Abstract
The subfornical organ (SFO) is a circumventricular organ recognized for its ability to sense and integrate hydromineral and hormonal circulating fluid balance signals, information which is transmitted to central autonomic nuclei to which SFO neurons project. While the role of SFO was once synonymous with physiological responses to osmotic, volumetric and cardiovascular challenge, recent data suggest that SFO neurons also sense and integrate information from circulating signals of metabolic status. Using microarrays, we have confirmed the expression of receptors already described in the SFO, and identified many novel transcripts expressed in this circumventricular organ including receptors for many of the critical circulating energy balance signals such as adiponectin, apelin, endocannabinoids, leptin, insulin and peptide YY. This transcriptome analysis also identified SFO transcripts, the expressions of which are significantly changed by either 72 h dehydration, or 48 h starvation, compared to fed and euhydrated controls. Expression and potential roles for many of these targets are yet to be confirmed and elucidated. Subsequent validation of data for adiponectin and leptin receptors confirmed that receptors for both are expressed in the SFO, that discrete populations of neurons in this tissue are functionally responsive to these adipokines, and that such responsiveness is regulated by physiological state. Thus, transcriptomic analysis offers great promise for understanding the integrative complexity of these physiological systems, especially with development of technologies allowing description of the entire transcriptome of single, carefully phenotyped, SFO neurons. These data will ultimately elucidate mechanisms through which these uniquely positioned neurons respond to and integrate complex circulating signals.
Introduction
Cardiovascular disease and hypertension are associated with the development of obesity, insulin resistance and diabetes. These comorbidities represent critical components of ‘metabolic syndrome’, diagnosed in nearly 25% of the North American population in 2002 (Ford et al. 2002). Since the discovery of leptin in the early 1990s, a number of other neuroactive peptides and adipokines associated with metabolic syndrome, including adiponectin, amylin, angiotensin II, glucagon‐like peptide‐1 (GLP‐1), oxytocin (OT), α‐melanocyte stimulating hormone (α‐MSH), peptide YY/neuropeptide Y (Buijs, 1990; Ferguson & Washburn, 1998; Cowley et al. 1999; Kadowaki et al. 2006), have been shown to act not only as peripheral hormones, but also as neural signalling molecules in critical autonomic control centres of the brain. Importantly, the majority of these neuroactive signalling molecules exert what are apparently diverse physiological effects on cardiovascular (blood pressure, heart rate, baroreflex sensitivity), metabolic (food intake, metabolic rate), immune and reproductive functions by acting in these autonomic control centres in the hypothalamus and medulla. Although such commonality suggests these CNS centres are potential sites at which pathological changes may underlie all of the comorbidities associated with the metabolic syndrome, an understanding of the CNS circuitry through which this may occur has yet to emerge. An additional and intriguing part of such models is the inclusion of feedback control circuitry, through which these same molecules (e.g. glucose, adiponectin, amylin, angiotensin, cholecystokinin (CCK), GLP‐1, insulin, leptin, peptide YY (PYY), ghrelin) act as circulating signals providing critical information regarding homeostatic status to the CNS, despite the fact that most of these messengers do not diffuse freely across the blood–brain barrier (BBB) (Abstract Figure). Peptide specific transporters, such as the leptin transporter, have been suggested to mediate blood to brain signalling (Banks & Kastin, 1987), but their roles are not well defined and for many important circulating signals they do not exist (Spranger et al. 2006; Price et al. 2007). Intriguingly, there are specific regions of the brain that lie outside of the BBB, collectively termed circumventricular organs (CVOs), and a well‐established body of evidence has shown that these specialized structures do play critical roles in sensing and responding to circulating signals associated with fluid balance, cardiovascular, metabolic and immune function (McKinley et al. 2003; Hoyda et al. 2009; Sisó et al. 2010; Mimee et al. 2013) (Abstract Figure).
Circumventricular organs as sensors of circulating signals
The CVOs are specialized, structurally unique CNS nuclei that lack the normal BBB (Weindl, 1986; Gross, 1992). The area postrema (AP), subfornical organ (SFO), and organum vasculosum of the lamina terminalis (OVLT) are the only CVOs containing neuronal cell bodies, as opposed to nerve terminals. These three regions are classified as the ‘sensory CVOs’ in view of their roles as critical integrative centres where circulating peptides act to regulate the cardiovascular, immune and neuroendocrine systems (Weindl & Sofroniew, 1981; Ferguson & Bains, 1996; McKinley et al. 2003; Cottrell & Ferguson, 2004; Fry & Ferguson, 2007). While the AP was initially known for its ability to sense circulating toxins and trigger nausea and emesis (Hornby, 2001), this CVO also plays roles in the regulation of energy balance (Ritter et al. 1981; Contreras et al. 1982; Hyde & Miselis, 1983), and in central cardiovascular regulation (Brody, 1988; Ferguson & Smith, 1991; Osborn et al. 2000). Receptor localization (Sexton et al. 1994), and electrophysiological (Riediger et al. 2001) and lesion studies (Rowland & Richmond, 1999), collectively identified the AP as the primary CNS site at which circulating amylin acts to inhibit food intake. Other studies have shown effects of GLP‐1 (Rowland et al. 1997), CCK (Edwards et al. 1986; Sun & Ferguson, 1997), adrenomedullin (Shan & Krukoff, 2000; Yang & Ferguson, 2003), orexin (Yang & Ferguson, 2002), adiponectin (Fry et al. 2006), PYY (Price et al. 2008) and ghrelin (Lawrence et al. 2002; Fry & Ferguson, 2009) on AP neurons. In contrast, the SFO was initially recognized for its pre‐eminent role as the primary CNS site at which circulating angiotensin acted to stimulate drinking (Simpson & Routtenberg, 1975), increase blood pressure (Mangiapane & Simpson, 1980), and modulate vasopressin secretion (Iovino & Steardo, 1984). However, this limited perspective of the physiological roles of the SFO has changed significantly over the past 5 years, in part a result of our ability to identify novel targets with transcriptomic technologies, and this evolution will be the focus of the remainder of this article.
Subfornical organ
The SFO is a forebrain midline structure located on the dorsal surface of the third ventricle below the ventral hippocampal commissure, and is primarily known for its well established roles in cardiovascular and neuroendocrine regulation (Ferguson & Bains, 1997; McKinley et al. 1998). Roles for the SFO in anorexia and emaciation (Trivedi et al. 2003) and immune function (Takahashi et al. 1997) have also been suggested. The SFO can been subdivided anatomically into ‘core’ and ‘shell’ zones with the primary efferent projections to the paraventricular nucleus (PVN), supraoptic nucleus (SON), median preoptic nucleus, OVLT and arcuate nucleus (Miselis, 1981; Lind, 1986; Gruber et al. 1987), while those originating from the shell project primarily to the bed nucleus of the stria terminalis (McKinley et al. 2003). These neural outputs position this CVO to effectively communicate with all of the critical hypothalamic autonomic control centres, and thus play important roles in the regulation of a much greater spectrum of homeostatic functions. SFO neurons have for some time been known to sense circulating signals involved in fluid (osmolarity, sodium, calcium, relaxin, atrial natriuretic peptide), cardiovascular (angiotensin, endothelin, vasopressin (VP)), and immune (interleukin 1β) regulation (Cottrell & Ferguson, 2004). Specific excitatory projections have been found to VP and oxytocin (OT) neurons in the SON and PVN, as well as to parvocellular areas of the PVN that in turn project either to the median eminence (corticotropin‐releasing hormone (CRH), thyrotropin‐releasing hormone (TRH) neurons), the medulla (OT, VP, TRH neurons), or the spinal cord (OT, VP neurons) (Ferguson & Bains, 1996). More recent single cell recordings have shown direct effects of metabolic signals such as calcitonin (Schmid et al. 1998), amylin (Riediger et al. 1999) and ghrelin (Pulman et al. 2006) on SFO neurons (Abstract Figure). Thus studies were slowly building a catalogue describing the SFO as a structure with neurons able to sense a variety of different signalling molecules present in the circulation, but progress was driven effectively by the analysis on one signalling molecule at a time, with the driving force originating in studies demonstrating receptors/binding sites for that molecule in this CVO.
Transcriptomic analysis
The development of reliable tools for whole transcriptomic analysis offered an alternative approach where single studies could describe relative expression levels of the entire genome. We utilized this technology not only to catalogue the expression levels of all transcripts represented on the Affymetrix gene chip (>30,000), but also to assess changes in expression associated with the challenges of fluid (72 h) or food (48 h) deprivation in the SFO (Hindmarch et al. 2008). While these studies confirmed the expression of many previously identified receptors in the SFO (AT1A, CaSR, ETB, CLCR), they also identified for the first time receptors for many of the critical circulating energy balance signals, including adiponectin, apelin, endocannabinoids (CB1), leptin, insulin and PYY, as well as peptides believed to play important roles in the regulation of feeding such as apelin, cholecystokinin (CCK) and brain derived neurotrophic factor (BDNF) (Fig. 1). Although these findings identify new targets and support the conclusion that the SFO plays important roles in monitoring these signals, and transmitting this information to critical autonomic control centres in the hypothalamus, in reality they represent only the first step in a detailed systematic analysis as highlighted in three examples described below. Importantly, the original microarray data for these SFO experiments are held in the NCBI Gene Expression Omnibus, a functional genomics data repository for Microarray and Sequence data (http://www.ncbi.nlm.nih.gov/geo; Accession number: GSE12978), and are thus freely accessible to all, for additional analysis.
Figure 1. Targets in SFO identified by microarray analysis .
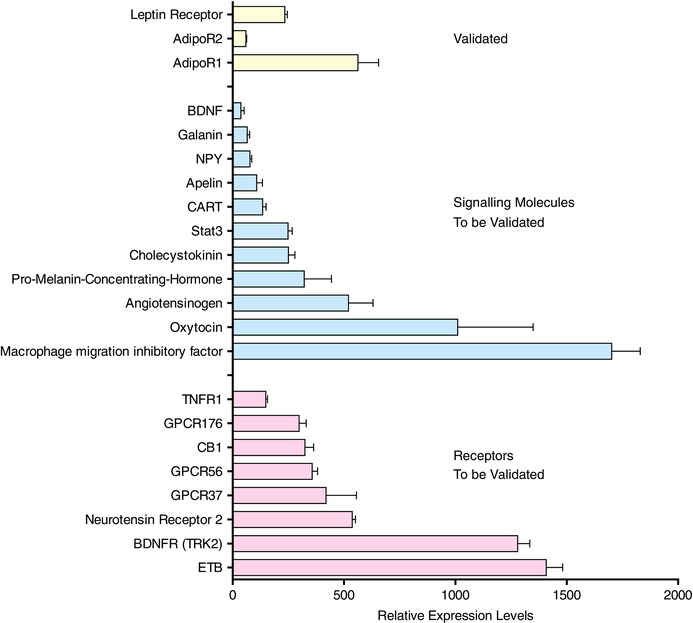
This histogram shows data from our microarray analysis (Hindmarch et al. 2008) highlighting the relative expression levels of transcripts from our microarray analysis, the validation of which we describe in this paper (yellow). In addition we show here some novel target transcripts identified in our array study which are known to be potential signalling molecules (blue), or receptors (pink) associated with the regulation of energy balance.
Validation: functional roles for SFO in energy balance?
The transcriptome of the SFO is dynamic and is modified by physiological state. Food deprivation (48 h) resulted in 687 transcripts regulated by greater than 2‐fold while fluid deprivation resulted in just 44 transcripts (Hindmarch et al. 2008). These data suggested to us that in addition to its well‐established roles in the regulation of fluid balance and cardiovascular regulation the SFO may also play important roles in the regulation of energy homeostasis. We have subsequently demonstrated that electrical activation of SFO neurons stimulates food intake in satiated animals (Smith et al. 2010). We have also shown that while lesion of either AP or SFO in isolation does not influence long term food intake or body weight, lesion of both CVOs reduces both parameters (Baraboi et al. 2010 b), suggesting complementary sensory functions of these CVOs. Such double lesions also result in reduced patterns of c‐fos activation in PVN, the hypothalamic location of adrenal (CRH) and thyroid (TRH) control neurons, and in the nucleus of the tractus solitarius (NTS) in response to systemic GLP‐1 and PYY receptor activation, confirming vital roles for these structures in sensing circulating signals (Baraboi et al. 2010 a,b).
Validation: adiponectin actions in the SFO – dynamic changes with food deprivation?
Our array studies not only identified adiponectin receptors in the SFO for the first time, but in addition suggested that the expression level of adipoR2 was in fact changed by food deprivation. In this case our initial approach to validation began with molecular confirmation that adipoR1 and R2 were expressed in SFO using standard PCR (Fig. 2 A), and selectively altered levels of AdipoR2 expression following food deprivation were then confirmed using quantitative RT‐PCR (Fig. 2 B). Patch clamp recordings confirmed functional roles for these receptors showing that adiponectin hyperpolarized 35% and depolarized 22% of SFO neurons as shown in Fig. 2 C (Alim et al. 2010). Intriguingly similar recordings showing that adiponectin depolarized 77% (compared to only 22% in controls) of SFO neurons from food deprived animals while no cells hyperpolarized (Alim et al. 2010) (Fig. 2 D), provided direct evidence of functional consequences associated with these transcriptome changes induced by physiological state.
Figure 2.
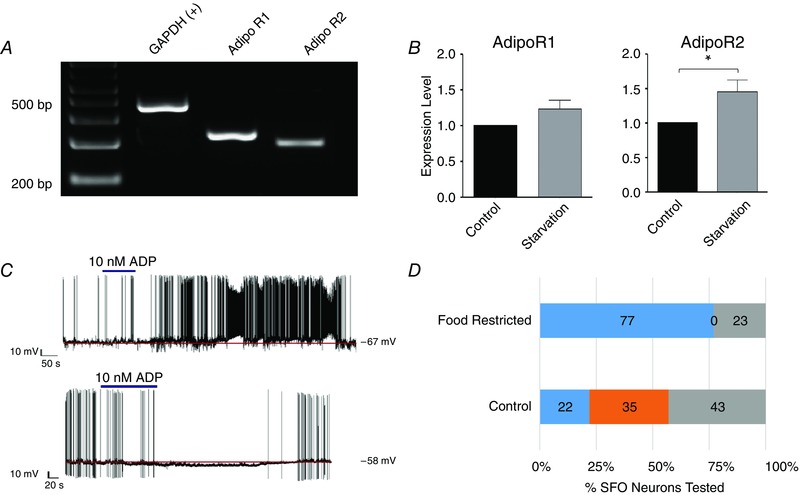
The process of adiponectin receptor validation (Alim et al. 2010) following our identification of these receptors in SFO
A shows an agarose gel from RT‐PCR analysis of whole tissue from SFO using primer sets for GAPDH, AdipoR1 and AdipoR2, all of which were seen to be clearly expressed in the SFO. B, the histograms show qRT‐PCR data confirming microarray analysis indicating that while AdipoR2 was significantly increased by 48 h of food restriction (*), AdipoR1 was not. C, patch clamp recordings from SFO neurons also validate functional roles for these receptors in that proportions of these cells are either depolarized or hyperpolarized by bath administration of adiponectin (ADP, 10 nm, indicated by the bar). D, the histogram summarizes proportions of dissociated SFO neurons from control and food deprived (48 h) groups showing depolarizations (blue bars) or hyperpolarizations (orange bars) in response to adiponectin and illustrate a large shift toward depolarizing effects in the latter group.
Validation: leptin actions in SFO – dynamic changes in obesity?
The leptin receptor (ObRb) was also reported as present in SFO by our microarray analysis, although the extensive literature on CNS distribution of leptin receptors had not identified expression in this CVO. Our follow‐up validation in this instance included PCR of SFO tissue with primer sets directed toward different regions of the leptin receptor which confirmed our array work (Fig. 3 A). Antibodies against the leptin receptors (despite concerns regarding specificity) also confirmed expression, as did the induction of p‐Stat3 by leptin (Smith et al. 2009). Functional roles for ObRb in SFO were confirmed both by electrophysiology showing depolarizing (39%) (the same neurons that were depolarized by amylin) and hyperpolarizing (25%) effects of leptin on SFO neurons (Smith et al. 2009) (Fig. 3 B), and by microinjection studies showing that direct administration into SFO caused changes in blood pressure (Fig. 3 C), effects that are no longer observed in the diet induced obesity (DIO) obese phenotype (aged matched chow fed and diet resistant animals retain responsiveness to leptin as illustrated in Fig. 3 D (Smith & Ferguson, 2012).
Figure 3. Leptin receptor validation in SFO .
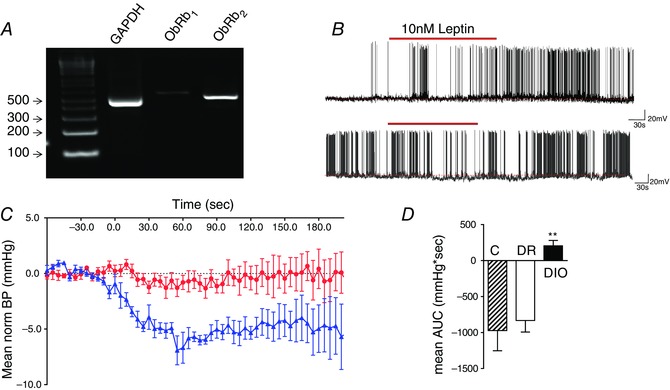
Data adapted from Smith et al. (2009) and Smith & Ferguson (2012). A shows an agarose gel from RT‐PCR analysis of whole tissue from SFO using primer sets for GAPDH, and two different sets for the long form of the leptin receptor, ObRb1, ObRb2, all of which were seen to be clearly expressed in the SFO. B, patch clamp recordings are shown from two different dissociated SFO neurons, one of which depolarized with an increase in action potential frequency (upper panel), and one which hyperpolarized with a decrease in spike frequency (lower panel) in response to bath administration of 10 nm leptin (red bar). C, normalized blood pressure recordings showing the hypotensive response to microinjection of 5 pm (in 0.5 μl) leptin into the SFO of control rats (blue) in contrast to the lack of effect of such microinjections in DIO animals (red). D, summary data showing mean area under the curve (AUC) for blood pressure changes in rats fed normal chow (control, C), and high fat diet fed animals which were either similar in weight to chow fed age matched animals (DR) or were obese (DIO). **P < 0.01, Newman–Keuls post hoc analysis.
Validation: untapped targets?
Additional SFO genes identified by our array studies which are associated with CNS regulation of energy balance, and have yet to be validated and pursued, include receptors for endocannabinoids (CB1), BDNF (BDNFR) and tumour necrosis factor α (TNFR1) (Abstract Figure), and signalling molecules such as cocaine and amphetamine related transcript (CART), OT, pro‐melanin‐concentrating hormone (PMCH) and signal transducer and activator of transcription 3 (STAT3) (Hindmarch et al. 2008). Finally, we have identified true glucose sensing neurons in the SFO (Medeiros et al. 2012), and have preliminary data showing that acute (<24 h) changes in glucose concentration modify the responsiveness of SFO neurons to CCK. These findings highlight the role of physiological state in modifying the sensory abilities of SFO in the regulation of energy balance. Thus, our initial array interrogation of the SFO has identified this CVO as a region of the CNS which performs critical roles in continually monitoring circulating metabolic, cardiovascular and immune signalling molecules. Intriguingly, the SFO, through its efferent connections to hypothalamic autonomic control centres, may then coordinate the integrated regulation of metabolic, cardiovascular, immune and neuroendocrine outputs.
One‐cell‐at‐a‐time
We have presented here data that firstly profile the transcriptome of the control, dehydrated and fasted SFO, and identified regulated targets that have been validated by additional studies. That said, it needs to be emphasized that such profiles of tissue composed of entire nuclei are based on the average expression of the entire population of discrete SFO neurons rather than being representative of the component subpopulations of cells of this tissue. Thus, describing the properties of these single neurons will also be critical to understanding the functional physiological roles of the SFO. We have already described that significant proportions (>25%) of SFO neurons respond to signals such as angiotensin (>60%), ghrelin (>25%), leptin (>60%), or adiponectin (>50%) and this suggests that each neuron has specific receptors (i.e. sensors) for a number of these molecules. Further, studies have confirmed that single SFO neurons can sense multiple signals (Anderson et al. 2001; Pulman et al. 2006; Smith et al. 2009). There is also overwhelming evidence that expression of a transcript can differ from cell to cell (Xi et al. 1999; Yamashita et al. 2002; Pulman et al. 2006; Hoyda et al. 2007) but until recently it has been technically challenging to describe the entire transcriptome of a single neuron. Technological advances have now paved the way for single‐cell profiling of the SFO to become a realistic ambition. Regardless of the technology used (Lee et al. 2013) Next Generation Sequencing (NGS) of the transcriptome (RNAseq) involves the preparation and subsequent sequencing of a library of short sequences that represent both coding and non‐coding transcripts within a particular tissue or cell. The read‐data that result from these experiments can be aligned back to the appropriate genome in order to identify the relative abundance of each transcript. Single cell transcriptomics is already changing the way we are able to describe populations of cells; while previous efforts to phenotype populations of individual neurons relied upon physiological profiling (electrophysiological, molecular), libraries of substantive numbers of individual cell transcriptomes can now be sequenced. This approach is not without its limitations since one must either destroy the cell (via patch) or dissociate and sort the cells in order to capture the nuclear material for sequencing. Single cell transcriptomic analysis is, however, moving within reach, and the recent identification of 47 molecularly distinct subclasses following a molecular census of 3005 single cell transcriptomes from somatosensory S1 cortex and hippocampus CA1 in mice (Zeisel et al. 2015) substantiates the potential value of this approach. In the SFO or other neuronal structures, we expect that the profiling of the control SFO transcriptome will reveal similar intra‐cell diversity as found in the S1 and CA1 cells. We anticipate that it will be possible to capture accurate signatures for those SFO neurons that respond to circulating signals such as angiotensin, leptin or adiponectin, or core compared to shell neurons, or interneurons compared to projection neurons, or neurons that project to one output nucleus compared to another. Such classification will then open the door to examination of how these individual subpopulations of SFO neurons are differentially regulated by specific physiological challenges.
Concluding remarks
In conclusion, we have described here an emerging literature supporting the idea that the SFO plays important roles in the regulation of energy balance in addition to its well established roles in the control of fluid balance, the cardiovascular system and immune regulation (Abstract Figure). We have described the role that transcriptomic profiling of the whole SFO has played in the development of these ideas. Transcriptome‐wide data analysis continues to tell us that changes in transcript expression associated with physiological challenges are complex, integrated and plastic. Many potential targets remain as subjects for future validation studies. Electrophysiological and molecular data also tell us that there is great diversity within a population of cells even within the same tissue. In order to properly describe the roles of neuronal tissue like the SFO in biological phenomena such as energy homeostasis it is important that we begin to describe the individual cells that make up these structures.
Additional information
Competing interests
None declared.
Funding
This work is supported by the Biotechnology and Biological Sciences Research Council (A.V.F., BB/J005452/1), a High Impact Research Chancellory Grant, University of Malaya (C.C.T.H., H‐20001‐E000086) and the Canadian Institutes for Health Research (A.V.F., MOP‐12192).
Biographies
Alastair Ferguson received his PhD at the University of Calgary and after a postdoctoral fellowship at McGill University joined the Department of Physiology as an Assistant Professor at Queen's University in 1984. The research programme focuses on understanding the CNS pathways involved in regulation of the integrated autonomic nervous system. A variety of techniques are directed toward elucidating the role of critical autonomic nuclei and multiple neuroregulatory signalling molecules in the integration and regulation of the autonomic nervous system.

Charles Hindmarch is a cardiovascular neuroscientist working at the University of Bristol as a Research Associate and at the University of Malaya as a Visiting Professor. The central theme of his research is to establish various models of hypertension exposed to a variety of physiological cues including dietary (salt/fat), age and activity (treadmill exercised). He employs high throughput transcriptomics such as RNAseq to profile both specific structures and single cells in the brain in order to establish appropriate gene targets for molecular, physiological and functional validation.
This review was presented at the symposium “Bristol‐São Paulo Research Summit” – Autonomic and neuroendocrine dysfunction in chronic diseases, which took place at the University of São Paulo, Brasil, between 7–10 August 2014.
References
- Alim I, Fry WM, Walsh MH & Ferguson AV (2010). Actions of adiponectin on the excitability of subfornical organ neurons are altered by food deprivation. Brain Res 1330, 72–82. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Smith PM & Ferguson AV (2001). Subfornical organ neurons projecting to paraventricular nucleus: whole‐cell properties. Brain Res 921, 78–85. [DOI] [PubMed] [Google Scholar]
- Banks WA & Kastin AJ (1987). Saturable transport of peptides across the blood‐brain barrier. Life Sci 41, 1319–1338. [DOI] [PubMed] [Google Scholar]
- Baraboi ED, Michel C, Smith P, Thibaudeau K, Ferguson AV & Richard D (2010. a). Effects of albumin‐conjugated PYY on food intake: the respective roles of the circumventricular organs and vagus nerve. Eur J Neurosci 32, 826–839. [DOI] [PubMed] [Google Scholar]
- Baraboi ED, Smith P, Ferguson AV & Richard D (2010. b). Lesions of area postrema and subfornical organ alter exendin‐4‐induced brain activation without preventing the hypophagic effect of the GLP‐1 receptor agonist. Am J Physiol Regul Integr Comp Physiol 298, R1098–R1110. [DOI] [PubMed] [Google Scholar]
- Brody MJ (1988). Central nervous system and mechanisms of hypertension. Clin Physiol Biochem 6, 230–239. [PubMed] [Google Scholar]
- Buijs RM (1990). Vasopressin and oxytocin localization and putative functions in the brain. Acta Neurochir Suppl (Wien) 47, 86–89. [DOI] [PubMed] [Google Scholar]
- Contreras RJ, Fox E & Drubovich ML (1982). Area postrema lesions produce feeding deficits in the rat: effects of preoperative dieting and 2‐deoxy‐D‐glucose. Physiol Behav 29, 875–884. [DOI] [PubMed] [Google Scholar]
- Cottrell GT & Ferguson AV (2004). Sensory circumventricular organs: central roles in integrated autonomic regulation. Regul Pept 117, 11–23. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF & Cone RD (1999). Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 24, 155–163. [DOI] [PubMed] [Google Scholar]
- Edwards GL, Ladenheim EE & Ritter RC (1986). Dorsomedial hindbrain participation in cholecystokinin‐induced satiety. Am J Physiol 251, R971–R977. [DOI] [PubMed] [Google Scholar]
- Ferguson AV & Bains JS (1996). Electrophysiology of the circumventricular organs. Front Neuroendocrinol 17, 440–475. [DOI] [PubMed] [Google Scholar]
- Ferguson AV & Bains JS (1997). Actions of angiotensin in the subfornical organ and area postrema: implications for long term control of autonomic output. Clin Exp Pharmacol Physiol 24, 96–101. [DOI] [PubMed] [Google Scholar]
- Ferguson AV & Smith P (1991). Autonomic mechanisms underlying area postrema stimulation‐induced cardiovascular responses in rats. Am J Physiol 261, R1–R8. [DOI] [PubMed] [Google Scholar]
- Ferguson AV & Washburn DLS (1998). Angiotensin II: a peptidergic neurotransmitter in central autonomic pathways. Prog Neurobiol 54, 169–192. [DOI] [PubMed] [Google Scholar]
- Ford ES, Giles WH & Dietz WH (2002). Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 287, 356–359. [DOI] [PubMed] [Google Scholar]
- Fry M & Ferguson AV (2007). The sensory circumventricular organs: brain targets for circulating signals controlling ingestive behavior. Physiol Behav 91, 413–423. [DOI] [PubMed] [Google Scholar]
- Fry M & Ferguson AV (2009). Ghrelin modulates electrical activity of area postrema neurons. Am J Physiol Regul Integr Comp Physiol 296, R485–R492. [DOI] [PubMed] [Google Scholar]
- Fry M, Smith PM, Hoyda TD, Duncan M, Ahima RS, Sharkey KA & Ferguson AV (2006). Area postrema neurons are modulated by the adipocyte hormone adiponectin. J Neurosci 26, 9695–9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross PM (1992). Circumventricular organ capillaries. Prog Brain Res 91, 219–233. [PubMed] [Google Scholar]
- Gruber K, McRae‐Degueurce A, Wilkin LD, Mitchell LD & Johnson AK (1987). Forebrain and brainstem afferents to the arcuate nucleus in the rat: potential pathways for the modulation of hypophyseal secretions. Neurosci Lett 75, 1–5. [DOI] [PubMed] [Google Scholar]
- Hindmarch C, Fry M, Yao ST, Smith PM, Murphy D & Ferguson AV (2008). Microarray analysis of the transcriptome of the subfornical organ in the rat: regulation by fluid and food deprivation. Am J Physiol Regul Integr Comp Physiol 295, R1914–R1920. [DOI] [PubMed] [Google Scholar]
- Hornby PJ (2001). Central neurocircuitry associated with emesis. Am J Med 111, Suppl. 8A, 106S–112S. [DOI] [PubMed] [Google Scholar]
- Hoyda TD, Fry M, Ahima RS & Ferguson AV (2007). Adiponectin selectively inhibits oxytocin neurons of the paraventricular nucleus of the hypothalamus. J Physiol 585, 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyda TD, Smith PM & Ferguson AV (2009). Gastrointestinal hormone actions in the central regulation of energy metabolism: potential sensory roles for the circumventricular organs. Int J Obes (Lond) 33, Suppl. 1, S16–S21. [DOI] [PubMed] [Google Scholar]
- Hyde TM & Miselis RR (1983). Effects of area postrema/caudal medial nucleus of solitary tract lesions on food intake and body weight. Am J Physiol 244, R577–R587. [DOI] [PubMed] [Google Scholar]
- Iovino M & Steardo L (1984). Vasopressin release to central and peripheral angiotensin II in rats with lesions of the subfornical organ. Brain Res 322, 365–368. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K & Tobe K (2006). Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116, 1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence CB, Snape AC, Baudoin FM & Luckman SM (2002). Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology 143, 155–162. [DOI] [PubMed] [Google Scholar]
- Lee CY, Chiu YC, Wang LB, Kuo YL, Chuang EY, Lai LC & Tsai MH (2013). Common applications of next‐generation sequencing technologies in genomic research. Transl Cancer Res 2; http://www.thetcr.org/article/view/962. [Google Scholar]
- Lind RW (1986). Bi‐directional, chemically specified neural connections between the subfornical organ and the midbrain raphe system. Brain Res 384, 250–261. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Allen AM, Burns P, Colvill LM & Oldfield BJ (1998). Interaction of circulating hormones with the brain: the roles of the subfornical organ and the organum vasculosum of the lamina terminalis. Clin Exp Pharmacol Physiol Suppl 25, S61–S67. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, McAllen RM, Davern P, Giles ME, Penschow J, Sunn N, Uschakov A & Oldfield BJ (2003). The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol 172, III–XII, 1–122. [DOI] [PubMed] [Google Scholar]
- Mangiapane ML & Simpson JB (1980). Subfornical organ: forebrain site of pressor and dipsogenic action of angiotensin II. Am J Physiol 239, R382–R389. [DOI] [PubMed] [Google Scholar]
- Medeiros N, Dai L & Ferguson AV (2012). Glucose‐responsive neurons in the subfornical organ of the rat – a novel site for direct CNS monitoring of circulating glucose. Neuroscience 201, 157–165. [DOI] [PubMed] [Google Scholar]
- Mimee A, Smith PM & Ferguson AV (2013). Circumventricular organs: Targets for integration of circulating fluid and energy balance signals? Physiol Behav 121, 96–102. [DOI] [PubMed] [Google Scholar]
- Miselis RR (1981). The efferent projections of the subfornical organ of the rat: a circumventricular organ within a neural network subserving water balance. Brain Res 230, 1–23. [DOI] [PubMed] [Google Scholar]
- Osborn JW, Collister JP & Carlson SH (2000). Angiotensin and osmoreceptor inputs to the area postrema: role in long‐term control of fluid homeostasis and arterial pressure. Clin Exp Pharmacol Physiol 27, 443–449. [DOI] [PubMed] [Google Scholar]
- Price CJ, Hoyda TD & Ferguson AV (2008). The area postrema: A brain monitor and integrator of systemic autonomic state. Neuroscientist 14, 182–194. [DOI] [PubMed] [Google Scholar]
- Price TO, Samson WK, Niehoff ML & Banks WA (2007). Permeability of the blood‐brain barrier to a novel satiety molecule nesfatin‐1. Peptides 28, 2372–2381. [DOI] [PubMed] [Google Scholar]
- Pulman KJ, Fry WM, Cottrell GT & Ferguson AV (2006). The subfornical organ: A central target for circulating feeding signals. J Neurosci 26, 2022–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riediger T, Schmid HA, Lutz T & Simon E (2001). Amylin potently activates AP neurons possibly via formation of the excitatory second messenger cGMP. Am J Physiol Regul Integr Comp Physiol 281, R1833–R1843. [DOI] [PubMed] [Google Scholar]
- Riediger T, Schmid HA, Young AA & Simon E (1999). Pharmacological characterisation of amylin‐related peptides activating subfornical organ neurones. Brain Res 837, 161–168. [DOI] [PubMed] [Google Scholar]
- Ritter RC, Slusser PG & Stone S (1981). Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 213, 451–452. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Crews EC & Gentry RM (1997). Comparison of Fos induced in rat brain by GLP‐1 and amylin. Regul Pept 71, 171–174. [DOI] [PubMed] [Google Scholar]
- Rowland NE & Richmond RM (1999). Area postrema and the anorectic actions of dexfenfluramine and amylin. Brain Res 820, 86–91. [DOI] [PubMed] [Google Scholar]
- Schmid HA, Rauch M & Koch J (1998). Effect of calcitonin on the activity of ANG II‐responsive neurons in the rat subfornical organ. Am J Physiol Regul Integr Comp Physiol 274, R1646–R1652. [DOI] [PubMed] [Google Scholar]
- Sexton PM, Paxinos G, Kenney MA, Wookey PJ & Beaumont K (1994). In vitro autoradiographic localization of amylin binding sites in rat brain. Neuroscience 62, 553–567. [DOI] [PubMed] [Google Scholar]
- Shan J & Krukoff TL (2000). Area postrema ablation attenuates activation of neurones in the paraventricular nucleus in response to systemic adrenomedullin. J Neuroendocrinol 12, 802–810. [DOI] [PubMed] [Google Scholar]
- Simpson JB & Routtenberg JB (1975). Subfornical organ lesions reduce intravenous angiotensin‐induced drinking. Brain Res 88, 154–161. [DOI] [PubMed] [Google Scholar]
- Sisó S, Jeffrey M & González L (2010). Sensory circumventricular organs in health and disease. Acta Neuropathol 120, 689–705. [DOI] [PubMed] [Google Scholar]
- Smith PM, Chambers AP, Price CJ, Ho W, Hopf C, Sharkey KA & Ferguson AV (2009). The subfornical organ: a central nervous system site for actions of circulating leptin. Am J Physiol Regul Integr Comp Physiol 296, R512–R520. [DOI] [PubMed] [Google Scholar]
- Smith PM & Ferguson AV (2012). Cardiovascular actions of leptin in the subfornical organ are abolished by diet‐induced obesity. J Neuroendocrinol 24, 504–510. [DOI] [PubMed] [Google Scholar]
- Smith PM, Rozanski G & Ferguson AV (2010). Acute electrical stimulation of the subfornical organ induces feeding in satiated rats. Physiol Behav 99, 534–537. [DOI] [PubMed] [Google Scholar]
- Spranger J, Verma S, Göhring I, Bobbert T, Seifert J, Sindler AL, Pfeiffer A, Hileman SM, Tschöp M & Banks WA (2006). Adiponectin does not cross the blood‐brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes 55, 141–147. [PubMed] [Google Scholar]
- Sun K & Ferguson AV (1997). Cholecystokinin activates area postrema neurons in rat brain slices. Am J Physiol 272, R1625–R1630. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Smith PM, Ferguson AV & Pittman QJ (1997). Circumventricular organs and fever. Am J Physiol 273, R1690–R1695. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Jiang M, Tamvakopoulos CC, Shen X, Yu H, Mock S, Fenyk‐Melody J, Van der Ploeg LH & Guan XM (2003). Exploring the site of anorectic action of peripherally administered synthetic melanocortin peptide MT‐II in rats. Brain Res 977, 221–230. [DOI] [PubMed] [Google Scholar]
- Weindl A (1986). Neuroendocrine aspects of circumventricular organs In Frontiers in Neuroendocrinology, ed. Ganong W. & Martin L, pp. 3–32. Oxford University Press, New York. [Google Scholar]
- Weindl A & Sofroniew MV (1981). Relation of neuropeptides to mammalian circumventricular organs. Adv Biochem Psychopharmacol 28, 303–320. [PubMed] [Google Scholar]
- Xi D, Kusano K & Gainer H (1999). Quantitative analysis of oxytocin and vasopressin messenger ribonucleic acids in single magnocellular neurons isolated from supraoptic nucleus of rat hypothalamus. Endocrinology 140, 4677–4682. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Glasgow E, Zhang BJ, Kusano K & Gainer H (2002). Identification of cell‐specific messenger ribonucleic acids in oxytocinergic and vasopressinergic magnocellular neurons in rat supraoptic nucleus by single‐cell differential hybridization. Endocrinology 143, 4464–4476. [DOI] [PubMed] [Google Scholar]
- Yang B & Ferguson AV (2002). Orexin‐A depolarizes dissociated rat area postrema neurons through activation of a nonselective cationic conductance. J Neurosci 22, 6303–6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B & Ferguson AV (2003). Adrenomedullin influences dissociated rat area postrema neurons. Regul Pept 112, 9–17. [DOI] [PubMed] [Google Scholar]
- Zeisel A, Munoz‐Manchado AB, Codeluppi S, Lonnerberg P, La MG, Jureus A, Marques S, Munguba H, He L, Betsholtz C, Rolny C, Castelo‐Branco G, Hjerling‐Leffler J & Linnarsson S (2015). Brain structure. Cell types in the mouse cortex and hippocampus revealed by single‐cell RNA‐seq. Science 347, 1138–1142. [DOI] [PubMed] [Google Scholar]


