Abstract
Several studies over the past 20 years have shown that carbon dioxide lasers operating at wavelengths between 9.3 and 9.6-μm with pulse durations near 20-μs are ideal for hard tissue ablation. Those wavelengths are coincident with the peak absorption of the mineral phase. The pulse duration is close to the thermal relaxation time of the deposited energy of a few microseconds which is short enough to minimize peripheral thermal damage and long enough to minimize plasma shielding effects to allow efficient ablation at practical rates. The desired pulse duration near 20-μs has been difficult to achieve since it is too long for transverse excited atmospheric pressure (TEA) lasers and too short for radio-frequency (RF) excited lasers for efficient operation. Recently, Coherent Inc. (Santa Clara, CA) developed the Diamond J5-V laser for microvia drilling which can produce laser pulses greater than 100-mJ in energy at 9.4-μm with a pulse duration of 26-μs and it can achieve pulse repetition rates of 3 KHz. We report the first results using this laser to ablate dental enamel. Efficient ablation of dental enamel is possible at rates exceeding 50-μm per pulse. This laser is ideally suited for the selective ablation of carious lesions.
Keywords: dental hard tissue ablation, carbon dioxide laser, enamel ablation rate
1. INTRODUCTION
Studies over the past 30 years have demonstrated that carbon dioxide lasers can be used for several unique treatment modalities in dentistry, including: soft tissue vaporization with hemostasis, removal (ablation) of carious and noncarious dental hard tissue, preventive caries inhibition treatments, and surface conditioning for improved adhesion to composite restorative materials.[1] Lasers offer practical advantages over the conventional dental handpiece. This includes achieving high aspect ratios (volume / area) for high precision tissue removal while minimizing pain, noise and vibration by operating in a non-contact mode. Additionally, laser pulses can be delivered rapidly and efficiently to tooth surfaces using high-speed scanning systems for selectively removing carious lesions and failed composite restorations through various feedback mechanisms.[2, 3]
However, a major concern is excessive heat deposition in the tooth which may lead to eventual loss of pulpal vitality, thus any viable laser-dental procedure has to minimize the accumulation of heat in the tooth. Several studies have demonstrated that microsecond pulsed 9–11-μm CO2 lasers are well-suited for dental hard-tissue applications.[4–6] CO2 lasers operate at wavelengths coincident with the strongest absorption in dental hard tissues due to the phosphate ion in hydroxyapatite. The stronger the absorption by a material, the less light penetrates/transmits and the heat deposition is more locally confined. Furthermore, ablation thresholds for dental hard tissue removal are lower than for other laser wavelengths. Accordingly, shorter wavelength CO2 laser pulses with high absorption coefficients are ideal for minimizing peripheral thermal damage and excessive heat accumulation (Fig. 1).
Fig. 1.

Infrared absorption spectrum of enamel. Optical properties of enamel and dentin at 9.3-μm (J5-V operates at 9.37-μm). Shown are the wavelength, absorption coefficient, (1/e) absorption depth, thermal relaxation time and the reflectance.
Typically, the laser pulse duration should be on the order of the thermal relaxation time for axial heat conduction to limit the peripheral thermal damage and decrease the total energy delivered to tissues. [7] However, laser-tissue ablation is more complicated and plasma and debris shielding may dramatically reduce ablation efficiency for short laser pulses.
Earlier studies employed 9.3 and 9.6-μm TEA lasers that utilize a simple high voltage discharge to excite the gas mixture. [8–14] In contrast, Radio Frequency (RF) excited slab lasers use a radiofrequency source to excite the CO2 gas and can be operated efficiently with a completely sealed gas mixture. The principal advantages of the RF slab laser over the TEA laser are small size and sealed gas mixture. Systems with output in the range of 10–30 W are now available at relatively low-cost that do not require water-cooling. One potential disadvantage of these RF-excited systems is the greater potential for peripheral thermal damage since these lasers are operated most efficiently with longer pulse durations greater than 60-μs, much longer than the thermal relaxation time of 1–2-μs for 9.3 and 9.6-μm CO2 laser wavelengths in enamel. The RF-excited slab laser operates most efficiently with pulse durations of 60-μs to 5-ms, and repetition rates in the tens of kHz are feasible as long as a 50% duty cycle is not exceeded. Visuri investigated a RF-excited slab laser operating at 9.4-μm with a pulse duration of 300-μs for laser ablation of dentin and enamel.[15] He observed ablation rates of ~80-μm per pulse for dentin and 44-μm per pulse for enamel. The maximum repetition rate investigated was limited to 10-Hz. Recent technological developments have reduced the laser pulse duration to the 25–35-μs range. In a study a few years ago, we investigated a low cost 9.3-μm RF system from Inlight Inc. with pulse durations as short as 36-μs but the single pulse energies were low. [16]
Recently, a commercial RF-excited CO2 laser the Diamond J5-V from Coherent (Santa Clara, CA) was developed for circuit board (microvia) drilling operating at 9.4-μm with a pulse duration of 26-μs. The purpose of this study was to determine if this laser is well-suited for enamel ablation.
2. MATERIALS AND METHODS
2.1 Sample Preparation
Twenty-nine sound human teeth were collected (CHR approved) and sterilized with gamma radiation. Teeth were sectioned into ~500-μm thick samples using a diamond saw, Isomet 5000, Buehler (Lake Bluff, IL) and stored in a 0.01% thymol solution to maintain tissue hydration and prevent bacterial growth. Samples were separated into two groups (n = 20 & n = 9). Group 1 (n = 20) was used to determine the enamel ablation rates for fluence ranging from 2 to 286-J/cm2. Group 2 was used to measure the ablation rates and surface roughness with respect to varying laser pulse overlap (25, 50, 100-μm).
2.2 Laser Irradiation Parameters
An RF-excited laser prototype, Diamond J5-V from Coherent (Santa Clara, CA) operating at a wavelength of 9.4 μm was used with a pulse duration of 26-μs and a pulse repetition rate of 100-Hz. The laser had a Gaussian output beam. The laser energy output was monitored using a power/energy meter, ED-200 from Gentec (Quebec, Canada). The beam profile was imaged using a Pyrocam Beam profilometer from Ophir-Spiricon (Logan, UT) (Fig. 2A). A room temperature HgCdTe detector Boston Electronics PD-10.6-3 (Boston, MA) with a response time of a few nanoseconds was used to measure the laser pulse temporal profile (Fig. 2B). The laser beam was focused to a beam diameter of 200-μm using an f = 125-mm ZnSe scanning lens. A razor blade was scanned across the beam to determine the diameter (1/e2) of the laser beam of 200-μm. A computer-controlled stage high-speed motion control system with Newport (Irvine, CA) UTM150 and 850G stages and an ESP300 controller was used to scan the samples across the laser beam.
Fig. 2.
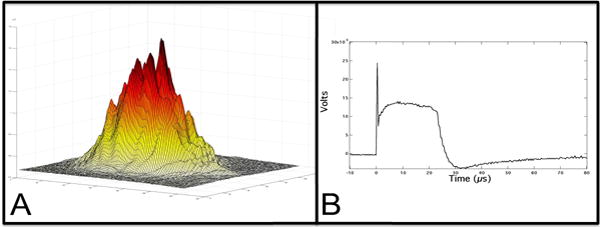
A) Spatial beam profile shows Gaussian beam. B) The temporal profile, 26-μs, was measured with a HgCdTe detector.
For the Group 1 samples (n = 20) a scanning rate of 5 mm/sec and a laser pulse repetition rate of 100-Hz was used to generate a spatial overlap of laser spots by ~ 50%. CaF2 attenuators were placed in front of the beam to vary fluence. Group 2 samples (n = 9) were scanned across the laser beam at the rates of 2.5, 5 and 10-mm/sec and irradiated with incident intensities of 7, 15 and 39-J/cm2 with a 100-Hz pulse repetition rate. Each sample from both groups was scanned 3 times with the same parameters. A low volume/low pressure air-actuated fluid spray delivery system consisting of a 780S spray valve, a Valvemate 7040 controller, and a fluid reservoir from EFD, Inc. (East Providence, RI) was used to provide a uniform spray of fine water mist onto the tooth surfaces at 2 mL/min.
2.3 3D Digital Microscopy
Tooth surfaces were examined after laser irradiation using an optical microscopy/3D surface profilometry system, the VHX-1000 from Keyence (Osaka, Japan). A VH-Z100R lens was used with a 100–1000 times magnification. 3D surface profiles were acquired by scanning the image plane of the microscope and reconstructing a 3D surface using an algorithm that determined the optimal focal depth at each pixel. The built-in Keyence 3-D shape measurement software, VHX-H3M, was used to correct the tilt of the sample and measure the ablation depth for samples in Group 1 & 2. Additionally, surface profiles along the ablation trough were acquired for samples in Group 2 to measure surface roughness. High magnification (600–800×) was used to visually assess peripheral thermal damage.
2.4 Analysis of 3D surface images
Image processing was carried out using Igor Pro, data analysis software from Wavemetrics Inc. (Lake Oswego, OR). Each surface roughness profile was normalized against its computed linear regression line to correct the tilt of each sample’s profile. The root mean square (RMS) was calculated for these normalized profiles taken in Group 2 and averaged to measure the amount of surface roughening. The height between the lowest point and the surface was measured with the VHX-H3M to determine the ablation depths in Groups 1 & 2. The ablation depths were averaged for each ablation profile.
3. RESULTS AND DISCUSSION
3.1 Enamel ablation rate
The fluence thresholds that produced microscopically observable surface changes on enamel were measured. The lowest fluence that produced an observable ablation crater was 4.4-J/cm2 and the mean ablation depth was 6.2-μm (Fig. 3). Above 100-J/cm2, the mean crater depths exceeded 150-μm. Given that the scanning overlap is 50% of the laser pulse diameter, the maximum ablative rate of enamel with this laser is ~ 50–56-μm/pulse. This ablation rate exceeds ablation rates of previous CO2 laser dental systems at 9.6-μm, 8-μs (~10-μm/pulse) and 9.3-μm, 18-μs (~20-μm/pulse).[14, 17] In addition, none of the samples exhibited any thermal damage (Fig. 4).
Fig. 3.
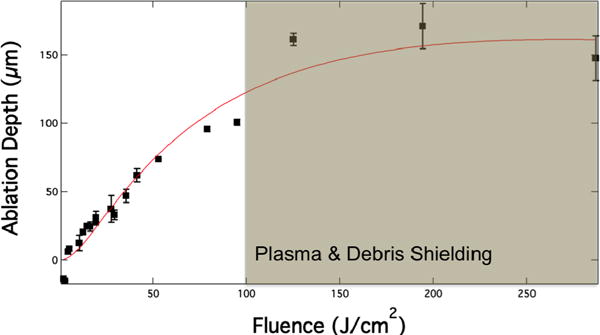
Enamel ablation rate versus fluence. The laser spot diameter was 200-μm and the spot overlap was ½ the spot diameter. The enamel ablation rate starts to plateau at fluence above 100-J/cm2 due to plasma and debris shielding.
Fig. 4.
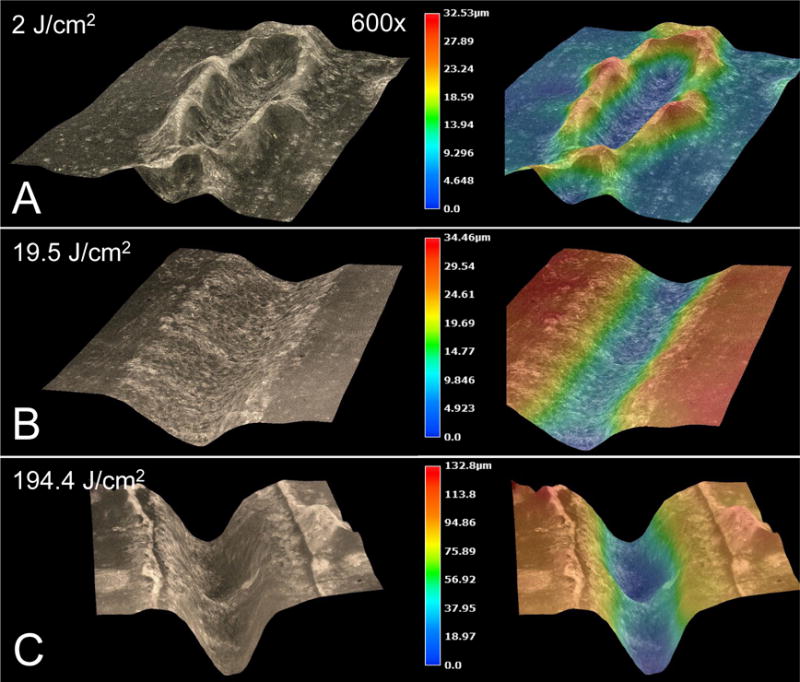
(Left) 3D microscopy images and (right) height-colored at the following fluences: (A) 2-J/cm2 swells up the enamel to a height of 13.5-μm, (B) 19.5 J/cm2 ablates enamel to an average depth of 28-μm, (C) 194.4-J/cm2 ablates enamel to a depth of 120-μm. Note the lack of carbonization at high laser pulse energies.
For incident fluence over 100-J/cm2, the ablation efficiency begins to decrease due to plasma and debris shielding (Fig. 3), however it is highly unlikely that a clinical laser will be used at this energy. An advantage of using CO2 lasers is the high pulse repetition rates. This laser is capable of reaching repetition rates up to 3-KHz. Moreover, it is far more efficient to rapidly scan an area at high repetition rates to delocalize thermal deposition and prevent laser stalling. It is interesting to note that at subablative fluence (2 & 3-J/cm2), irradiated enamel surfaces exhibit raised enamel structures (Fig. 4A).
3.2 Pulse overlap and surface roughness
We have found that near-IR reflectance can be used for the image-guided ablation of dental decay. [18] Surface roughening can scatter light which may increase the reflectivity and confound image guided ablation, moreover it also can be aesthetically displeasing.[18] Therefore, it is important to choose laser scanning parameters that minimize surface roughening. The influence of varying the fluence and the spatial overlap of the laser pulses was also investigated. In Fig. 5 the surface roughness and ablation rate is shown for various scanning parameters. As anticipated the roughness increases with increasing fluence while it decreases with increasing overlap.
Fig. 5.
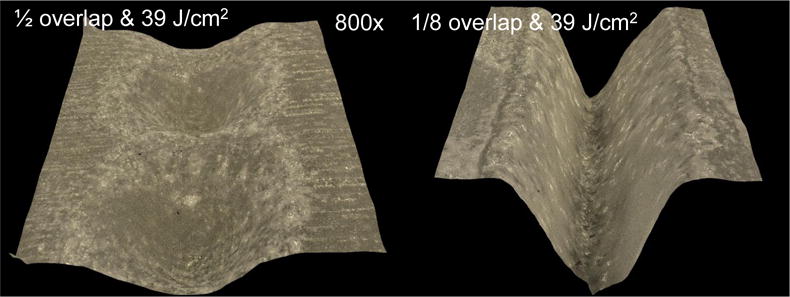
3D microscopy images showing variation of the spatial overlap of the laser pulses and the roughness (RMS).
In summary, a 9.4-μm RF CO2 laser operating with a pulse duration of 26-μs ablates enamel at high rates without causing any visible thermal damage. Future studies will focus on developing a selective caries ablation system with this laser using a novel near-infrared caries detection system.
Fig. 6.
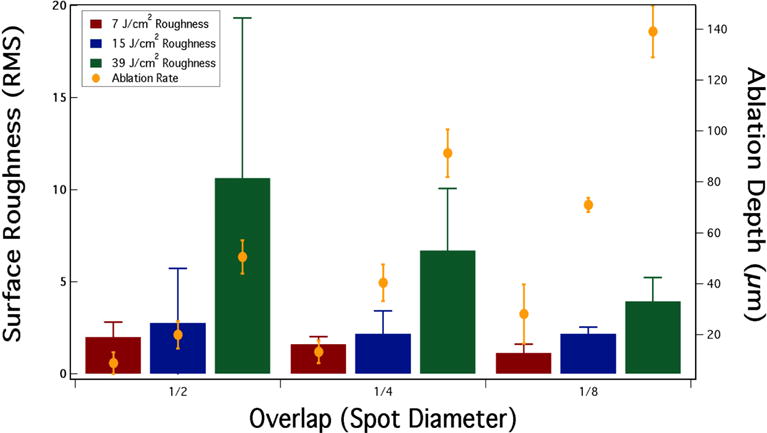
Surface roughness (RMS) and the ablation depth for varying incident fluence and spatial overlap of the laser pulses.
Acknowledgments
We would like to thank Jin W. Kim, Cynthia L. Darling, Robert Lee, Jacob C. Simon, and Andrew Jang for their contributions and acknowledge the support of NIH/NIDCR grant R01-DE019631.
References
- 1.Wigdor HA, Walsh JT, Jr, Featherstone JD, Visuri SR, Fried D, Waldvogel JL. Lasers in dentistry. Lasers Surg Med. 1995;16(2):103–33. doi: 10.1002/lsm.1900160202. [DOI] [PubMed] [Google Scholar]
- 2.Chan KH, Hirasuna K, Fried D. Rapid and Selective Removal of Composite from Tooth Surfaces with a 9.3-μm CO2 Laser using Spectral Feedback. Lasers Surg Med. 2011;43(8):824–832. doi: 10.1002/lsm.21111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tao YC, Fried D. Near-infrared image-guided laser ablation of dental decay. J Biomed Opt. 2009;14(5):054045. doi: 10.1117/1.3253390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staninec M, Darling CL, Goodis HE, Pierre D, Cox DP, Fan K, Larson M, Parisi R, Hsu D, Manesh SK, Ho C, Hosseini M, Fried D. Pulpal effects of enamel ablation with a microsecond pulsed lambda = 9.3-microm CO2 laser. Lasers Surg Med. 2009;41(4):256–63. doi: 10.1002/lsm.20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodis HE, Fried D, Gansky S, Rechmann P, Featherstone JD. Pulpal safety of 9.6 microm TEA CO2 laser used for caries prevention. Lasers Surg Med. 2004;35(2):104–10. doi: 10.1002/lsm.20043. [DOI] [PubMed] [Google Scholar]
- 6.Wigdor HA, Walsh JT., Jr Histologic analysis of the effect on dental pulp of a 9.6-microm CO(2) laser. Lasers Surg Med. 2002;30(4):261–6. doi: 10.1002/lsm.10051. [DOI] [PubMed] [Google Scholar]
- 7.van Gemert MJC, Welch AJ. Time constants in thermal laser medicine. Lasers Surg Med. 1989;9:405–421. doi: 10.1002/lsm.1900090414. [DOI] [PubMed] [Google Scholar]
- 8.Krapchev VB, Rabii CD, Harrington JA. “Novel CO2 laser system for hard tissue ablation,” Lasers in Surgery: Advanced Characterization, Therapeutics, and Systems IV. Proc SPIE. 1994;2128:341–348. [Google Scholar]
- 9.Ertl T, Muller G. “Hard tissue ablation with pulsed CO2 lasers,” Lasers in orthopedic, dental, and veterinary medicine II. Proc SPIE. 1993;1880:176–181. [Google Scholar]
- 10.Lukac M, Hocevar F, Cencic S, Nemes K, Keller U, Hibst R, Sustercic D, Gaspirc B, Skaleric U, Funduk N. Effects of pulsed CO2 and Er:YAG lasers on enamel and dentin,” Lasers in orthopedic, dental, and veterinary medicine II. Proc SPIE. 1993;1880:169–175. [Google Scholar]
- 11.Forrer M, Frenz M, Romano V, Altermatt HJ, Weber HP, Silenok A, Istomyn M, Konov VI. Bone-ablation mechanism using CO2 lasers of different pulse duration and wavelength. Appl Phys B. 1993;56:104–112. [Google Scholar]
- 12.Romano V, Rodriguez R, Altermatt HJ, Frenz M, Weber HP. “Bone microsurgery with IR-lasers: a comparative study of the thermal action at different wavelengths,” Laser interaction with hard and soft tissue. Proc SPIE. 1994;2077:87–96. [Google Scholar]
- 13.Fried D. “Laser Processing of Dental Hard Tissues” Laser Applications in Microelectronics and Optoelectronic Manufacturing X. Proc SPIE. 2005;5713:259–269. [Google Scholar]
- 14.Fried D, Murray MW, Featherstone JDB, Akrivou M, Dickenson KM, Duhn C. Dental hard tissue modification and removal using sealed TEA lasers operating at λ=9.6 μm. J Biomedical Opt. 2001;6(2):231–238. doi: 10.1117/1.1344192. [DOI] [PubMed] [Google Scholar]
- 15.Visuri SR. Laser Irradiation of Dental Hard Tissues. Northwestern University; Evanston, IL: 1996. [Google Scholar]
- 16.Assa S, Meyer S, Fried D. “Ablation of dental hard tissues with a microsecond pulsed carbon dioxide laser operating at 9.3-μm with an integrated scanner,” Lasers in Dentistry XIV. Proc SPIE. 2008;6843(E):1–7. doi: 10.1117/12.778799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan K, Bell P, Fried D. Rapid and conservative ablation and modification of enamel, dentin, and alveolar bone using a high repetition rate transverse excited atmospheric pressure CO2 laser operating at lambda=9.3 micro. J Biomed Opt. 2006;11(6):064008. doi: 10.1117/1.2401151. [DOI] [PubMed] [Google Scholar]
- 18.LaMantia NR, Tom H, Chan KH, Simon JC, Darling CL, Fried D. High contrast optical imaging methods for image guided laser ablation of dental caries lesions,” Lasers in Dentistry XX. Proc SPIE. 2014;8929(P):1–7. doi: 10.1117/12.2045683. [DOI] [PMC free article] [PubMed] [Google Scholar]


