The oversupply of nutrients to skeletal muscle produces metabolic by‐products that drive insulin resistance, predisposing people to diabetes, atherosclerosis and heart disease. Ceramides, reactive oxygen species, diacylglycerols, branched chain amino acids, acylcarnitines, citric acid cycle intermediates and other metabolites have all been implicated as antagonists of insulin action, but their relative importance is a source of contention. Here we advance the position that ceramides are both sufficient and necessary for obesity‐induced insulin resistance.
Ceramides antagonize insulin signalling by blocking activation of the anabolic enzyme Akt/PKB, inhibiting glucose uptake and impairing storage of nutrients as glycogen or triglyceride (Chaurasia & Summers, 2015) (Fig. 1). These sphingolipids also disrupt lipid metabolism, particularly in the liver, by inhibiting oxidation and stimulating fatty acid uptake (Chaurasia & Summers, 2015). Three lines of evidence support roles in insulin resistance in vivo. First, ceramide reduction interventions invariably improve insulin sensitivity in mice, rats and hamsters. Second, lipidomic profiling studies reveal strong relationships between tissue ceramides and insulin resistance in humans, non‐human primates and rodents. Third, glucocorticoids, inflammatory agonists and adiponectin modulate insulin sensitivity by positively or negatively altering ceramide synthesis or metabolism. Here we highlight a subset of prominent studies conducted in model systems and humans revealing roles for the sphingolipid in muscle insulin resistance and metabolic disorders.
Figure 1. Ceramide production and action in insulin‐responsive tissues .
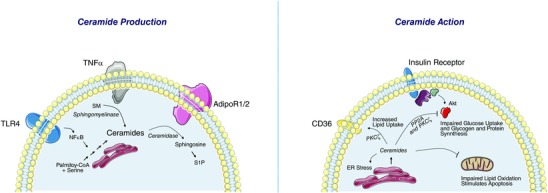
Left, ceramide biosynthesis requires saturated fatty acids and palmitate in a four‐step biosynthetic pathway. Intervening in this pathway invariably improves insulin sensitivity in rodents. Inflammatory modulators stimulate this biosynthesis and/or promote the conversion of sphingomyelin back into ceramides. Adiponectin further modulates ceramide levels by controlling its rates of degradation. Right, once ceramide levels rise above a critical threshold level, they antagonize insulin signalling, increase lipid uptake, and inhibit lipid oxidation. These contribute to the tissue dysfunction that underlies metabolic disorders.
Ceramides are requisite for insulin resistance in rodent and cell culture models of insulin resistance
Ceramide production occurs in the endoplasmic reticulum through a biosynthetic pathway that requires four, evolutionarily conserved enzymatic reactions. Ceramides (and its precursor dihydroceramides) then traffic to the Golgi apparatus, where they can be converted into complex sphingolipids (sphingomyelins, glucosylceramides, etc.), or to other compartments (e.g. lysosomes) where they are deacylated by ceramidases. Pharmaceutical or genetic interventions to slow ceramide synthesis or accelerate its degradation are invariably insulin‐sensitizing in rodents.
Pharmacological inhibition or genetic haploinsufficiency of serine palmitoyltransferase (Spt), the first enzyme in the biosynthetic pathway, ameliorates insulin resistance in various models of obesity, including obese mice, fructose‐fed hamsters, or leptin/leptin receptor‐deficient mice/rats (Holland et al. 2007; Yang et al. 2009; Ussher et al. 2010; Li et al. 2011; Dekker et al. 2013). In the absence of obesity, the insulin resistance caused by lard infusion or dexamethasone injection can be negated through these interventions (Holland et al. 2007).
Genetic ablation of ceramide synthase 6, which is involved in the third step in the biosynthetic pathway, prevents insulin resistance in mice fed a high fat diet (Turpin et al. 2014). Moreover, pharmacological inhibition of ceramide synthases prevents insulin resistance caused by lard infusion (Holland et al. 2007).
Pharmacological or genetic inhibition of the fourth enzyme, dihydroceramide desaturase‐1 (Des1), confers protection from diet‐ or dexamethasone‐induced insulin resistance (Holland et al. 2007; Bikman et al. 2012).
Overexpression of an inducible acid ceramidase transgene in adipose tissue or the liver leads to an acute reduction in ceramides and restoration of insulin sensitivity in mice fed a high fat diet (Xia et al. 2015).
Studies in isolated muscle or cultured myotubes reveal that the effects can be tissue‐autonomous. Exogenous ceramides antagonize insulin signalling or action in cultured myocytes or isolated fibres. Moreover, inhibition of ceramide synthesis (i.e. myriocin, fumonisin B1, or siRNA‐mediated knockdown of Spt subunits or Des‐1) or stimulation of ceramide degradation (e.g. acid ceramidase overexpression) negates lipid‐antagonism of insulin signalling in these systems (Chaurasia & Summers, 2015).
A provocative hypothesis that is gaining traction is that muscle ceramides may also be also delivered through the circulation. Infusion of ceramide‐enriched low density lipoproteins reduced whole‐body glucose owing to a reduction in glucose disposal in skeletal muscle (Boon et al. 2013). Depletion of ceramides from these lipoproteins rendered them ineffectual as insulin antagonists. These data may explain why tissue‐specific reduction of sphingolipid synthesis in adipose tissue or the liver reduces serum ceramides and markedly improves whole body and/or muscle insulin sensitivity (Turpin et al. 2014; Xia et al. 2015).
Tissue ceramide levels predict the severity of insulin resistance in man
The preponderance of lipid profiling studies in humans demonstrate relationships between ceramides and insulin resistance. For example, elevations in muscle ceramides were reported in individuals with general or abdominal obesity (Amati et al. 2011; Coen et al. 2013; de la Maza et al. 2015) in association with muscle insulin resistance (Adams et al. 2004; Coen et al. 2010; Amati et al. 2011). These cross‐sectional observations are not universal (Skovbro et al. 2008), and this discordance is the source of the existing controversy. Associations between liver ceramides and hepatic insulin resistance (Longato et al. 2012) and between adipose ceramides and fatty liver disease (Kolak et al. 2007) are also reported.
Insulin‐sensitizing treatments (metformin, pioglitazone, exercise, bariatric surgery, etc.) reduce ceramides. Both diet‐induced weight loss (Dube et al. 2011) and exercise training (Dube et al. 2008, 2011; Amati et al. 2011) lower muscle ceramide levels and enhance muscle insulin sensitivity. Profound weight loss caused by bariatric surgery also reduced muscle ceramides in conjunction with improved insulin sensitivity (Coen et al. 2015). Superimposing exercise after this surgery‐induced weight loss further reduced specific ceramide species (Coen et al. 2015). In contrast, levels of diacylglycerols in skeletal muscle were not altered with bariatric surgery‐induced weight loss (Coen et al. 2015), nor were they decreased by chronic exercise training (Amati et al. 2011). To the contrary, muscle diacylglcyerol levels were actually higher in endurance‐trained athletes who have markedly high insulin sensitivity in skeletal muscle (Amati et al. 2011).
Infusion of a lipid emulsion into humans induces insulin resistance. The intervention increases muscle diacylglycerols, but not ceramides (Itani, 2002; Nowotny et al. 2013; Szendroedi et al. 2014). This triglyceride emulsion is comprised primarily of unsaturated lipids, which could explain the lack of observed increases in ceramides. Indeed, rodent studies using lipid infusion cocktails reveal that saturated fats induce insulin resistance via ceramide‐dependent mechanisms, while unsaturated fats antagonize insulin action through a different mechanism (Holland et al. 2007). Highly trained athletes exposed to this unsaturated lipid emulsion also become insulin resistant, but their insulin resistance (and associated decreased glucose oxidation) is compensated by an increase in fatty acid oxidation, i.e. their greater metabolic flexibility (Dube et al. 2014). Thus we argue that this lipid infusion model induces a physiological insulin resistance that does not resemble pathobiology.
Circulating sphingolipids are also associated with insulin resistance and type 2 diabetes in humans. Plasma ceramides are higher in obese children (Lopez et al. 2013) and diabetic adults (Haus et al. 2009) and correlate with the severity of insulin resistance (Haus et al. 2009). Plasma ceramides also correlate with characteristics of the metabolic syndrome in non‐human primates fed a Western diet (Brozinick et al. 2013). Studies exploring the effects of insulin‐sensitizing pioglitazone treatment on plasma ceramides also demonstrate a correlation between the decrease in plasma ceramides and improved insulin sensitivity (Warshauer et al. 2015). Bergman and colleagues reported that an acute bout of exercise decreased plasma ceramide levels during recovery consistent with the insulin‐sensitizing effects of exercise (Bergman et al. 2015). Taken together, the majority of human studies demonstrate a consistent role for tissue and circulating ceramides in insulin resistance, obesity and type 2 diabetes.
Adiponectin elicits its broad spectrum of metabolic benefits by degrading ceramides
The Scherer group recently attributed the broad spectrum of anti‐diabetic and cardioprotective actions of adiponectin to the activation of a ceramidase (Holland et al. 2011). Adiponectin receptors contain a domain with high homology to ceramidase enzymes, and substitution for residues predicted to be important for ceramidase activity negates adiponectin action. Moreover, increasing circulating adiponectin levels in mice selectively depletes ceramides in various tissues, while genetic ablation of adiponectin receptors exacerbates sphingolipid‐dependent toxicity. These findings suggest that ceramide depletion could be a unifying mechanism to explain the pleiotropic actions of the adipokine. Subsequent studies revealed that FGF21, a member of the fibroblast growth factor superfamily, enhances insulin sensitivity through a mechanism involving adiponectin‐dependent reductions in tissue ceramide levels (Holland et al. 2013).
Conclusions
A panoply of interventional studies in rodents and profiling studies in humans reveal likely roles for sphingolipids in insulin resistance in skeletal muscle, as well as liver and adipose tissue. These data clearly reveal the promise of ceramide reduction therapies to treat metabolic disorders resulting from obesity and dyslipidaemia.
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief (250 word) comment. Comments may be submitted up to 6 weeks after publication of the article, at which point the discussion will close and the CrossTalk authors will be invited to submit a ‘Last Word’. Please email your comment, including a title and a declaration of interest, to jphysiol@physoc.org. Comments will be moderated and accepted comments will be published online only as ‘supporting information’ to the original debate articles once discussion has closed.
Additional information
Competing interests
Professor Summers is a shareholder with Centaurus Therapeutics, Inc.
Author contributions
Both authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work is supported by NIH grants R01AG20128 and R01DK078192 to B.G., an NHMRC research fellowship APP1112502 (to S.A.S.), and by the Victorian State Government OIS scheme.
Supporting information
Comments.
Last words by Summers and Goodpaster.
Last words by Petersen and Jurczak.
Acknowledgements
The authors thank the members of their laboratories for helpful discussion. The figure in this article was partially adapted from Servier Medical Art.
Biographies
Scott Summers directs the Translational Metabolic Health laboratory and Bioenergetics Core at the Baker IDI Heart and Diabetes Institute. His lab endeavours to elucidate the metabolic basis of diabetes and heart disease, with recent emphasis on the role of ceramides modulators of nutrient homeostasis. He is also the co‐founder and scientific advisor of Centaurus Therapeutics Inc., a USA‐based biotechnology company developing new therapeutics to combat the metabolic underpinnings of chronic diseases.
Bret Goodpaster conducts clinical translational ‘bench to bedside’ investigations of skeletal muscle and its role in human health, ageing and disease. He reported the Athlete's Paradox in 2001, demonstrating that endurance athletes have markedly high insulin sensitivity despite having high levels of muscle triglycerides. This helped move the field towards a better understanding of lipotoxicity within skeletal muscle, including the potential role for ceramides in insulin resistance.

Linked articles This article is part of a CrossTalk debate. Click the links to read the other articles in this debate: http://dx.doi.org/10.1113/JP271677, http://dx.doi.org/10.1113/JP272136, http://dx.doi.org/10.1113/JP272137.
References
- Adams JM 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC & Mandarino LJ (2004). Ceramide content is increased in skeletal muscle from obese insulin‐resistant humans. Diabetes 53, 25–31. [DOI] [PubMed] [Google Scholar]
- Amati F, Dube JJ, Alvarez‐Carnero E, Edreira MM, Chomentowski P, Coen PM, Switzer GE, Bickel PE, Stefanovic‐Racic M, Toledo FG & Goodpaster BH (2011). Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance‐trained athletes? Diabetes 60, 2588–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman BC, Brozinick JT, Strauss A, Bacon S, Kerege A, Bui HH, Sanders P, Siddall P, Kuo MS & Perreault L (2015). Serum sphingolipids: relationships to insulin sensitivity and changes with exercise in humans. Am J Physiol Endocrinol Metab 309, E398–E408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikman BT, Guan Y, Shui G, Siddique MM, Holland WL, Kim JY, Fabrias G, Wenk MR & Summers SA (2012). Fenretinide prevents lipid‐induced insulin resistance by blocking ceramide biosynthesis. J Biol Chem 287, 17426–17437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon J, Hoy AJ, Stark R, Brown RD, Meex RC, Henstridge DC, Schenk S, Meikle PJ, Horowitz JF, Kingwell BA, Bruce CR & Watt MJ (2013). Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes 62, 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozinick JT, Hawkins E, Hoang Bui H, Kuo MS, Tan B, Kievit P & Grove K (2013). Plasma sphingolipids are biomarkers of metabolic syndrome in non‐human primates maintained on a Western‐style diet. Int J Obes (Lond) 37, 1064–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia B & Summers SA (2015). Ceramides – lipotoxic inducers of metabolic disorders. Trends Endocrinol Metab 26, 538–550. [DOI] [PubMed] [Google Scholar]
- Coen PM, Dube JJ, Amati F, Stefanovic‐Racic M, Ferrell RE, Toledo FG & Goodpaster BH (2010). Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes 59, 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen PM, Hames KC, Leachman EM, DeLany JP, Ritov VB, Menshikova EV, Dube JJ, Stefanovic‐Racic M, Toledo FG & Goodpaster BH (2013). Reduced skeletal muscle oxidative capacity and elevated ceramide but not diacylglycerol content in severe obesity. Obesity (Silver Spring) 21, 2362–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen PM, Menshikova EV, Distefano G, Zheng D, Tanner CJ, Standley RA, Helbling NL, Dubis GS, Ritov VB, Xie H, Desimone ME, Smith SR, Stefanovic‐Racic M, Toledo FG, Houmard JA & Goodpaster BH (2015). Exercise and weight loss improve muscle mitochondrial respiration, lipid partitioning and insulin sensitivity following gastric bypass surgery. Diabetes 64, 3737–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker MJ, Baker C, Naples M, Samsoondar J, Zhang R, Qiu W, Sacco J & Adeli K (2013). Inhibition of sphingolipid synthesis improves dyslipidemia in the diet‐induced hamster model of insulin resistance: evidence for the role of sphingosine and sphinganine in hepatic VLDL‐apoB100 overproduction. Atherosclerosis 228, 98–109. [DOI] [PubMed] [Google Scholar]
- de la Maza MP, Rodriguez JM, Hirsch S, Leiva L, Barrera G & Bunout D (2015). Skeletal muscle ceramide species in men with abdominal obesity. J Nutr Health Aging 19, 389–396. [DOI] [PubMed] [Google Scholar]
- Dube JJ, Amati F, Stefanovic‐Racic M, Toledo FG, Sauers SE & Goodpaster BH (2008). Exercise‐induced alterations in intramyocellular lipids and insulin resistance: the athlete's paradox revisited. Am J Physiol Endocrinol Metab 294, E882–E888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube JJ, Amati F, Toledo FG, Stefanovic‐Racic M, Rossi A, Coen P & Goodpaster BH (2011). Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia 54, 1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube JJ, Coen PM, DiStefano G, Chacon AC, Helbling NL, Desimone ME, Stafanovic‐Racic M, Hames KC, Despines AA, Toledo FG & Goodpaster BH (2014). Effects of acute lipid overload on skeletal muscle insulin resistance, metabolic flexibility, and mitochondrial performance. Am J Physiol Endocrinol Metab 307, E1117–E1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA & Kirwan JP (2009). Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 58, 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, Bauer SM, Wade M, Singhal E, Cheng CC, Volk K, Kuo MS, Gordillo R, Kharitonenkov A & Scherer PE (2013). An FGF21‐adiponectin‐ceramide axis controls energy expenditure and insulin action in mice. Cell Metab 17, 790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ & Summers SA (2007). Inhibition of ceramide synthesis ameliorates glucocorticoid‐, saturated‐fat‐, and obesity‐induced insulin resistance. Cell Metab 5, 167–179. [DOI] [PubMed] [Google Scholar]
- Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA & Scherer PE (2011). Receptor‐mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 17, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itani SI, Rudderman NB, Schmieder F & Boden G (2002). Lipid‐induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C and IkB‐a. Diabetes 51, 2005–2011. [DOI] [PubMed] [Google Scholar]
- Kolak M, Westerbacka J, Velagapudi VR, Wagsater D, Yetukuri L, Makkonen J, Rissanen A, Hakkinen AM, Lindell M, Bergholm R, Hamsten A, Eriksson P, Fisher RM, Oresic M & Yki‐Jarvinen H (2007). Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes 56, 1960–1968. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang H, Liu J, Liang CP, Li Y, Li Y, Teitelman G, Beyer T, Bui HH, Peake DA, Zhang Y, Sanders PE, Kuo MS, Park TS, Cao G & Jiang XC (2011). Reducing plasma membrane sphingomyelin increases insulin sensitivity. Mol Cell Biol 31, 4205–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longato L, Ripp K, Setshedi M, Dostalek M, Akhlaghi F, Branda M, Wands JR & de la Monte SM (2012). Insulin resistance, ceramide accumulation, and endoplasmic reticulum stress in human chronic alcohol‐related liver disease. Oxid Med Cell Longev 2012, 479348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez X, Goldfine AB, Holland WL, Gordillo R & Scherer PE (2013). Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. J Pediatr Endocrinol Metab 26, 995–998. [DOI] [PubMed] [Google Scholar]
- Nowotny B, Zahiragic L, Krog D, Nowotny PJ, Herder C, Carstensen M, Yoshimura T, Szendroedi J, Phielix E, Schadewaldt P, Schloot NC, Shulman GI & Roden M (2013). Mechanisms underlying the onset of oral lipid‐induced skeletal muscle insulin resistance in humans. Diabetes 62, 2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovbro M, Baranowski M, Skov‐Jensen C, Flint A, Dela F, Gorski J & Helge JW (2008). Human skeletal muscle ceramide content is not a major factor in muscle insulin sensitivity. Diabetologia 51, 1253–1260. [DOI] [PubMed] [Google Scholar]
- Szendroedi J, Yoshimura T, Phielix E, Koliaki C, Marcucci M, Zhang D, Jelenik T, Muller J, Herder C, Nowotny P, Shulman GI & Roden M (2014). Role of diacylglycerol activation of PKCtheta in lipid‐induced muscle insulin resistance in humans. Proc Natl Acad Sci USA 111, 9597–9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, Wunderlich CM, Mauer J, Xu E, Hammerschmidt P, Bronneke HS, Trifunovic A, LoSasso G, Wunderlich FT, Kornfeld JW, Bluher M, Kronke M & Bruning JC (2014). Obesity‐induced CerS6‐dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab 20, 678–686. [DOI] [PubMed] [Google Scholar]
- Ussher JR, Koves TR, Cadete VJ, Zhang L, Jaswal JS, Swyrd SJ, Lopaschuk DG, Proctor SD, Keung W, Muoio DM & Lopaschuk GD (2010). Inhibition of de novo ceramide synthesis reverses diet‐induced insulin resistance and enhances whole‐body oxygen consumption. Diabetes 59, 2453–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshauer JT, Lopez X, Gordillo R, Hicks J, Holland WL, Anuwe E, Blankfard MB, Scherer PE & Lingvay I (2015). Effect of pioglitazone on plasma ceramides in adults with metabolic syndrome. Diabetes Metab Res Rev 31, 734–744. [DOI] [PubMed] [Google Scholar]
- Xia JY, Holland WL, Kusminski CM, Sun K, Sharma AX, Pearson MJ, Sifuentes AJ, McDonald JG, Gordillo R & Scherer PE (2015). Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab 22, 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA & Samad F (2009). Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab 297, E211–E224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comments.
Last words by Summers and Goodpaster.
Last words by Petersen and Jurczak.


