Abstract
Robinow syndrome is a rare congenital disorder characterized by mesomelic limb shortening, genital hypoplasia, and distinctive facial features. Recent reports have identified, in individuals with dominant Robinow syndrome, a specific type of variant characterized by being uniformly located in the penultimate exon of DVL1 and resulting in a −1 frameshift allele with a premature termination codon that escapes nonsense-mediated decay. Here, we studied a cohort of individuals who had been clinically diagnosed with Robinow syndrome but who had not received a molecular diagnosis from variant studies of DVL1, WNT5A, and ROR2. Because of the uniform location of frameshift variants in DVL1-mediated Robinow syndrome and the functional redundancy of DVL1, DVL2, and DVL3, we elected to pursue direct Sanger sequencing of the penultimate exon of DVL1 and its paralogs DVL2 and DVL3 to search for potential disease-associated variants. Remarkably, targeted sequencing identified five unrelated individuals harboring heterozygous, de novo frameshift variants in DVL3, including two splice acceptor mutations and three 1 bp deletions. Similar to the variants observed in DVL1-mediated Robinow syndrome, all variants in DVL3 result in a −1 frameshift, indicating that these highly specific alterations might be a common cause of dominant Robinow syndrome. Here, we review the current knowledge of these peculiar variant alleles in DVL1- and DVL3-mediated Robinow syndrome and further elucidate the phenotypic features present in subjects with DVL1 and DVL3 frameshift mutations.
Main Text
Robinow syndrome is a genetically heterogeneous disorder that can segregate as either an autosomal-dominant (DRS1 [MIM: 180700]) or an autosomal-recessive (RRS [MIM: 268310]) trait. DRS can result from hypomorphic mutations in WNT5A1, 2 (MIM: 164975), whereas RRS is caused by biallelic loss-of-function variants in ROR23, 4 (MIM: 602337). The original clinical description of Robinow syndrome included mesomelia, normal intellect, genital hypoplasia, and distinctive facial features comprising frontal bossing, prominent eyes, and a depressed nasal bridge, which are collectively referred to as a “fetal face.”5
Recently, studies on cohorts of individuals presenting with DRS have identified an intriguing mutational mechanism, uniformly located frameshift mutations within DVL1 (MIM: 601365), as a cause of DRS6, 7 (DRS2 [MIM: 616331]). The nature of these rare variants is remarkable: all cluster within the penultimate exon, consistently resulting in a −1 frameshift, and all share an identical premature termination codon. The mutant alleles are predicted to escape nonsense-mediated decay8 (NMD) and thus result in a protein product containing a mutant C terminus that is rich in proline, highly basic, and greater than 100 amino acids in length.6, 7 However, a large fraction of individuals with DRS remain without an etiologic molecular diagnosis; they do not have variants in genes known to be associated with Robinow syndrome. It is noteworthy that all of the current known genic causes of Robinow syndrome, including heterozygous hypomorphic alleles in WNT5A, biallelic loss-of-function variants in ROR2, and frameshifts in DVL1, occur within genes involved in non-canonical Wnt signaling. WNT5A acts as an extracellular soluble ligand that is recognized by ROR2, and together they employ the dishevelled (DVL) family of proteins to further transduce the non-canonical signal.9
The complexity of human development requires coordination of distinct cellular signaling pathways. Core pathways, including hedgehog, TGF-β, and Wnt signaling, have been studied extensively. Wnt-β-catenin signaling consists of highly conserved machinery that coordinates cell fate, proliferation, and differentiation during development and tissue homeostasis.10, 11 Non-canonical, β-catenin-independent Wnt signaling is involved in orchestrating cell migration and tissue morphogenesis, including convergent-extension movements in vertebrate gastrulation.12, 13 Emerging data suggest that the three human homologs of dishevelled, DVL1, DVL2 (MIM: 602151), and DVL3 (MIM: 601368), are core components in the correct routing and transmission of canonical and non-canonical Wnt signals, most likely by acting in large multiprotein complexes.14, 15 Collectively, Robinow syndrome appears to be a result of dysregulation in the WNT5A-ROR2-DVL pathway. Because a majority of individuals with Robinow syndrome do not receive a molecular diagnosis, we hypothesized that affected individuals have mutations residing in distinct genes within this branch of the non-canonical Wnt pathway, including the dishevelled human paralogs, DVL1, DVL2, and DVL3.
Because of the uniform location of disease-associated variants in DVL1-mediated Robinow syndrome and the knowledge that DVL1, DVL2, and DVL3 share 59%–67% amino acid homology,16, 17 we elected to perform direct Sanger sequencing of the penultimate and final exons of DVL1, DVL2, and DVL3 in a cohort of 34 individuals referred to Radboud University Medical Center for diagnostic testing. All individuals had received a clinical diagnosis of possible Robinow syndrome by a clinical geneticist. DNA was obtained from the subjects and unaffected family members after they provided written informed consent. This study was approved by the Radboud University Medical Center review board and by the institutional review board at Baylor College of Medicine (protocol no. H-29697). Individuals were not pre-selected for similarity of their traits or possible modes of inheritance. From this cohort, 24 individuals had been screened for variants in ROR2, but no candidate variants were identified. WNT5A had been tested in one individual. Dominant inheritance was suspected for 16 individuals, and recessive inheritance was suspected in one individual. The mode of inheritance was undetermined for the remaining individuals. One individual from this large cohort, 015902, was found to have a frameshift deletion within the final exon of DVL3.
To confirm and assess the contribution of dishevelled to the phenotype, we then Sanger sequenced the penultimate and final exons of DVL1, DVL2, and DVL3 from our in-house database of subjects (n = 17) with Robinow syndrome. These subjects were ascertained on the basis of clinical similarity to those with DVL1-mediated Robinow syndrome. Of the ten affected individuals, two were screened for variants in WNT5A, and two were screened for variants in ROR2. In concordance with the observations underlying DVL1-mediated Robinow syndrome,7 one affected individual had a frameshift in the penultimate exon of DVL1 (Figure 1). Remarkably, four additional individuals harbored variants in DVL3, including two frameshifts and two splice acceptor mutations affecting the penultimate or last exon (Figure 2). In total, one individual with a variant in DVL1 and five individuals with variants in DVL3 were identified from the above-described approach. All DVL1 and DVL3 variants identified from Sanger sequencing were annotated and analyzed with conceptual translation from Mutalyzer version 2.0.8.18
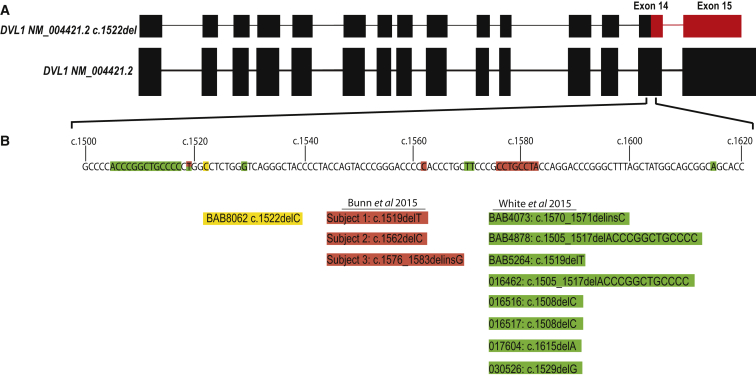
Figure 1.
Locations of All Currently Described Variants Resulting in DVL1-Mediated Robinow Syndrome
(A) Depiction of the resulting mutant transcript in BAB8062. Black represent regions identical to the wild-type DVL1, and red indicates the mutant coding region.
(B) Locations of all variants currently described in the literature. Orange indicates nucleotides deleted in individuals reported by Bunn et al.,6 green represents those from White et al.,7 and yellow indicates those in BAB8062, reported here. All the variants are located within a stretch of ∼110 nucleotides in the penultimate exon, and all are deletions that result in a −1 frameshift.
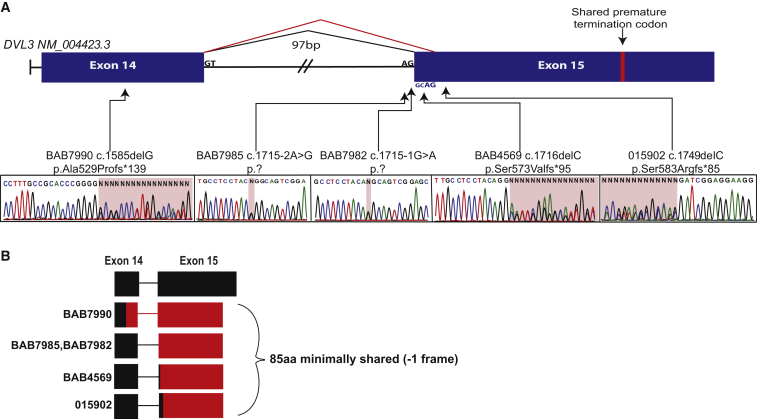
Figure 2.
Robinow-Associated DVL3 Variants
Overview of the variants identified in DVL3 in individuals with autosomal-dominant Robinow syndrome.
(A) Location of the identified variants within the final two exons, including three frameshifts in coding regions and two splice acceptor variants. The red bar within exon 15 represents the shared premature termination codon of all individuals within our cohort.
(B) Representation of the predicted mutant C-terminal tail from Robinow-syndrome-affected individuals in our cohort. Black regions represent amino acids encoded by exons of the wild-type DVL3. Red regions represent mutant amino acids predicted to result from translation in the −1 frame.
Individual BAB8062, who is of Turkish ancestry, harbored a de novo heterozygous 1 bp deletion in the penultimate exon of DVL1: c.1522delC (p.Pro508Leufs∗141) (GenBank: NM_004421.2). To exclude the presence of this variant in other family members, we used standard PCR amplification. DVL1 primer sequences consisted of 5′-GGGGAAGGGCAGGTAGGG-3′ (forward) and 5′-CAGTGAGTGGGGGCTTCG-3′ (reverse). Amplified PCR products were purified with ExoSAP-IT (Affymetrix) and sequenced with di-deoxynucleotide Sanger sequencing at the DNA Sequencing and Gene Vector Core at Baylor College of Medicine. The mutant allele was not observed in any family members, including the unaffected brother (BAB8065), unaffected mother (BAB8063), and unaffected father (BAB8064). In accordance with our previous observations for DVL1-mediated Robinow syndrome, this variant is located in the penultimate exon and is predicted to generate a premature termination codon within the last exon.
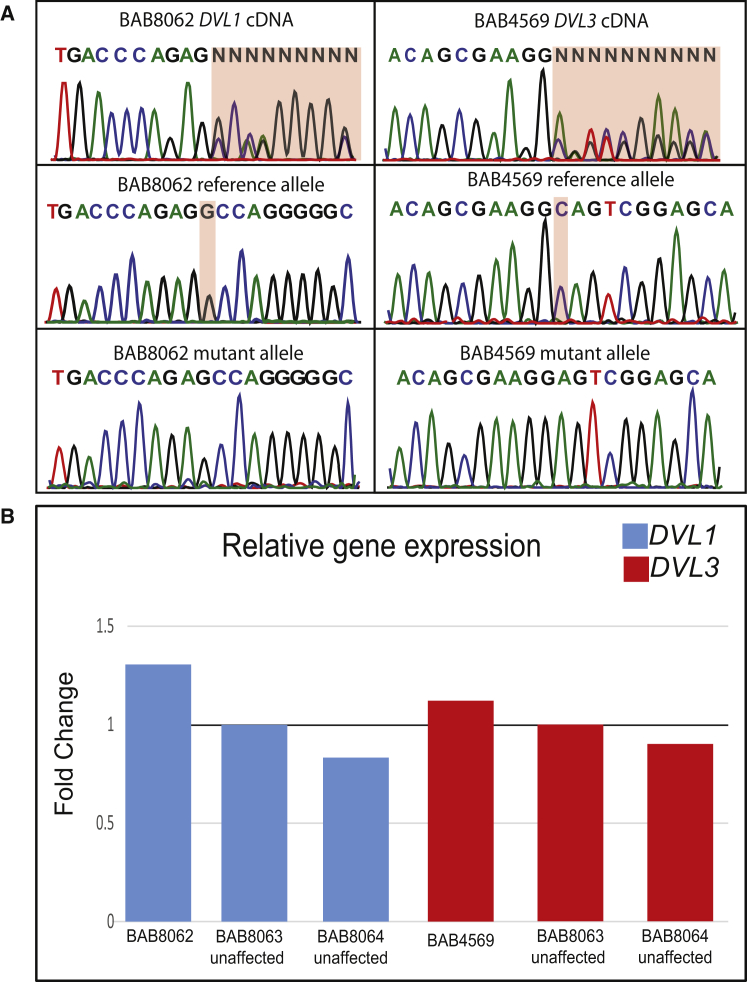
Figure 1 demonstrates the surprisingly uniform distribution of all previously reported DVL1 variants found in subjects from four continents, including the variant identified in the Turkish subject (BAB8062) reported herein. Conceptual translation of the mutant allele in BAB8062 predicts that the variant will escape NMD and generate a C-terminally truncated protein from the −1 reading frame. The mutant allele is predicted to encounter a stop codon after the creation of a highly basic, proline-rich mutant C-terminal tail consisting of 140 amino acids while truncating the final 23 C-terminal amino acids. The presence of both mutant and wild-type mRNA in the lymphoblastoid cells from BAB8062 supports the hypothesis that the DVL1 mutant transcript escapes NMD (Figure 3A). Furthermore, a TaqMan gene-expression assay of DVL1 confirmed that the mutant allele’s mRNA is expressed at levels similar to those in the unaffected parents (Figure 3B). These data are in agreement with previous reports6, 7 and further support the contention that DVL1 −1 frameshift mutations that escape NMD are an important molecular cause of DRS.
Figure 3.
mRNA Expression of DVL1 and DVL3 Transcripts in Robinow Syndrome
(A) Chromatograms display Sanger sequencing results of cloned cDNA transcripts in BAB8062 (DVL1) and BAB4569 (DVL3). Mutant and reference alleles were cloned and sequenced independently for confirming mutant mRNA expression.
(B) Depiction of the relative change in gene expression according to a TaqMan gene-expression assay for DVL1 and DVL3 with exon-spanning probes Hs00737028_m1 and Hs00610263_m1, respectively. For evaluating relative expression, the ΔΔct method19 was used with TBP as the endogenous control and unaffected individual BAB8063 as the control subject.
Five individuals contained frameshift variants in DVL3, including two splice acceptor mutations and three 1 bp deletions; all of the identified variants are predicted to result in a frameshift to the −1 reading frame and a premature termination codon in the last exon. All identified variants in DVL3 are represented in Figure 2 according to GenBank: NM_004423.3. Primers used to amplify the final two exons of DVL3 were 5′-ACCACGGTCTCTCTCATCCA-3′ (forward) and 5′-AAGACGGACGGATGGAGAGA-3′ (reverse). BAB7990 harbored a frameshift deletion (c.1585delG [p.Ala529Profs∗139]) located in the penultimate exon of DVL3, similar to the variants observed in DVL1. BAB4569 and 015902 contained 1 bp deletions located in the first several nucleotides of the final exon (c.1716delC [p.Ser573Valfs∗95] and c.1749delC [p.Ser583Argfs∗85], respectively). For confirming the resulting mutant reading frame, the frameshift deletions in BAB7990 and BAB4569 were manually cloned, and both alleles were sequenced independently. BAB4569 had previously undergone whole-exome sequencing as part of the Baylor-Hopkins Center for Mendelian Genomics initiative, but heterogeneous coverage, presumably due to poor capture resulting from high GC content of the region, prevented identification of this variant allele in our analytical informatics pipeline. Similar to those of DVL1, both mutant and wild-type transcripts of DVL3 were expressed (Figure 3A) in carrier lymphoblastoid cell lines (BAB4569). In addition, DVL3 did not show altered mRNA expression (Figure 3B). In aggregate, our data support the prediction that DVL3 mutants escape NMD. Additionally, individuals BAB7982 and BAB7985 contained single-nucleotide variants (SNVs) in the canonical splice acceptor site of the final exon (c.1715−1G>A and c.1715−2A>G, respectively). In silico prediction of the splice-altering SNVs by the Human Splicing Finder20 web tool predicts the activation of a cryptic splice acceptor site 2 bp downstream of the canonical splice acceptor site in both mutant transcripts. Activation of this cryptic splice site would result in the use of the −1 frame, which is predicted to create a mutant protein product similarly to all the observed DVL3 variants reported herein. None of the described variants are reported in the 1000 Genomes Project, NHLBI Exome Sequencing Project, dbSNP, the Atherosclerosis Risk in Communities database of ∼4,000 individuals, or our in-house database of >4,200 exomes, which include more than 600 individuals of Turkish ancestry. The identified Robinow-associated variants are not present in the Exome Aggregation Consortium (ExAC) Browser. However, analysis of the ExAC Browser indicated that three distinct frameshift indels are located in the 3′ coding region of DVL3 and are predicted to escape NMD. Those variants have not been confirmed by Sanger sequencing. Furthermore, none of the alleles in the ExAC Browser create a protein product identical to those identified in our study.
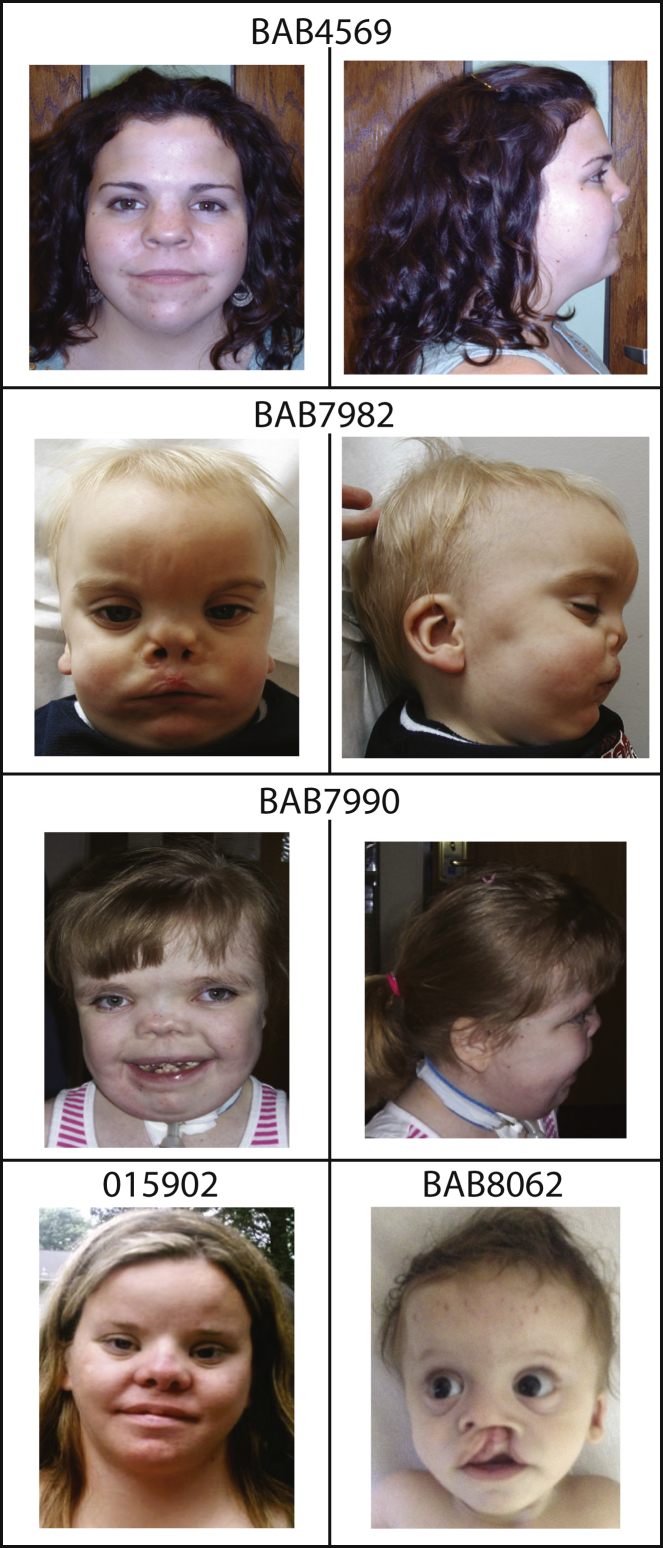
Detailed clinical information was available for four subjects with Robinow syndrome due to DVL3 frameshift alleles. The clinical phenotype of individuals harboring DVL3 mutations is similar to that of individuals with DVL1 mutations and concordant with earlier clinical descriptions of DRS (Table 1, Figure 4, Table S1; see Supplemental Data for detailed clinical descriptions).21 Macrocephaly was found in two of four individuals with DVL3 mutations and therefore cannot be used to distinguish between DVL1- and DVL3- mediated forms of Robinow syndrome. Curiously, two out of four individuals had telecanthus noted instead of true hypertelorism. Congenital heart defects and cleft lip and/or cleft palate, which can be a major clinical-management concern at birth, were observed in three out of four individuals. It is noteworthy that all individuals with DVL3 mutations have short stature, whereas individuals with DVL1 mutations have a final stature in the low-normal range.7 None of the individuals with DVL3 variants have been reported to have osteosclerosis, but no specific investigation was conducted to exclude it.
Table 1.
Clinical Features of Individuals with DVL3-Mediated Robinow Syndrome
|
Individualsa |
||||
|---|---|---|---|---|
| BAB7990 | BAB7982 | BAB4569 | 015902 | |
| Current age | 14 years | 12 years | 35 years | 38 years |
| Age at last examination | 10 years | 19 months | 33 years | 27 years |
| Genotype | c.1585delG | c.1715−1G>A | c.1716delC | c.1749delC |
| De novo | affected father | + | + | affected mother |
| Gender | female | male | female | female |
| Growth Features | ||||
| Height percentile | <3rd | <3rd | <3rd | <3rd |
| Head-circumference percentile | 50th–75th | 50th | >98th | >98th |
| Facial Features | ||||
| Frontal bossing | − | + | − | + |
| High forehead | − | + | − | − |
| Midface hypoplasia | + | + | + | + |
| Hypertelorism | + | + | − | − |
| Telecanthus | − | − | + | + |
| Upslanting palpebral fissures | + | + | − | + |
| Long eyelashes | + | + | − | − |
| Prominent eyes | + | + | + | − |
| Blue sclerae | + | ND | + | − |
| Epicanthal folds | − | ND | + | − |
| Anteverted nares | + | + | + | + |
| Wide, low nasal bridge | + | + | not low | + |
| Short nose | + | + | + | + |
| Long philtrum | + | − | + | + |
| Triangular mouth | − | − | + | − |
| Thin upper lip | − | − | − | − |
| Gingival hyperplasia | + | ND | + | + |
| Absent uvula | − | − | − | − |
| Cleft lip and/or palate | cleft palate | cleft lip and palate | cleft palate | − |
| Bilobed tongue | + and short | ND | + | + |
| Dental anomalies | misaligned | ND | missing | missing |
| Short neck | − | − | − | webbed |
| Micrognathia | + | + | + | − |
| Abnormally shaped or positioned ears | − | low set | − | − |
| Skeletal Features | ||||
| Mesomelia | + | + | + | + |
| Brachydactyly | + | + | + | + |
| Clinodactyly | + | + | + | + |
| Syndactyly | + | − | − | − |
| Camptodactyly | + | − | − | − |
| Broad thumb | + | − | − | − |
| Nail dysplasia | − | − | − | − |
| Bifid first and second phalanges | + | − | − | − |
| Hypoplastic phalanges | + | + (fifth) | − | − |
| Broad first toe | + | − | − | + |
| Scoliosis or kyphosis | + | − | + | − |
| Pectus anomaly | + | ND | + | − |
| Urogenital Features | ||||
| Sacral dimple | + | − | − | − |
| Cryptorchidism | NA | + | NA | NA |
| Hypospadias | NA | − | NA | NA |
| Micropenis | NA | buried | NA | NA |
| Agenesis of the labia minora | − | NA | − | − |
| Small clitoris | + | NA | − | − |
| Urinary reflux | + | − | − | − |
| Inguinal hernia | − | − | − | − |
| Heart defects | VSD, PA, HRH | PDA, PFO, TR | VSD | − |
| Umbilical hernia | − | − | − | + |
| Seizures | − | − | − | − |
| Hearing loss | − | − | + | − |
| Omphalocele | + | − | − | − |
| Hepatomegaly | − | − | − | − |
Abbreviations are as follows: +, present; −, absent; NA, not applicable; ND, no data; PA, pulmonary atresia; HRH, hypoplastic right heart; PDA, patent ductus arteriosus; PFO, patent foramen ovale; TR, tricuspid regurgitation; and VSD, ventricular septal defect.
Clinical data were not available for subject BAB7985.
Figure 4.
Facial Features of the Individuals with DRS in This Study
Individuals BAB4569, BAB7982, BAB7990, and 015902 have DVL3 variants, and BAB8062 has a de novo variant in DVL1.
Dishevelled (dsh), originally discovered in Drosophila melanogaster, is necessary for tissue patterning. Drosophila dsh has evolved three mammalian orthologs: the homologous genes DVL1, DVL2, and DVL3. Our data support the growing evidence that fly genes with greater than one human homolog are more likely to be associated with human Mendelian diseases than are fly genes with a single human homolog, indicating possible specialization during evolution.22 In mice, DVL1 and DVL3 co-localize within the developing neural tube, and all three DVL proteins have similar localization patterns.23 Whereas Dvl1−/− mice have a mild phenotype including social abnormalities, Dvl2−/− and Dvl3−/− mice independently demonstrate partial lethality and conotruncal defects. Double knockout of either Dvl1 and Dvl2 or Dvl2 and Dvl3 results in neural-tube defects, suggesting redundant roles between the DVL homologs.24 However, Dvl1−/−Dvl3−/− mice do not display neural-tube defects, indicating functional divergence between the DVL homologs;24, 25 one study has estimated that DVL2 contributes more than 95% of the total DVL pool in several mouse cell types.26
Our data strongly support previous studies reporting that specific −1 frameshift variants in the penultimate exon of DVL1 cause autosomal-dominant Robinow syndrome. The current literature available on DVL1-mediated Robinow syndrome indicates that these variants are highly specific and tightly cluster in the penultimate exon and lead to a mutant C-terminal peptide tail that is highly basic and proline rich (Figure 1). Our data from a subject of Turkish origin, with the same clinically recognizable specific diagnosis of Robinow syndrome, further support the notion that DVL1-mediated Robinow syndrome is a product of the mutant C-terminal tail, a direct result of −1 frameshift variants, and causes the mutant DVL1 to act in a dominant-negative or gain-of-function manner.
As a result of the high similarity among paralogous DVLs, DVL1, DVL2, and DVL3 in mice and humans have been proposed to have functional redundancy.24, 27, 28 All variants observed thus far to underly DVL-mediated Robinow syndrome result in a mutant C-terminal tail, the presence of which most likely alters protein folding, potentially affecting numerous C-terminal phosphorylation sites and obstructing C-terminal interactors. The three DVL homologs are capable of forming large dynamic multiprotein complexes observed as cellular puncta,14 and these self-associated forms of DVL are critical for its role in signaling the canonical pathway.15 The self-associated DVL puncta can be dispersed by the hyperphosphorylation of DVL. Casein kinase 1ε (CK1ε) is the major kinase responsible for Wnt-induced DVL3 phosphorylation.29 Interestingly, the hyperphosphorylation of DVL3 requires C-terminal serine clusters, the absence of which alters the DVL3 subcellular localization and DVL polymerization.29 It is possible that the C termini of DVLs have an ability to suppress canonical Wnt-β-catenin signaling and promote non-canonical Wnt transduction. Therefore, DVL-mediated Robinow syndrome might be a result of specific mutations that affect the phosphorylation of the C-terminal tails of DVL1 and DVL3. Further evidence of this is supported by the interaction between phosphorylated DVL3 and the non-canonical Wnt receptor ROR2.30 Stimulated by the hyperphosphorylation of DVL3 by CK1ε, ROR2 has been shown to interact with DVL3. Interestingly, the DVL3-ROR2 interaction is dependent on the DVL C terminus, which is lost in all observed DVL mutants we have identified thus far.31 Curiously, a DVL1 truncated allele was observed to increase canonical signaling only when co-expressed in an equal ratio with the wild-type DVL16 in vitro, perhaps indicating a dominant-negative interaction given the regulated stoichiometry between the homologs of DVL within the large signaling complex and its dynamic polymerization, which might be partially regulated by ROR2.30 Further work is necessary to elucidate the mechanism by which these specific mutations in DVL1 and DVL3 result in DRS and their interactions within the global Wnt signalosome.
We conclude that Sanger sequencing of the penultimate and final exons of DVL1 and DVL3 in individuals with a suspected diagnosis of autosomal-dominant Robinow syndrome is a prudent means for potentially establishing a molecular diagnosis. In addition to identifying truncating variants in DVL3 as a cause of Robinow syndrome, we have shown that these parallel the distinct −1 frameshift mutational mechanism elucidated in DVL1-mediated Robinow syndrome. Additionally we have shown that the phenotypic features of DVL1- and DVL3-mediated Robinow syndrome are largely concordant, but possible distinguishing features include head circumference and stature. Clinical investigations of subjects with Robinow syndrome are required for exploring the associated increased bone mineral density and the potential underlying Wnt-signaling differences related to bone mineralization.
Conflicts of Interest
J.R.L. holds stock ownership in 23andMe Inc. and Lasergen Inc., is a paid consultant for Regeneron Pharmaceuticals, and is a co-inventor on multiple United States and European patents related to molecular diagnostics. The Department of Molecular and Human Genetics at Baylor College of Medicine derives revenue from molecular genetic testing offered in the Baylor-Miraca Medical Genetics Laboratories (BMGL). J.R.L. is on the Scientific Advisory Board of the BMGL.
Acknowledgments
This work was supported in part by the Baylor Hopkins Center for Mendelian Genomics (U54HG006542), jointly funded by the National Human Genome Research Institute (NHGRI) and National Heart, Lung, and Blood Institute (NHLBI). J.J.W. is funded in part by the Smith-Magenis Syndrome Research Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHGRI, NHLBI, or NIH. The authors would like to thank the Exome Aggregation Consortium and the groups that provided exome variant data for comparison. A full list of contributing groups can be found at http://exac.broadinstitute.org/about.
Published: February 25, 2016
Footnotes
Supplemental Data include one table and a Supplemental Note and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2016.01.005.
Contributor Information
Han G. Brunner, Email: han.brunner@radboudumc.nl.
Claudia M.B. Carvalho, Email: cfonseca@bcm.edu.
Accession Numbers
The accession number for the whole-exome sequencing DNA sequences reported in this paper is dbGaP: phs000711, under the Baylor Hopkins Center for Mendelian Genomics study. Sample identifiers are SRS915722, SRS915721, and SRS915720. Additionally, the accession numbers for all variants identified in DVL1 and DVL3 are ClinVar: SCV000257455, SCV000257456, SCV000257457, SCV000257458, SCV000257459, and SCV000257460.
Web Resources
The URLs for data presented herein are as follows:
1000 Genomes, http://www.1000genomes.org
Atherosclerosis Risk in Communities Study, http://www2.cscc.unc.edu/aric
Baylor Miraca Genetics Laboratories, http://www.bcm.edu/geneticlabs/
Clinvar, http://www.ncbi.nlm.nih.gov/clinvar/
Exome Aggregation Consortium (ExAC) Browser, http://exac.broadinstitute.org
NHLBI Exome Sequencing Project (ESP) Exome Variant Server, http://www.evs.gs.washington.edu/EVS
OMIM, http://www.omim.org
Supplemental Data
References
- 1.Person A.D., Beiraghi S., Sieben C.M., Hermanson S., Neumann A.N., Robu M.E., Schleiffarth J.R., Billington C.J., Jr., van Bokhoven H., Hoogeboom J.M. WNT5A mutations in patients with autosomal dominant Robinow syndrome. Dev. Dyn. 2010;239:327–337. doi: 10.1002/dvdy.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roifman M., Marcelis C.L.M., Paton T., Marshall C., Silver R., Lohr J.L., Yntema H.G., Venselaar H., Kayserili H., van Bon B., FORGE Canada Consortium De novo WNT5A-associated autosomal dominant Robinow syndrome suggests specificity of genotype and phenotype. Clin. Genet. 2015;87:34–41. doi: 10.1111/cge.12401. [DOI] [PubMed] [Google Scholar]
- 3.Afzal A.R., Rajab A., Fenske C.D., Oldridge M., Elanko N., Ternes-Pereira E., Tüysüz B., Murday V.A., Patton M.A., Wilkie A.O., Jeffery S. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat. Genet. 2000;25:419–422. doi: 10.1038/78107. [DOI] [PubMed] [Google Scholar]
- 4.van Bokhoven H., Celli J., Kayserili H., van Beusekom E., Balci S., Brussel W., Skovby F., Kerr B., Percin E.F., Akarsu N., Brunner H.G. Mutation of the gene encoding the ROR2 tyrosine kinase causes autosomal recessive Robinow syndrome. Nat. Genet. 2000;25:423–426. doi: 10.1038/78113. [DOI] [PubMed] [Google Scholar]
- 5.Robinow M., Silverman F.N., Smith H.D. A newly recognized dwarfing syndrome. Am. J. Dis. Child. 1969;117:645–651. doi: 10.1001/archpedi.1969.02100030647005. [DOI] [PubMed] [Google Scholar]
- 6.Bunn K.J., Daniel P., Rösken H.S., O’Neill A.C., Cameron-Christie S.R., Morgan T., Brunner H.G., Lai A., Kunst H.P.M., Markie D.M., Robertson S.P. Mutations in DVL1 cause an osteosclerotic form of Robinow syndrome. Am. J. Hum. Genet. 2015;96:623–630. doi: 10.1016/j.ajhg.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White J., Mazzeu J.F., Hoischen A., Jhangiani S.N., Gambin T., Alcino M.C., Penney S., Saraiva J.M., Hove H., Skovby F., Baylor-Hopkins Center for Mendelian Genomics DVL1 frameshift mutations clustering in the penultimate exon cause autosomal-dominant Robinow syndrome. Am. J. Hum. Genet. 2015;96:612–622. doi: 10.1016/j.ajhg.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khajavi M., Inoue K., Lupski J.R. Nonsense-mediated mRNA decay modulates clinical outcome of genetic disease. Eur. J. Hum. Genet. 2006;14:1074–1081. doi: 10.1038/sj.ejhg.5201649. [DOI] [PubMed] [Google Scholar]
- 9.Oishi I., Suzuki H., Onishi N., Takada R., Kani S., Ohkawara B., Koshida I., Suzuki K., Yamada G., Schwabe G.C. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 10.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald B.T., Tamai K., He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamanaka H., Moriguchi T., Masuyama N., Kusakabe M., Hanafusa H., Takada R., Takada S., Nishida E. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sokol S. A role for Wnts in morpho-genesis and tissue polarity. Nat. Cell Biol. 2000;2:E124–E125. doi: 10.1038/35017136. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama N., Golebiewska U., Wang H.Y., Malbon C.C. Wnt-dependent assembly of supermolecular Dishevelled-3-based complexes. J. Cell Sci. 2010;123:3693–3702. doi: 10.1242/jcs.075275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwarz-Romond T., Fiedler M., Shibata N., Butler P.J.G., Kikuchi A., Higuchi Y., Bienz M. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat. Struct. Mol. Biol. 2007;14:484–492. doi: 10.1038/nsmb1247. [DOI] [PubMed] [Google Scholar]
- 16.Pizzuti A., Amati F., Calabrese G., Mari A., Colosimo A., Silani V., Giardino L., Ratti A., Penso D., Calzà L. cDNA characterization and chromosomal mapping of two human homologues of the Drosophila dishevelled polarity gene. Hum. Mol. Genet. 1996;5:953–958. doi: 10.1093/hmg/5.7.953. [DOI] [PubMed] [Google Scholar]
- 17.Semënov M.V., Snyder M. Human dishevelled genes constitute a DHR-containing multigene family. Genomics. 1997;42:302–310. doi: 10.1006/geno.1997.4713. [DOI] [PubMed] [Google Scholar]
- 18.Wildeman M., van Ophuizen E., den Dunnen J.T., Taschner P.E. Improving sequence variant descriptions in mutation databases and literature using the Mutalyzer sequence variation nomenclature checker. Hum. Mutat. 2008;29:6–13. doi: 10.1002/humu.20654. [DOI] [PubMed] [Google Scholar]
- 19.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Desmet F.O., Hamroun D., Lalande M., Collod-Béroud G., Claustres M., Béroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:e67. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazzeu J.F., Pardono E., Vianna-Morgante A.M., Richieri-Costa A., Ae Kim C., Brunoni D., Martelli L., de Andrade C.E., Colin G., Otto P.A. Clinical characterization of autosomal dominant and recessive variants of Robinow syndrome. Am. J. Med. Genet. A. 2007;143:320–325. doi: 10.1002/ajmg.a.31592. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto S., Jaiswal M., Charng W.L., Gambin T., Karaca E., Mirzaa G., Wiszniewski W., Sandoval H., Haelterman N.A., Xiong B. A drosophila genetic resource of mutants to study mechanisms underlying human genetic diseases. Cell. 2014;159:200–214. doi: 10.1016/j.cell.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J., Hamblet N.S., Mark S., Dickinson M.E., Brinkman B.C., Segil N., Fraser S.E., Chen P., Wallingford J.B., Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etheridge S.L., Ray S., Li S., Hamblet N.S., Lijam N., Tsang M., Greer J., Kardos N., Wang J., Sussman D.J. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008;4:e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lijam N., Paylor R., McDonald M.P., Crawley J.N., Deng C.-X., Herrup K., Stevens K.E., Maccaferri G., McBain C.J., Sussman D.J., Wynshaw-Boris A. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- 26.Lee Y.N., Gao Y., Wang H.Y. Differential mediation of the Wnt canonical pathway by mammalian Dishevelleds-1, -2, and -3. Cell. Signal. 2008;20:443–452. doi: 10.1016/j.cellsig.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamblet N.S., Lijam N., Ruiz-Lozano P., Wang J., Yang Y., Luo Z., Mei L., Chien K.R., Sussman D.J., Wynshaw-Boris A. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–5838. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- 28.Wang H.Y., Malbon C.C. Dishevelled C-terminus: prolyl and histidinyl motifs. Acta Physiol. (Oxf.) 2012;204:65–73. doi: 10.1111/j.1748-1716.2011.02291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernatík O., Šedová K., Schille C., Ganji R.S., Červenka I., Trantírek L., Schambony A., Zdráhal Z., Bryja V. Functional analysis of dishevelled-3 phosphorylation identifies distinct mechanisms driven by casein kinase 1ϵ and frizzled5. J. Biol. Chem. 2014;289:23520–23533. doi: 10.1074/jbc.M114.590638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishita M., Itsukushima S., Nomachi A., Endo M., Wang Z., Inaba D., Qiao S., Takada S., Kikuchi A., Minami Y. Ror2/Frizzled complex mediates Wnt5a-induced AP-1 activation by regulating Dishevelled polymerization. Mol. Cell. Biol. 2010;30:3610–3619. doi: 10.1128/MCB.00177-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witte F., Bernatik O., Kirchner K., Masek J., Mahl A., Krejci P., Mundlos S., Schambony A., Bryja V., Stricker S. Negative regulation of Wnt signaling mediated by CK1-phosphorylated Dishevelled via Ror2. FASEB J. 2010;24:2417–2426. doi: 10.1096/fj.09-150615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.