Abstract
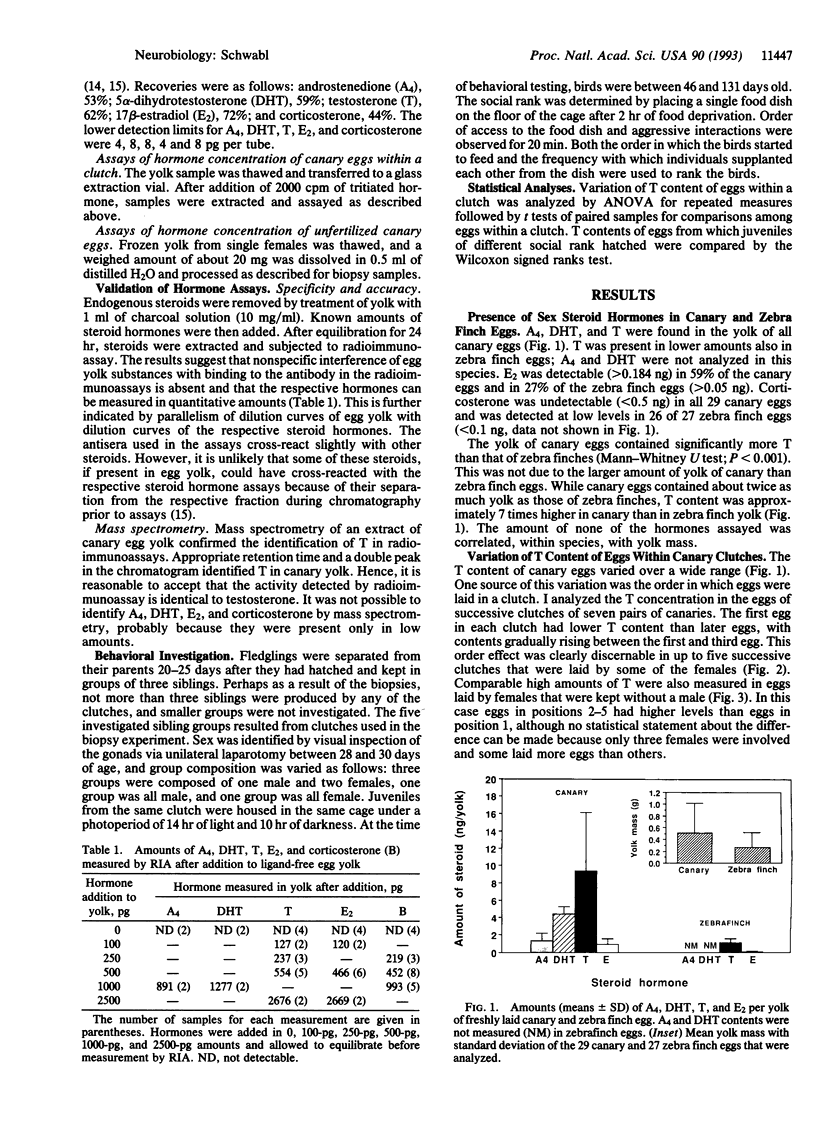
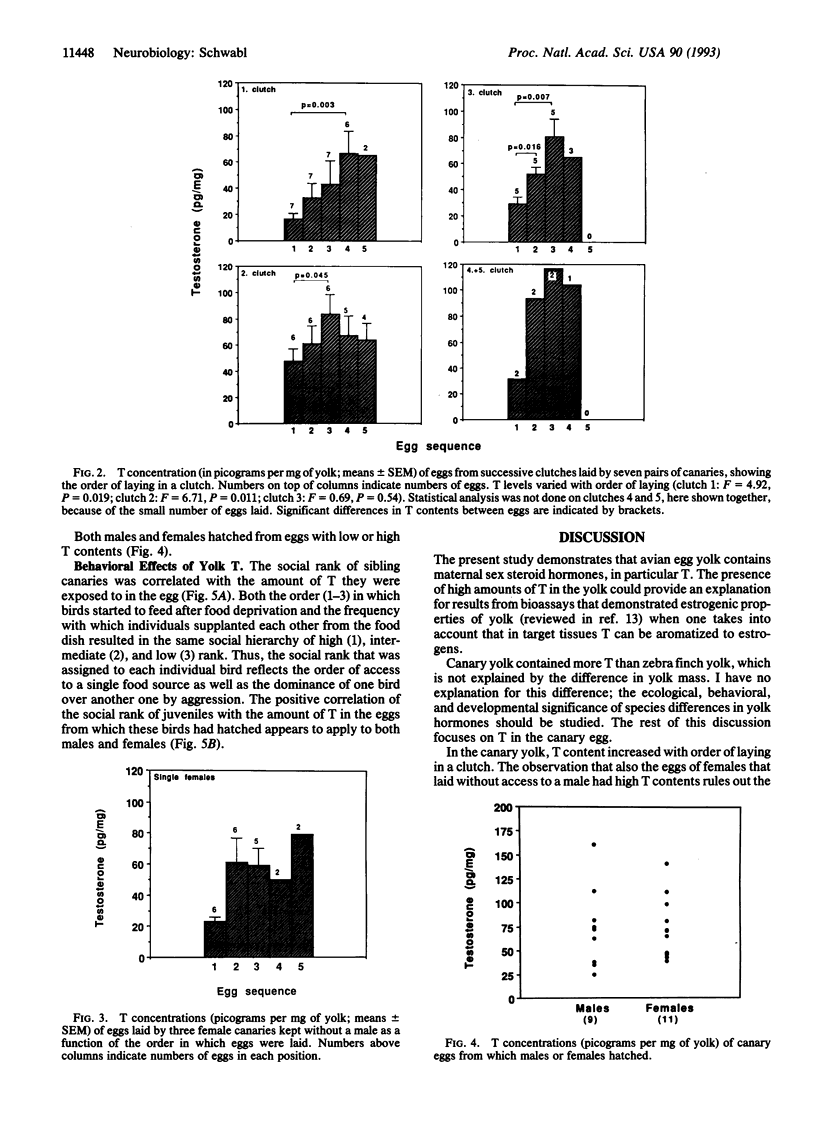
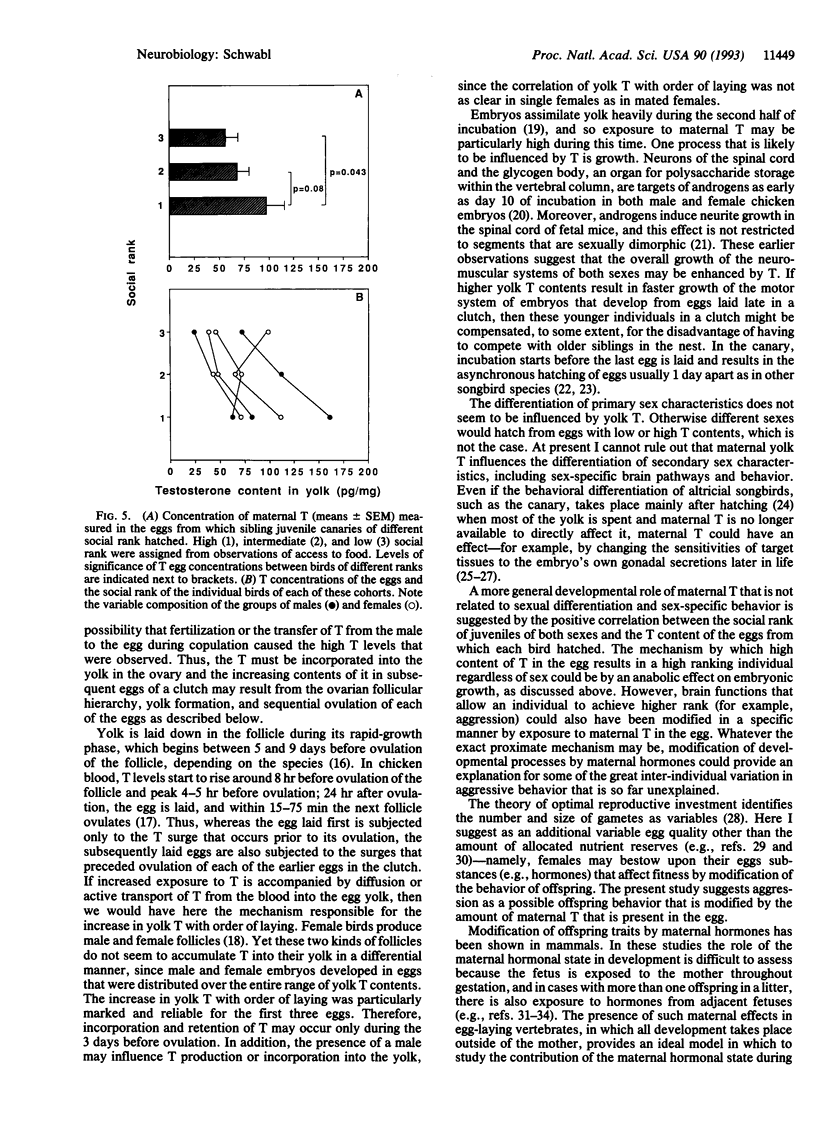
The sex steroid hormones that affect development in birds have been thought to be produced exclusively by the embryo or neonate. I used radioimmunoassay to measure the amounts of androstenedione, 5 alpha-dihydrotestosterone, testosterone, 17 beta-estradiol, and corticosterone in the yolk of freshly laid canary (Serinus canaria) and zebra finch (Poephila guttata) eggs. Testosterone was found in both canary and zebra finch eggs, but its contents were much higher in the former than in the latter. The testosterone content of canary eggs in a same clutch increased with the order of laying, regardless of the genetic sex of the offspring that hatched from these eggs. Yolk testosterone was also present in the eggs of female canaries that were kept without a male, indicating that it is of maternal origin. The social rank of juvenile canaries was positively correlated with the concentration of yolk testosterone in the eggs from which they hatched, suggesting that the development of aggressive behavior of offspring might be subject to modification by maternal testosterone. These findings indicate that female songbirds can bestow upon their eggs a dose of hormone that modifies the behavior of offspring. Variable doses of these hormones might explain some of the individual variation in offspring behavior.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins-Regan E., Abdelnabi M., Mobarak M., Ottinger M. A. Sex steroid levels in developing and adult male and female zebra finches (Poephila guttata). Gen Comp Endocrinol. 1990 Apr;78(1):93–109. doi: 10.1016/0016-6480(90)90051-m. [DOI] [PubMed] [Google Scholar]
- Adkins E. K. Embryonic exposure to an antiestrogen masculinizes behavior of female quail. Physiol Behav. 1976 Aug;17(2):357–359. doi: 10.1016/0031-9384(76)90088-3. [DOI] [PubMed] [Google Scholar]
- Balthazart J., De Clerck A., Foidart A. Behavioral demasculinization of female quail is induced by estrogens: studies with the new aromatase inhibitor, R76713. Horm Behav. 1992 Jun;26(2):179–203. doi: 10.1016/0018-506x(92)90041-s. [DOI] [PubMed] [Google Scholar]
- Clemens L. G., Gladue B. A., Coniglio L. P. Prenatal endogenous androgenic influences on masculine sexual behavior and genital morphology in male and female rats. Horm Behav. 1978 Feb;10(1):40–53. doi: 10.1016/0018-506x(78)90023-5. [DOI] [PubMed] [Google Scholar]
- Elbrecht A., Smith R. G. Aromatase enzyme activity and sex determination in chickens. Science. 1992 Jan 24;255(5043):467–470. doi: 10.1126/science.1734525. [DOI] [PubMed] [Google Scholar]
- Even M. D., Dhar M. G., vom Saal F. S. Transport of steroids between fetuses via amniotic fluid in relation to the intrauterine position phenomenon in rats. J Reprod Fertil. 1992 Nov;96(2):709–716. doi: 10.1530/jrf.0.0960709. [DOI] [PubMed] [Google Scholar]
- Hauser K. F., Toran-Allerand C. D. Androgen increases the number of cells in fetal mouse spinal cord cultures: implications for motoneuron survival. Brain Res. 1989 Apr 17;485(1):157–164. doi: 10.1016/0006-8993(89)90677-x. [DOI] [PubMed] [Google Scholar]
- Hertelendy F., Common R. H. A chromatographic investigation of egg yolk for the presence of steroid estrogens. Poult Sci. 1965 Sep;44(5):1205–1209. doi: 10.3382/ps.0441205. [DOI] [PubMed] [Google Scholar]
- Hutchison J. B., Wingfield J. C., Hutchison R. E. Sex differences in plasma concentrations of steroids during the sensitive period for brain differentiation in the zebra finch. J Endocrinol. 1984 Dec;103(3):363–369. doi: 10.1677/joe.0.1030363. [DOI] [PubMed] [Google Scholar]
- Licht P., Frank L. G., Pavgi S., Yalcinkaya T. M., Siiteri P. K., Glickman S. E. Hormonal correlates of 'masculinization' in female spotted hyaenas (Crocuta crocuta). 2. Maternal and fetal steroids. J Reprod Fertil. 1992 Jul;95(2):463–474. doi: 10.1530/jrf.0.0950463. [DOI] [PubMed] [Google Scholar]
- Reid F. A., Gasc J. M., Stumpf W. E., Sar M. Androgen target cells in spinal cord, spinal ganglia, and glycogen body of chick embryos. Autoradiographic localization. Exp Brain Res. 1981;44(3):243–248. doi: 10.1007/BF00236561. [DOI] [PubMed] [Google Scholar]
- Schlinger B. A., Arnold A. P. Plasma sex steroids and tissue aromatization in hatchling zebra finches: implications for the sexual differentiation of singing behavior. Endocrinology. 1992 Jan;130(1):289–299. doi: 10.1210/endo.130.1.1727704. [DOI] [PubMed] [Google Scholar]
- Schumacher M., Sulon J., Balthazart J. Changes in serum concentrations of steroids during embryonic and post-hatching development of male and female Japanese quail (Coturnix coturnix japonica). J Endocrinol. 1988 Jul;118(1):127–134. doi: 10.1677/joe.0.1180127. [DOI] [PubMed] [Google Scholar]
- Shahabi N. A., Norton H. W., Nalbandov A. V. Steroid levels in follicles and the plasma of hens during the ovulatory cycle. Endocrinology. 1975 Apr;96(4):962–968. doi: 10.1210/endo-96-4-962. [DOI] [PubMed] [Google Scholar]
- Sinervo B., Huey R. B. Allometric engineering: an experimental test of the causes of interpopulational differences in performance. Science. 1990 Jun 1;248(4959):1106–1109. doi: 10.1126/science.248.4959.1106. [DOI] [PubMed] [Google Scholar]
- Tanabe Y., Saito N., Nakamura T. Ontogenetic steroidogenesis by testes, ovary, and adrenals of embryonic and postembryonic chickens (Gallus domesticus). Gen Comp Endocrinol. 1986 Sep;63(3):456–463. doi: 10.1016/0016-6480(86)90146-2. [DOI] [PubMed] [Google Scholar]
- Tanabe Y., Yano T., Nakamura T. Steroid hormone synthesis and secretion by testes, ovary, and adrenals of embryonic and postembryonic ducks. Gen Comp Endocrinol. 1983 Jan;49(1):144–153. doi: 10.1016/0016-6480(83)90018-7. [DOI] [PubMed] [Google Scholar]
- West-Eberhard M. J. Alternative adaptations, speciation, and phylogeny (A Review). Proc Natl Acad Sci U S A. 1986 Mar;83(5):1388–1392. doi: 10.1073/pnas.83.5.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield J. C., Farner D. S. The determination of five steroids in avian plasma by radioimmunoassay and competitive protein-binding. Steroids. 1975 Sep;26(3):311–321. doi: 10.1016/0039-128x(75)90077-x. [DOI] [PubMed] [Google Scholar]
- vom Saal F. S., Bronson F. H. In utero proximity of female mouse fetuses to males: effect on reproductive performance during later life. Biol Reprod. 1978 Nov;19(4):842–853. doi: 10.1095/biolreprod19.4.842. [DOI] [PubMed] [Google Scholar]



