deasi & Harris, 2015).
In addition to the mechanisms that may involve the control of the transcription of target genes requiring time for leptin to produce effects like the phosphorylation of STAT3, alternative mechanisms that directly affect ion channels and membrane potential were also suggested for the acute modulation of cardiorespiratory responses by leptin acting in the NTS (Cowley et al. 2001; Cirielo & Moreau, 2010). It was proposed that leptin may facilitate the activation of some of the NTS neurons that were at rest promoting a potentiation of the response (Cirielo & Moreau, 2013). On the other hand, leptin may have an excitatory or inhibitory effect in different neurons and increased firing rate of preproglucagon neurons of the NTS or hyperpolarization of rat NTS neurons by the action of leptin was demonstrated in different studies (Williams & Smith, 2006; Hisadome et al. 2010). More studies are necessary to confirm the NTS as the site of action of leptin to facilitate respiratory responses and the mechanisms by which leptin alters the activity of cardiorespiratory NTS neurons.
Effects of leptin on the ventral surface of the medulla in modulating breathing and cardiovascular responses
The breathing rhythm and pattern generation depend on the coordinated activity of neurons grouped in a long column located laterally in the brainstem, extending from the dorsolateral pons to the caudal ventrolateral medulla (Nattie, 1999; Feldman et al. 2003; Guyenet et al. 2005; Moreira et al. 2006). In this region, the retrotrapezoid nucleus (RTN) is considered a primary site for central chemoreception and integration of signals from peripheral chemoreceptors that ascend from the NTS (Guyenet et al. 2008; Takakura et al. 2011). Microinjections of leptin for three consecutive days into the rostral ventrolateral medulla increased baseline ventilation and the ventilatory response to CO2 in ob/ob mice (Table 1 and Fig. 3) (Bassi et al. 2014 a). These results suggest that leptin may facilitate the central chemoreflex acting in the rostral ventrolateral surface of the medulla, which among other centres includes the RTN and RVLM (Bassi et al. 2014 a). However, acute exposure to leptin did not change the activity of RTN chemosensitive neurons in brainstem slices from rat pups (7–12 days postnatal). Bath application of leptin to these chemosensitive RTN neurones from rat pups, identified by increased firing rate to hypercapnia (≥1.5 Hz response to 10% CO2) (Mulkey et al. 2006; Wenker et al. 2012), produced negligible effects on basal neuronal activity and CO2/H+ sensitivity of the neurones (Fig. 2 A and B; Bassi et al. 2013). Astrocytes can also contribute to chemoreception in the RTN (Wenker et al. 2010; Gourine et al. 2010; Huckstepp et al. 2010) and LRs were found in astrocytes in the hypothalamus (Kim et al. 2014). Tests in vitro also demonstrated that leptin did not affect basal conductance and CO2/H+‐sensitive current of chemosensitive RTN astrocytes (Fig. 2 C and D; Bassi et al. 2013). Furthermore, we found no evidence that leptin receptors are present on chemosensitive RTN neurons or astrocytes. Therefore, these results suggest that the activity of RTN chemoreceptors (neurones or astrocytes) is not modulated by leptin on a short time scale.
Figure 3. Potential sites of leptin action to modulate breathing .
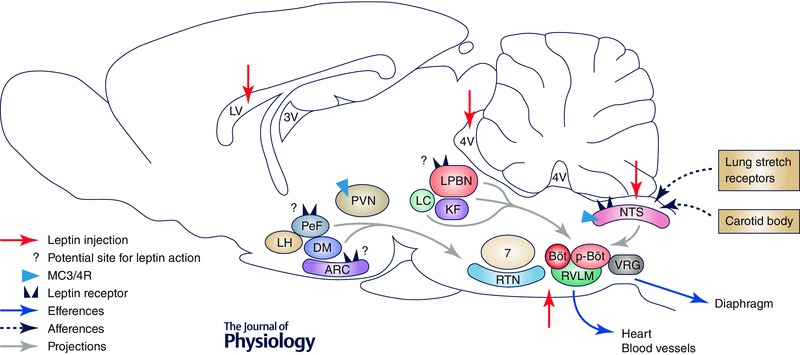
Schematic view showing sites where leptin was administered (red arrows) into the CNS in previous studies (Inyushkin et al. 2009, 2010; Bassi et al. 2012, 2013, 2014; Ciriello & Moreau, 2012) and possible sites of leptin action for breathing modulation. Leptin administered into the lateral ventricle (LV) might act in different nuclei of the hypothalamus, including in perifornical (PeF), dorsomedial (DM) and arcuate (ARC) nuclei that express leptin receptors (LR) and MC3/4R and which are known to affect ventilation via projections to nuclei in the pons and medulla involved in respiratory control, among them the lateral parabrachial nuclei (LBPN) and the retrotrapezoid nuclei (RTN). In addition, leptin may also directly act in brainstem nuclei that control breathing and chemoreflex function, such as the nucleus of the solitary tract (NTS), the rostral ventrolateral medulla (RVLM) and the RTN. However, the respiratory network is complex and leptin effects on respiratory control are still poorly understood. Other respiratory nuclei that leptin might act on are locus coeruleus (LC), Kölliker‐Fuse (KF), Bötzinger complex (Böt), pre‐Bötzinger complex (p‐Böt) and ventral respiratory group (VRG). Other abbreviations: 7, facial nuclei; LH, lateral nucleus of hypothalamus; PVN, paraventricular nucleus of hypothalamus; 3V, third ventricle; 4V, fourth ventricle.
In summary, in vivo experiments demonstrated that leptin stimulated the ventilatory response to CO2 when chronically administered into the rostral ventrolateral region of the medulla in mice, suggesting that leptin may facilitate chemorespiratory mechanisms located in this region. On the other hand, tests in vitro showed no effect of acute treatment with leptin on the basal level of the CO2/H+ sensitivity of chemosensitive RTN neurones or astrocytes in slices of rat pups. These contradictory results might be related to the different species tested or because the effects of leptin on chemorespiratory mechanisms might depend on its chronic long time scale action in the ventrolateral region of the medulla. It is also possible that leptin does not directly act in the chemosensitive RTN neurons or astrocytes. Leptin may act in other neurones (not chemosensitive) that are part of the neural circuitry involved in chemoreflex, such as those located in the RVLM and Bötzinger complex in which LRs are present (Barnes et al. 2010; Bassi et al. 2012) (Fig. 3). The potential action of leptin in the ventrolateral medulla to facilitate chemoreflex and the effects of leptin on central chemoreception are questions that need more studies in the future.
Leptin also increases RSNA and, therefore, may affect blood pressure contributing to the development of hypertension (Hall et al. 2010). Leptin may affect blood pressure and RSNA by directly acting on LRs present on adrenergic/noradrenergic C1/A1 cells located in the ventrolateral medulla, which possibly are part of the pre‐autonomic neurones of the RVLM involved in cardiovascular regulation (Barnes & McDougal, 2014). Activation of either the hypoxic or hypercapnic chemoreflex elicits hyperventilation combined with sympathetic activation. These responses are controlled by a neuronal network within the ventrolateral medulla and depend on the modulation of the pre‐sympathetic neurons in the RVLM by the respiratory pattern generator (Haselton & Guyenet, 1989; Dempsey et al. 2002). Considering that leptin administered into the ventrolateral medulla enhances basal ventilation and the hypercapnic ventilatory response (see above), it is possible that leptin acting on the ventral surface of the medulla might also influence blood pressure by contributing to this coupling of respiratory and cardiovascular modulation (Figs 2 and 3). This is a further area ripe for investigation.
Leptin actions in the hypothalamus may also contribute to the chemoreflex
The brain melanocortin system mediates the majority of leptin effects on appetite, metabolism and cardiovascular function, especially in the hypothalamus (Greenfield, 2011; Dubinion et al. 2013; Li et al. 2013). Leptin acts on POMC neurons in the ARC and promotes the release of α‐melanocyte‐stimulating hormone (α‐MSH) that, in turn, activates the melanocortin 3 and 4 receptors (MC3/4R) (Fig. 1) (Cowley et al. 2001; Morton & Schwartz, 2011.
Leptin acting on POMC neurones and recruiting melanocortin system also appears to be involved in leptin effects on breathing function. Agouti yellow (Ay) mice, a model overexpressing the MC3/4R‐inhibiting agouti protein, exhibited a reduced CO2 ventilatory responsiveness during non‐REM sleep, but not during wakefulness (Polotsky et al. 2004). Similarly, central MC3/4R blockade reduced the stimulatory effects of intracerebroventricular leptin administration on the ventilatory response to central chemoreceptor activation (Bassi et al. 2014 b), supporting the involvement of the melanocortin system in mediating leptin effects on ventilation (Table 1). In addition, mice with LR deletion in the entire CNS (Lept‐Nestin‐cre) or specifically in POMC neurons (LepR/POMC‐cre) also exhibited attenuated baseline and ventilatory responses to CO2 (Bassi et al. 2014 b). Taken together, these results suggest that leptin facilitates ventilatory function, at least in part, via the brain melanocortin system, including the POMC–MC4R pathway (Figs 1, 2, 3).
The MC3/4R is a major pathway in the hypothalamus and the expression of LR and/or MC4R is present in many hypothalamic nuclei, including the paraventricular nucleus (PVN), dorsomedial nucleus (DMH) and peri‐fornical region (PeF) of the hypothalamus (Kishi et al. 2003). Activation of the PVN region with bicuculline or glutamate increased phrenic and hypoglossal outflows in some studies (Yeh et al. 1997; Mack et al. 2002; Fortuna et al. 2009). Additionally, disinhibition of neurones in the DMH increased phrenic nerve activity (McDowall et al. 2007), whereas microinjection of gabazine into the PeF increased blood pressure, phrenic nerve discharge and firing rate of the chemosensitive RTN neurones in isoflurane‐anaesthetized rats (Fortuna et al. 2009; Li et al. 2013). Therefore, perhaps leptin might also modulate respiratory responses activating the melanocortin system in these hypothalamic nuclei (Fig. 3), a hypothesis that needs future investigation.
Besides this hypothalamic effect of leptin and MC4R, extra‐hypothalamic autonomic and respiratory control by leptin–MC4R has been suggested. MC4R‐specific hybridization was evident in the central nucleus of the amygdala, the periaqueductal grey, the lateral parabrachial nucleus (LPBN), the NTS, the dorsal motor nucleus of the vagus (DMV), and the intermediolateral nucleus of the spinal cord (IML), among others (Kishi et al. 2003). In addition, the presence of MC4R in the RVLM is also suggested. The direct administration of picomolar concentrations of MC3/4R agonist (5 pmol of MTII) into the RVLM elicited hyperthermia, tachycardia, hyperactivity and body weight loss (Skibicka & Grill, 2009). In the same study, the effects of the activation of melanocortin receptors in other hindbrain nuclei, such as the NTS and LPBN, indicate that the effects of CNS MC3/4R stimulation are mediated by an anatomical distributed network of melanocortin receptors. However, the involvement of MC3/4R on chemorespiratory control in the ventral area of medulla has to be investigated.
It is important to note that in some studies leptin was injected in a relatively high concentration and, therefore, it is not possible to exclude actions outside the sites of injection such as the NTS and RTN and/or that the actions are more pharmacological than physiological. Thus, more studies are necessary to clarify this potential physiological action of leptin in specific areas of the hindbrain involved in cardiorespiratory control.
Perspectives and conclusions
Emerging evidence clearly shows that leptin is a relevant modulator of breathing. In the last few years, an increasing number of studies have investigated the effects of this peptide in the modulation of the respiratory activity, especially on central chemorespiratory control as recent results have shown. Leptin may act in different areas of the CNS modulating breathing by acute and chronic mechanisms. The rapid effects of leptin were demonstrated in the NTS and attributed to a direct effect of leptin on membrane potential. Conversely, in ventral areas of the medulla, such as the RTN and RVLM, it seems that the modulation of breathing depends on chronic effects of leptin. The POMC–MC3/4Rs are also a potential mechanism activated by leptin to modulate breathing. However, more studies are necessary for a definitive conclusion about the involvement of these mechanisms on the effects of leptin on respiratory responses.
Although few studies about the action of leptin in the complex network involved with breathing control can be found in the literature, the information that currently exists is enough to emphasize the important relationship between obesity and excessive production of leptin, which can generate dysfunction in the action of this peptide in the CNS (leptin resistance), promoting not only an excess accumulation of fat tissue and cardiovascular injury, but also serious respiratory disorder. Given this, a range of possibilities is open for future experiments to clarify the role of leptin in respiratory function.
Additional information
Competing interests
The authors have no conflicts of interest to disclose.
Funding
The authors’ work was supported by grants from FAPESP (thematic and post‐doc), CNPq, Capes and UNESP‐PROPE (Brazil), and the National Heart, Lung and Blood Institute (HL104101; USA).
Biography
Mirian Bassi earned her PhD at the University of São Paulo, supervised by Professor ML Glass and received training in integrative respiratory physiology. Subsequently, she joined the Guyton Research Center, Mississippi, USA supervised by Professor JE Hall. Most recently she joined School of Dentistry of Araraquara – UNESP, supervised by Professor E Colombari. Her research has been focus in the central respiratory control and obesity, investigating new mechanisms mediated by CNS in the mediation of cardiorespiratory function, specially the role of leptin on arterial and central chemoreflex. Eduardo Colombari obtained his DDS degree at the School of Dentistry of Araraquara – UNESP and his PhD at the University of São Paulo, supervised by Professor BH Machado and Professor WT Talman (The University of Iowa) and received classical training in whole animal integrative physiology regarding cardiovascular and body fluid homeostasis. Subsequently, he worked in Federal University of São Paulo (UNIFESP) from Assistant to Associate Professor. As Professor of Physiology rejoined School of Dentistry of Araraquara – UNESP. He has trained many PhD students and his current research focuses on central neural control of cardiovascular and integrative physiology on normal and pathophysiologycal condition.

This review was presented at the symposium “Bristol‐São Paulo Research Summit” – Autonomic and neuroendocrine dysfunction in chronic diseases, which took place at the University of São Paulo, Brasil, between 7–9 August 2014.
References
- Arnold AC & Diz DI (2014). Endogenous leptin contributes to baroreflex suppression within the solitary tract nucleus of aged rats. Am J Physiol Heart Circ Physiol 307, H1539–H1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AC, Shaltout HA, Gallagher PE & Diz DI (2009). Leptin impairs cardiovagal baroreflex function at the level of the solitary tract nucleus. Hypertension 54, 1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes MJ & McDougal DH (2014). Leptin into the ventral lateral medulla (RVLM) augments renal sympathetic nerve activity and blood pressure. Front Neurosci 8, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes MJ, Rogers RC, Van Meter MJ & Hermann GE (2010). Co‐localization of TRHR1 and LepRb receptors on neurons in the hindbrain of the rat. Brain Res 1355, 70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi M, Furuya WI, Menani JV, Colombari DS, do Carmo JM, da Silva AA, Hall JE, Moreira TS, Wenker IC, Mulkey DK & Colombari E (2014. a). Leptin into the ventrolateral medulla facilitates chemorespiratory response in leptin deficient (ob/ob) mice. Acta Physiol (Oxf) 211, 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi M, Furuya WI, Menani JV, Colombari DSA, do Carmo JM, da Silva AA, Hall JE, Wenker IC, Mulkey DK & Colombari E (2013). Effects of leptin in the retrotrapezoid nucleus (RTN) on CO2‐sensitivity and respiration. FASEB J 27, 1137.12 (abstract).23288930 [Google Scholar]
- Bassi M, Giusti H, Leite CM, Anselmo‐Franci JA, do Carmo JM, da Silva AA, Hall JE, Colombari E & Glass ML (2012). Central leptin replacement enhances chemorespiratory responses in leptin‐deficient mice independent of changes in body weight. Pflugers Arch 464, 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi M, Nakamura NB, Furuya WI, Colombari DSA, Menani JV, do Carmo JM, da Silva AA, Hall JE & Colombari E (2014. b). Activation of the brain melanocortin system is required for leptin‐induced modulation of chemorespiratory function. Acta Physiol (Oxf) 213, 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Z, Ballou E, Jiao W, McKenna KE, Morrison SF & McCrimmon DR (2013). Systemic leptin produces a long‐lasting increase in respiratory motor output in rats. Front Physiol 4, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriello J & Caverson MM (2014). Carotid chemoreceptor afferent projections to leptin receptor containing neurons in nucleus of the solitary tract. Peptides 58, 30–35. [DOI] [PubMed] [Google Scholar]
- Ciriello J & Moreau JM (2012). Leptin signaling in the nucleus of the solitary tract alters the cardiovascular responses to activation of the chemoreceptor reflex. Am J Physiol Regul Integr Comp Physiol 303, R727–R736. [DOI] [PubMed] [Google Scholar]
- Ciriello J & Moreau JM (2013). Systemic administration of leptin potentiates the response of neurons in the nucleus of the solitary tract to chemoreceptor activation in the rat. Neuroscience 229, 88–99. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD & Low MJ (2001). Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484. [DOI] [PubMed] [Google Scholar]
- Cundrle I Jr, Somers VK, Singh P, Johnson BD, Scott CG & Olson LJ (2013). The relationship between leptin and ventilatory control in heart failure. J Card Fail 19, 756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Sheel AW, St Croix CM & Morgan BJ (2002). Respiratory influences on sympathetic vasomotor outflow in humans. Respir Physiol Neurobiol 130, 3–20. [DOI] [PubMed] [Google Scholar]
- Desai BN & Harris RBS (2015). Leptin in the hindbrain facilitates phosphorylation of STAT3 in the hypothalamus. American Journal of Physiology Endocrinology and Metabolism 308, E351–E361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo JM, da Silva AA, Ebaady SE, Sessums PO, Abraham RS, Elmquist JK, Lowell BB & Hall JE (2014). Shp2 signaling in POMC neurons is important for leptin's actions on blood pressure, energy balance, and glucose regulation. Am J Physiol Regul Integr Comp Physiol 307, R1438–R1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo JM, da Silva AA, Sessums PO, Ebaady SH, Pace BR, Rushing JS, Davis MT & Hall JE (2013). Role of Shp2 in forebrain neurons in regulating metabolic and cardiovascular functions and responses to leptin. Int J Obes (Lond) 38, 775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinion JH, do Carmo JM, Adi A, Hamza S, da Silva AA & Hall JE (2013). Role of proopiomelanocortin neuron Stat3 in regulating arterial pressure and mediating the chronic effects of leptin. Hypertension 61, 1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias CF, Kelly JF, Lee CE, Ahima RS, Drucker DJ, Saper CB & Elmquist JK (2000). Chemical characterization of leptin‐activated neurons in the rat brain. J Comp Neurol 423, 261–281. [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS & Nattie EE (2003). Breathing: rhythmicity plasticity chemosensitivity. Annu Rev Neurosci 26, 239–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuna MG, Stornetta RL, West GH & Guyenet PG (2009). Activation of the retrotrapezoid nucleus by posterior hypothalamic stimulation. J Physiol 587, 5121–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghilardi N & Skoda RC (1997). The leptin receptor activates janus kinase 2 and signals for proliferation in a factor‐dependent cell line. Mol Endocrinol 11, 393–399. [DOI] [PubMed] [Google Scholar]
- Gilat H, Vinker S, Buda I, Soudry E, Shani M & Bachar G (2014). Obstructive sleep apnea and cardiovascular comorbidities: a large epidemiologic study. Medicine (Baltimore) 93, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K & Kasparov S (2010). Astrocytes control breathing through pH‐dependent release of ATP. Science 329, 571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield JR (2011). Melanocortin signaling and the regulation of blood pressure in human obesity. J Neuroendocrinol 23, 186–193. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J & Baskin DG (2002). Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143, 239–246. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL & Bayliss DA (2005). Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci 25, 8938–8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL & Bayliss DA (2008). Retrotrapezoid nucleus and central chemoreception. J Physiol 586, 2043–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G & Stec DE (2010). Obesity‐induced hypertension: role of sympathetic nervous system, leptin and melanocortins. J Biol Chem 285, 17271–17276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan SM & Rahmouni K (2013). PI3K signaling: A key pathway in the control of sympathetic traffic and arterial pressure by leptin. Molecular Metabolism 2, 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselton JR & Guyenet PG (1989). Central respiratory modulation of medullary sympathoexcitatory neurons in rat. Am J Physiol 256, R739–R750. [DOI] [PubMed] [Google Scholar]
- Hisadome K, Reimann F, Gribble FM & Trapp S (2010). Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon‐like peptide 1 neurons. Diabetes 59, 1890–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi T, Kawagishi T, Okuma Y, Tanaka J & Nomura Y (2002). Brain stem is a direct target for leptin's action in the central nervous system. Endocrinology 143, 3498–3504. [DOI] [PubMed] [Google Scholar]
- Huckstepp RT, id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV & Dale N (2010). Connexin hemichannel‐mediated CO2‐dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol 588, 3901–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo L, Grill HJ & Bjørbæk C (2006). Divergent regulation of proopiomelanocortin neurons by leptin in the nucleus of the solitary tract and in the arcuate hypothalamic nucleus. Diabetes 55, 567–573. [DOI] [PubMed] [Google Scholar]
- Inyushkin AN, Inyushkina EM & Merkulova NA (2009). Respiratory responses to microinjections of leptin into the solitary tract nucleus. Neurosci Behav Physiol 39, 231–240. [DOI] [PubMed] [Google Scholar]
- Inyushkina EM, Merkulova NA & Inyushkin AN (2010). Mechanisms of the respiratory activity of leptin at the level of the solitary tract nucleus. Neurosci Behav Physiol 40, 707–713. [DOI] [PubMed] [Google Scholar]
- Kim JG, Suyama S, Koch M, Jin S, Argente‐Arizon P, Argente J, Liu ZW, Zimmer MR, Jeong JK, Szigeti‐Buck K, Gao Y, Garcia‐Caceres C, Yi CX, Salmaso N, Vaccarino FM, Chowen J, Diano S, Dietrich MO, Tschöp MH & Horvath TL (2014). Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat Neurosci 17, 908–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB & Elmquist JK (2003). Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol 457, 213–235. [DOI] [PubMed] [Google Scholar]
- Leung RS & Bradley TD (2001). Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 164, 2147–2165. [DOI] [PubMed] [Google Scholar]
- Li P, Cui BP, Zhang LL, Sun HJ, Liu TY & Zhu GQ (2013). Melanocortin 3/4 receptors in paraventricular nucleus modulate sympathetic outflow and blood pressure. Exp Physiol 98, 435–443. [DOI] [PubMed] [Google Scholar]
- McDowall LM, Horiuchi J & Dampney RA (2007). Effects of disinhibition of neurons in the dorsomedial hypothalamus on central respiratory drive. Am J Physiol Regul Integr Comp Physiol 293, R1728–R1735. [DOI] [PubMed] [Google Scholar]
- Mack SO, Kc P, Wu M, Coleman BR, Tolentino‐Silva FP & Haxhiu MA (2002). Paraventricular oxytocin neurons are involved in neural modulation of breathing. J Appl Physiol (1985) 92, 826–834. [DOI] [PubMed] [Google Scholar]
- Makinodan K, Yoshikawa M, Fukuoka A, Tamaki S, Koyama N, Yamauchi M, Tomoda K, Hamada K & Kimura H (2008). Effect of serum leptin levels on hypercapnic ventilatory response in obstructive sleep apnea. Respiration 75, 257–264. [DOI] [PubMed] [Google Scholar]
- Malhotra A & White DP (2002). Obstructive sleep apnoea. Lancet 360, 237–245. [DOI] [PubMed] [Google Scholar]
- Malli F, Papaioannou AI, Gourgoulianis KI & Daniil Z (2010). The role of leptin in the respiratory system: an overview. Respir Res 111, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansukhani MP, Kara T, Caples SM & Somers VK (2014). Chemoreflexes, sleep apnea, and sympathetic dysregulation. Curr Hypertens Rep 16, 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark AL, Agassandian K, Morgan DA, Liu X, Cassel MD & Rahmouni K (2009). Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension 53, 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JG, Moar KM & Hoggard N (1998). Localization of leptin receptor (Ob‐R) messenger ribonucleic acid in the rodent hindbrain. Endocrinology 139, 29–34. [DOI] [PubMed] [Google Scholar]
- Morris DL & Rui L (2009). Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab 297, E1247–E1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira TS, Takakura AC, Colombari E & Guyenet PG (2006). Central chemoreceptors and sympathetic vasomotor outflow. J Physiol 577, 369–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ & Schwartz MW (2011). Leptin and the central nervous system control of glucose metabolism. Physiol Rev 91, 389–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Mistry AM, Guyenet PG & Bayliss DA (2006). Purinergic P2 receptors modulate excitability but do not mediate pH sensitivity of RTN respiratory chemoreceptors. J Neurosci 26, 7230–7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaishi VS, Cardinali LI, Zampieri TT, Furigo IC, Metzger M & Donato JJr (2014). Possible crosstalk between leptin and prolactin during pregnancy. Neuroscience 14, 71–83. [DOI] [PubMed] [Google Scholar]
- Nattie EE (1999). CO2 brainstem chemoreceptors and breathing. Prog Neurobiol 59, 299–331. [DOI] [PubMed] [Google Scholar]
- O'Donnell CP, Tankersley CG, Polotsky VP, Schwartz AR & Smith PL (2000). Leptin, obesity, and respiratory function. Respir Physiol 119, 173–180. [DOI] [PubMed] [Google Scholar]
- Olson AL & Zwillich C (2005). The obesity hypoventilation syndrome. Am J Med 118, 948–956. [DOI] [PubMed] [Google Scholar]
- Polotsky VY, Smaldone MC, Scharf MT, Li J, Tankersley CG, Smith PL, Schwartz AR & O'Donnell CP (2004). Impact of interrupted leptin pathways on ventilatory control. J Appl Physiol (1985) 96, 991–998. [DOI] [PubMed] [Google Scholar]
- Rahmouni K & Morgan DA (2007). Hypothalamic arcuate nucleus mediates sympathetic and arterial pressure responses to leptin. Hypertension 49, 647–652. [DOI] [PubMed] [Google Scholar]
- Skibicka KP & Grill HJ (2009). Hypothalamic and hindbrain melanocortin receptors contribute to the feeding, thermogenic, and cardiovascular action of melanocortins. Endocrinology 150, 5351–5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Colombari E, Menani JV & Moreira TS (2011). Ventrolateral medulla mechanisms involved in cardiorespiratory responses to central chemoreceptor activation in rats. Am J Physiol Regul Integr Comp Physiol 300, 501–510. [DOI] [PubMed] [Google Scholar]
- Wenker IC, Kréneisz O, Nishiyama A & Mulkey DK (2010). Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1–Kir5.1‐like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol 104, 3042–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker IC, Sobrinho CR, Takakura AC, Moreira TS & Mulkey DK (2012). Regulation of ventral surface CO2/H+‐sensitive neurons by purinergic signalling. J Physiol 590, 2137–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KW & Smith BN (2006). Rapid inhibition of neural excitability in the nucleus tractus solitarii by leptin: implications for ingestive behaviour. J Physiol 573, 395–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh ER, Erokwu B, LaManna JC & Haxhiu MA (1997). The paraventricular nucleus of the hypothalamus influences respiratory timing and activity in the rat. Neurosci Lett 232, 63–66. [DOI] [PubMed] [Google Scholar]


