Abstract
Canine leishmaniosis (CanL) is caused by Leishmania infantum, an obligate intracellular protozoan parasite, endemic in U.S. hunting dog populations. CanL has been found in dogs in 28 states and two Canadian provinces. Previous studies by our group, (Boggiatto, 2011), demonstrated that vertical transmission of Leishmania was the predominant means of transmission within U.S. dogs. Very little is known regarding how this alternative means of transmission, alters the long-term immunity and clinical presentation of leishmaniosis in dogs born to a positive bitch. This study follows the immunological progression of CanL in three pups after birth to an infected bitch. During the course of the study, these dogs were tested every six months over the course of six years. Both immunologic (IFN-γ, T cell proliferation, antibody production) and parasitological parameters (qPCR) of vertically-infected dogs were measured. Within the six years after birth to an L. infantum-infected, oligosymptomatic bitch, all dogs had at least one L. infantum PCR-positive test. Interestingly, despite living in the same location for their entire lives and being full siblings, these pups demonstrate three different disease progression patterns of L. infantum infection. One dog progressed to oligosymptomatic disease, maintaining a positive titer and had intermittent positive PCR results. One asymptomatic dog had positive serological titers and demonstrated a robust CD4+ immune response to infection. The third dog had a negligible response to L. infantum antigen and was healthy. This work demonstrates the biologic variability associated with vertically-transmitted infection similar to the variety of presentations observed during vector-borne leishmaniosis.
Keywords: leishmaniasis, vertical transmission, progression, immune response, canine, qPCR, serology
Introduction
Leishmaniasis is a spectral vector-borne disease caused by intracellular protozoan parasites of the genus Leishmania. Natural hosts include dogs and humans (Dantas-Torres et al., 2012; Millan et al., 2014; Ravendra and Anuradha, 2006). Leishmania is endemic in human and canine populations in more than 98 countries affecting more than 1 million people per year and countless dogs (Alvar et al., 2013). Global canine mortality due to leishmaniosis is unknown. Data generated based on CanL seropositive dog culling in multiple countries including Brazil, suggests that canine mortality associated with CanL is 20-30 times greater than that of humans (Alvar et al., 2013; Costa et al., 2007; Esch et al., 2012; Palatnik-de-Sousa et al., 2001). Visceralizing leishmaniasis, due to its systemic presentation, is commonly termed leishmaniosis. Signs of CanL include: weight loss, lethargy, decreased appetites, enlarged lymph nodes, kidney failure and immune compromise (Oliveira et al., 2010; Solano-Gallego et al., 2011). Leishmaniosis is not commonly found in people within the U.S. but has become endemic within the hunting dog population. The first documented autochthonous hunting dog infection was reported in 1980 in Oklahoma (Anderson et al., 1980). The first large outbreak of CanL occurred in 1999 in the Northeastern U.S. Follow-up studies demonstrated that more than 18 U.S. states and 2 Canadian provinces had serologically positive hunting dogs (Duprey et al., 2006; Gaskin et al., 2002). Since then, CanL has spread further within these hunting dog populations to 35 states and 2 Canadian provinces (Petersen, 2009; Schantz et al., 2005). The current prevalence of leishmaniosis in a specific breed of U.S. hunting dogs is greater than 3 per 1000 dogs (Toepp et al in preparation).
Multiple diagnostic techniques can be used to assess presence or absence of Leishmania infection. For the purposes of this study, real time quantitative polymerase chain reaction (qPCR) was used to identify and quantitate parasitic infection. Immune response to infection was evaluated by antibody titer, T cell proliferation and IFN-γ production. Measurement of canine IFN-γ-producing CD4+ T cells was shown previously to predict T helper 1-based immune control of infection, or loss thereof (Boggiatto et al., 2010).
To date, no naturally L. infantum-infected sand fly within the U.S. has been found and therefore no hunting dog leishmaniosis case has been determined to be secondary to sand fly transmission (Duprey et al., 2006). Instead vertical transmission was shown to be the predominant means of transmission in U.S. hunting dogs (Boggiatto et al., 2011). This study found that 10 of 12 naturally infected puppies had L.infantum parasites in multiple organs, including bone marrow, liver, lymph node, lung, spleen, thymus, blood, and umbilicus (Boggiatto et al. 2011). Multiple other studies have demonstrated that vertical transmission is not unique to these dogs or the U.S. (da Silva et al., 2009; Masucci et al., 2003; Naucke and Lorentz, 2012; Pangrazio et al., 2009; Rosypal et al., 2005).
Although there have been cross-sectional and case studies of vertically transmitted L. infantum infection in dogs, CanL progression from birth after vertical transmission has not been evaluated (Avila-Garcia et al., 2013; da Silva et al., 2009; Esch and Petersen, 2013; Gibson-Corley et al., 2008; Osorio et al., 2012; Pangrazio et al., 2009; Tanczos et al., 2012). This study follows three hunting dogs from birth as part of the litter described in (Boggiatto et al., 2011). These pups were born to a naturally infected, oligosymptomatic, dam within a controlled laboratory facility then returned to their kennel of origin and followed over six years. We hypothesized that by observing this cohort over time we would see similar immune progression and development of clinical leishmaniosis as previously described after classical, vector-borne transmission.
1 Materials and Methods
Animals
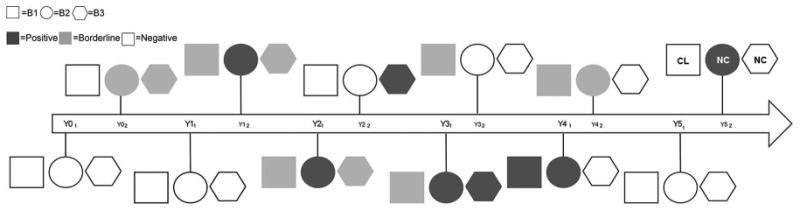
Three pups born to an L. infantum infected, oligosymptomatic dam from a litter of 15 (12 euthanized as part of Boggiatto et al 2011) were followed from birth until age 6. These dogs were sampled every 6 months to evaluate L. infantum infection status (Figure 1). Animals were enrolled with informed consent of the owner and followed with approval by the Iowa State University and University of Iowa Animal Control and Use Committees (IACUC). Animals were housed according to AAALAC accreditation standards.
Fig. 1. Timeline of diagnostic progression of Leishmania-infected dogs.
Dog B1 = square, B2 = circle, and B3 = hexagon. Positive qPCR or serology = dark grey. Borderline qPCR or a CDC IFAT titer of 1:16 or 1:32 = light grey. Negative for all diagnostic tests = white. Clinical status provided at last time point; CL indicates dog had multiple signs of clinical CanL, NC indicates one or no signs of disease based on physical exam.
Serology (IFA)
Canine serum samples were stored at -20°C and sent to the Centers for Disease Control and Prevention (CDC) for IFAT and in vitro promastigote culture as previous described (Badaro et al., 1983; Duprey et al., 2006).
PCR
Quantitative real-time qPCR was used to assess parasite ribosomal and/or kinetoplast DNA isolated from whole blood as previous (Boggiatto et al., 2011; Esch et al., 2013). L. infantum kinetoplastid specific primer sets and probe were used for qPCR diagnostic tests run from 2009-2011 F,5′-CCGCCCGCCTCAAGAC and R, 5′-TGCTGAATATTGGTGGTTTTGG (Integrated DNA Technologies, Coralville, IA), probe [6-FAM]-AGCCGCGAGGACC-3′ MGB (Applied Biosystems, Foster City, CA). Ribosomal specific Leishmania spp. primer and probe were used in 2010 through present F 5′-AAGCCACCCCAGAGGTAAAAA, R 5′ GACGGGTCTGACCCTTGGTT, (Integrated DNA Technologies, Coralville, IA), probe 5′ 6FAM-CGGTTCGGTGTGTGGCGCC-MGBNFQ (Applied Biosystems, Foster City, CA). Results were analyzed using ABI 7000 System SDS Software (Applied Biosystems, Life Technologies, Grand Island, NY).
CD4± T cell Proliferation and IFN-v Production via FACS analysis
Canine CD4+ T cell IFN-γ production and proliferation were assessed by intracellular flow cytometry labeling and BrdU or EdU incorporation, respectively, as previously described by (Esch et al., 2013). Briefly, peripheral blood mononuclear cells were isolated plated in 96-well tissue culture, round bottom plates at 5 × 10 cells per well. Cells were stimulated with 1 μg/mL of freeze-thawed L. infantum antigen for 7 days. Brefeldin-A (Sigma Aldrich, St. Louis, MO) was added to each well at a final 1X concentration per manufacturer's recommendation 6 hours prior to intracellular labeling. Interferon-γ was labeled with anti-Bovine-RPE clone CC302 (AbD Serotec, Raleigh, NC). Cells were fixed with BD™ Stabilizing Fixative (BD Biosciences, Franklin Lakes, NJ). Cells were collected for analysis utilizing an LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo vX (Treestar, Ashland, OR).
Statistical Analysis
Statistical analyzes were completed using Excel (Microsoft, Redmond, WA) and Graph Pad Prism version 6.05 (Graph Pad Software Inc., La Jolla, CA).
2 Results and Discussion
Results
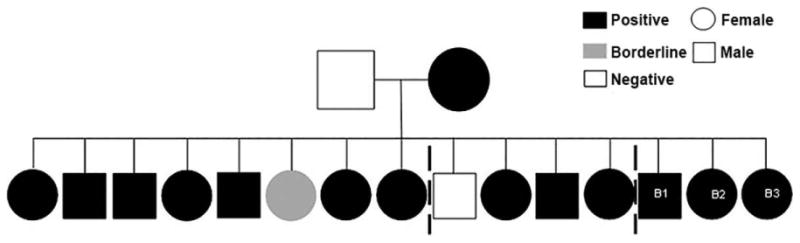
Vertical transmission of CanL was previously demonstrated to propagate infection within US hunting dogs (Boggiatto et al., 2011). Ten of twelve vertically infected puppies had qPCR evidence of Leishmania infantum parasitism in multiple organs (Figure 2, left, full data in Boggiatto et al., 2011). Despite presence of L. infantum while in utero during immune development, there was robust T cell responses to Leishmania antigens; these dogs were not centrally tolerant to parasite antigen. It was unknown how infection at birth altered CanL immune progression over multiple years of canine life. Three pups with in utero Leishmania exposure (Figure 2, B1-3) were followed from birth for six years. We expected in utero parasite exposure would induce these dogs to develop clinical disease over their working lifetime.
Fig. 2. Leishmaniosis pedigree of litter.
Litter including dogs followed in this study (B1, B2, B3) designated as infected based on any diagnostic test: T-cell proliferation assay, IFN-γ production, qPCR, and IFAT. Infected black, grey inconclusive data and white no evidence of infection. From left to right: the first 8 pups euthanized within 24h of birth, next 4 dogs followed for 3 months (these 12 dogs featured in Boggiatto et al, 2011), last 3 dogs followed in this study.
The three pups enrolled in this study were adopted back to the original kennel as they had no diagnostic evidence of L. infantum infection as measured by qPCR and IFAT at three months old. Through multiple years of sampling, these dogs exhibited fluctuating parasitemia, as demonstrated by positive PCR from blood (Figure 3). All three dogs had PCR detectable parasites within two to three years of life and within six months of each other. One PCR positive dog, B3, did not progress in a step-wise manner from negative to borderline then to positive but rather went directly from negative to positive (Figure 3).
Fig. 3. qPCR data on B-pup dogs from 2009-2014.
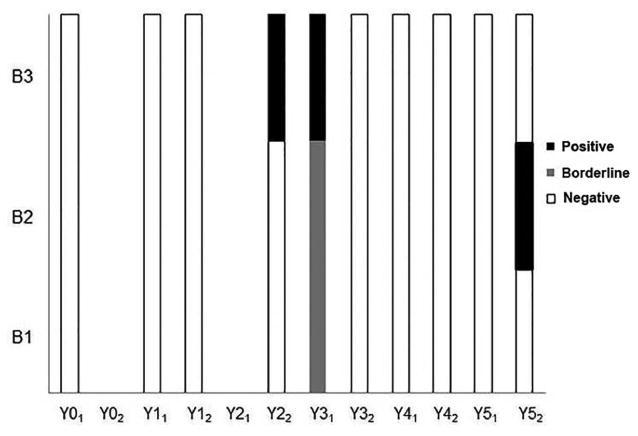
Dogs tested via Leishmania ribosomal or kinetoplastid-targeted qPCR twice per year. Each sample was run as neat or 1:10 dilution in duplicate. Ct less than 40 cycles for 2 plus runs was positive, one= borderline, zero =negative. Dogs B1, B2, and B3 with positive qPCR=black, borderline qPCR=light grey, negative qPCR=white.
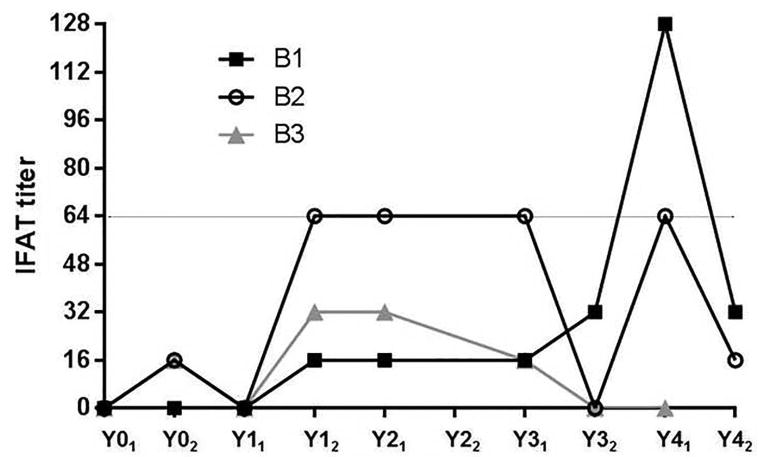
Utilizing qPCR detection as an approximation of parasitemia in each dog, we were interested in assessing whether qPCR-detectable presence of parasites was mirrored by seroconversion to Leishmania. Serology was measured via IFAT as performed at the Centers for Disease Control (CDC) (Figure 4). Serological patterns were quite different for each of the three dogs, not matching or echoing their qPCR results. Dog B1 had a suggestive titer of 1:16 consistently for three sampling periods before increasing to 1:32 and to a positive titer of 1:128 six months later. Dog B2 reported a very different pattern of positive 1:64 titers consistently before dropping to a titer of 1:16. The last dog, B3, had suspect titers, 1:32 twice and 1:16, before becoming seronegative in the last two years. Low titers were indicative of exposure and associated with maintenance of a subclinical disease state. These low titers did not correlate with clinical progression (Boggiatto et al., 2010; Solano-Gallego et al., 2011; Solano-Gallego et al., 2001).
Fig. 4. Serological responses to L. infantum infection.
Titers determined via IFAT performed at CDC. Titers based on two-fold dilution of sera, dilutions of 1:16 and 1:32 were considered suggestive and dilutions ≥1:64 were considered positive as designated by grey line.
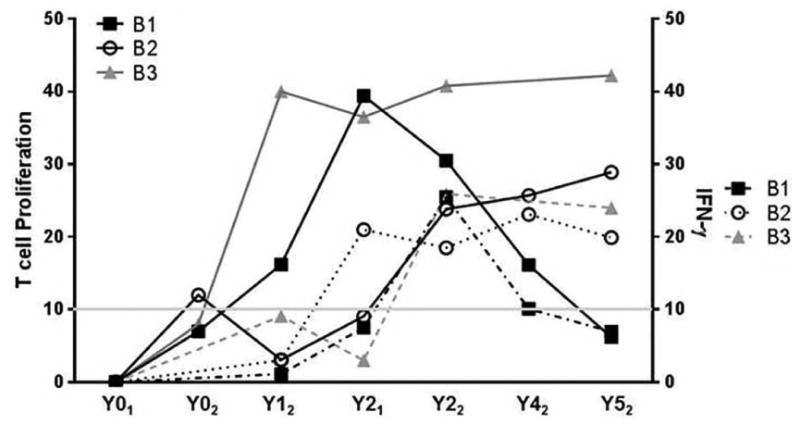
Based on these completely different PCR and serological profiles from three littermates all cohoused in the same environment, we were interested to determine whether their immunologic responses may be more informative regarding disease progression than qPCR or serologic diagnostic values over time. To do this we assessed two critical features of the protective T helper 1 (Th1) response (Boggiatto, 2010), proliferation to parasite antigen and production of IFN-γ via FACS analysis. Each dog had a gradual increase in T cell proliferation and IFN-γ production over the course of the study (Figure 5). Each dog responded to infection differently allowing us to observe three immune manifestations of CanL. Dog B1 had robust, increased populations of proliferative and IFN-γ-producing T cells between Y01-Y21 (Figure 5). After a dramatic rise in the population of IFN-γ producing cells, this dog had a dramatic crash of these T cell responses (Y22-Y52). A dramatic rise in IFAT serology followed the drop in T cell proliferation and IFN-γ production in this dog. qPCR did not provide informative data regarding progression of this dog. Based on physical examination, dog B1 had clinical signs of CanL at the last blood collection. While this dog was initially able to produce an effective CanL immune response, it is not able to maintain this response with subsequent clinical disease.
Fig. 5. L. infantum-specific T cell proliferation and IFN-γ production over six years of life with vertically-transmitted L. infantum infection.
Proliferation of L. infantum antigen-specific T cell populations from whole blood was measured via BrdU or EdU incorporation (left axis). CD4+ T cell populations positive for intracellular IFN-γ from each dog assessed via FACS (right axis).
Dog B2 had lower initial populations of proliferative and IFN-γ producing T cells than B1, Y01-Y22, but both of these T cell populations increased over Y42-Y52, suggesting this littermate was slower in mounting a productive Th1 immune response. Serological and qPCR results from this dog both moderately increased over the same timespan as T-cell proliferation and IFN-γ. Dog B2 was borderline via qPCR, suggesting that the dog was infected, maintaining a lower parasite load perhaps secondary to the presence of antigen-responsive T cell populations.
Dog B3 had a population of IFN-γ producing cells at Y3, and has maintained a robust population of L. infantum-specific proliferating T-cells since Y0. Serology and qPCR correlate with the presence of a population of IFN-γ producing cells maintaining a negative titers from Y32 to Y52. B3 had positive qPCR at Y22-Y31, but returned negative qPCR Y32-Y52. These results suggest this dog's immune response was controlling CanL.
3 Discussion
Although there is a good understanding of immune responses to vector-borne CanL very little is known about how vertical transmission alters responses to infection (Oliveira et al., 2010; Solano-Gallego et al., 2001). Lack of continuous qPCR parasite detection suggests that the parasite was only intermittently present or more importantly detectable in peripheral blood, and had visceralized. Consistent positive serological results suggest that the dogs had antibody-based immune responses to Leishmania over the course of six years but more closely correlated with clinical progression than immune control of disease. Variation in antibody titers could represent varying degrees to susceptibility to infection (Quinnell et al., 2003;Solano-Gallego et al., 2001).
Detectable to strong CD4+ T cell proliferation after birth suggests this litter of dogs was indeed able to mount specific cellular immune responses to Leishmania antigens and they were not centrally tolerant to L. infantum antigen. This is in contrast to other vertically-transmitted diseases, e.g. Bovine Viral Diarrhea Virus (Schweizer et al., 2006). As time passes without control of infection, canine L. infantum specific CD4+ T cells were shown to lose antigen proliferation and IFN-γ production, known as T cell exhaustion (Esch et al., 2013).
Other than CD4+ T cells as a source for IFNγ, CD8+ T cells, TH0 cells, and NKT cells also can secrete IFN-γ during Leishmania infection (Esch et al., 2013; Lehmann et al., 2000), however have only a minor role in the IFN-γ cytokine pool. Rising IFN-γ –producing populations in these dogs mirror increases in CD4+ T cell proliferation. These results indicate, particularly in dog B2 and 3, productive Th1 immune responses, critical for control of Leishmania and other intracellular pathogens (Sacks and Noben-Trauth, 2002). Dog B1 had an early productive immune response against Leishmania, but populations of IFN-γ-positive cells waned with progression to clinical disease.
Dogs with transplacental L. infantum infection had productive immune responses early after infection. Over time dog B1 experienced immune exhaustion and failed to control infection, with clinical disease consequences. To serve their working purpose, working dogs must maintain strong immune responses throughout infection despite the many other physical demands placed upon them to ward off clinical disease. While dogs with CanL may diagnostically appear to have controlled infection, the potential for progression to clinical disease after coinfection(s) or with increased age and resultant immune senescence is high.
4 Conclusions
This immunologic and diagnostic description of three dogs over six years of life with L. infantum infection provides a novel, prospective characterization of progressive canine leishmaniosis. This unique insight into the natural progression of leishmaniosis after vertical transmission indicates that while a dog born to an infected dam may not present with disease early in life, this does not absolve a dog from clinical disease later in life. Characterization of immunological and diagnostic changes that occurred in hunting dogs infected with L. infantum in utero, indicates that much like vector-borne infection, there are many different manifestations of CanL. While all three dogs controlled Leishmania infection for the first 2-3 years of life, over time each dog had positive PCR and serology, as well as developed a CD4+ T cell immune response to Leishmania antigen. More than five years later, one dog has become oligosymptomatic. Based on this slow progression to disease, it is imperative that breeding precautions be discussed with at-risk dog owners or handlers and management methods employed to reduce zoonotic exposure to people during whelping and other blood exposures to prevent transmission of this CanL.
Acknowledgments
The authors would like to thank the collaborating hunting dog hunts for their support of this study. This work was funded by D13CA-501 from the Morris Animal Foundation, the American Kennel Club Canine Health Foundation (ACORN grants 799-A, 1220-A to CAP) and the National Institutes of Health R21AI074711 to CAP.
Footnotes
Department of Epidemiology, S429 CPHB 145 N. Riverside Dr Iowa City, IA, 52241 Tel.: 319 384 1579, fax: 319 384 4155
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
4.1 References
- Alvar J, Croft SL, Kaye P, Khamesipour A, Sundar S, Reed SG. Case study for a vaccine against leishmaniasis. Vaccine. 2013;31:B244–B249. doi: 10.1016/j.vaccine.2012.11.080. [DOI] [PubMed] [Google Scholar]
- Anderson DC, Buckner RG, Glenn BL, MacVean DW. Endemic canine leishmaniasis. Veterinary Pathology. 1980;17:94–96. doi: 10.1177/030098588001700110. [DOI] [PubMed] [Google Scholar]
- Avila-Garcia M, Mancilla-Ramirez J, Segura-Cervantes E, Farfan-Labonne B, Ramirez-Ramirez A, Galindo-Sevilla N. Transplacental transmission of cutaneous Leishmania mexicana strain in BALB/c mice. American Journal of Tropical Medicine & Hygiene. 2013;89:354–358. doi: 10.4269/ajtmh.12-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badaro R, Reed SG, Carvalho EM. Immunofluorescent antibody test in American visceral leishmaniasis: sensitivity and specificity of different morphological forms of two Leishmania species. Am J Trop Med Hyg. 1983;32:480–484. doi: 10.4269/ajtmh.1983.32.480. [DOI] [PubMed] [Google Scholar]
- Boggiatto PM, Gibson-Corley KN, Metz K, Gallup JM, Hostetter JM, Mullin K, Petersen CA. Transplacental transmission of Leishmania infantum as a means for continued disease incidence in North America. PLoS Negl Trop Dis. 2011;5:e1019. doi: 10.1371/journal.pntd.0001019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggiatto PM, Ramer-Tait AE, Metz K, Kramer EE, Gibson-Corley K, Mullin K, Hostetter JM, Gallup JM, Jones DE, Petersen CA. Immunologic indicators of clinical progression during canine Leishmania infantum infection. Clin Vaccine Immunol. 2010;17:267–273. doi: 10.1128/CVI.00456-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa CH, Tapety CM, Werneck GL. [Control of visceral leishmaniasis in urban areas: randomized factorial intervention trial] Rev Soc Bras Med Trop. 2007;40:415–419. doi: 10.1590/s0037-86822007000400009. [DOI] [PubMed] [Google Scholar]
- da Silva SM, Ribeiro VM, Ribeiro RR, Tafuri WL, Melo MN, Michalick MS. First report of vertical transmission of Leishmania (Leishmania) infantum in a naturally infected bitch from Brazil. Vet Parasitol. 2009;166:159–162. doi: 10.1016/j.vetpar.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F, Solano-Gallego L, Baneth G, Ribeiro VM, de Paiva-Cavalcanti M, Otranto D. Canine leishmaniosis in the Old and New Worlds: unveiled similarities and differences. Trends Parasitol. 2012;28:531–538. doi: 10.1016/j.pt.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Duprey ZH, Steurer FJ, Rooney JA, Kirchhoff LV, Jackson JE, Rowton ED, Schantz PM. Canine visceral leishmaniasis, United States and Canada, 2000-2003. Emerg Infect Dis. 2006;12:440–446. doi: 10.3201/eid1203.050811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch KJ, Juelsgaard R, Martinez PA, Jones DE, Petersen CA. Programmed death 1-mediated T cell exhaustion during visceral leishmaniasis impairs phagocyte function. Journal of Immunology. 2013;191:5542–5550. doi: 10.4049/jimmunol.1301810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch KJ, Petersen CA. Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clin Microbiol Rev. 2013;26:58–85. doi: 10.1128/CMR.00067-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch KJ, Pontes NN, Arruda P, O'Connor A, Morais L, Jeronimo SM, Petersen CA. Preventing zoonotic canine leishmaniasis in northeastern Brazil: pet attachment and adoption of community leishmania prevention. Am J Trop Med Hyg. 2012;87:822–831. doi: 10.4269/ajtmh.2012.12-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin AA, Schantz P, Jackson J, Birkenheuer A, Tomlinson L, Gramiccia M, Levy M, Steurer F, Kollmar E, Hegarty BC, Ahn A, Breitschwerdt EB. Visceral leishmaniasis in a New York foxhound kennel. J Vet Intern Med. 2002;16:34–44. doi: 10.1892/0891-6640(2002)016<0034:vliany>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Gibson-Corley KN, Hostetter JM, Hostetter SJ, Mullin K, Ramer-Tait AE, Boggiatto PM, Petersen CA. Disseminated Leishmania infantum infection in two sibling foxhounds due to possible vertical transmission. Can Vet J. 2008;49:1005–1008. [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Enssle KH, Lehmann I, Emmendorfer A, Lohmann-Matthes ML. The capacity to produce IFN-gamma rather than the presence of interleukin-4 determines the resistance and the degree of susceptibility to Leishmania donovani infection in mice. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2000;20:63–77. doi: 10.1089/107999000312748. [DOI] [PubMed] [Google Scholar]
- Masucci M, De Majo M, Contarino RB, Borruto G, Vitale F, Pennisi MG. Canine leishmaniasis in the newborn puppy. Vet Res Commun. 2003;27(Suppl 1):771–774. doi: 10.1023/b:verc.0000014268.61966.69. [DOI] [PubMed] [Google Scholar]
- Millan J, Ferroglio E, Solano-Gallego L. Role of wildlife in the epidemiology of Leishmania infantum infection in Europe. Parasitol Res. 2014;113:2005–2014. doi: 10.1007/s00436-014-3929-2. [DOI] [PubMed] [Google Scholar]
- Naucke TJ, Lorentz S. First report of venereal and vertical transmission of canine leishmaniosis from naturally infected dogs in Germany. Parasit Vectors. 2012;5:67. doi: 10.1186/1756-3305-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AM, Diaz S, Santos C, Bourdeau P, da Fonseca IP. Geographical distribution, clinical presentation, treatment and prevention of canine leishmaniosis in Portugal: a 2007 field survey. Revista Portuguesa de Ciencias Veterinarias. 2010;105:21–29. [Google Scholar]
- Osorio Y, Rodriguez LD, Bonilla DL, Peniche AG, Henao H, Saldarriaga O, Travi BL. Congenital transmission of experimental leishmaniasis in a hamster model. American Journal of Tropical Medicine & Hygiene. 2012;86:812–820. doi: 10.4269/ajtmh.2012.11-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatnik-de-Sousa CB, dos Santos WR, Franca-Silva JC, da Costa RT, Reis AB, Palatnik M, Mayrink W, Genaro O. Impact of canine control on the epidemiology of canine and human visceral leishmaniasis in Brazil. Am J Trop Med Hyg. 2001;65:510–517. doi: 10.4269/ajtmh.2001.65.510. [DOI] [PubMed] [Google Scholar]
- Pangrazio KK, Costa EA, Amarilla SP, Cino AG, Silva TM, Paixao TA, Costa LF, Dengues EG, Diaz AA, Santos RL. Tissue distribution of Leishmania chagasi and lesions in transplacentally infected fetuses from symptomatic and asymptomatic naturally infected bitches. Vet Parasitol. 2009;165:327–331. doi: 10.1016/j.vetpar.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Petersen CA. Leishmaniasis, an emerging disease found in companion animals in the United States. Top Companion Anim Med. 2009;24:182–188. doi: 10.1053/j.tcam.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinnell RJ, Courtenay O, Garcez LM, Kaye PM, Shaw MA, Dye C, Day MJ. IgG subclass responses in a longitudinal study of canine visceral leishmaniasis. Vet Immunol Immunopathol. 2003;91:161–168. doi: 10.1016/s0165-2427(02)00311-2. [DOI] [PubMed] [Google Scholar]
- Ravendra G, Anuradha D. Animal models for vaccine studies for visceral leishmaniasis. Indian Journal of Medical Research. 2006;123:439–454. [PubMed] [Google Scholar]
- Rosypal AC, Troy GC, Zajac AM, Frank G, Lindsay DS. Transplacental transmission of a North American isolate of Leishmania infantum in an experimentally infected beagle. J Parasitol. 2005;91:970–972. doi: 10.1645/GE-483R.1. [DOI] [PubMed] [Google Scholar]
- Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- Schantz PM, Steurer FJ, Duprey ZH, Kurpel KP, Barr SC, Jackson JE, Breitschwerdt EB, Levy MG, Fox JC. Autochthonous visceral leishmaniasis in dogs in North America. J Am Vet Med Assoc. 2005;226:1316–1322. doi: 10.2460/javma.2005.226.1316. [DOI] [PubMed] [Google Scholar]
- Schweizer M, Matzener P, Pfaffen G, Stalder H, Peterhans E. “Self” and “nonself” manipulation of interferon defense during persistent infection: bovine viral diarrhea virus resists alpha/beta interferon without blocking antiviral activity against unrelated viruses replicating in its host cells. J Virol. 2006;80:6926–6935. doi: 10.1128/JVI.02443-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano-Gallego L, Miro G, Koutinas A, Cardoso L, Pennisi MG, Ferrer L, Bourdeau P, Oliva G, Baneth G, The LeishVet, G LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors. 2011;4:86. doi: 10.1186/1756-3305-4-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano-Gallego L, Riera C, Roura X, Iniesta L, Gallego M, Valladares JE, Fisa R, Castillejo S, Alberola J, Ferrer L, Arboix M, Portus M. Leishmania infantum-specific IgG, IgG1 and IgG2 antibody responses in healthy and ill dogs from endemic areas. Evolution in the course of infection and after treatment. Vet Parasitol. 2001;96:265–276. doi: 10.1016/s0304-4017(00)00446-5. [DOI] [PubMed] [Google Scholar]
- Tanczos B, Balogh N, Kiraly L, Biksi I, Szeredi L, Gyurkovsky M, Scalone A, Fiorentino E, Gramiccia M, Farkas R. First record of autochthonous canine leishmaniasis in Hungary. Vector Borne Zoonotic Dis. 2012;12:588–594. doi: 10.1089/vbz.2011.0906. [DOI] [PMC free article] [PubMed] [Google Scholar]


