SUMMARY
Sex differences in physiology and disease susceptibility are commonly attributed to developmental and/or hormonal factors, but there is increasing realisation that cell-intrinsic mechanisms play important and persistent roles1,2. Here we use the Drosophila melanogaster intestine to investigate the nature and significance of cellular sex in an adult somatic organ in vivo. We find that the adult intestinal epithelium is a cellular mosaic of different sex differentiation pathways, and displays extensive sex differences in expression of genes with roles in growth and metabolism. Cell-specific reversals of the sexual identity of adult intestinal stem cells uncover its key roles in controlling organ size, its reproductive plasticity and its response to genetically induced tumours. Unlike previous examples of sexually dimorphic somatic stem cell activity, the sex differences in intestinal stem cell behaviour arise from intrinsic mechanisms, which control cell cycle duration and involve a new doublesex- and fruitless-independent branch of the sex differentiation pathway downstream of transformer. Together, our findings indicate that the plasticity of an adult somatic organ is reversibly controlled by its sexual identity, imparted by a new mechanism that may be active in more tissues than previously recognised.
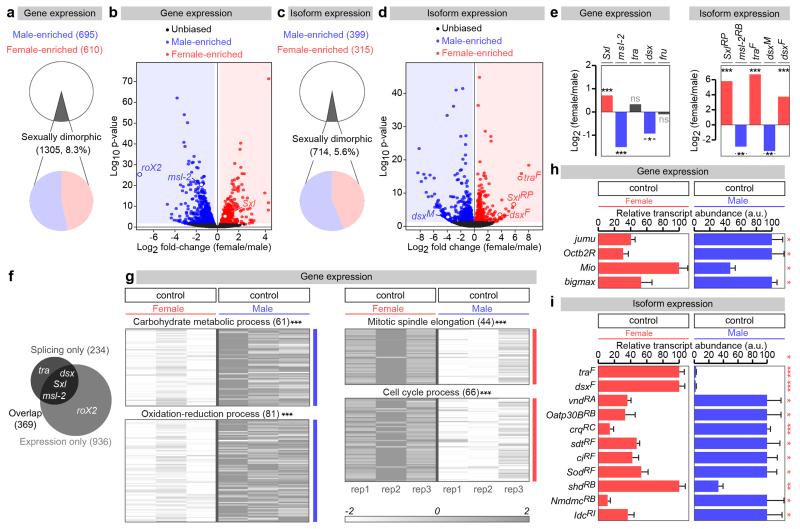
Sex differences in intestinal physiology3 prompted us to investigate possible molecular underpinnings. RNA-seq transcriptional profiling of virgin adult midguts (GEO accession number GSE74775) indicated significant sexual dimorphism in gene expression and/or splicing, with 8.3% of all genes and 5.6% of all isoforms expressed in the midgut displaying sex differences in expression (Extended Data Fig. 1a-f and GutSexRNAseq.xls). Sex-biased expression or splicing was confirmed for a subset of genes by real time qRT-PCR (Extended Data Fig. 1h, i). Genes with sex differences in expression cluster into distinct biological processes; genes assigned to cell division-related processes are more abundantly expressed in females, whereas genes coding for proteins that function in carbohydrate metabolism and redox processes are preferentially expressed in males (Extended Data Fig. 1g).
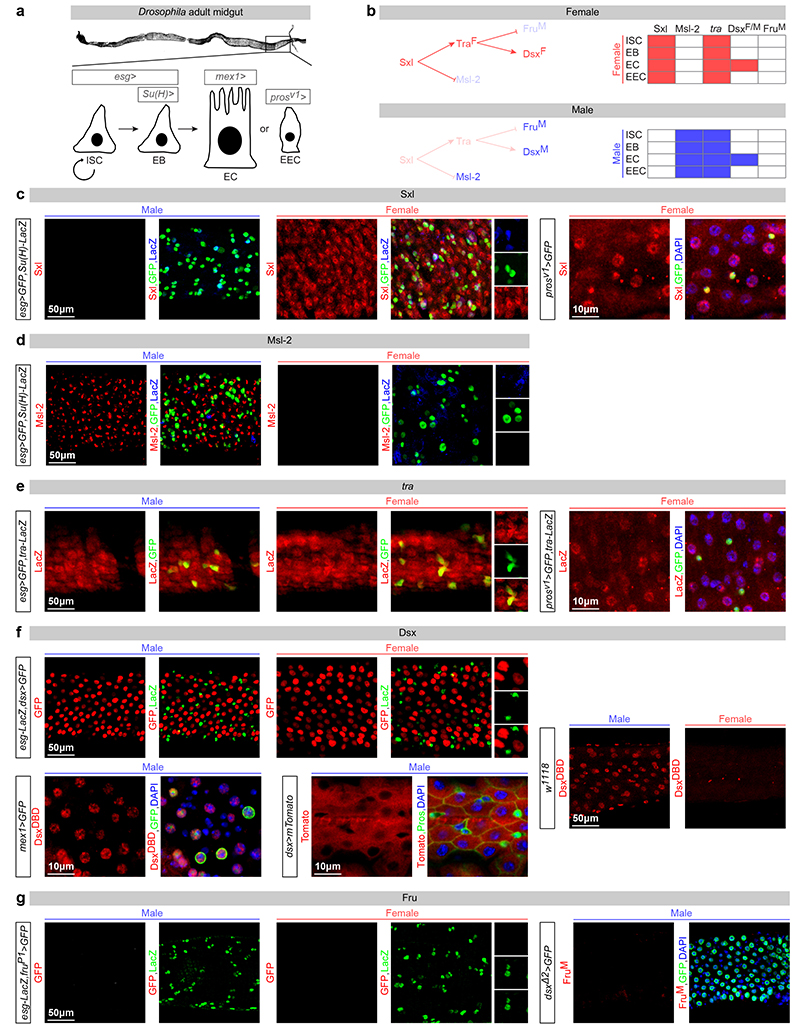
The above results suggested active expression of sex determinants in the adult intestine. In Drosophila, sex chromosome sensing leads to sexually dimorphic expression of Sex lethal (Sxl): the master regulator of both sexual development and dosage compensation (DC, an epigenetic process by which transcription of the single male X chromosome is upregulated twofold4). Sxl controls the sex-specific splicing of its downstream target gene transformer (tra)5, leading to functional Tra protein expression only in females, and to the sex-specific splicing of two Tra direct targets – the transcription factors doublesex (dsx) and fruitless (fru) – that sculpt sexually dimorphic anatomical features, reproductive systems and behaviour6-8 (Extended Data Fig. 2b). As may be expected from their roles in DC4, Sxl and the sexually dimorphic DC complex (DCC) member Male-specific lethal 2 (Msl-2) are ubiquitously expressed in the intestinal epithelium of females and males, respectively, in both adult intestinal progenitors (intestinal stem cells (ISCs) and postmitotic enteroblasts (EBs)) and their differentiated progeny (enterocytes (ECs) and enteroendocrine cells (EECs)) (Extended Data Fig. 2a-d). Consistent with our RNA-seq analysis, a newly generated tra-LacZ reporter is also expressed in all four epithelial cell types of the adult intestine (Extended Data Fig. 2a, e) - suggesting that, downstream of Sxl, the sex differentiation pathway remains active in this adult epithelium. However, the two targets of Tra-mediated sex-specific splicing are either absent from all midgut epithelial cells (FruM), or are expressed in only a subset of epithelial cells (DsxF/M, expressed in ECs but absent from ISCs, EBs and EECs) (Extended Data Fig. 2a, f, g). Hence, the expression of sex determination genes is maintained in the adult midgut, but displays cell type specificity; while adult-born enterocytes express all members of the canonical sex determination pathway, their siblings (the EECs) and both types of adult intestinal progenitors (ISCs and EBs) express the early (Sxl, Tra), but not the late (DsxF/M, FruM), effectors of the pathway.
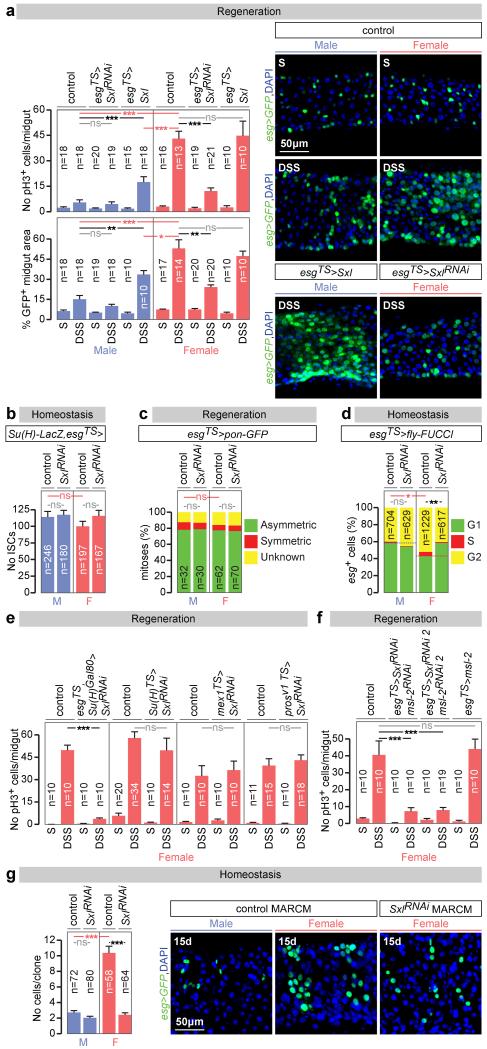
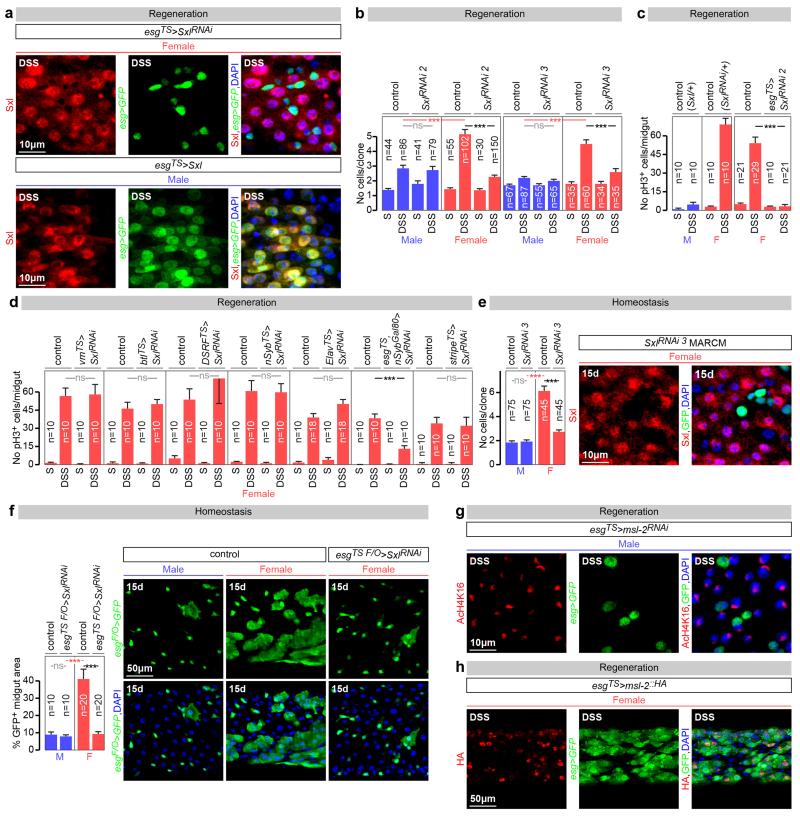
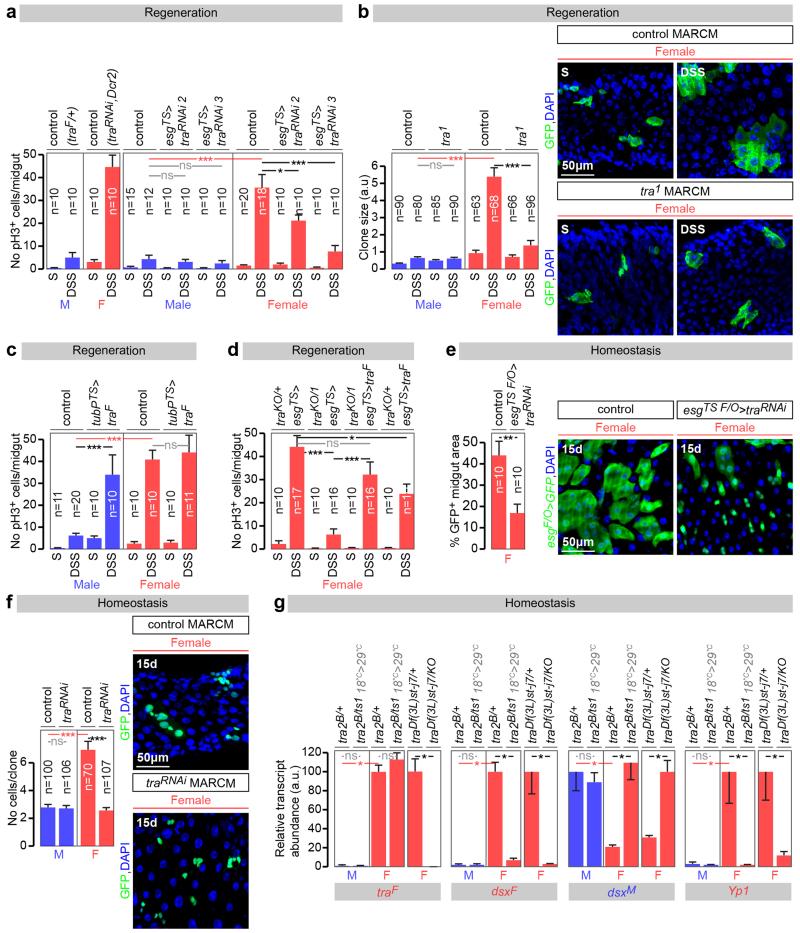
Both the enrichment analysis and the presence of Sxl/tra in adult ISCs pointed to sexually dimorphic ISC proliferation. Female flies exhibit a rapid proliferative response to dextran sodium sulphate (DSS)-induced damage of the intestinal epithelium (Fig. 1a and 9). This response was less pronounced in male midguts (Fig. 1a), or in female (but not male) midguts following adult-restricted Sxl downregulation in intestinal progenitors (Fig. 1a and Extended Data Fig. 3a, c). Conversely, ectopic expression of Sxl in adult intestinal progenitors enhanced proliferation in male (but not female) midguts (Fig. 1a and Extended Data Fig. 3a). Additional cell type- and adult-specific Sxl downregulation experiments indicated that Sxl acts in ISCs, and not in other cells, to control sexually dimorphic proliferation rather than differentiation (Fig. 1e and Extended Data Figs. 3d and 4a). Mechanistically, females do not have a significantly higher density of ISCs than male flies (Fig. 1b) or a higher ratio of symmetric vs asymmetric divisions (Fig. 1c), suggesting that the proliferative capacity of female ISCs is intrinsically enhanced by their expression of Sxl. Consistent with this idea, a higher percentage of their adult progenitors are found in G2/S phase at the expense of G1 during in homeostatic conditions (Fig. 1d), suggestive of shorter cell cycles, and adult-specific downregulation of Sxl in intestinal progenitors abrogated the sexual dimorphism in G2/S to G1 ratio, without affecting the number of ISCs or their division mode (Figs. 1b-d). Clonal analyses further confirmed the intrinsic nature of the sexual dimorphism in proliferation, its Sxl control and adult reversibility both during regeneration and homeostasis (Fig. 1g and Extended Data Fig. 3b, e and f).
Figure 1. Sxl controls intrinsic sex differences in adult ISC proliferation independently of dosage compensation.
a, Number of mitoses (phospho-histone H3 (pH3)-positive cells, top graph) and percentage of cells positive for intestinal progenitor markers (escargot (esg)-positive/total area, bottom graph) in controls and flies with adult-restricted downregulation or mis-expression of Sxl in ISCs/EBs (achieved by esg-Gal4, tub-Gal80TS-driven Sxl RNAi or UAS-Sxl, respectively). Flies were exposed to control (sucrose, S) or damage-inducing (DSS) diets. Representative images are shown to the right (DNA: DAPI, in blue; ISC/EB marker: GFP, in green). b, Stem cell number (esg+, Supressor of Hairless (Su(H))− cells) in the posterior midgut following 20 days of adult ISC/EB-specific Sxl downregulation. c, Quantifications of symmetric (red) vs asymmetric (green) ISC divisions based on cortical Partner of Numb (Pon)-GFP distribution in metaphase and telophase reveal no differences between the sexes or upon adult ISC/EB-specific Sxl downregulation. Mitoses with lack of clear Pon-GFP signal are displayed in yellow. d, Percentage of progenitors in G1, S or G2 as revealed by ISC/EB-driven expression of the cell cycle indicator Fly-FUCCI. e, Number of mitoses in DSS-treated flies with adult-specific downregulation of Sxl in ISCs (esg-Gal4, Su(H)-Gal80 driver), EBs (Su(H)-Gal4), ECs (midgut expression 1 (mex1-Gal4) or EECs (prospero (pros)V1-Gal4). f, pH3 quantifications following adult-specific msl-2 downregulation or mis-expression in ISCs/EBs. g, MARCM clone size quantifications (graph) and representative images (labelled in green with GFP) reveal that clones expressing Sxl-RNAi are smaller than control clones in females, but not in males. n denotes the number of midguts (a, e, f), ISCs/EBs (b, d), mitoses (c) or clones (g) that were analysed for each genotype. Results combined from at least two independent experiments. See Supplementary Information for full genotypes. In this and all subsequent figures, error bars correspond to standard error of the mean (SEM).
To investigate whether the reported Sxl effects result from deregulated DC, we first confirmed that DC can be functionally inactivated in adults by observing loss of histone H4 lysine 16 acetylation of the X chromosome upon adult-specific downregulation of msl-2 in male intestinal progenitors (Extended Data Fig. 3g). We then investigated whether ectopic msl-2 expression accounted for the reduced proliferation resulting from Sxl downregulation by co-downregulating both genes in adult intestinal progenitors. This did not reinstate female proliferation (Fig. 1f). The converse experiment - mis-expression of msl-2 in adult female intestinal progenitors using a newly generated transgene coding for HA-tagged Msl-2 - did not reduce their proliferation (Fig. 1f) despite efficient Msl-2 protein expression and function (Extended Data Fig. 3h and data not shown). Hence, DC does not account for the ISC sex differences.
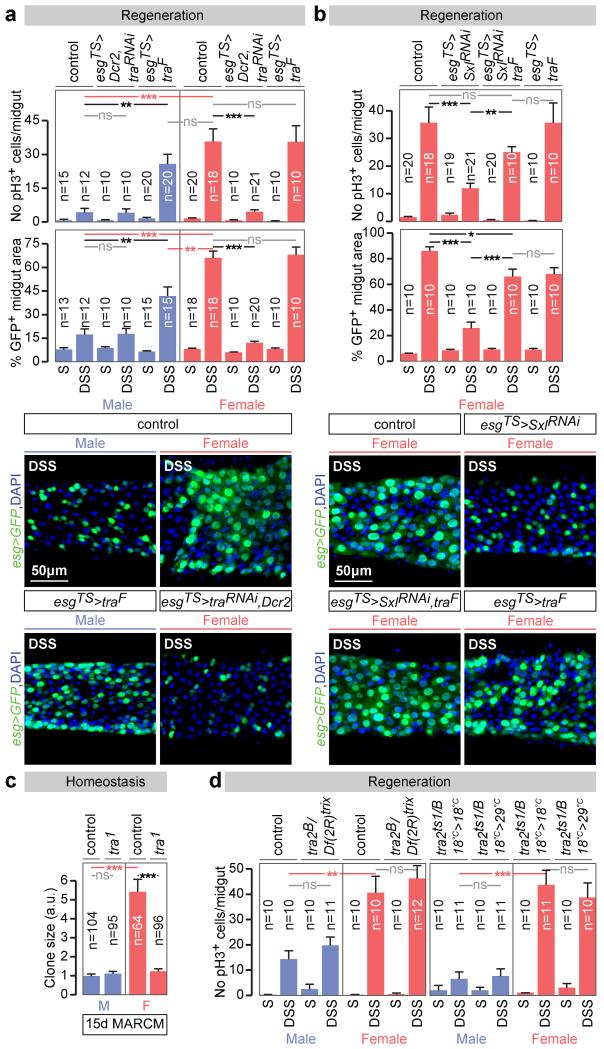
This focused our attention on the sex differentiation pathway and its main effector tra. Like Sxl, tra downregulation reduced DSS-induced proliferation in females to levels comparable to those seen in male midguts, but did not affect proliferation in male midguts (Fig. 2a and Extended Data Fig. 5a). Conversely, tra mis-expression – either ubiquitous (Extended Data Fig. 5c) or confined to adult intestinal progenitors (Fig. 2a and Extended Data Fig. 5a) - increased the proliferative response of ISCs to DSS in adult males, but not in females. Clonal and tra mutant rescue experiments further confirmed the adult, cell-intrinsic requirement for tra in regulating sexually dimorphic proliferation, both during regeneration (Extended Data Fig. 5b, d) and in normal homeostasis (Fig. 2c and Extended Data Fig. 5e, f). Strikingly, reintroduction of a tra transgene specifically in adult intestinal progenitors fully rescued the reduced proliferation resulting from Sxl downregulation (Fig. 2b). Together with the Sxl experiments, these results show that the sex of the midgut is actively specified in adult flies. Unlike other adult somatic cell types6,10,11, adult ISCs have a plastic sexual identity manifested by an intrinsic, Sxl/tra-controlled and DC-independent sexual dimorphism in both their basal and regenerative proliferation. These findings extend previous work in both mouse and Drosophila12-16 by showing that sexual identity not only needs to be actively maintained, but can also be reversed bidirectionally in the adult cells of a nongonadal organ.
Figure 2. tra, but not tra2, controls intrinsic sex differences in adult ISC proliferation.
a, Mitoses (top graph) and intestinal progenitor area (bottom graph) in flies exposed to control (sucrose, S) or damage-inducing (DSS) diets, in both controls and flies with adult ISC/EB-restricted tra downregulation or mis-expression. Representative images are shown below the graphs (DNA: DAPI, in blue; ISC/EB marker: GFP, in green). b, Comparable quantifications of the Sxl downregulation phenotypes in females, and their rescue by re-expression of traF. c, Clone size quantifications (in arbitrary units of GFP fluorescence, see Methods) reveal that tra null mutant MARCM clones are smaller than control clones only in females. d, No significant differences in ISC proliferation in the midguts of DSS-treated males or females lacking tra2 (tra2B/Df(2R)trix), or lacking tra2 specifically in adults (achieved by shifting flies with the thermosensitive allele tra2ts1 from 18°C to 29°C in the adult stage), vs controls. n denotes the number of midguts (a, e, f), ISCs/EBs (b, d), mitoses (c) or clones (g) that were analysed for each genotype. Results combined from at least two independent experiments. See Supplementary Information for full genotypes.
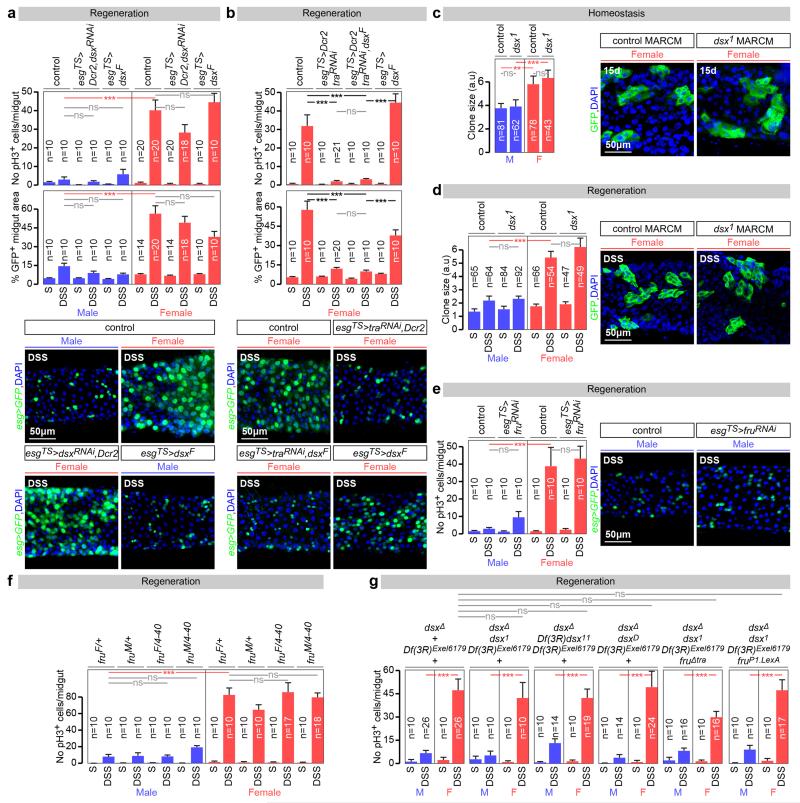
Unexpectedly (and in contrast to tra), mutation of the canonical Tra binding partner Transformer 2 (Tra2)5,7 failed to affect the sex differences in ISC proliferation (Fig. 2d), despite interfering with the sex-specific expression of dsxF and Yolk protein 1 (Yp1) transcripts as anticipated (Extended Data Fig. 5g). Together with our finding that ISCs do not express Dsx or FruM (Extended Data Fig. 2a, f, g), this result suggested that a non-canonical Tra2-, Dsx-, FruM-independent sex determination pathway drives sexually dimorphic proliferation in adult intestinal stem cells. We conducted a series of rescue, gain- and loss-of-function experiments using dsxF transgenes, dsx/fru mutants, RNAi transgenes and mutants in which dsx/fru splicing was biased towards either male or female isoforms (Extended Data Fig. 6a-g). These ruled out both a direct action of dsx or fruM in intestinal progenitors as well as indirect contributions to ISC proliferation from other dsx/fruM-expressing cells such as the neighbouring ECs, thus confirming that the sex of adult somatic stem cells in the intestine is specified by a novel branch of the sex determination pathway.
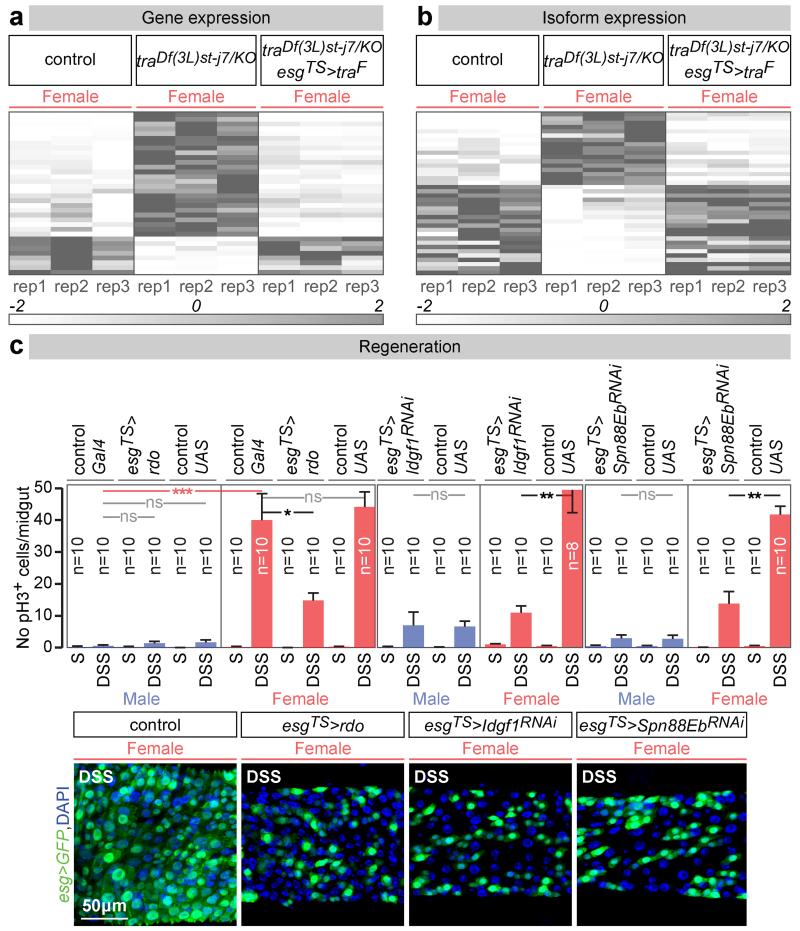
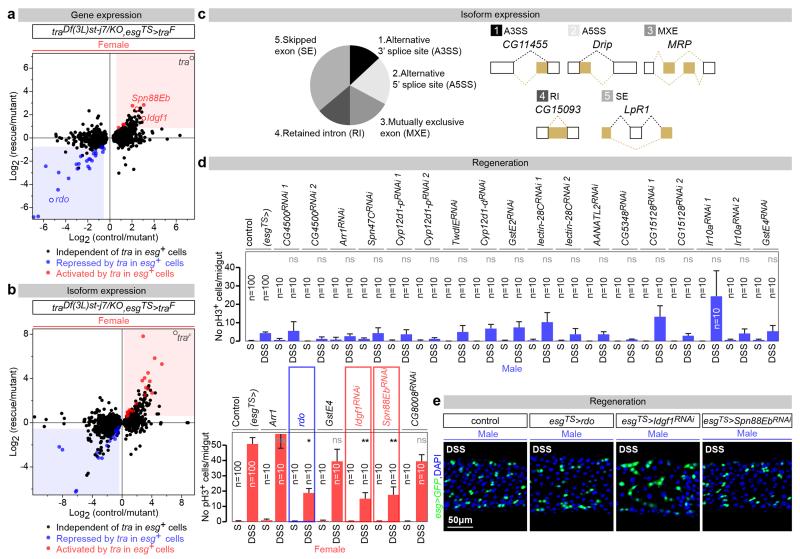
We then combined new RNA-seq and genetic experiments to identify tra target genes in adult ISCs, defined as genes the expression or splicing of which was different between control and tra mutant females, but returned to levels comparable to those of controls upon adult ISC-specific re-introduction of tra (see Methods, GEO accession number GSE74775 and GutSexRNAseq.xls for details). This yielded 72 genes with tra-regulated expression (34) or splicing (38) (Fig. 3a, b and Extended Data Fig. 7a-c). A genetic screen (Extended Data Fig. 7d, see Methods for details) identified three of them as negative or positive regulators of proliferation accounting for the sexual dimorphism in ISC proliferation. Indeed, downregulation of Imaginal disc growth factor 1 (Idgf1) or Serpin 88Eb (Spn88Eb), normally activated by tra in adult intestinal progenitors, reduced proliferation only in females. By contrast, over-expression of reduced ocelli (rdo), normally repressed by tra in the same cells, reduced proliferation only in females (Fig. 3c and Extended Data Fig. 7e). These three targets are regulated at the expression – rather than splicing – level: consistent with novel, tra2- and, possibly, splicing-independent roles of tra. Once produced, they may be secreted or targeted to the cell surface17-19, suggesting that sex differences may result from modulation of how ISCs interact with their local environment. All three proteins also appear to be expressed in other tissues and/or during development, and belong to protein families also represented in mammals: leucine-rich repeat (rdo), inhibitory serpins (Spn88Eb) and secreted glycoproteins (Idgf1)17,19,20. It will therefore be interesting to explore whether they account for how organisms attain their sexually dimorphic body size during development.
Figure 3. tra targets in adult intestinal progenitors.
Heat maps of the genes with tra-regulated expression (a) or splicing (b) in ISCs/EBs, displaying their normalized abundance in females, tra null mutant females and tra null mutant females with feminized ISCs/EBs (esgTS>traF) (see Methods for details). c, Mitoses (pH3) quantifications of adult ISC/EB-confined manipulations of rdo, Idgf1 and Spn88Eb expression. Representative images for each genotype are shown below the graphs (DNA: DAPI, in blue; ISC/EB marker: GFP, in green). n = 10 midguts per genotype/condition. Results combined from at least two independent experiments. See GutSexRNAseq.xls file for a full list of names and quality scores, and Supplementary Information for full genotypes.
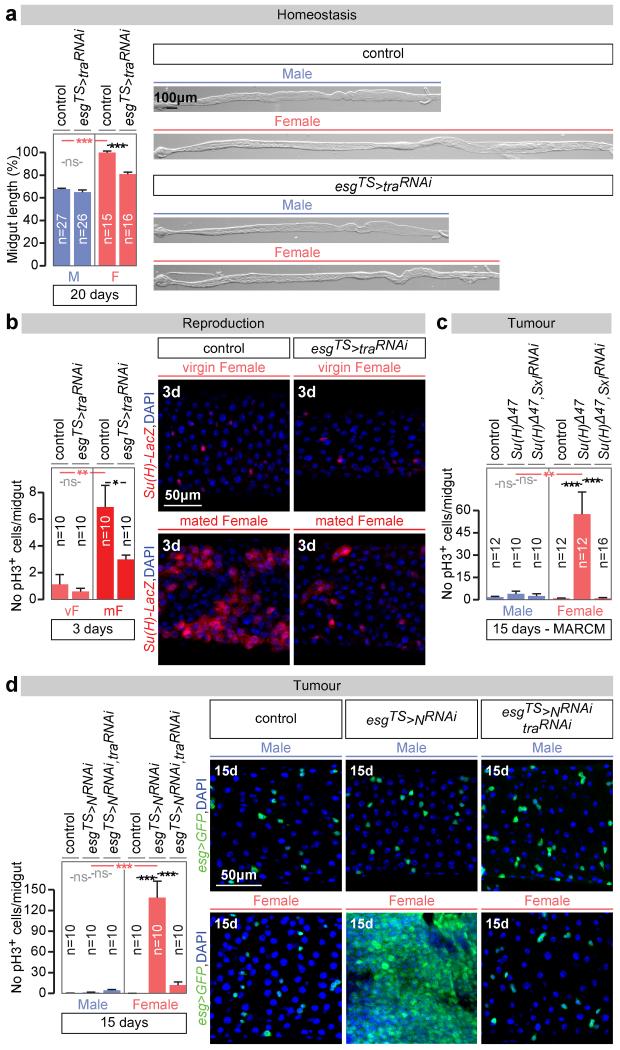
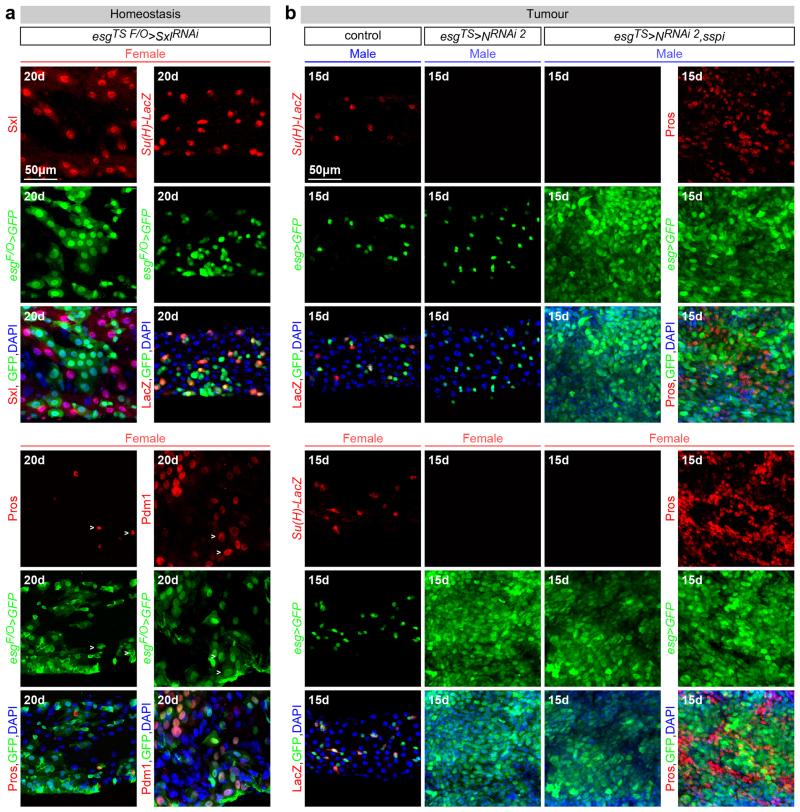
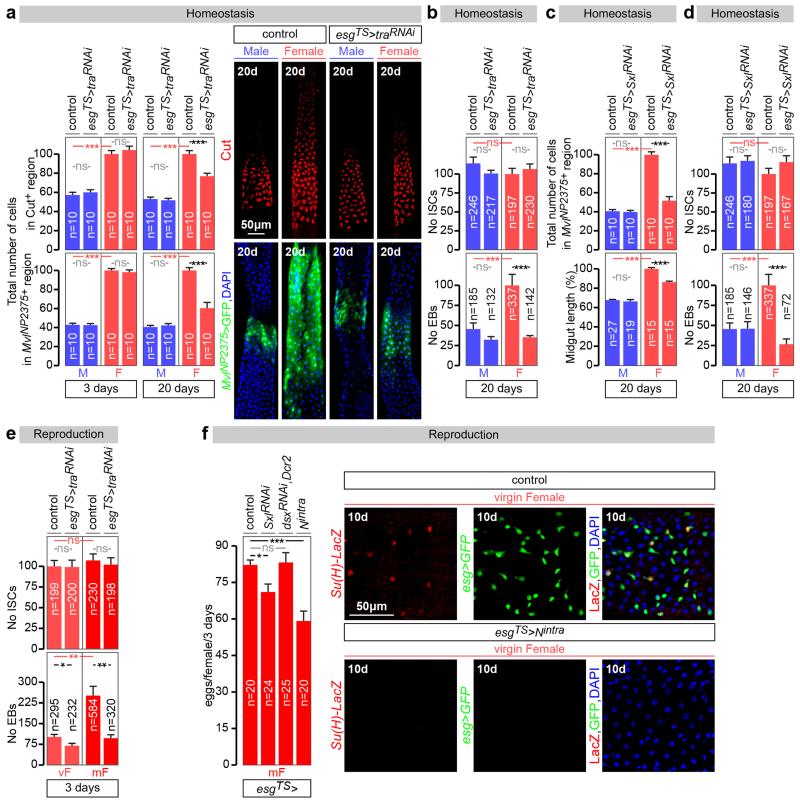
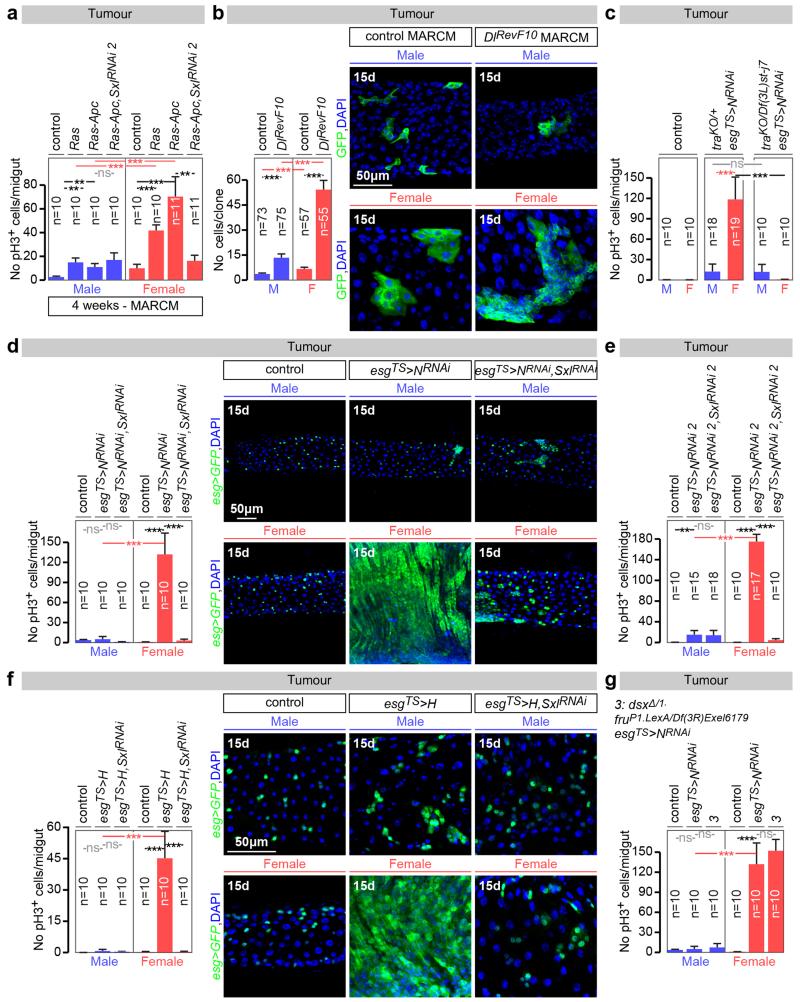
Are the sex differences in ISCs physiologically significant? Wild-type female midguts are longer than male midguts at days 3, 5 and 20 of adult life (Fig. 4a and data not shown). Adult-specific and cell-autonomous masculinisation of ISCs (achieved by tra or Sxl downregulation) dramatically shrank the female (but not the male) midgut towards a male-like size (Fig. 4a and Extended Data Fig. 8c). Cell type- and region-specific quantifications indicated that a reduction in ISC proliferation and, consequently, the number of differentiated progeny accounted for the observed shrinkage (Extended Data Fig. 8a-d). Female flies with masculinised adult ISCs also failed to undergo the recently described midgut resizing triggered by mating21 (Fig. 4b and Extended Data Fig. 8e) and had slightly reduced fecundity (Extended Data Fig. 8f), indicating that sex differences endow females with enhanced stem cell plasticity to optimise reproduction. Females are, however, more prone to genetically induced tumours; indeed, adult-specific interference with Notch (N) or Apc-ras signalling, previously shown to lead to tissue overgrowth reminiscent of gastrointestinal tumours in females22,23, resulted in hyperplasia of female (but not male) midguts (Fig. 4c, d and Extended Data Fig. 9a-f). Hyperplasia resulted from sex differences in proliferation rather than sex-specific differentiation defects (Extended Data Fig. 4b), and could be prevented by simultaneously masculinising female adult ISCs by downregulating or mutating Sxl/tra, but not dsx or fruM (Fig. 4c, d and Extended Data Fig. 9a, c-g). Increased susceptibility of female flies to genetically induced tumours was also observed after mating (data not shown). Thus, the intrinsic sexual identity of adult intestinal stem cells plays key roles in adult life, both in maintaining organ size and in modulating its plasticity.
Figure 4. Physiological significance of the sex differences in intestinal progenitors.
a, Midgut length quantifications and representative images of phenotypes resulting from adult-specific masculinization of ISC/EB-specific masculinization of ISCs (achieved by esgTS-driven tra downregulation initiated after the phase of midgut post-eclosion growth, see Methods for details). b, The number of mitoses (pH3-positive cells) is higher in control female flies 3 days after mating. The postmating increase is abrogated upon adult ISC/EB-specific tra downregulation. An EB marker (Su(H)LacZ, in red in image panels) reveals that the EB expansion seen in females after mating is reduced upon adult ISC/EB-specific tra downregulation. See also Extended Data Fig. 8 for quantifications. c, pH3 quantifications inside MARCM clones of control flies, Su(H) mutants and Su(H) mutants in which Sxl has been downregulated inside the clone. Su(H) mutation only leads to increased pH3 counts in females, and this increase is Sxl dependent. d, Hyperplasia (quantified by the number of pH3-positive cells) resulting from adult ISC/EB-driven N downregulation and its modulation by tra in female and male midguts. Confocal images show intestinal progenitor coverage of representative midgut portions for each genotype (DNA: DAPI, in blue; ISC/EB marker: GFP, in green). n denotes the number of midguts (a, b, c, d) that were analysed for each genotype. Virgin flies were used in all experiments unless otherwise indicated. Results combined from at least two independent experiments. See Supplementary Information for full genotypes.
Previous observations had pointed to additional branches of the sex determination pathway24,25. Our discovery of this new branch has important implications for organs such as the nervous system, where sexual identity was thought to be confined to FruM and/or Dsx-expressing neurons26, and raises the possibility that every cell has a sexual identity that actively regulates its plasticity and physiology. While early sex specification in Drosophila differs from that of mammals, there is increasing evidence that it converges on common effectors - such as the Dsx/Dmrt family of transcription factors and their targets12. Hence, the recently reported sex differences in both intestinal gene expression and microbiota in the mammalian intestine27,28 could at least partly result from similar, so far unexplored, intrinsic sex differences. Similarly, the possible contribution of the intrinsic sexual identity of adult somatic stem cells to both organ plasticity and to the well documented sex differences in susceptibility to many types of cancer29,30 deserves further investigation.
METHODS
Fly husbandry
Fly stocks were reared on a standard cornmeal/agar diet (6.65% cornmeal, 7.15% dextrose, 5% yeast, 0.66% agar supplemented with 2.2% nipagin and 3.4 mL/L propionic acid). All experimental flies were kept in incubators at 25°C, 65% humidity and on a 12 hr light/dark cycle, except for those containing tub-Gal80TS transgenes, which were set up at 18°C (restrictive temperature) and transferred to 29°C (permissive temperature) at the time when Gal4 induction was required. This depended on the specific experimental requirements but typically, for loss-of-function (RNAi) or gain-of-function (UAS) experiments, flies were raised and aged as adults for 3 days at 18°C, were then shifted to 29°C to induce transgene expression, and adult midguts were dissected after 10-20 days (as indicated in each figure panel). Flies were transferred to fresh vials every 3 days and fly density was kept to a maximum of 15 flies per vial. Virgin flies were used for all experiments unless indicated otherwise.
For mutant ISC clonal analyses (MARCM clones), 3-day-old adults (raised and aged at 25°C) were heat-shocked for 1 hr at 37°C to induce clones, and were then kept at 25°C (or 29°C for MARCM RNAi clones) until dissection (10 days, 15 days or 4 weeks thereafter as indicated in each figure panel). Flies were transferred to fresh vials every 3 days.
For damage-induced regeneration assays, virgin flies were collected over 72 hrs at 18°C, and were then shifted to 29°C for 7 days on standard media. Flies were then transferred in an empty vial containing a piece of 3.75 cm × 2.5cm paper. 500mL of 5% sucrose solution (control) or 5% sucrose + 3 dextran sulfate sodium (DSS) solution was used to wet the paper, used as feeding substrate. Flies were transferred to new vial with fresh feeding paper every day for 3 days prior dissection.
For fecundity experiments, females were raised and aged as virgins for 3 days at 18°C, and were then shifted to 29°C to induce transgene expression for 10 days. Females were then mated overnight to OregonR males (10 males, 10 females per vial). Males were then removed, and single female flies were transferred individual vials every 24h for a 3-day period. Eggs were counted from the vacated vials.
Fly stocks
UAS transgenes
UAS-SxlRNAi (VDRC: GD 3131), UAS-Sxl (generated by 31), UAS-pon.GFP (BDSC:42741, generated by 32), UAS-GFP.E2f1.1-230, UAS-mRFP1.NLS.CycB.1-266 (BDSC: 55121, generated by 33), UAS-GFP (BDSC: 35786), UAS-Dicer2 (VDRC: 60007), UAS-traRNAi (BDSC: 28512, TRiP.JF03132), UAS-traF (BDSC: 4590, generated by 34), UAS-msl-2RNAi (VDRC: GD 29356), UAS-SxlRNAi 2 (VDRC: KK 109221), UAS-msl-2RNAi 2 (BDSC:35390, TRiP.GL00309), UAS-msl-2HA (this study), UAS-dsxRNAi (BDSC: 26716, TRiP.JF02256), UAS-dsxF (BDSC: 44223, generated by 35), UAS-Arr1 (generated by 36), UAS-rdo (generated by 17), UAS-GstE4 (generated by 37), UAS-NotchRNAi (VDRC: KK 100002), UAS-SxlRNAi 3 (BDSC: 34393, TRiP.HMS00609), UAS-traRNAi 2 (VDRC: GD 2560), UAS-traRNAi 3 (NIG: 16724-R2), UAS-fruRNAi (BDSC: 31593, TRiP.JF01182), UAS-NotchRNAi 2 (VDRC: GD 27229), UAS-Hairless (generated by 38), UAS-Nintra (generated by 39), UAS-sspitz (gift from J. Treisman, generated by 40), UAS-RasV12; FRT82B line (generated by 41).
Mutants
tra1, FRT2A, fruP1-Gal4 chromosome (generated by 42), FRT82b, dsx1 chromosome (generated by 43), dsxΔ, Df(3R)Exel6179 chromosome (generated by 44), Df(3R)dsx11 (BDSC: 1865, generated by 45), In(3R)dsx23 (BDSC: 1849, generated by 46), FRT82b, dsx1, fruΔtra chromosome (gift from B. Baker, generated by 47), dsx1, fruP1-LexA chromosome (gift from B. Prud’homme, generated by 48), traKO (this study), Df(3L)st-j7 (BDSC:5416, generated by 49), tra1 (BDSC: 675, generated by 50), tra2B (BDSC: 25137, generated by 51), Df(2R)trix (BDSC:1896, generated by 52), tra2ts1 (BDSC: 2413, generated by 52), dsxD (BDSC: 840, generated by 53, fruF and fruM (gift from G. Jefferis, generated by 47, fru4-40 (gift from G. Jefferis, generated by 54), FRT82b, DIRevF10 chromosome (gift from S. Bray, generated by 55), Su(H)Δ47, FRT40A chromosome (gift from S. Bray, generated by 56), FRT82B Apc2N175K, ApcQ8 chromosome and hs-flp;UAS-RasV12; FRT82B Apc2N175K, ApcQ8 line (both generated by 41).
Reporters and Gal4 drivers
Su(H)GBE-LacZ (generated by 57), esg-GFPP01986 (gift from L. Jones, Flytrap), esg-LacZk00606 (BDSC : 10359), dsx-Gal4 (generated by 58), fruP1-Gal4 (gift from G. Jefferis, generated by 59), esg-Gal4NP7397, UAS-GFP, Tub-Gal80TS chromosome (gift from J. de Navascués), Su(H)GBE-Gal80 (generated by 60), Su(H)GBE-Gal4 (generated by 61), mex1-Gal4 (generated by 62), prosV1-Gal4 (generated by 63), Mvl-Gal4NP2375(Kyoto: 104178), dsxΔ2-Gal4 (generated by 64), vm-Gal4 (BDSC: 48547, GMR13B09-GAL4), btl-Gal4 (generated by 65), nSyb-Gal4 (BDSC: 51941), elav-Gal4 (BDSC: 8765), nSyb-Gal80 (gift from J. Simpson), stripe-Gal4 (BDSC: 26663).
MARCM stocks
FRT40A: w, hs-flp,Tub-Gal4, UAS-GFP; tub-Gal80, FRT40A (gift from J. de Navascués), FRT2A: y, w, hs-flp; Tub-Gal4,UAS-GFP; Tub-Gal80, FRT2A (gift from I. Salecker), FRT2A (BDSC: 1997), FRT82b: w, hs-flp; UAS-mCD8GFP, Tub-Gal4; FRT82b, Tub-Gal80 (gift from M. Vidal), FRT82b (BDSC:7369).
Generation of traKO and traLacZ transgenic lines
To generate the transgenic reporter of tra promoter activity traLacZ, the upstream intergenic region between spd-2 and tra was cloned using the following primer pair: 5’-AAAATCTAGAAACTAATAAAGTATATGAG-3’ and 5’-AAAAGGTACCCGGAAAATGCTGGAAATTAAATGATGC-3’. PCR was performed with Q5 high-fidelity polymerase from New England Biolabs (M0491S). The PCR product was digested with XbaI and KpnI prior to cloning into the pH-Pelican attb vector (66, gift from C. Thummel). The construct was sequence-verified and a transgenic line was established through ΦC-31 integrase mediated transformation (Bestgene, attP site: attP40).
The new amorphic allele of tra, traKO, was generated using the accelerated homologous recombination method recently developed by Baena-Lopez67. The 5’ (4302nt) and 3’ (2928nt) homology arms were produced from a tra BAC (BAC-PAC resources: CH322-140P01) with the following primer pairs respectively: 5’-AAAAGCGGCCGCCATTCTACTCTTGAATTGGCTAGC-3’/5’-AAAAGTACCATGATGCACTTTCCTCAGTGTGA-3’ and 5’-AAAAGGCGCGCCAAGAGAATACCATGG-3’/5’-AAAAGGCGCGCCATTGTCGACACAATCAAACTG-3’. PCRs were performed with Q5 high-fidelity polymerase from New England Biolabs (M0491S). The PCR products were digested with NotI/KpnI or AscI respectively prior to cloning into the pTVCherry vector to generate pTVCherry[tra]. The vector was sequence-verified and inserted into random genomic locations by P-element-mediated transformation (Bestgene). Transformants (not necessarily mapped or homozygosed) were crossed to hs-FLP, hs-SceI flies (DBSC: 25679) and the resulting larvae were heat-shocked at 48, 72, 96 and 120 hrs after egg laying for 1 hr at 37°C. Approximately 200 adult females with mottled eyes (indicating the presence of pTVCherry[tra] and the transgenes carrying hs-FLP and hs-SceI) were crossed in pools of 15 to ubiquitin-Gal4[3xP3-GFP] males and the progeny was screened for the presence of red-eyed flies. The ubiquitin-Gal4[3xP3-GFP] transgene was subsequently removed by selecting against the presence of GFP in the ocelli. The generated deletion removed 342 nucleotides starting 13 nucleotides upstream of the transcription start site.
Generation of the UAS-msl-2HA line
To overexpress msl-2, a transgenic UAS line was generated from msl-2 cDNA adding an HA tag at the C-terminal (BDGP Gold cDNAs, clone ID: GH22488) with the following primer pair: 5’-AAA AAGATCTATGGCTCAGACGGCATACTTG and 5’-AAAATCTAGATTAAGCGTAATCTGGAACATCGTATGGGTACAAGTCATCCGAGCCCGAC-3’. PCR was performed with Q5 high-fidelity polymerase from New England Biolabs (M0491S). The PCR product was digested with BglII and XbaI prior to cloning into the pUASTattb vector68. The construct was sequence-verified and a transgenic line was established through ΦC-31 integrase mediated transformation (Bestgene, attP site ZH-86Fb, DBSC: 24749).
Immunohistochemistry
Intact guts were fixed at room temperature for 20 min in PBS, 3.7% formaldehyde. All subsequent incubations were done in PBS, 4% horse serum, 0.2% Triton X-100 at 4°C following standard protocols.
The following primary antibodies were used: mouse anti-Sxl (M114, DSHB Hydridoma) 1/50, mouse anti-Sxl (M18, DSHB Hydridoma) 1/50, goat anti-Msl-2 (dC-20, sc-32459, Santa Cruz Biotechnology) 1/50, chicken anti-beta galactosidase (ab9361, Abcam) 1/200, rabbit anti-phospho-Histone H3 Ser10 (9701L, Cell Signalling Technology) 1/500, rabbit anti-fruM (Male-2, generated by 69) 1/500, mouse anti-DsxDBD (DSHB Hybridoma) 1/100, mouse anti-Prospero (MR1A, DSHB Hybridoma) 1/50, goat anti-ac-Histone H4 Lys16 (sc-8662, Santa Cruz Biotechnology) 1/ 500, rat anti-HA (11867423001, Roche) 1/500, mouse anti-GFP (11814460001, Roche) 1/1000, mouse anti-Pdm1 (kind gift of Steve Cohen, generated by 70) 1/20. Fluorescent secondary antibodies (FITC-, Cy3- and Cy5-conjugated) were obtained from Jackson Immunoresearch. Vectashield with DAPI (Vector Labs) was used to stain DNA.
Cell, clone and midgut length quantifications
Mitotic indices were quantified by counting phospho-Histone H3-positive cells in >10 midguts per genotype, time point, and/or condition (e.g. male/female), and are displayed as means ± standard error of the mean (SEM). For posterior midgut cell counts, a midgut portion immediately anterior to the junction with the tubules and hindgut was imaged at 20× magnification. Cells were counted using ImageJ in areas of identical size across all genotypes to control for size differences. Threshold was adjusted for the GFP channel (ImageJ function: Image>Adjust>Threshold) to subtract background, and the percentage of area above the threshold were considered (ImageJ function: analyse particles). Data was collected from at least 10 midguts per genotype and/or condition, and is displayed as mean of % of GFP-positive area ± SEM.
MARCM clones were quantified as number of cells per clone (when the GFP reporter was expressed in the nucleus) or by the size of the GFP area/clone (in arbitrary units, when the reporter was membrane-tagged GFP).
To measure midgut length, guts were dissected and were then straightened on polyLysine-coated slides. After imaging, a line was draw between the most anterior point of the proventriculus and the midgut-hindgut junction using ImageJ. The number of pixels contained in the line was used as a proxy for midgut length. For display purposes, the value obtained for a control female midgut selected at random was used as reference to normalise all the other values, which are shown as percentage of control female length value.
Functional RNAi screen of tra targets
To test the functional significance of tra targets, only those with female/tra null mutant female fold differences in transcript abundance >±2 that were also under tra control in adult intestinal progenitors were selected for functional validation. The contribution of these tra targets to sex differences in adult ISC proliferation was investigated using publicly available RNAi and UAS lines (see below for details of the RNAi lines, and Methods section ‘Fly stocks’ for details of the UAS lines) expressed from esg-Gal4ts. Results obtained with 11 out of the 16 lines belonging to the VDRC KK collection were not considered and are not shown in Extended Data Fig. 7 because their expression in adult ISCS from esgTS-Gal4 resulted in a recurrent ISC differentiation phenotype in both males and females. We attribute this effect to the previously reported dominant Gal4-dependent toxicity issue with this VDRC KK collection71).
| Gene | Transformant ID | Library |
|---|---|---|
| Idgf1 | 12416 | GD |
| Snp88Eb | 28425 | GD |
| CG17470 | 100414 | KK |
| CG8008 | 4159 | GD |
| Klp54D | 100140 | KK |
| CG4500 | 34852 | GD |
| CG4500 | 106260 | KK |
| Arr1 | 22196 | GD |
| Arr1 | 109860 | KK |
| Arr1 | UAS | |
| rdo | 107213 | KK |
| rdo | UAS | |
| Spn47C | 25534 | GD |
| Spn47C | 105933 | KK |
| Cyp12d1-p | 49269 | GD |
| Cyp12d1-p | 109256 | KK |
| TwdlE | 24867 | GD |
| TwdlE | 107483 | KK |
| Cyp12d1-d | 109248 | KK |
| Cyp12d1-d | 50507 | GD |
| GstE2 | 32945 | GD |
| GstE4 | 100986 | KK |
| GstE4 | 20472 | GD |
| GstE4 | UAS | |
| lectin-28C | 45634 | GD |
| lectin-28C | 104290 | KK |
| AANATL2 | 44677 | GD |
| AANATL2 | 102802 | KK |
| CG5348 | 1698 | GD |
| CG5348 | 106565 | KK |
| CG15128 | 100238 | KK |
| CG15128 | 19302 | GD |
| CG15236 | 101045 | KK |
| Ir10a | 45403 | GD |
| Ir10a | 100181 | KK |
Statistics and data presentation
All statistical analyses were carried out in the R environment72. Comparisons between two genotypes/conditions were analyzed with the Mann-Whitney-Wilcoxon rank sum test (R function wilcox.test). All graphs were generated using Adobe Illustrator. All confocal and bright field images belonging to the same experiment and displayed together in our figure were acquired using the exact same settings. For visualization purposes, level and channel adjustments were applied using ImageJ to the confocal images shown in the figure panels (the same correction was applied to all images belonging to the same experiment), but all quantitative analyses were carried out on unadjusted raw images or maximum projections. In all figures, n denotes the number of midguts, ISCs/EBs, mitoses or clones that were analysed for each genotype. Values are presented as average ± standard error of the mean (SEM), p-values from Mann-Whitney-Wilcoxon test (non-significant (ns): p>0.05; *: 0.05>p>0.01; **: 0.01>p>0.001; ***: p>0.001). Lines and asterisks highlighting significant comparisons across sexes are displayed in red, whereas those highlighting significant comparisons within same-sex datasets are displayed in black.
RNA-seq experiments
RNA extraction
RNA from 30 pooled dissected midguts was extracted using Trizol (Invitrogen), and 3 such samples were used for each sex and genotype. RNA-seq libraries were prepared from 500ng of total RNA using the Illumina Truseq mRNA stranded library prep kit (Illumina Inc. San Diego, USA) according to the manufacturer’s protocol. Library quality was checked on a Bioanalyser HS DNA chip and concentrations were estimated by Qubit measurement. Libraries were pooled in equimolar quantities and sequenced on a Hiseq2500 using paired end 100bp reads. At least 30 million reads passing filter were achieved per sample. After demultiplexing, raw RNASeq reads were aligned with Tophat splice junction mapper73, version 2.0.11 against Ensembl Drosophila genome reference sequence assembly (dm3) and transcript annotations.
For differential gene expression analysis, gene-based read counts were then obtained using HTSeq count module (version 0.5.3p9)74. Differential expression analysis was performed on the counts data using DESeq2 Bioconductor package75. The analysis was run with the default parameters. DESeq2 package uses negative binomial model to model read counts and then performs statistical tests for differential expression of genes. Raw p values were then adjusted for multiple testing with the Benjamini-Hochberg procedure. GO enrichment analysis was performed using FlyMine v40.1.b. In Fig. 3a, b and Extended Data Fig. 1g, each column corresponds to one of three different replicates (30 midguts each) for each sex, and transcript abundance for each gene was normalized to a scale of −2 (white) to +2 (gray).
Isoform expression analysis
Reads were first aligned to the transcript sets using Bowtie software (version 1.0.0)76. Transcript annotations for cDNA and non-coding RNAs were obtained from Ensembl (release 75). Isoform abundances were then calculated using mmseq R package (version 1.0.8)77. Differential expression analysis was performed on these abundances using the DESeq2 Bioconductor package (version 1.42.1)78. Isoforms not expressed in any of the samples were not included in the differential expression analysis. Raw p values were adjusted for multiple testing with the Benjamini-Hochberg procedure. Initial isoform analysis identified differential expression for 1379 isoforms between male and female midguts (p<0.05 cutoff), which, following subtraction of those corresponding to single-isoform genes, yielded 704 isoforms (see main text for subsequent analysis).
RNA-seq data displays
For the volcano plots in Extended Data Fig. 1, log 2 fold change values were plotted against log 10 of adjusted p values. Genes and isoforms significantly upregulated in males are coloured blue, while those significantly upregulated in females are coloured red. Selected genes and isoforms are further highlighted as empty circles. For the scatter charts with quadrants in Extended Data Fig. 7, log 2 fold change values (between control and tra mutant females) were plotted against log 2 fold change values (between tra mutant females and tra mutant females and female with adult-specific, progenitor-specific tra expression). Genes and isoforms significantly repressed by tra are coloured blue, whereas those significantly activated by tra are coloured red. Selected genes and isoforms are further highlighted as empty circles. Volcano plots and scatter charts were generated using Adobe Illustrator. To generate the heat maps, a matrix containing the relative values of gene/isoform expression was built. Transcript abundance for each gene/isoform was normalized to a scale ranging from −2 to +2. A hierarchical clustering algorithm (with Euclidian distance and average linking) was applied to the matrix using the MeV software suite79. Area-proportional Venn diagrams were generated using BioVenn (http://www.cmbi.ru.nl/cdd/biovenn/).
Real time qRT-PCRs
RNAs were extracted from 30 dissected midguts using Trizol (Invitrogen). RNAs were cleaned using RNAeasy mini Kit (QIAGEN), and cDNAs were synthesized using the iScript cDNA synthesis kit (Bio-Rad) from 300ng of total RNAs. Quantitative PCRs were performed by mixing cDNA samples (5ng) with iTaq™ Universal SYBR® Green Supermix (Bio-Rad, #172-5124) and the relevant primers in a 96-well plate. Expression values were normalized to eIF4G. For each gene/isoform, at least 3 independent biological replicates were used, and 2 technical replicates were performed. See Supplementary Information for a list of all primers used.
Extended Data
Extended Data Fig. 1. Sexually dimorphic transcription and splicing in the adult midgut.
a, Number and percentage of genes with sexually dimorphic gene expression, as revealed by RNA-seq transcriptional profiling of virgin male and female dissected midguts (p<0.05 cutoff). b, Volcano plot displaying all genes with detectable midgut expression. Female/male ratio of gene expression is shown on the X axis (in log2 scale) and significance is displayed on the Y axis as the negative logarithm (log10 scale) of the adjusted p-value. Genes with significantly upregulated (p<0.05 cutoff) expression in males and females are coloured in blue and red, respectively. Other genes are displayed in black. Genes with known sex-specific transcription are displayed as red (female-enriched) or blue (male-enriched) open circles. c and d, Comparable analyses for sex-biased isoforms belonging to genes with multiple transcripts. We identity 714 sex-biased isoforms belonging to a total of 603 genes. Isoforms resulting from known sex-specific alternative splicing are displayed as in panel b. e, Female/male ratios of overall transcript abundance (left graph) and abundance of sex-biased isoforms (right graph) for the members of the Drosophila sex determination pathway as revealed by RNA-seq analysis of the adult midgut. We note a sexual dimorphism in dsx transcript levels. f, Venn diagram illustrating the overlap between the genes showing sex-biased expression (overall transcript abundance, light grey, 1305 genes) and sex-biased alternative splicing (sex-biased isoforms, dark grey, 603 genes) in the adult midgut. Known members of the sex determination pathway are displayed as examples. g, Heat maps displaying genes with sexually dimorphic expression clustered by enrichment in specific biological processes, as revealed by Gene Ontology enrichment analysis. Genes with sexually dimorphic expression belonging to the top 4 enriched biological processes are shown. h and i, Real-time qRT-PCR data for a subset of genes for which RNA-seq transcriptional profiling experiments revealed sexually dimorphic expression (h) or isoforms (i). RNA was obtained from midguts from virgin male and female samples (same genotypes as for the RNA-seq experiments). For each gene/isoform, expression abundance was arbitrarily set up at 100% for the sex with the highest expression level, and percentage of that expression is displayed for the other sex. See Methods for details, GutSexRNAseq.xls for a full list of names and quality scores, and Supplementary Information for full genotypes.
Extended Data Fig. 2. Cell type-specific expression of sex determinants in the adult intestinal epithelium of virgin flies.
a, In the adult Drosophila midgut, resident stem cells (ISCs) and their postmitotic daughter cells (EBs) maintain the adult intestinal epithelium during normal homeostasis and regenerate it after injury by giving rise to two types of differentiated progeny: ECs and EECs80,81. The posterior midgut area used to visualise and quantify phenotypes is boxed. The following Gal4 drivers were used to label and/or genetically manipulate these four different cell types present in the adult intestinal epithelium: midgut expression 1 (mex1) for ECs, prospero (prosv1) for EECs, escargot (esg) to target both ISCs and EBs, and Supressor or Hairless (Su(H)) for EBs alone. b, The canonical sex determination pathway in somatic cells of Drosophila melanogaster, consisting of a cascade of sex-specific alternative splicing events culminating in the production of sex-specific transcription factors encoded by Dsx and FruM. In females, Sex lethal (Sxl) is activated and regulates the splicing of transformer (tra) pre-mRNA, resulting in the production of TraF. TraF regulates the female-specific splicing of dsx pre-mRNA (dsxF) and fru transcript coming from the P1 promoter (fruP1, giving rise to fruM). In males, Sxl is not expressed and no functional Tra is produced, resulting in default splicing of dsx and fru pre-mRNAs, leading to FruM and DsxM proteins, respectively. The resulting male- and female-specific Dsx and Fru isoforms confer sexual identity to the cells in which they are produced. In addition, in females, Sxl represses dosage compensation by inhibiting Msl-2 expression. The tables summarize the cell-specific expression profiles of the sex determinants in adult midguts of virgin males and females, shown in the panels below. c, Sxl protein (in red) is expressed only in female midguts. Co-staining with ISC/EB reporters indicates that Sxl is found esg-positive progenitors (ISCs: GFP-positive and LacZ-negative cells, and EBs: GFP-positive and LacZ-positive cells). It is also expressed in female polyploid ECs (GFP- (in green) and LacZ- (in blue) negative cells). Co-staining with prosV1 reporter indicates that it is also expressed in EECs. d, Msl-2 protein is found in the same cell types only in males (staining is confined to the X chromosome, consistent with the signal observed in non-intestinal tissues82. e, A new reporter of tra promoter activity (traLacZ, see Methods for details) is broadly expressed in the epithelium of both male and female midguts, including ISCs and EBs (as revealed by co-staining with esg-Gal4-driven GFP) and ECs (GFP-negative cells with large nuclei). Co-staining with prosV1 reporter indicates that it is also expressed in EECs. f, A dsx-Gal4 reporter (visualised with a GFP reporter that has been false-coloured in red for consistency with the other panels) is active in male and female polyploid ECs (LacZ-negative cells), and is inactive in esg-positive progenitors (LacZ-positive cells, in green). Dsx protein (visualised using a DsxDBD-specific antibody in red) is expressed strongly only in males bot not females, indicating that the sexual dimorphism in dsx transcript levels found in our RNA-seq analyses (Extended Data Fig. 1e) is further enhanced at the protein level. Co-staining of the same antibody with the EC marker mex1-Gal4 confirms expression in ECs. Cytoplasmic dsx-Gal4-driven expression of an mTomato reporter is apparent in ECs (visualised as large cells by co-staining with the membrane-enriched marker Armadillo), but is absent from EECs (as revealed by the gaps in mTomato expression in cells that are labelled with Pros). g, The fruP1-Gal4 reporter (which labels the only sexually dimorphic fru transcript that gives rise to FruM protein) is inactive in both male and female midguts, as revealed by lack of GFP signal (false-coloured in red for consistency with other panels). Consistent with the lack of fruP1-Gal4 expression, a FruM-specific antibody (in red) is not expressed in the male midgut. An independent dsx reporter (dsxΔ2-Gal4) is expressed in polyploid ECs, consistent with the data displayed in f. See Supplementary Information for full genotypes.
Extended Data Fig. 3. Sxl controls intrinsic sex differences in adult ISC proliferation independently of dosage compensation.
a, Immunocytochemistry using a Sxl-specific antibody indicates that adult-restricted downregulation of Sxl in intestinal progenitors (ISCs/EBs) - achieved by Gal80TS-controlled expression of a Sxl RNAi transgene – efficiently downregulates Sxl expression in progenitors, but not large polyploid ECs. Conversely, efficient ectopic Sxl protein expression is obtained by expression of a UAS-Sxl transgene in adult ISCs/EBs of male flies. In all panels Sxl antibody is in red; DNA: DAPI, in blue; ISC/EB marker: GFP, in green. b, Quantifications of the number of cells inside control MARCM83 clones, or MARCM clones expressing RNAi transgenes directed against Sxl following DSS treatment. 5 days after clone induction by heat shock, female clones are larger than male clones only when Sxl is present, confirming the cell autonomy of Sxl action. c, Additional controls for Fig. 1a, and confirmation of phenotypes using an independent RNAi transgene. This second RNAi transgene against Sxl (different from the one used in Fig. 1a) reduces the number of pH3-positive cells in DSS-treated female midguts when expressed from esgTS in adults ISCs/EBs, confirming an adult progenitor-specific requirement for Sxl in promoting damage-induced cell divisions in female flies. d, Adult-specific downregulation of Sxl in adult visceral muscle (using the vm driver), trachea (btl-Gal4 and DSRF-Gal4), neurons (nSyb-Gal4, Elav-Gal4), or fat body (stripe-Gal4) does not reduce DSS-induced ISC proliferation in females. By contrast, Sxl downregulation using an ISC/EB driver with suppressed neuronal expression (esg-Gal4 combined with nSyb-Gal80) effectively reduces DSS-induced ISC proliferation in females. Together, these results indicate that Sxl does not control sexually dimorphic DSS-induced ISCs proliferation from non-ISC cells. e, MARCM clones expressing a third RNAi transgene against Sxl (distinct from those used in Fig. 1 and above) are smaller than control clones in females, whereas their size is comparable to that of wild-type or Sxl-RNAi clones in males. This confirms that, during normal homeostasis, female ISCs divide more often than male ISCs because of the cell-autonomous action of Sxl. The graph shows quantifications of the number of cells within each clone 15 days after clone induction by heat shock, and the confocal images show representative clones (labelled in green with GFP) for each genotype. f, Clonal analyses of homeostatic proliferation using the inducible esg flip-out system - which labels progenitors and their progeny generated within a defined temporal window84 - in midguts of control males, control females and females in which Sxl downregulation has been confined to adult progenitors. 15 days after induction, the size (assessed as the percentage of GFP-positive area) of control female clones was significantly larger than that of male clones, but both became comparable upon adult-specific Sxl downregulation using Sxl RNAi transgenes. The graph shows area quantifications for each sex/genotype, and the confocal images show representative clones for each genotype. g, Immunohistochemical detection of histone H4 lysine 16 (H4Lys16) acetylation (in red) indicates that adult-specific downregulation of msl-2 in male intestinal progenitors (ISCs/EBs marker: GFP, in green) results in loss of H4Lys16 acetylation of the X chromosome. h, Efficient Msl-2 mis-expression in adult female intestinal progenitors (ISCs/EBs marker: GFP, in green) is confirmed by immunocytochemistry using an HA-specific antibody (in red). n denotes the number of guts (c, d, f), or clones (b, e) that were analysed for each genotype. Results combined from at least two independent experiments. See Supplementary Information for full genotypes.
Extended Data Fig. 4. Sexually dimorphic proliferation does not result from sex differences in differentiation.
a, Markers for all four intestinal cell types are still apparent following adult-specific downregulation of Sxl in the intestinal progenitors of females - achieved by Gal80TS-controlled expression of a Sxl RNAi transgene. Indeed, expression of esg-Gal4 (ISC/EBs), Su(H)-LacZ (EBs), Pdm1 (ECs) and Pros (EECs) can be readily detected, suggesting that Sxl downregulation in females (which results in reduced ISC proliferation) does not have a major effect on differentiation. Sxl staining confirms efficient downregulation in ISCs/EBs, but not neighbouring cells. b, The same markers reveal that the differentiation defect resulting from N downregulation, previously reported in females, is also apparent in males (note loss of Su(H)-LacZ following N downregulation in both males and females), suggesting that sex differences in differentiation do not contribute to the sex differences in susceptibility to N-induced tumours. Co-expression of a mitogen (secreted Spitz, sSpi) abrogates the sex differences in tumour susceptibility by efficiently triggering hyperplasia also in males, as revealed by an expanded progenitor (GFP-positive) area in both males and females. The identity of these tumours in males is also comparable to that previously show for N tumours in females (consisting of high Pros-positive EEC-like cells and low Pros-positive neoplastic ISC-like cells85. This further suggests that the sex differences in N-induced tumour susceptibility do not arise from sexually dimorphic differentiation effects, but result from sex differences in ISC proliferation. See Supplementary Information for full genotypes.
Extended Data Fig. 5. tra, but not tra2, controls intrinsic sex differences in adult ISC proliferation.
a, Additional controls for Fig. 2a, and confirmation of phenotypes using independent RNAi transgenes. Two additional RNAi transgenes against tra reduce the number of pH3-positive cells in DSS-treated female – but not male - midguts when expressed from esgTS in adults ISCs/EBs, confirming an adult progenitor-specific requirement for tra in promoting damage-induced cell divisions in female flies. b, tra1 MARCM mutant clones are smaller than control clones in females, whereas their size is comparable to that of wild-type or tra1 clones in males. This confirms that, during normal homeostasis, female ISCs divide more often than male ISCs because of the cell-autonomous action of tra. The graph shows quantifications of clone size (in arbitrary units of GFP fluorescence, as described in Methods) 15 days after clone induction by heat shock, and the confocal images show representative clones (labelled in green with GFP) for each genotype. c, Ubiquitous, adult-restricted traF expression from tubPTS in males increases the number of pH3-positive cells following DSS treatment to levels comparable to those of female flies. d, Re-introduction of this traF transgene specifically in adult ISCs/EBs rescues the reduced, male-like intestinal proliferation (as assessed by the number of pH3-positive cells) of tra null mutant females entirely lacking the tra gene from all their tissues (traKO/tra1) to levels comparable to those of control females. Expression of this transgene in control heterozygous female flies (traKO/+ esgTS>traF) does not significantly increase their proliferation (in fact, it reduces it slightly relative to traKO/+ esgTS> controls, likely as a consequence of its over-expression). e, Clonal analyses of homeostatic proliferation using the inducible esg flip-out system - which labels progenitors and their progeny generated within a defined temporal window84 - in midguts of control females and females in which tra downregulation has been confined to adult progenitors. 15 days after induction, the size (assessed as the percentage of GFP-positive area) of control clones is significantly larger than that of tra-RNAi clones. The graph shows area quantifications for each genotype, and the confocal images show representative clones for each genotype. f, Consistent with the tra mutant clonal analysis in Fig. 2c, quantifications of clone size (number of cells per clone) reveal that MARCM clones in which tra has been downregulated are smaller than control clones only in females. Their size is comparable to that of wild-type or tra-downregulated mutant clones in males. The confocal images show representative clones (labelled in green with GFP) for each genotype in females. g, qRT-PCR quantifications of relative abundance of traF, dsxF, dsxM and Yp1 transcripts in adult-specific tra2 mutants (tra2B/ts1 grown at permissive temperatures, then switched to the restrictive temperature 4 days after eclosion and transcriptionally profiled following 10 additional days at the restrictive temperature) and controls (tra2B/+). In tra2 mutant females, dsxF is lost, dsxM is upregulated to levels comparable to those of control males and Yp1 (a DsxF target) is lost (to levels also comparable to those of males). tra mutants (traDf(3L)st-j7/KO) were also used as a positive control. n denotes the number of guts (a, c, d, e), or clones (b, f) that were analysed for each genotype. Results combined from at least two independent experiments. See Supplementary Information for full genotypes.
Extended Data Fig. 6. dsx- and fruM-independent control of sexually dimorphic proliferation in adult intestinal stem cells.
a, Adult-restricted downregulation of dsx (achieved by co-expression of a dsx-RNAi transgene and Dicer-2 (Dcr-2) in ISCs/EBs) has no effect on the compensatory ISC proliferation observed upon DSS treatment in neither males nor females. dsxF expression in the same conditions does not increase ISC proliferation in either males or females. b, dsxF expression does not rescue the reduced proliferation resulting from tra downregulation in females. Representative images for each genotype are shown in both a and b (DNA: DAPI, in blue; ISC/EB marker: GFP, in green). c, The size of dsx null mutant (dsx1) MARCM clones (quantified in arbitrary units of GFP fluorescence as described in Methods) is comparable to that of controls in both sexes 15 days after clone induction by heat shock. Confocal images show representative clones (labelled in green with GFP) for each genotype. d, Quantifications of clone size in control and dsx null mutant (dsx1) MARCM clones in the midguts of DSS-treated males and females. 5 days after clone induction by heat shock, there are no significant differences in clone size (quantified in arbitrary units of GFP fluorescence as described in Methods) between control and mutants clones in either males or females. Confocal images show representative clones (labelled in green with GFP) for each genotype. e, An RNAi transgene against fru does not reduce the number of pH3-positive cells in DSS-treated midguts when expressed from esgTS in the adult ISCs/EBs of either males or females. Confocal images show that number of intestinal progenitors (esg-positive cells in green) is also unaffected by this manipulation. f, Quantifications of the number of pH3-positive cells upon DSS treatment indicates that the sexual dimorphism in ISC proliferation is unaffected in females with forced fruM expression (fruM/fru4-40) or in males with forced fruF expression (fruF/fru4-40). g, ISC proliferation is unaffected in the migduts of DSS-treated males and females entirely lacking dsx (dsxΔ/dsx1), producing only DsxF (dsxΔ/dsx11) or DsxM (dsxΔ/dsxD). ISC proliferation is also unaffected in dsx, fruM double null mutant males and females (dsxΔ,Df(3R)Exel6179/dsx1, fruP1.LexA), and in dsx null mutants in which fruM is ectopically produced in females (dsxΔ,Df(3R)Exel6179/dsx1, fruΔtra). n denotes the number of guts (a, b, e, f, g), or clones (c, d) that were analysed for each genotype. Results combined from at least two independent experiments. See Supplementary Information for full genotypes.
Extended Data Fig. 7. tra targets in adult ISCs.
a, Scatter plot of all 1346 genes with tra-dependent expression in the adult fly midgut. For each gene, control female/tra null mutant female (Df(3L)st-j7/traKO) fold differences in transcript abundance (X axis, log2 scale) are plotted against tra mutant female with feminized ISCs (adult-restricted rescue of traF in ISCs/EBs)/tra mutant female fold differences (Y axis, log2 scale). Genes with tra-sensitive expression and significantly repressed by traF in ISC/EBs (p<0.05 cutoff) are therefore found in the left-bottom quadrant and are displayed in blue, whereas those significantly activated by traF are found in the top-right quadrant and are displayed in red. Genes with tra-dependent transcription, independent of the action of traF in intestinal precursors are displayed in black. b, Comparable analysis of tra-dependent alternative splicing. c, tra expression in adult ISCs affects splicing of 38 transcripts by at least 5 different mechanisms. The outcome of each of the alternative splicing mechanisms is shown in yellow for a representative gene. d, Adult-restricted downregulation (RNAi lines) or mis-expression (UAS lines) of tra targets in adult ISCs/EBs by means of esg-Gal4, tubGal80TS. Genes normally repressed in female progenitors in a traF-dependent manner were downregulated in males (top row) and/or misexpressed in females (bottom row). Genes upregulated in female progenitors in a traF-dependent manner were downregulated in females (bottom row). Adult-restricted downregulation of Idgf1 and Spn88Eb reduces the number of mitoses (pH3-positive cells) in DSS-treated females. Conversely, rdo mis-expression inhibits DSS-induced ISC proliferation in females. Adult-restricted downregulation of other traF targets in the same conditions does not affect ISC proliferation in either males or females. e, Male controls for Fig. 3c. In contrast to their effects on females, adult ISC/EB-restricted mis-expression of rdo or downregulation of Idgf1 and Spn88Eb does not reduce the percentage of midgut area covered by esg-positive cells in DSS-treated males (DNA: DAPI, in blue; ISC/EB marker: GFP, in green). n denotes the number of guts (d) that were analysed for each genotype. Results combined from at least two independent experiments. See Supplementary Information for full genotypes.
Extended Data Fig. 8. Effects of the sexual identity of adult ISCs on midgut size and reproductive plasticity.
a, The number of cells in the R3a-b and R4a midgut regions, defined by expression of Cut and MvlNP2375 respectively (as described in 86), is higher in females, and can be significantly reduced in females to numbers comparable to those found in males after 20 (but not 3) days of adult-specific downregulation of tra in intestinal progenitors (achieved by esgTS-driven tra downregulation initiated after the phase of midgut post-eclosion growth, see Methods for details). No effects are apparent following downregulation in males. Representative images of these midgut regions (labelled in red with Cut or in green with MvlNP2375,esgTS–driven GFP) are shown to the right for each genotype. b, Adult ISC/EB-specific tra downregulation does not affect the number of ISCs (esg-positive, Su(H)-negative cells) in either males or females, but strongly reduces EB (esg-positive, Su(H)-positive cells) production in females. c and d, Quantifications as in a and b for midguts with adult-specific downregulation of Sxl in intestinal progenitors. c, Reduced number of cells in the R4a midgut region (top graph) and total midgut length (bottom graph) in female flies following 20 days of adult-specific and cell-autonomous masculinization of their intestinal progenitors (achieved by-down regulation of Sxl over 20 days with esg-Gal4). The same manipulation has no discernible effects in males. d, The same genetic manipulation does not affect the number of ISCs (esg-positive, Su(H)-negative cells) in either males or females, but strongly reduces EB (esg-positive, Su(H)-positive cells) production in females. e, The number of EBs (esg-and Su(H)-positive cells, bottom graph), but not ISCs (esg-positive only cells, top graph) is higher in control female flies 3 days after mating. Adult ISC/EB-specific tra downregulation abrogates the postmating increase in EBs in females without affecting EB number in males, or ISC number in males or females. f, Adult-specific Sxl downregulation in intestinal progenitors leads to a small, but significant, reduction in egg production. An unrelated manipulation that also reduces ISC proliferation by inducing differentiation of ISCs (esgTS>Notchintra, images to the right of the graph and 87) also results in reduced egg production, whereas downregulation of dsx (which does not control sex differences in progenitor proliferation) has no such effect. It should, however, be noted that esg-Gal4 is expressed in a subset of cells in the ovary niche21. Hence, the possibility that these cells contribute to the observed phenotype cannot entirely be ruled out. Images to the right show loss of intestinal progenitor cell makers esg-Gal4 and Su(H)-LacZ following expression of Nintra in adult intestinal progenitors, indicative of loss of progenitor identity. n denotes the number of guts (a, c), ISCs/EBs (b, d, e), or female flies (f) that were analysed for each genotype. Results combined from at least two independent experiments. See Supplementary Information for full genotypes.
Extended Data Fig. 9. Effects of the sexual identity of adult ISCs on the susceptibility to genetically induced tumours.
a, The number of mitoses in Apc-ras mutant midguts is larger than that of control clones in both sexes, but it is higher and dependent on Sxl in females. b, The size of Delta (Dl, the N ligand) null mutant (DlRevF10) MARCM clones is larger than that of control clones in both sexes, but female mutant clones are larger than male mutant clones. The graph shows quantifications of the number of cells within each clone 15 days after clone induction by heat shock, and the confocal images show representative clones (labelled in green with GFP) for each genotype. c, In tra “female” mutant flies (traKO and traKO/Df(3L)st-j7) reduced N signalling in intestinal progenitors fails to induce the hyperplasia (quantified by the number of pH3 cells) normally observed in control females. d, Following 15 days of adult-specific downregulation of Notch (N) in intestinal progenitors, hyperplasia (quantified by the number of pH3-positive cells and also shown in representative images) is observed in female, but not male midguts. Adult-specific and cell-autonomous reversal of ISC/EB female identity – achieved by esgTS-driven downregulation of Sxl – fully prevents the hyperplasia induced by Notch downregulation in females, but has no effects on males. Confocal images show intestinal progenitor coverage of representative midgut portions for each genotype (DNA: DAPI, in blue; ISC/EB marker: GFP, in green). e, pH3 quantifications show a comparable effect for an independent RNAi transgene against Sxl. f, Adult-specific downregulation of Notch (N) signalling by ectopic expression of the downstream N signalling antagonist Hairless (H)38 leads to hyperplasia (quantified by the number of pH3-positive cells and also shown in representative images) in female, but not male midguts. Adult-specific and cell-autonomous reversal of ISC/EB female identity – achieved by esgTS-driven downregulation of Sxl – fully prevents the hyperplasia induced by H overexpression in females, but has no effects on males. Confocal images show intestinal progenitor coverage of representative midgut portions for each genotype (DNA: DAPI, in blue; ISC/EB marker: GFP, in green). g, The number of pH3-positive cells 15 days after N downregulation in adult intestinal progenitors of double null mutant flies lacking dsx and fruM (dsxΔ,Df(3R)Exel6179/dsx1, fruP1.LexA) is comparable to that of controls in both males and female flies. Like control flies, it is significantly higher in female flies. Virgin flies were used in all these experiments. n denotes the number of guts (a, c, d, e, f, g), or clones (b) that were analysed for each genotype. Results combined from at least two independent experiments. See Supplementary Information for full genotypes.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Konstantinos Vanezis and Laurence Game for technical assistance, and Cayetano Gonzalez, Tom Carroll and Daniel Perea for discussions. Bruce Baker, Allison Bardin, Alberto Baena-Lopez, Sarah Bray, Andreu Casali, Thomas Cline, Steve Cohen, Daniel Eberl, Stephen Goodwin, Greg Jefferis, Leanne Jones, Ryusuke Niwa, Benjamin Prud’homme, Iris Salecker, Lucas Sanchez, Carl Thummel, Jessica Treisman, Marcos Vidal, Daisuke Yamamoto and Laurence Zwiebel shared reagents. Dafni Hadjieconomou, Jake Jacobson, George King and Esmeralda Parra-Peralbo provided comments on the manuscript. This work was funded by an ERC Starting Grant to I.M-A. (ERCStG 310411) and MRC intramural funding. B.H. holds an EMBO long-term fellowship and I.M-A. is a member of, and is supported by, the EMBO Young Investigator Programme.
Footnotes
Competing financial interests
The authors have no competing financial interests.
REFERENCES
- 1.Arnold AP. The end of gonad-centric sex determination in mammals. Trends in genetics : TIG. 2012;28:55–61. doi: 10.1016/j.tig.2011.10.004. doi:10.1016/j.tig.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nature reviews. Genetics. 2008;9:911–922. doi: 10.1038/nrg2415. doi:10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cognigni P, Bailey AP, Miguel-Aliaga I. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell metabolism. 2011;13:92–104. doi: 10.1016/j.cmet.2010.12.010. doi:10.1016/j.cmet.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucchesi JC, Kuroda MI. Dosage compensation in Drosophila. Cold Spring Harbor perspectives in biology. 2015;7 doi: 10.1101/cshperspect.a019398. doi:10.1101/cshperspect.a019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boggs RT, Gregor P, Idriss S, Belote JM, McKeown M. Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell. 1987;50:739–747. doi: 10.1016/0092-8674(87)90332-1. [DOI] [PubMed] [Google Scholar]
- 6.Camara N, Whitworth C, Van Doren M. The creation of sexual dimorphism in the Drosophila soma. Current topics in developmental biology. 2008;83:65–107. doi: 10.1016/S0070-2153(08)00403-1. doi:10.1016/S0070-2153(08)00403-1. [DOI] [PubMed] [Google Scholar]
- 7.Christiansen AE, Keisman EL, Ahmad SM, Baker BS. Sex comes in from the cold: the integration of sex and pattern. Trends in genetics : TIG. 2002;18:510–516. doi: 10.1016/s0168-9525(02)02769-5. [DOI] [PubMed] [Google Scholar]
- 8.Villella A, Hall JC. Neurogenetics of courtship and mating in Drosophila. Advances in genetics. 2008;62:67–184. doi: 10.1016/S0065-2660(08)00603-2. doi:10.1016/S0065-2660(08)00603-2. [DOI] [PubMed] [Google Scholar]
- 9.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell stem cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. doi:10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arthur BI, Jr., Jallon JM, Caflisch B, Choffat Y, Nothiger R. Sexual behaviour in Drosophila is irreversibly programmed during a critical period. Current biology : CB. 1998;8:1187–1190. doi: 10.1016/s0960-9822(07)00491-5. [DOI] [PubMed] [Google Scholar]
- 11.Ferveur JF, et al. Genetic feminization of pheromones and its behavioral consequences in Drosophila males. Science. 1997;276:1555–1558. doi: 10.1126/science.276.5318.1555. [DOI] [PubMed] [Google Scholar]
- 12.Clough E, et al. Sex- and tissue-specific functions of Drosophila doublesex transcription factor target genes. Developmental cell. 2014;31:761–773. doi: 10.1016/j.devcel.2014.11.021. doi:10.1016/j.devcel.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Q, Wawersik M, Matunis EL. The Jak-STAT target Chinmo prevents sex transformation of adult stem cells in the Drosophila testis niche. Developmental cell. 2014;31:474–486. doi: 10.1016/j.devcel.2014.10.004. doi:10.1016/j.devcel.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matson CK, et al. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature. 2011;476:101–104. doi: 10.1038/nature10239. doi:10.1038/nature10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shapiro-Kulnane L, Smolko AE, Salz HK. Maintenance of Drosophila germline stem cell sexual identity in oogenesis and tumorigenesis. Development. 2015;142:1073–1082. doi: 10.1242/dev.116590. doi:10.1242/dev.116590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uhlenhaut NH, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–1142. doi: 10.1016/j.cell.2009.11.021. doi:10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Caldwell JC, Fineberg SK, Eberl DF. reduced ocelli encodes the leucine rich repeat protein Pray For Elves in Drosophila melanogaster. Fly. 2007;1:146–152. doi: 10.4161/fly.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Handke B, et al. The hemolymph proteome of fed and starved Drosophila larvae. PloS one. 2013;8:e67208. doi: 10.1371/journal.pone.0067208. doi:10.1371/journal.pone.0067208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawamura K, Shibata T, Saget O, Peel D, Bryant PJ. A new family of growth factors produced by the fat body and active on Drosophila imaginal disc cells. Development. 1999;126:211–219. doi: 10.1242/dev.126.2.211. [DOI] [PubMed] [Google Scholar]
- 20.Brown JB, et al. Diversity and dynamics of the Drosophila transcriptome. Nature. 2014;512:393–399. doi: 10.1038/nature12962. doi:10.1038/nature12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiff T, et al. Endocrine remodelling of the adult intestine sustains reproduction in Drosophila. eLife. 2015;4:e06930. doi: 10.7554/eLife.06930. doi:10.7554/eLife.06930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. doi:10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 23.Patel PH, Edgar BA. Tissue design: how Drosophila tumors remodel their neighborhood. Seminars in cell & developmental biology. 2014;28:86–95. doi: 10.1016/j.semcdb.2014.03.012. doi:10.1016/j.semcdb.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Evans DS, Cline TW. Drosophila switch gene Sex-lethal can bypass its switch-gene target transformer to regulate aspects of female behavior. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E4474–4481. doi: 10.1073/pnas.1319063110. doi:10.1073/pnas.1319063110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finley KD, Taylor BJ, Milstein M, McKeown M. dissatisfaction, a gene involved in sex-specific behavior and neural development of Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:913–918. doi: 10.1073/pnas.94.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinett CC, Vaughan AG, Knapp JM, Baker BS. Sex and the single cell. II. There is a time and place for sex. PLoS biology. 2010;8:e1000365. doi: 10.1371/journal.pbio.1000365. doi:10.1371/journal.pbio.1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markle JG, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. doi:10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 28.Steegenga WT, et al. Sexually dimorphic characteristics of the small intestine and colon of prepubescent C57BL/6 mice. Biology of sex differences. 2014;5:11. doi: 10.1186/s13293-014-0011-9. doi:10.1186/s13293-014-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cook MB, et al. Sex disparities in cancer incidence by period and age. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:1174–1182. doi: 10.1158/1055-9965.EPI-08-1118. doi:10.1158/1055-9965.EPI-08-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siudeja K, et al. Frequent Somatic Mutation in Adult Intestinal Stem Cells Drives Neoplasia and Genetic Mosaicism during Aging. Cell stem cell. 2015 doi: 10.1016/j.stem.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
ADDITIONAL REFERENCES
- 31.Ruiz MF, Sarno F, Zorrilla S, Rivas G, Sanchez L. Biochemical and functional analysis of Drosophila-sciara chimeric sex-lethal proteins. PloS one. 2013;8:e65171. doi: 10.1371/journal.pone.0065171. doi:10.1371/journal.pone.0065171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu B, Ackerman L, Jan LY, Jan YN. Modes of protein movement that lead to the asymmetric localization of partner of Numb during Drosophila neuroblast division. Molecular cell. 1999;4:883–891. doi: 10.1016/s1097-2765(00)80218-x. [DOI] [PubMed] [Google Scholar]
- 33.Zielke N, et al. Fly-FUCCI: A versatile tool for studying cell proliferation in complex tissues. Cell reports. 2014;7:588–598. doi: 10.1016/j.celrep.2014.03.020. doi:10.1016/j.celrep.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Ferveur JF, Stortkuhl KF, Stocker RF, Greenspan RJ. Genetic feminization of brain structures and changed sexual orientation in male Drosophila. Science. 1995;267:902–905. doi: 10.1126/science.7846534. [DOI] [PubMed] [Google Scholar]
- 35.Lee G, Hall JC, Park JH. Doublesex gene expression in the central nervous system of Drosophila melanogaster. Journal of neurogenetics. 2002;16:229–248. doi: 10.1080/01677060216292. [DOI] [PubMed] [Google Scholar]
- 36.Merrill CE, Sherertz TM, Walker WB, Zwiebel LJ. Odorant-specific requirements for arrestin function in Drosophila olfaction. Journal of neurobiology. 2005;63:15–28. doi: 10.1002/neu.20113. doi:10.1002/neu.20113. [DOI] [PubMed] [Google Scholar]
- 37.Daborn PJ, et al. Evaluating the insecticide resistance potential of eight Drosophila melanogaster cytochrome P450 genes by transgenic over-expression. Insect biochemistry and molecular biology. 2007;37:512–519. doi: 10.1016/j.ibmb.2007.02.008. doi:10.1016/j.ibmb.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Bardin AJ, Perdigoto CN, Southall TD, Brand AH, Schweisguth F. Transcriptional control of stem cell maintenance in the Drosophila intestine. Development. 2010;137:705–714. doi: 10.1242/dev.039404. doi:10.1242/dev.039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Celis JF, Bray S. Feed-back mechanisms affecting Notch activation at the dorsoventral boundary in the Drosophila wing. Development. 1997;124:3241–3251. doi: 10.1242/dev.124.17.3241. [DOI] [PubMed] [Google Scholar]
- 40.Schweitzer R, Shaharabany M, Seger R, Shilo BZ. Secreted Spitz triggers the DER signaling pathway and is a limiting component in embryonic ventral ectoderm determination. Genes & development. 1995;9:1518–1529. doi: 10.1101/gad.9.12.1518. [DOI] [PubMed] [Google Scholar]
- 41.Martorell O, et al. Conserved mechanisms of tumorigenesis in the Drosophila adult midgut. PloS one. 2014;9:e88413. doi: 10.1371/journal.pone.0088413. doi:10.1371/journal.pone.0088413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kohl J, Ostrovsky AD, Frechter S, Jefferis GS. A bidirectional circuit switch reroutes pheromone signals in male and female brains. Cell. 2013;155:1610–1623. doi: 10.1016/j.cell.2013.11.025. doi:10.1016/j.cell.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mellert DJ, Robinett CC, Baker BS. doublesex functions early and late in gustatory sense organ development. PloS one. 2012;7:e51489. doi: 10.1371/journal.pone.0051489. doi:10.1371/journal.pone.0051489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cande J, Stern DL, Morita T, Prud’homme B, Gompel N. Looking under the lamp post: neither fruitless nor doublesex has evolved to generate divergent male courtship in Drosophila. Cell reports. 2014;8:363–370. doi: 10.1016/j.celrep.2014.06.023. doi:10.1016/j.celrep.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker BS, Hoff G, Kaufman TC, Wolfner MF, Hazelrigg T. The doublesex locus of Drosophila melanogaster and its flanking regions: a cytogenetic analysis. Genetics. 1991;127:125–138. doi: 10.1093/genetics/127.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duncan IW, Kaufman TC. Cytogenic analysis of chromosome 3 in Drosophila melanogaster: mapping of the proximal portion of the right arm. Genetics. 1975;80:733–752. doi: 10.1093/genetics/80.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Demir E, Dickson BJ. fruitless splicing specifies male courtship behavior in Drosophila. Cell. 2005;121:785–794. doi: 10.1016/j.cell.2005.04.027. doi:10.1016/j.cell.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 48.Mellert DJ, Knapp JM, Manoli DS, Meissner GW, Baker BS. Midline crossing by gustatory receptor neuron axons is regulated by fruitless, doublesex and the Roundabout receptors. Development. 2010;137:323–332. doi: 10.1242/dev.045047. doi:10.1242/dev.045047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belote JM, Hoffmann FM, McKeown M, Chorsky RL, Baker BS. Cytogenetic analysis of chromosome region 73AD of Drosophila melanogaster. Genetics. 1990;125:783–793. doi: 10.1093/genetics/125.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sturtevant AH. A gene in Drosophila melanogaster that transforms females into males. Genetics. 1945;30:297–299. doi: 10.1093/genetics/30.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mattox W, Baker BS. Autoregulation of the splicing of transcripts from the transformer-2 gene of Drosophila. Genes & development. 1991;5:786–796. doi: 10.1101/gad.5.5.786. [DOI] [PubMed] [Google Scholar]
- 52.Belote JM, Baker BS. Sexual behavior: its genetic control during development and adulthood in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:8026–8030. doi: 10.1073/pnas.84.22.8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gowen JW, Fung STC. Determination of sex through genes in a major sex locus in Drosophila melanogaster. Heredity. 1957;11:397–402. [Google Scholar]
- 54.Anand A, et al. Molecular genetic dissection of the sex-specific and vital functions of the Drosophila melanogaster sex determination gene fruitless. Genetics. 2001;158:1569–1595. doi: 10.1093/genetics/158.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haenlin M, Kramatschek B, Campos-Ortega JA. The pattern of transcription of the neurogenic gene Delta of Drosophila melanogaster. Development. 1990;110:905–914. doi: 10.1242/dev.110.3.905. [DOI] [PubMed] [Google Scholar]
- 56.Morel V, Schweisguth F. Repression by suppressor of hairless and activation by Notch are required to define a single row of single-minded expressing cells in the Drosophila embryo. Genes & development. 2000;14:377–388. [PMC free article] [PubMed] [Google Scholar]
- 57.Furriols M, Bray S. A model Notch response element detects Suppressor of Hairless-dependent molecular switch. Current biology : CB. 2001;11:60–64. doi: 10.1016/s0960-9822(00)00044-0. [DOI] [PubMed] [Google Scholar]
- 58.Rideout EJ, Dornan AJ, Neville MC, Eadie S, Goodwin SF. Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nature neuroscience. 2010;13:458–466. doi: 10.1038/nn.2515. doi:10.1038/nn.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manoli DS, et al. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436:395–400. doi: 10.1038/nature03859. doi:10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- 60.Wang L, Zeng X, Ryoo HD, Jasper H. Integration of UPRER and oxidative stress signaling in the control of intestinal stem cell proliferation. PLoS genetics. 2014;10:e1004568. doi: 10.1371/journal.pgen.1004568. doi:10.1371/journal.pgen.1004568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeng X, Chauhan C, Hou SX. Characterization of midgut stem cell- and enteroblast-specific Gal4 lines in drosophila. Genesis. 2010;48:607–611. doi: 10.1002/dvg.20661. doi:10.1002/dvg.20661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Phillips MD, Thomas GH. Brush border spectrin is required for early endosome recycling in Drosophila. Journal of cell science. 2006;119:1361–1370. doi: 10.1242/jcs.02839. doi:10.1242/jcs.02839. [DOI] [PubMed] [Google Scholar]
- 63.Balakireva M, Stocker RF, Gendre N, Ferveur JF. Voila, a new Drosophila courtship variant that affects the nervous system: behavioral, neural, and genetic characterization. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:4335–4343. doi: 10.1523/JNEUROSCI.18-11-04335.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan Y, Robinett CC, Baker BS. Turning males on: activation of male courtship behavior in Drosophila melanogaster. PloS one. 2011;6:e21144. doi: 10.1371/journal.pone.0021144. doi:10.1371/journal.pone.0021144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shiga Y, Tanaka-Matakatsu M, Hayashi S. A nuclear GFP/ beta-galactosidase fusion protein as a marker for morphogenesis in living Drosophila. Dev Growth Differ. 1996;38:99–106. [Google Scholar]
- 66.Misra JR, Horner MA, Lam G, Thummel CS. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes & development. 2011;25:1796–1806. doi: 10.1101/gad.17280911. doi:10.1101/gad.17280911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baena-Lopez LA, Alexandre C, Mitchell A, Pasakarnis L, Vincent JP. Accelerated homologous recombination and subsequent genome modification in Drosophila. Development. 2013;140:4818–4825. doi: 10.1242/dev.100933. doi:10.1242/dev.100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. doi:10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kimura K, Ote M, Tazawa T, Yamamoto D. Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature. 2005;438:229–233. doi: 10.1038/nature04229. doi:10.1038/nature04229. [DOI] [PubMed] [Google Scholar]
- 70.Yeo SL, et al. On the functional overlap between two Drosophila POU homeo domain genes and the cell fate specification of a CNS neural precursor. Genes & development. 1995;9:1223–1236. doi: 10.1101/gad.9.10.1223. [DOI] [PubMed] [Google Scholar]
- 71.Green EW, Fedele G, Giorgini F, Kyriacou CP. A Drosophila RNAi collection is subject to dominant phenotypic effects. Nature methods. 2014;11:222–223. doi: 10.1038/nmeth.2856. doi:10.1038/nmeth.2856. [DOI] [PubMed] [Google Scholar]
- 72.R Development Core Team . R Foundation for Statistical Computing. 2014. [Google Scholar]
- 73.Kim D, et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome biology. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. doi:10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. doi:10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anders S, Huber W. Differential expression analysis for sequence count data. Genome biology. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. doi:10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome biology. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. doi:10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turro E, et al. Haplotype and isoform specific expression estimation using multi-mapping RNA-seq reads. Genome biology. 2011;12:R13. doi: 10.1186/gb-2011-12-2-r13. doi:10.1186/gb-2011-12-2-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome biology. 2014;15:550. doi: 10.1186/s13059-014-0550-8. doi:10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saeed AI, et al. TM4 microarray software suite. Methods in enzymology. 2006;411:134–193. doi: 10.1016/S0076-6879(06)11009-5. doi:10.1016/S0076-6879(06)11009-5. [DOI] [PubMed] [Google Scholar]
- 80.Jiang H, Edgar BA. Intestinal stem cell function in Drosophila and mice. Current opinion in genetics & development. 2012;22:354–360. doi: 10.1016/j.gde.2012.04.002. doi:10.1016/j.gde.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lemaitre B, Miguel-Aliaga I. The digestive tract of Drosophila melanogaster. Annual review of genetics. 2013;47:377–404. doi: 10.1146/annurev-genet-111212-133343. doi:10.1146/annurev-genet-111212-133343. [DOI] [PubMed] [Google Scholar]
- 82.Kelley RL, et al. Expression of msl-2 causes assembly of dosage compensation regulators on the X chromosomes and female lethality in Drosophila. Cell. 1995;81:867–877. doi: 10.1016/0092-8674(95)90007-1. [DOI] [PubMed] [Google Scholar]
- 83.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 84.Jiang H, et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. doi:10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patel PH, Dutta D, Edgar BA. Niche appropriation by Drosophila intestinal stem cell tumours. Nature cell biology. 2015;17:1182–1192. doi: 10.1038/ncb3214. doi:10.1038/ncb3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buchon N, et al. Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell reports. 2013;3:1725–1738. doi: 10.1016/j.celrep.2013.04.001. doi:10.1016/j.celrep.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 87.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. doi:10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.