Abstract
Objectives
To investigate cognitive and attentional function in adolescents and young adults with operated congenital heart disease.
Background
Previous research has indicated that children with congenital heart disease have deficits in broad areas of cognitive function. However, less attention has been given to survivors as they grow into adolescence and early adulthood.
Method
Participants included 18 non-syndromic adolescents and young adults with Tetralogy of Fallot and d-Transposition of the Great Arteries that required cardiac surgery before the age of five and 18 healthy, unaffected siblings (age 11–22 years for both groups). Cases with congenital heart disease and their siblings were administered Wechsler Intelligence scales and reported attention problems using the Achenbach System of Empirically Based Assessments. Cases were compared to both healthy siblings and established norms.
Key Findings
Cases performed significantly lower than siblings on Full Scale IQ and processing speed, and significantly lower than norms on perceptual reasoning. Cases also reported more attention problems compared to both siblings and norms. Effect sizes varied with medium to large effects for processing speed, perceptual reasoning, working memory, and attention problems.
Conclusions
Findings suggest that neurocognitive function may continue to be affected for congenital heart disease survivors in adolescence and young adulthood and that comparisons to established norms may underestimate neurocognitive vulnerabilities.
Keywords: Congenital Heart Disease, Tetralogy of Fallot, d-Transposition of the Great Arteries, Neurocognitive function, Attention
Every year 40,000 children in the U.S. are diagnosed with congenital heart disease (CHD) and 25% of these children with critical CHD require surgery during infancy (Hoffman & Kaplan, 2002). Advances in surgical and medical care have led to dramatic increases in survival rates, as over one million adults now have some form of CHD (Sable et al., 2011; Warnes et al., 2008). But much like children with a range of chronic systemic health conditions including sickle cell disease and diabetes (Berkelhammer et al., 2007; Desrocher & Rovet, 2004), CHD, as well as related cyanosis and early cardiac surgery, often has significant adverse effects on brain development and neurocognitive function (Wray, 2006). Emerging evidence suggests that as children with CHD grow into adolescence and young adulthood, they may be at risk for long-term neuropsychological consequences (Marino et al., 2012).
Several studies have focused on cognitive function in children with CHD. A recent meta-analysis by Karsdorp, Everaerd, Kindt, and Mulder (2007) identified deficits in broad areas of cognitive function. Results indicated that full scale IQ and performance IQ, but not verbal IQ, are reduced in CHD. However, the effect sizes vary according to severity of the CHD, with large effect sizes for Hypoplastic Left Heart Syndrome (HLHS) and small effect sizes for both Tetralogy of Fallot (TOF) and d-Transposition of the Great Arteries (d-TGA). This meta-analysis suggests that the most significantly impacted are children with a severe form of CHD, HLHS. However, less severe CHD, such as TOF and d-TGA, are more common and affect over three times as many children each year (Hoffman & Kaplan, 2002). Less is known about cognitive outcomes in this population.
Karsdorp et al. (2007) noted that future research should move beyond broad measures of verbal and performance IQ toward the examination of other domains of cognitive functioning, including visual-spatial skills and self-reports of functioning. Recent studies of children with d-TGA and TOF have found effects in several areas of cognitive function including attention and inhibition (Bellinger, Wypij, Rappaport, Jonas, Wernovsky, & Newburger, 2003; Calderon, Bonnet, Courtin, Concordet, Plumet, & Angeard, 2010; Miatton, De Wolf, François, Thiery, & Vingerhoets, 2007; Shillingford, Glanzman, Ittenbach, Clancy, Gaynor, & Wernovsky, 2008), working memory (Calderon et al., 2010), and visual-spatial processing (Bellinger et al., 2003, 2011). A recent study by Calderon, Jambaqué, Bonnet, and Angeard, (2014) examining the emergence of executive dysfunction in children with d-TGA found significant delays in the areas of inhibition and cognitive flexibility and suggested that deficits in processing speed may underlie these delays. Taken together, there is evidence that children with CHD are at risk for deficits or relative weaknesses in the multiple domains of cognitive function, but this has been studied less frequently during the period of adolescence and young adulthood (see Bellinger & Newburger, 2011 for exception). Given that children with CHD are now living past childhood, it is important to understand if these difficulties follow them into adolescence and young adulthood, or if cognitive difficulties remit over time (Bellinger & Newburger, 2010).
Another important question is how to quantify cognitive functioning in CHD. Most studies included in the Karsdorp et al. (2007) meta-analysis compared children with CHD to either established test norms based on national normative samples or local healthy controls, which can include same age children from the patient’s peer group or siblings. A common practice in other pediatric conditions is to use siblings as an additional, informative comparison (e.g., Campbell et al., 2007; Donders & Taneja, 2009; Reeves et al., 2007; Smith & Wilkins, 2015). Sibling controls have several strengths, in that they have the same family socioeconomic status and parent education level that come from sharing a home environment and may differ from the characteristics of nationally representative normative samples. However, established norms based on nationally representative samples provide essential information for determining eligibility for special education services and the development of Individualized Educational Plans (IEPs) and 504 plans. Therefore, comparisons that include both established norms and sibling controls can help researchers and clinicians better understand cognitive functioning relative to both normative samples of peers and family members.
Our goal was to continue to address the question of neuropsychological consequences in CHD by focusing on the time period of adolescence and young adulthood, expanding the cognitive domains assessed in addition to the two Wechsler indices, and using both national norms and sibling controls as comparisons in two forms of operated CHD: TOF and d-TGA. We aimed to characterize cognitive and attentional functioning in adolescents and young adults with TOF and d-TGA and compare function to healthy siblings and established norms. We hypothesized that functioning in the areas of perceptual reasoning, processing speed, working memory, and attention would be lower in adolescents and young adults with CHD, compared to sibling controls as well as national norms.
METHOD
Participants
Participants were recruited from the pediatric and adult cardiology clinics at a tertiary medical center with a CHD program for children and adults in the Southern United States as part of a larger study investigating cognitive function in adolescents and young adults with TOF and d-TGA. Cases with CHD were on average 16.1 years old (SD=3.5) and 61% were female; sibling controls were on average 16.2 years old (SD=3.5) and 56% were female. Sixteen (89%) sibling pairs were Caucasian, 1 (5.6%) African-American, 1 (5.6%) Asian; 1 (5.6%) was Hispanic/Latino. Twelve cases (67%) had TOF and 6 (33%) had d-TGA. All cases had surgery during infancy. Median age at first cardiac surgery was 91 days [interquartile range (IQR): 7–182]; median cardiopulmonary bypass time was 127 minutes (IQR: 68–148), bypass time data available in 61% of the operative and anesthesia records obtained. A range of parent education levels (28% high school diploma/GED, 28% some college, 17% college degree, 22% graduate or professional school) and family income levels (22%: $25,000 or less; 22%: $25,001–50,000; 6%: $50,001–75,000; 17%: $75,000–100,000; 28%: $100,000 or above; 1 chose not to answer) were reported. The sample was representative of the median income from the state in which the study was conducted but represented a higher level of education.
Procedures
Potential participants were identified through medical record systems and recruited in person during a routine visit to the pediatric or adult CHD cardiology clinic. Inclusion criteria were (a) diagnosis of either d-TGA or TOF requiring cardiac bypass surgery before 5 years of age; (b) age from 10 up to 29 years (the oldest participant enrolled was 22); and (c) no known genetic, cognitive, or developmental disorders. Inclusion criteria for sibling controls were the same as above but no serious medical condition. Of the 23 adolescents and young adults who were approached to participate and spoke to a research assistant about the study, 18 case-sibling dyads were enrolled in the study. Reasons for declining included: not interested in research (2), anxiety about research procedures (2), and time demands of the study (1). Nine of 18 study participants had only one sibling. Of the nine that had more than one sibling, the sibling closest in age was recruited for six families. Two families chose to have a different sibling enrolled due to the siblings’ interests or convenience for the family (e.g., the sibling did not live within the same state). One family had a different sibling enrolled because the sibling closest in age had a significant developmental disorder that was part of the exclusion criteria for study enrollment. Of the 18 sibling controls, eight were younger than the children with CHD, nine were older, and one was a twin. The 18 cases and 18 controls did not significantly differ in age or gender (p’s <.50)
Cases and siblings completed questionnaire measures online and were administered cognitive tests at the hospital by a licensed clinical psychologist and a doctoral student in clinical psychology. Parents completed online questionnaires about their child with CHD. Informed consent and assent was obtained from participants and from parents when participants were <18 years. Participating cases and controls were reimbursed for their time. Cognitive testing feedback letters with results and interpretation were mailed to families after participation. This study was approved by the Institutional Review Board.
Measures
Cognitive function
Wechsler Intelligence Scales were administered to all cases and sibling controls. Participants aged 10–16 years were administered the Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV; Wechsler et al., 2004) and participants aged 17–22 years were administered the Wechsler Adult Intelligence Scale – Fourth Edition (WAIS-IV; Wechsler, 2008). These are considered equivalent measures with the same indices of intelligence. These are widely used and well-validated measures of cognitive function and intelligence and have been used previously with pediatric cardiology populations (see Karsdorp et al., 2007). For the current analyses, Full Scale IQ (FSIQ) and the four indices, Verbal Comprehension (VCI), Perceptual Reasoning (PRI), Processing Speed (PSI) and Working Memory (WMI), were examined.
Attention
Cases and sibling controls self-reported attention symptoms on the Youth Self Report (YSR) or Adult Self Report (ASR) and mothers reported on cases’ attention symptoms on the Child Behavior Checklist (CBCL) or Adult Behavior Checklist (ABCL; Achenbach & Rescorla, 2001, 2003). Mothers’ reports of sibling control’s attention were not collected, due to concerns about non-independence of data, as mothers within the same family would report on both cases and sibling controls. Three young adults did not have parents report on their attention, as they lived outside their parents’ home. Participants reported the frequency of behaviors along a three-point Likert Scale (0 = “Not True”, 1 = “Somewhat or Sometimes True”, 2 = “Very True or Often True”). Two subscales were used: Attention Problems and Attention Deficit/Hyperactivity (AD/H). Normalized T-scores (M=50, SD=10) based on age and sex were used in current analyses. These measures are widely used and reliability and validity are well established.
Statistical power and data analyses
Statistical analyses were conducted with the Statistical Package for Social Sciences 19th edition. Examination of multivariate outliers was conducted with Mahalanobis Distances; none exceeded the cutoff so none were excluded. Means and standard deviations, bivariate correlations, and paired-sample and one-sample t-tests were conducted. All t-tests were two-tailed. Power analyses indicated that with n = 18, β = .80, and α = .05, significant differences of medium to large effect sizes (d > .45) could be detected.
RESULTS
Preliminary analyses
Means and standard deviations of cognitive function outcomes can be found in Table 1. Mean FSIQ was 96 (SD = 11.44) for cases and 102.83 (SD = 10.89) for sibling controls. Mean index scores ranged from 93.83 (PRI) to 101.94 (VCI) for cases and 99.39 (PRI) to 105.17 (VCI) for sibling controls.
Table 1.
Cognitive Outcomes in Cases and Sibling Controls
| Case | Sibling | Sibling comparison | Norm comparison | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean (SD) | Mean (SD) | t | d | t | d | |
| FSIQ | 96.00 (11.44) | 102.83 (10.89) | −2.66* | −.61 | −1.48 | −.35 |
| VCI | 101.94 (11.79) | 105.17 (11.05) | −1.11 | −.28 | .70 | .16 |
| PRI | 93.83 (11.39) | 99.39 (11.90) | −1.85+ | −.48 | −2.30* | −.54 |
| WMI | 93.11 (14.91) | 100.56 (14.40) | −1.96+ | −.52 | −1.96+ | −.46 |
| PSI | 95.94 (10.33) | 103.17 (11.06) | −2.53* | −.73 | −1.67 | −.39 |
Notes: FSIQ = Full Scale IQ, VCI = Verbal Comprehension Index, PRI = Perceptual Reasoning Index, WMI = Working Memory Index, PSI = Processing Speed Index
p<.05
p<.10
FSIQ scores for CHD cases ranged from 68 to 119 (see Figure 1). Two (11%) had FSIQs in the High Average range (110–119), 12 (67%) in the Average range (90–109), 3 (17%) in the Below Average range (80–89), and 1 (5%) in the Extremely Low range (60–69). FSIQ scores for sibling controls ranged from 86 to 121. One (5%) had a FSIQ in the Superior range (120–129), 5 (28%) in the High Average range (110–119), 10 (56%) in the Average range (90–109), and 2 (11%) in the Below Average range (80–89). Because of the variance in IQ data, we compared the cases and siblings both with and without the highest and lowest cases (i.e., univariate outliers), and the pattern of results was the same with effect sizes in the same range. Because none of the cases exceeded the Mahalanobis Distance cutoff, only findings from comparisons with the complete sample are reported below.
Figure 1.
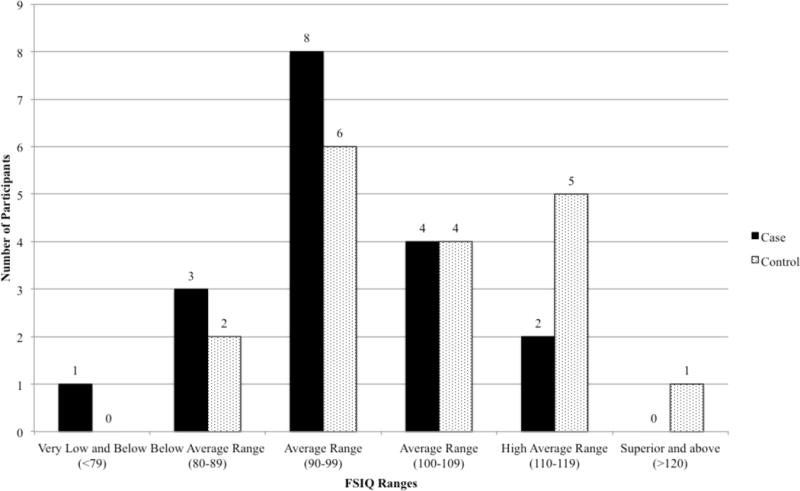
Full Scale Intelligence Quotient (FSIQ) in Cases and Sibling Controls.
Means and standard deviations of attention outcomes can be found in Table 2. According to case self-reports, 18% of cases were within the borderline clinical range on the Attention Problems and AD/H scales as compared to 16% that would be expected in a normative sample (i.e., T > 65); T scores ranged from 50 to 66 for Attention Problems and 50 to 64 for AD/H problems. According to parent reports, 21% were within the borderline clinical range on the Attention Problems scale and 14% were within the borderline clinical range for the AD/H scale; T scores ranged from 50 to 67 for Attention Problems and 50 to 65 for AD/H problems. According to sibling control self-report, 5% of siblings were within the borderline clinical range on the Attention Problems and AD/H scales; T-scores ranged from 50 to 64 for Attention Problems and 50 to 60 for AD/H problems. Cases’ self-reports and mothers’ reports were significantly and positively correlated for Attention Problems (r = .53, p = .05) and AD/H Problems (r = .57, p = .05)
Table 2.
Self-reports and Parent Reports of Attention
| Case self-report | Self-report to norm comparison | Mother report of case | Mother report to norm comparison | Sibling self-report | Case to sibling comparison | |
|---|---|---|---|---|---|---|
|
| ||||||
| M (SD) | t | M (SD) | t | M (SD) | t | |
| Attention Problems | 54.47 (4.96) | 3.71** | 55.14 (5.83) | 3.30** | 53.18 (3.81) | 1.65 |
| AD/H | 55.82 (4.16) | 5.78** | 53.86 (4.79) | 3.01* | 53.24 (3.09) | 2.62* |
Notes: Scores are age- and sex-adjusted T-scores from the ABCL, CBCL, YSR, and ASR
AD/H = Attention Deficit/Hyperactivity Scale
n = 15 mother report
p<.01,
p<.05,
p<.10
Exploratory analyses indicated that age was not significantly related to measures of cognitive function or reports of attention. In addition, reports of attention were not significantly related to FSIQ or index scores for either cases or siblings.
Cognitive function compared to sibling controls and norms
Cases with CHD demonstrated lower mean scores than sibling controls across FSIQ and all index scores (see Table 1). Scores were significantly lower on the Full Scale IQ (t = −2.66, p < .05) and PSI (t = −2.53, p < .05). Differences approached significance on the PRI (t = −1.85, p = .08) and WMI (t = −1.96, p = .07). Effect sizes as assessed by Cohen’s d ranged from small-to-medium for VCI and PRI and to medium-to-large for FSIQ, WMI, and PSI. Of the 18 case-sibling pairs, siblings outperformed cases on FSIQ in 13 cases, while cases outperformed siblings in 5 cases.
Cases with CHD demonstrated lower scores than norms across index scores, and significantly lower scores compared to norms on the PRI (t = −2.30, p < .05). Differences approached significance on the WMI (t = −1.96, p = .07). Effect sizes ranged from small to medium for FSIQ, PRI, WMI, and PSI (see Table 1).
Attention problems
Self-reports for adolescents and young adults with CHD reflected significantly higher scores on both the Attention Problems (t = 3.71, p < .01) and AD/H (t = 5.78, p < .001) scales compared to norms (see Table 2). Mother report on cases with CHD also reflected significantly higher scores on both the Attention Problems (t = 3.30, p < .01) and AD/H (t = 3.01, p < .05) scales compared to norms. Cases with CHD also demonstrated significantly higher self-reported AD/H symptoms than sibling controls (t = 2.62, p < .05). Effect sizes ranged from small to medium for self and parent report of Attention Problems (d = .45 to .51) and AD/H (d = .39 to .58).1
DISCUSSION
Results indicated that adolescents and young adults with operated TOF and d-TGA CHD demonstrate relative weaknesses across a range of domains of neurocognitive function. Effect sizes ranged from small to large across indices, with medium to large effects for processing speed, perceptual reasoning, and working memory indices from WISC-IV and WAIS-IV, and reports of attention problems. These findings are especially important given that the study focused on adolescents and young adults with moderate, not severe single ventricle CHD. This group has been given less research attention, but represents a large and growing number of patients moving into adolescence and young adulthood (Sable et al., 2011; Warnes et al., 2008).
This study focused on the period of adolescence and young adulthood, and although this was a single time point study, our results suggest that differences in neurocognitive function observed in childhood may continue to be salient for many older CHD survivors. Indeed, the effect sizes in the current study were larger than in the Karsdorp et al., (2007) meta-analysis, which primarily focused on children in early and middle childhood. This could be an artifact of the relatively small sample size in the current study, or may suggest progressive worsening over time. However, the findings in the current study suggest that future research should more closely examine cognitive trajectories as children with CHD age. Adolescence and young adulthood represents a developmental period when continued medical monitoring is required (Sable et al., 2011), functioning may worsen, and additional surgical interventions may be required with associated risk for cerebrovascular events. These factors combined with our results suggest that patients’ cognitive functioning should also continue to be monitored.
The results from the current study are similar to the findings from previous research that focused on school-aged children with CHD, particularly those involving the PRI, which reflects visual-spatial skills (Bellinger & Newburger, 2003), and the WMI, which represents auditory working memory (Calderon et al., 2010). Although processing speed is understudied in this population, it appears that vulnerabilties in this domain could underlie poor performance in some tasks of executive function as suggested in a previous study (Calderon et al., 2014). Findings regarding attention problems expand upon previous studies by utilizing both patient- and parent-reported symptoms as well as using clinically relevant measures (Calderon et al., 2010; McCusker, Armstrong, Mullen, Doherty, & Casey, 2013). Verbal IQ appears to be unaffected in this sample, which is also consistent with previous studies of children with CHD (Karsdorp et al., 2007), although some studies have found deficits in higher-order language skills, such as use of complex language in social communication (Bellinger et al., 2003). Taken together, these findings suggest a cognitive profile with relative weaknesses in the areas of visual-spatial processing and cognitive proficiency and relative strength in verbal comprehension. A preserved VCI remains a provocative but unexplained phenomenon. It has clinical implications, as verbal comprehension as an area of relative strength can be leveraged for educational planning. Preserved verbal comprehension also suggests that differences in cognitive function may not be primarily due to poor educational environments during periods of hospitalization, but that other causal processes may underlie neurocognitive functioning in this population.
These findings are qualified by differences depending on the comparison group. When compared to established norms, patients demonstrated significantly lower performance on tasks of perceptual reasoning, and reduction in working memory that approached significance. Effect sizes ranged from small to medium. The findings are different when examining sibling controls. Notably, when compared to their healthy siblings, patients demonstrated significantly lower scores on full scale IQ and processing speed, and trending differences on perceptual reasoning and working memory, with medium to large effect sizes. Indeed, Figure 1 suggests a shift in the cases’ distribution of FSIQ scores to the left of the siblings’ distribution reflecting lower scores. This indicates that comparisons to national norms, which are the traditional benchmark, may underestimate differences in performance. Sibling controls are more closely matched demographically and take into account the expected cognitive potential given the family environment. The correlation between siblings on measures of cognitive function is typically in the moderate range (Deary, 2012; Nesbitt et al., 2012), and siblings provide an interesting comparison beyond a healthy control recruited from the community. For example, the current sample represented a higher level of parental education than regional demographics and siblings performed higher than established norms on several indices. However, it is also important to note that cases with CHD did not uniformly perform lower than their siblings. This suggests that the population as a whole may not demonstrate overall cognitive impairments or weaknesses. Instead, it appears that the majority of adolescents and young adults with CHD (72% of our sample) demonstrate relative weaknesses compared to siblings. This variability may have clinical implications for screening, intervention, and future research.
Comparisons to established norms provide useful information about how a patient is performing relative to typically developing, same-aged peers across the country and are compelling for educational purposes, disability classification, and determining eligibility for special education services. However, local controls such as siblings are compelling for individual patients and their families; functioning at a cognitive level below their own expectations or those of family members is relevant for individual concerns and impressions of patients and parents. Some previous CHD research has also characterized children as falling below expectation, although still within the average range (Bellinger & Newburger, 2010); this is similar to findings in other pediatric populations at risk for neurocognitive problems, such as children who have had strokes (Everts et al., 2008; O’Keefe et al., 2014). Expanding comparisons to sibling controls may help elucidate subtle cognitive weaknesses associated with CHD. Future studies should therefore consider utilizing comparisons with both normative samples and local controls.
A limitation of the current study was a small sample size, which impacted our ability to detect significant effects. Given this reduction in power, effect sizes (Cohen’s d) may better elucidate areas of significant impairment. Results above should also be interpreted with caution, given that correction for multiple tests reduced significant effects. As noted in the Methods, there was some inconsistency in the recruitment of sibling controls, as testing the nearest aged sibling was not always possible. And although the sample represented a range of income and parental education levels, it was relatively racially and ethnically homogenous which may limit generalizability of findings. In addition, future studies should continue to examine different domains of functioning in this population, such as social competence, and performance-based measures of attention. Future studies should also examine performance-based measures beyond the Wechsler intelligence tests and their indices. Although the current study examined working memory and processing speed, future research and clinical work with this population should include more extensive neuropsychological assessment.
The current study also had several strengths. First, it focused on an emerging but under-studied age group of adolescents and young adults with CHD. Another strength was the study population, as TOF and d-TGA comprise a large proportion of children born with CHD and therefore present a clinically important group (Hoffman & Kaplan, 2002). In addition, this study examined cognitive domains beyond full scale, verbal, and performance IQ to include other indices from the WISC-IV and WAIS-IV, such as working memory and processing speed, as well as reports of attention. Researchers need to continue to expand the domains of function assessed in order to better understand cognitive strengths and weaknesses in this population, which will help for clinical screening and educational planning as well as elucidation of potential causal mechanisms.
There is a growing body of evidence for neurocognitive vulnerabilities in CHD especially for children with severe defects such as single ventricles (e.g., HLHS; Karsdorp et al., 2007). Findings from the current sample, as well as from similar studies (Bellinger et al., 2003; Bellinger et al., 2011), suggest that there are also cognitive vulnerabilities for patients with moderate biventricular CHD (such as TOF and d-TGA). Previous studies have also found that these populations are at risk for poor outcomes beyond cognition, including school performance, restricted quality of life, and poor psychosocial functioning (see Marino et al., 2012). However, attempts to ameliorate cognitive problems via changes in surgical care (deep hypothermia with total circulatory arrest versus continuous low-flow bypass; Bellinger et al., 2011) and medical care (e.g. tight glycemic control after cardiac surgery; Agus et al., 2012) have been only moderately to minimally successful. Children with moderate and severe CHD will continue to require surgery early in life, and the cognitive risks of cyanosis, surgery, and anesthesia will always be significant. While a portion of this patient population may fall below expectation, many of those children and adolescents are not eligible for school services. Should future studies elucidate neuropsychological deficits on a range of measures of attention, working memory, and processing speed, intervention with cognitive remediation may hold promise for a subgroup of patients who display the greatest deficits. A recent review of interventions for children with a broad range of neurological disorders, acquired brain injuries, and neurodevelopmental disorders found positive treatment effects across studies, including large effects for attention, working memory, and memory tasks, and small effects for academic achievement and behavior rating scales (Robinson, Kaizar, Catroppa, Godfrey, & Yeates, 2014). Future research should examine the potential efficacy of cognitive remediation programs for children with CHD, as these children continue to demonstrate differences in cognitive and attentional functioning as they age into adolescence and young adulthood.
Acknowledgments
This study was supported by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
After correcting for multiple comparisons with the Benjamini-Hochberg False Discovery Rate (statistical significance defined as p < 0.05), attentional outcomes retained significance while Wechsler outcomes did not. In addition, after running a Multivariate Analysis of Variance (MANOVA) on the cognitive outcomes, the multivariate F was not significant.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms and profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA adult forms and profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2003. [Google Scholar]
- Agus MS, Steil GM, Wypij D, Costello JM, Laussen PC, Langer M, Gaies MG. Tight glycemic control versus standard care after pediatric cardiac surgery. New England Journal of Medicine. 2012;367(13):1208–1219. doi: 10.1056/NEJMoa1206044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC, Wypij D, Rappaport LA, Jonas RA, Wernovsky G, Newburger JW. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. The Journal of Thoracic and Cardiovascular Surgery. 2003;126(5):1385–1396. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Newburger JW. Neuropsychological, psychosocial, and quality-of-life outcomes in children and adolescents with congenital heart disease. Progress in Pediatric Cardiology. 2010;29(2):87–92. [Google Scholar]
- Bellinger DC, Wypij D, Rivkin MJ, DeMaso DR, Robertson RL, Dunbar-Masterson C, Newburger JW. Adolescents With d-Transposition of the Great Arteries Corrected With the Arterial Switch Procedure Neuropsychological Assessment and Structural Brain Imaging. Circulation. 2011;124(12):1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkelhammer LD, Williamson AL, Sanford SD, Dirksen CL, Sharp WG, Margulies AS, Prengler RA. Neurocognitive sequelae of pediatric sickle cell disease: a review of the literature. Child Neuropsychology. 2007;13(2):120–131. doi: 10.1080/09297040600800956. [DOI] [PubMed] [Google Scholar]
- Calderon J, Bonnet D, Courtin C, Concordet S, Plumet MH, Angeard N. Executive function and theory of mind in school-aged children after neonatal corrective cardiac surgery for transposition of the great arteries. Developmental Medicine & Child Neurology. 2010;52(12):1139–1144. doi: 10.1111/j.1469-8749.2010.03735.x. [DOI] [PubMed] [Google Scholar]
- Calderon J, Jambaqué I, Bonnet D, Angeard N. Executive Functions Development in 5-to 7-Year-Old Children With Transposition of the Great Arteries: A Longitudinal Study. Developmental Neuropsychology. 2014;39(5):365–384. doi: 10.1080/87565641.2014.916709. [DOI] [PubMed] [Google Scholar]
- Campbell LK, Scaduto M, Sharp W, Dufton L, Van Slyke D, Whitlock JA, Compas B. A meta-analysis of the neurocognitive sequelae of treatment for childhood acute lymphocytic leukemia. Pediatric Blood & Cancer. 2007;49(1):65–73. doi: 10.1002/pbc.20860. [DOI] [PubMed] [Google Scholar]
- Desrocher M, Rovet J. Neurocognitive correlates of type 1 diabetes mellitus in childhood. Child Neuropsychology. 2004;10(1):36–52. doi: 10.1076/chin.10.1.36.26241. [DOI] [PubMed] [Google Scholar]
- Donders J, Taneja C. Neurobehavioral characteristics of children with Duchenne muscular dystrophy. Child Neuropsychology. 2009;15(3):295–304. doi: 10.1080/09297040802665777. [DOI] [PubMed] [Google Scholar]
- Everts R, Pavlovic J, Kaufmann F, Uhlenberg B, Seidel U, Nedeltchev K, Steinlin M. Cognitive functioning, behavior, and quality of life after stroke in childhood. Child Neuropsychology. 2008;14(4):323–338. doi: 10.1080/09297040701792383. [DOI] [PubMed] [Google Scholar]
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. Journal of the American College of Cardiology. 2002;39(12):1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- Karsdorp PA, Everaerd W, Kindt M, Mulder BJ. Psychological and cognitive functioning in children and adolescents with congenital heart disease: a meta-analysis. Journal of Pediatric Psychology. 2007;32(5):527–541. doi: 10.1093/jpepsy/jsl047. [DOI] [PubMed] [Google Scholar]
- King AA, Strouse JJ, Rodeghier MJ, Compas BE, Casella JF, McKinstry RC, DeBaun MR. Parent education and biologic factors influence on cognition in sickle cell anemia. American journal of hematology. 2014;89(2):162–167. doi: 10.1002/ajh.23604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, Mahle WT. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management a scientific statement from the American Heart Association. Circulation. 2012;126(9):1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- McCusker CG, Armstrong MP, Mullen M, Doherty NN, Casey FA. A sibling-controlled, prospective study of outcomes at home and school in children with severe congenital heart disease. Cardiology in the Young. 2013;23(04):507–516. doi: 10.1017/S1047951112001667. [DOI] [PubMed] [Google Scholar]
- Miatton M, De Wolf D, François K, Thiery E, Vingerhoets G. Intellectual, neuropsychological, and behavioral functioning in children with tetralogy of Fallot. The Journal of thoracic and cardiovascular surgery. 2007;133(2):449–455. doi: 10.1016/j.jtcvs.2006.10.006. [DOI] [PubMed] [Google Scholar]
- O’Keeffe F, Liégeois F, Eve M, Ganesan V, King J, Murphy T. Neuropsychological and neurobehavioral outcome following childhood arterial ischemic stroke: Attention deficits, emotional dysregulation, and executive dysfunction. Child Neuropsychology. 2014;20(5):557–582. doi: 10.1080/09297049.2013.832740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves CB, Palmer S, Gross AM, Simonian SJ, Taylor L, Willingham E, Mulhern RK. Brief report: sluggish cognitive tempo among pediatric survivors of acute lymphoblastic leukemia. Journal of Pediatric Psychology. 2007;32(9):1050–1054. doi: 10.1093/jpepsy/jsm063. [DOI] [PubMed] [Google Scholar]
- Robinson KE, Kaizar E, Catroppa C, Godfrey C, Yeates KO. Systematic Review and Meta-Analysis of Cognitive Interventions for Children With Central Nervous System Disorders and Neurodevelopmental Disorders. Journal of pediatric psychology. 2014;39(8):846–865. doi: 10.1093/jpepsy/jsu031. [DOI] [PubMed] [Google Scholar]
- Sable C, Foster E, Uzark K, Bjornsen K, Canobbio MM, Connolly HM, Williams RG. Best Practices in Managing Transition to Adulthood for Adolescents With Congenital Heart Disease: The Transition Process and Medical and Psychosocial Issues A Scientific Statement From the American Heart Association. Circulation. 2011;123(13):1454–1485. doi: 10.1161/CIR.0b013e3182107c56. [DOI] [PubMed] [Google Scholar]
- Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW, Wernovsky G. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. 2008;121(4):e759–e767. doi: 10.1542/peds.2007-1066. [DOI] [PubMed] [Google Scholar]
- Smith R, Wilkins M. Perinatally acquired HIV infection: Long-term neuropsychological consequences and challenges ahead. Child Neuropsychology. 2015;21(2):234–268. doi: 10.1080/09297049.2014.898744. [DOI] [PubMed] [Google Scholar]
- Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, Webb GD. ACC/AHA 2008 Guidelines for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease) Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and …. Journal of the American College of Cardiology. 2008;52(23):e143–e263. doi: 10.1016/j.jacc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale–Fourth Edition (WAIS–IV) San Antonio, TX: NCS Pearson; 2008. [Google Scholar]
- Wechsler D, Kaplan E, Fein D, Kramer J, Morris R, Delis D, Maerlender A. WISC-IV: Wechsler intelligence scale for children 2004 [Google Scholar]
- Wray J. Intellectual development of infants, children and adolescents with congenital heart disease. Developmental Science. 2006;9(4):368–378. doi: 10.1111/j.1467-7687.2006.00502.x. [DOI] [PubMed] [Google Scholar]


