Abstract
BACKGROUND: Recent clinical trial results have suggested that programmed cell death ligand 1 (PD-L1) expression measured by immunohistochemistry may predict response to anti–programmed cell death 1 (PD-1) therapy. Results on the association between PD-L1 expression and survival among patients with advanced non–small cell lung cancer (NSCLC) treated with chemotherapy are inconsistent. MATERIAL AND METHODS: We evaluated the relationship between PD-L1 expression and overall survival (OS) among 204 patients with advanced NSCLC treated at Aarhus University Hospital, Aarhus, Denmark, from 2007 to 2012. PD-L1 expression was measured using a prototype immunohistochemistry assay with the anti–PD-L1 22C3 antibody (Merck). PD-L1 strong positivity and weak positivity were defined to be traceable to the clinical trial version of the assay. RESULTS: Twenty-five percent of patients had PD-L1 strong-positive tumors, and 50% had PD-L1 weak-positive tumors. No statistically significant association was found between PD-L1 expression and survival; adjusted hazard ratio of 1.34 (95% confidence interval, 0.88-2.03; median OS, 9.0 months) for the PD-L1 strong-positive group and 1.07 (0.74-1.55; median OS, 9.8 months) for the PD-L1 weak-positive group compared with the PD-L1–negative group (median OS, 7.5 months). No association was seen between PD-L1 expression and OS when PD-L1 expression levels were stratified by median or tertiles. CONCLUSIONS: In concordance with previous studies, we found PD-L1 measured by immunohistochemistry to be frequently expressed in patients with advanced NSCLC. However, PD-L1 expression is not a strong prognostic marker in patients with advanced NSCLC treated with chemotherapy.
Introduction
Non–small cell lung cancer (NSCLC) has a poor prognosis and is a leading cause of cancer death worldwide [1]. The majority of patients are diagnosed when their disease has reached an advanced stage, which leaves palliative treatment as the only option for therapy. Recent years have seen improvements in treatment options, particularly for subgroups of patients harboring specific drug-treatable genetic tumor alterations such as mutations in the epidermal growth factor receptor or the EML4-ALK translocation [2]. Therapy with monoclonal antibodies directed against programmed cell death 1 (PD-1) or its corresponding ligand, programmed cell death ligand 1 (PD-L1), has yielded impressive results in recent clinical trials and is a promising new treatment option for patients with advanced NSCLC [3], [4], [5]. PD-L1 expression measured by immunohistochemistry appears to be a predictive marker for most patients receiving this therapy [6]. Platinum-based doublet chemotherapy remains the cornerstone of palliative systemic treatment of NSCLC, but 50% to 60% of patients experience progressive disease while on this therapy [7], [8]. Investigations have explored the use of various biomarkers for response to chemotherapy, such as excision repair cross-complementation group 1, β-tubulin class III, expression of epidermal growth factor receptor, and genetic expression profiles. None of these have, as yet, reached clinical usage [9]. Continuous investigation in biomarkers to optimize patient selection is therefore needed.
The PD-1/PD-L1 interaction is one of the major pathways used by some tumors to escape immune surveillance. PD-1 is an immunoglobulin superfamily member that has been shown to negatively regulate antigen receptor signaling upon engagement of its ligands (PD-L1 and/or PD-L2) [10], [11], [12]. Studies using various PD-L1 detection antibodies and immunohistochemistry assays have found that a high level of PD-L1 expression in tumor cells correlates with poor prognosis in several human cancers, including breast, lung, renal, and melanoma [13], [14], [15], [16], [17], [18], [19], [20], [21]. Other studies have suggested a positive or no correlation between PD-L1 expression and survival among patients with cancer [22], [23]. Three recently published meta-analyses evaluated the prognostic significance of PD-L1 expression on tumor cells. Zhang et al. analyzed the results from 29 studies concerning PD-L1 expression in cancer originating from epithelium, including 6 studies in patients with NSCLC [24]. The authors concluded that positive PD-L1 expression (compared with PD-L1–negative expression) measured by immunohistochemistry was associated with shorter overall survival (OS) (hazard ratio [HR], 1.81; 95% confidence interval [CI], 1.33-2.46). However, a subgroup analysis discovered no association in six studies of patients with NSCLC (HR, 1.35; 95% CI, 0.81-2.23) [24]. Reviewing data from 1157 and 877 patients with NSCLC, in cancer stages I to IV from 6 and 5 published studies, respectively, the 2 other meta-analyses concluded that high PD-L1 expression is associated with worse survival (HR, 1.75; 95% CI, 1.40-2.20 and HR, 1.43; 95% CI, 1.24-1.63) [25], [26]. A recent study found PD-L1–positive expression, particularly PD-L1 strong-positive expression, to be associated with worse survival among Korean patients with early-stage NSCLC treated with surgery [27]. However, the negative prognostic value was not statistically significant after adjusting for postsurgical chemotherapy or radiotherapy. Currently, data are limited on whether PD-L1 expression is associated with survival among patients with advanced NSCLC treated with palliative chemotherapy.
The aim of the present study was to characterize PD-L1 expression levels measured by immunohistochemistry among patients with advanced NSCLC. A secondary aim was to investigate the association between PD-L1 expression and OS in patients with NSCLC receiving standard chemotherapy as their initial treatment.
Materials and Methods
Patients
Patients with advanced NSCLC who were treated at the Department of Oncology, Aarhus University Hospital, in Aarhus, Denmark, from 2007 to 2012 were eligible. The inclusion criteria were as follows: stage IIIa, IIIb, or IV NSCLC; first-line palliative treatment with platinum-based doublet chemotherapy; World Health Organization (WHO) performance status 0 to 2; and sufficient tumor tissue available for immunohistochemical analysis. Concomitant palliative radiotherapy was allowed. Clinicopathologic data were obtained by review of medical records. A computed tomographic scan of the thorax and upper abdomen with intravenous (IV) contrast was performed every 2 to 3 months during treatment and follow-up. This retrospective study was conducted in concordance with relevant local and regional guidelines and was approved by the regional ethics committee (reference number: 1-10-72-578-12) and the regional data protecting authority (reference number: 1-16-02-29-13).
PD-L1 Immunohistochemistry
PD-L1 expression was measured on the formalin-fixed, paraffin-embedded tumor samples acquired from diagnostic tumor biopsies. A prototype immunohistochemistry assay with the anti–PD-L1 22C3 antibody (Merck & Co., Inc., Kenilworth, NJ) was used. This assay uses the same primary antibody and ancillary reagents as the assay used in clinical trials of pembrolizumab [3] but uses a staining instrument that is no longer commercially available, uses an overnight incubation of the primary antibody, and includes both a low pH and proteinase K antigen retrieval. A brief overview of the prototype assay methodology is as follows: 4- to 5-μm sections of each sample were cut onto positively charged slides. Slides were then baked, deparaffinized in xylene, and rehydrated via an ethanol series. A low-pH target-retrieval solution (#S1700, Dako, Carpinteria, CA) was used for antigen retrieval, along with a 10-minute proteinase K incubation (#S3020, Dako) following a 1 × wash buffer rinse (#K8012 Envision FLEX + kit, Dako) as part of automated staining. Automated staining (QualTek TekMate 500, Newtown, PA) was followed by peroxidase blocking (#K8012, Dako). Slides were incubated overnight with the 22C3 antibody with a primary antibody diluent (#S0908 Primary Antibody Diluent, Dako). Secondary detection and chromogen deposition (#K8012-EnVision FLEX + Mouse, horseradish peroxidase–polymer reagent, and diaminobenzidine chromogen; Dako) were performed with 1 × wash buffer rinse between each incubation. Slides were counterstained with hematoxylin, washed in 1 × wash buffer, rinsed in distilled water, rehydrated through an ethanol series, and rinsed in xylene changes. Slides were then mounted with Cytoseal mounting media (#8312, Thermo Scientific, Waltham, MA) and coverslipped. Each staining run contained positive and negative controls along with a negative isotype-matched antibody control for each sample.
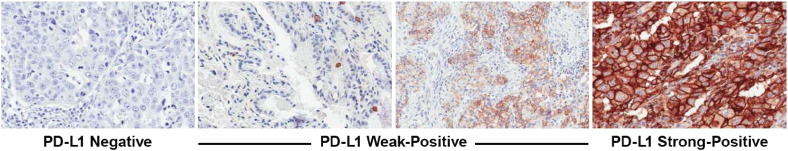
A board-certified pathologist evaluated all stained slides for PD-L1 membrane staining. Using the clinical trial assay to identify levels of PD-L1 expression that maximally predict clinical response to pembrolizumab, PD-L1 weak positive is defined as membranous PD-L1 expression in 1% to 49% of tumor cells, and PD-L1 strong positive is defined as expression in ≥ 50% of tumor cells [3]. Using data from 242 independent NSCLC tumors that were tested with both the prototype and clinical trial assays, the corresponding cutoffs using the prototype assay are 1% to 95% for weak positive and ≥ 96% for strong positive. Using these cutoffs, the “positive versus negative” and “strong positive versus not strong positive” concordances were maximized between the assays (89% concordance). Of note, the higher cutoffs identified for the prototype assay are likely the result of the darker stain produced with the prototype assay, which is likely due to the longer antibody incubation or the double antigen retrieval. Figure 1 shows examples of PD-L1 staining using the prototype assay.
Figure 1.
Examples of PD-L1 immunohistocehmistry staining in NSCLC tumor specimens. PD-L1 staining is evident from the presence of the chromogranin (brown); the counterstain is hematoxylin (blue). PD-L1 strong positivity and weak positivity were defined as traceable to the clinical trial version of the assay, which is currently used in the pembrolizumab clinical trials.
Statistical Analysis
The prevalence of PD-L1 strong and weak positivity was compared in subgroups based on age, sex, smoking status, histology, and cancer stages using the χ2 test. OS was defined as the time from the date of first chemotherapy cycle to the date of death or last follow-up. Kaplan-Meier methods, the log-rank test, and Cox proportional hazard models were used for survival analysis. For these analyses, the PD-L1–negative group was used as the reference group, and adjustment was made for age, sex, histology, smoking, and performance status. We also explored the association between PD-L1 expression and survival when PD-L1 expression levels were stratified by median or tertiles, or used as continuous variables.
Results
Patient Characteristics
The medical records of 449 patients with advanced NSCLC were reviewed, and 204 patients fulfilled the eligibility criteria. Clinicopathologic baseline characteristics are presented in Table 1. The median age at diagnosis was 65 years (range, 33-86 years), and 112 (55%) patients were women. Adenocarcinoma was the most frequent histologic subtype (72%). A total of 139 (68%) patients were current smokers, 63 (31%) patients were former or never smokers, and 2 (1%) patients had no information on smoking status. Most patients (88%) were diagnosed with stage IV NSCLC. The WHO performance status was 0 in 92 (45%) patients and 1 or 2 in 106 (52%) patients. Six (3%) patients had missing information on performance status at baseline. All patients received platinum-doublet chemotherapy as initial therapy, and 83% were treated with up to 4 cycles of carboplatin (area under the time-concentration curve 5, IV)/vinorelbine (60-80 mg/m2, oral). Four cycles of carboplatin/vinorelbine/bevacizumab (7.5 mg/kg, IV), followed by bevacizumab maintenance (if response or disease stabilization was obtained) until progression was administered to 15% of the patients. Two percent of patients were treated with other platinum-doublet regimens. Palliative radiotherapy given concomitantly with initial chemotherapy was administered to 32% of the patients. Median follow-up duration was 10.6 months (95% CI, 8.5-11.7).
Table 1.
Baseline Characteristics and Prevalence of Strong-Positive, Weak-Positive, and Negative PD-L1 Expression
| Subgroup | N | PD-L1 Expression |
P Value⁎ | ||
|---|---|---|---|---|---|
| Strong Positive |
Weak Positive |
Negative |
|||
| n (%) | n (%) | n (%) | |||
| Overall | 204 | 51 (25.0) | 102 (50.0) | 51 (25.0) | |
| Sex | |||||
| Women | 112 | 25 (22.3) | 57 (50.9) | 30 (26.8) | .58 |
| Men | 92 | 26 (28.3) | 45 (48.9) | 21 (22.8) | |
| Age | |||||
| < 65 years | 96 | 23 (24.0) | 46 (47.9) | 27 (28.1) | .65 |
| ≥ 65 years | 108 | 28 (25.9) | 56 (51.9) | 24 (22.2) | |
| Smoking status | |||||
| Current | 139 | 33 (23.7) | 74 (53.2) | 32 (23.0) | .49 |
| Former or never | 65 | 18 (26.7) | 28 (43.1) | 19 (29.2) | |
| Histology | |||||
| Squamous | 44 | 9 (20.5) | 26 (59.1) | 9 (20.5) | .40 |
| Nonsquamous | 160 | 42 (26.3) | 76 (47.5) | 42 (26.3) | |
| WHO performance status | |||||
| 0 | 92 | 23 (25.0) | 52 (56.5) | 17 (18.5) | .08 |
| 1/2 | 106 | 25 (23.6) | 47 (44.3) | 34 (32.1) | |
| Unknown | 6 | 3 (50.0) | 3 (50.0) | 0 (0.0) | |
Fisher exact test.
PD-L1 Expression
Representative examples of PD-L1–negative, weak-positive, and strong-positive immunohistochemical staining are shown in Figure 1. PD-L1 strong-positive staining and weak-positive staining were observed in 25% and 50% of patients, respectively. The remaining 25% of patients showed staining in < 1% of cells and were considered PD-L1 negative. There was no statistically significant association between PD-L1 expression and age, sex, histology, or smoking status in these patients with predominantly stage IV NSCLC (Table 1).
Survival Analysis
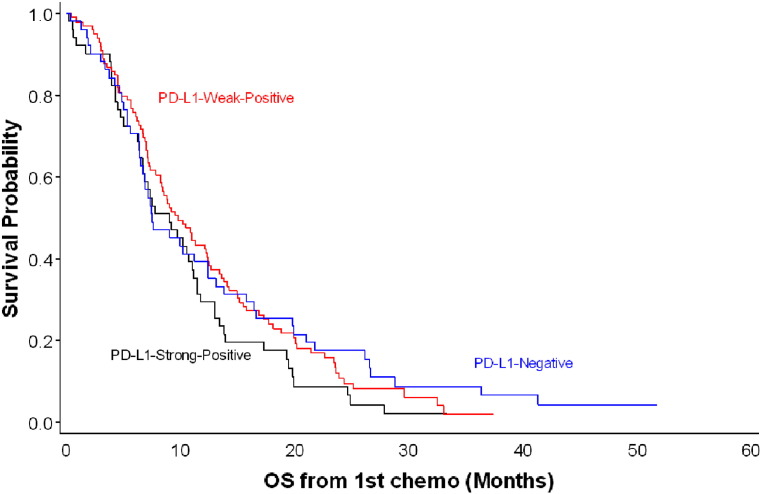
No statistically significant association was observed between PD-L1 expression and survival (log-rank test, P = 0.33; Figure 2). The crude HR was 1.31 (95% CI, 0.88-1.97; median OS, 9.0 months) for the PD-L1 strong-positive group and 1.04 (95% CI, 0.73-1.49; median OS, 9.8 months) for the PD-L1 weak-positive group when compared with the PD-L1–negative group (median OS, 7.5 months) (Table 2). The adjusted HR was 1.36 (95% CI, 0.90-2.06) for the PD-L1 strong-positive group and 1.09 (95% CI, 0.76-1.58) for the PD-L1 weak-positive group compared with the PD-L1–negative group. No association was observed between PD-L1 expression and OS when PD-L1 expression levels were stratified by the median or tertiles, or when the PD-L1 immunohistochemistry score was treated as a continuous variable. Similar results were observed when calculating OS from time when patients received the second line of systemic treatment (log-rank test, P = 0.38). In the subgroup analysis by histology, we observed a stronger association between PD-L1 expression and OS among patients with squamous cell histology than among patients with adenocarcinoma. However, the sample size in this subgroup was small (Table 2).
Figure 2.
Kaplan-Meier curves of overall survival since the start of chemotherapy, stratified by PD-L1 expression. n = 204; log-rank test, P = 0.33.
Table 2.
Cox Proportional Hazards Model for OS since the Start of Chemotherapy, Stratified by Histologic Subtype
| PD-L1 Expression | All Patients⁎ | Adenocarcinoma | Squamous Cell Carcinoma |
|---|---|---|---|
| PD-L1 negative | |||
| N/Deaths | 51/48 | 30/29 | 9/7 |
| Median OS (95% CI), months | 7.5 (6.4-12.4) | 10.7 (7.1-16.4) | 19.9 (3.3-NR) |
| PD-L1 weak positive | |||
| N/Deaths | 99/91 | 51/46 | 24/24 |
| Median OS (95% CI), months | 9.8 (8.2-12.3) | 12.1 (8.5-15.0) | 8.8 (6.1-12.2) |
| Crude HR (95% CI) | 1.04 (0.73-1.49) | 0.86 (0.54-1.36) | 2.76 (1.09-7.03) |
| Adjusted HR (95% CI)† | 1.09 (0.76-1.58) | 0.84 (0.53-1.34) | 2.36 (0.84-6.63) |
| PD-L1 strong positive | |||
| N/Deaths | 51/49 | 24/24 | 9/9 |
| Median OS (95% CI), months | 9.0 (6.4-11.1) | 10.9 (6.9-13.3) | 7.2 (0.9-10.2) |
| Crude HR (95% CI) | 1.31 (0.88-1.97) | 1.27 (0.74-2.19) | 4.88 (1.56-15.14) |
| Adjusted HR (95% CI)† | 1.36 (0.90-2.06) | 1.31 (0.76-2.27) | 3.87 (1.05-14.26) |
| PD-L1 strong or weak positive | |||
| N/Death | 150/140 | 75/70 | 33/33 |
| Median OS (95% CI), months | 9.3 (7.8-11.0) | 11.1 (9.2-13.3) | 8.4 (6.6-9.8) |
| Crude HR (95% CI) | 1.12 (0.80-1.57) | 0.96 (0.62-1.48) | 3.01 (1.21-7.51) |
| Adjusted HR (95% CI)† | 1.17 (0.83-1.66) | 0.96 (0.62-1.48) | 2.40 (0.87-6.60) |
NR, not reached.
The date of the first dose of chemotherapy was missing for 3 patients; therefore, only 201 patients are included in survival analysis.
HR: adjusting for age, sex, smoking status, histology, and performance status, with the PD-L1–negative group as the reference.
Discussion
Immunotherapy for lung cancer is evolving quickly and has the potential to revolutionize cancer treatment. Previous clinical trials have shown impressive overall response rates in heavily pretreated patients with NSCLC, and several anti–PD-1 and PD-L1 antibodies are currently under investigation [3], [4], [5]. Currently, the best predictive biomarker for response to immunotherapy is protein expression of PD-L1 in tumor samples measured by immunohistochemistry. Several immunohistochemistry assays that use different antibodies and various cutoff values are in current use. The role of PD-L1 expression as a prognostic biomarker in patients with advanced NSCLC treated with chemotherapy remains unknown, and further clinical studies are needed to evaluate the prognostic impact and prevalence of PD-L1 expression in this group of patients.
We retrospectively assessed PD-L1 expression and its correlation with clinicopathologic parameters and survival in a large cohort of patients with advanced NSCLC who were offered first-line treatment with standard platinum-doublet chemotherapy. PD-L1 expression, measured by use of a prototype immunohistochemistry assay, could be detected in 75% of the patients; 25% had strong staining intensity in the tumor sample. Unlike a previous analysis of PD-L1 expression using the same prototype immunohistochemistry assay and 22C3 antibody in Korean patients with predominately early-stage NSCLC [27], we detected no significant statistical correlation between clinicopathologic parameters and PD-L1 expression in our study. A reasonable explanation for this discrepancy could be that 88% of the patients had stage IV disease in our study. However, the prevalence of PD-L1 strong positivity (25%) was similar in both studies. Studies of surgically resected NSCLC tumors showed comparable expression rates of PD-L1 [14], [20], [23]. PD-L1 expression was not statistically associated with OS in our study.
In a study by Velcheti et al., the anti–PD-L1 5H1 antibody was used in the immunohistochemistry assay. PD-L1 positivity was defined as the automated quantitative analysis score of first signal detection beyond the signal intensity in formalin-fixed, paraffin-embedded samples from normal lung samples and negative controls. Separate cohorts of patients with stage I to IV NSCLC treated at hospitals in Greece or at Yale University were analyzed. Strong PD-L1 expression was detected in 25% (n = 75) of patients in the Greek cohort and in 36% (n = 56) in the Yale cohort. However, the outcome data differed from our results because positive PD-L1 expression was associated with better clinical outcomes (HR = 0.61 for the Greek cohort and HR = 0.63 for the Yale cohort) compared with negative PD-L1 expression [22]. In our study, we focused on PD-L1 expression in patients with stage IIIb to IV NSCLC initially treated with chemotherapy. In contrast, Velcheti et al. included all stages of patients with NSCLC and provided no information concerning the type of anticancer treatment. These differences in study design and patient selection criteria could explain the discrepancy between the reported survival results. A recently published study by D’Incecco et al. evaluated PD-1 and PD-L1 immunohistochemical expression in 125 patients with advanced NSCLC [28]. Immunohistochemistry was performed using the anti–PD-L1 ab58810 detection antibody, and samples staining in > 5% of tumor cells were considered to be PD-L1 positive. Sixty-eight (55%) patients were classified as PD-L1 positive. This prevalence is similar to what we observed in our study, where 75% of patients were either PD-L1 strong positive or PD-L1 weak positive. The lower cutoff limit used in our immunohistochemistry assay could explain the higher prevalence found in our study.
In concordance with our study, the authors of one meta-analysis found no association between PD-L1 expression and survival (HR, 1.35; 95% CI, 0.81-2.23). However, two other recently published meta-analyses found a high PD-L1 protein expression to be associated with poor survival. The analyses also indicated that the study results vary markedly primarily because of different immunohistochemistry antibodies, evaluation methods for PD-L1 positivity (cutoff % or H-score), and patient selection [24], [25], [26].
High PD-L1 expression on tumor cells indicates the presence of an antitumor immune response. On the other hand, the role of this antitumor immune response might not be such an important factor for survival outcomes in patients treated with chemotherapy because chemotherapy induces immunosuppression and suppresses the antitumor immune response. Ongoing clinical trials are investigating the efficacy of concomitant or sequential treatment with immunotherapy and conventional chemotherapy.
Different PD-L1 immunohistochemistry assays, using various detection antibodies and cutoffs, are currently being developed and evaluated clinically [[29], [30]]. Because of timing and logistic considerations, this study was conducted using a prototype immunohistochemistry assay. This prototype uses the same primary antibody (22C3) and the same ancillary reagents as the clinical trial assay used in clinical trials of pembrolizumab. However, the prototype assay also uses a staining platform that is no longer commercially available, and it employs a longer (overnight) antigen retrieval step than the clinical trial assay. The prototype assay produces a darker stain (likely due to the overnight antibody incubation and possibly due to the double antigen retrieval), which makes stained cells more noticeable but which also leads to higher estimates of the percentage of cells stained. Fortunately, appropriately adjusting the threshold for “strong positive” corrects for the higher estimates of cell staining. The darker staining sometimes also obscures cell morphology because of bleeding of the developer chromogen. In addition to defining PD-L1 strong-positive and weak-positive using prespecified criteria, we also performed sensitivity analyses for defining PD-L1 strong and weak-positive using other criteria in this study (i.e., by quintile, quartile, or tertile of the proportional score) or treating the IHC proportional score as a continuous variable. The results were consistent in all of the sensitivity analyses (data not shown).
Limitations associated with the present study relate to its retrospective design and its small sample size. On the other hand, the patients included in our study were all treated at the same institution and were uniformly followed with computed tomographic scans at regular intervals both during and after treatment. The completeness of all the clinical data leads to reliable survival analysis. The eligibility criteria were broad and reflect the patient cohorts seen in the everyday clinic given that, in Denmark, health care is almost entirely public and free of charge and that all patients are diagnosed and treated per established standards. The primary reason for exclusion of patients was lack of sufficient remaining tumor tissue for immunohistochemistry analysis.
The results of our study should pave the way for new prospective studies of the predictive and prognostic role of PD-L1 expression measured by immunohistochemistry in patients with NSCLC treated with chemotherapy; such studies may bring interesting results, especially concerning the efficacy of immunotherapy combined with chemotherapy. The immune system is very dynamic, and future studies should therefore optimally include repeat biopsies during treatment and at progression or consecutive analysis of circulating biomarkers.
Conclusion
The prevalence of PD-L1 expression detected in the present study is in concordance with previous studies. This indicates that a large proportion of patients with advanced NSCLC have positive PD-L1 expression, which makes them potentially responsive to immunotherapy. There was no association between PD-L1 expression and age, sex, smoking, or histology among these predominantly stage-IV patients with NSCLC. Based on a prototype IHC assay and cutoff, the results did not suggest that PD-L1 expression is a strong prognostic marker among patients with advanced NSCLC treated with chemotherapy.
Acknowledgements
The authors acknowledge the following individuals for their contribution to this study: Alise Reicin, Eric Rubin, Roger Dansey, Gregory M. Lubiniecki, Cong Chen, Jared Lunceford, Linda Sun, Jay Pearson, Mary Anne Rutkowski, Yuan Zhuang, Margaret Hodgson, Qing Shao, and Russell Weiner (Merck & Co., Inc., Kenilworth, NJ, USA) and QualTek Molecular Laboratories (Santa Barbara, CA, USA) for PD-L1 immunohistochemistry assay testing. Editorial assistance was provided by Melanie Leiby, PhD, of the ApotheCom Merck oncology team, Yardley, PA, USA, and funded by Merck & Co., Inc., Kenilworth, NJ, USA.
Footnotes
Disclosure: The study was supported by Merck & Co., Inc., Kenilworth, NJ, USA.
Wei Zhou, Marisa Dolled-Filhart, Kenneth Emancipator, and Dianna Wu are current or former employees of Merck & Co., Inc., Kenilworth, NJ, USA. Michael Busch-Sørensen is an employee of MSD Denmark, Ballerup, Denmark. Zhen Wang is an employee of MSD China, Shanghai, China. Marisa Dolled-Filhart, Kenneth Emancipator, and Dianna Wu are coauthors of a pending patent on PD-L1 immunohistochemistry and scoring (patent number WO 2014165422 A1). Marisa Dolled-Filhart and Wei Zhou own stock in Merck & Co., Inc. Kenneth Emancipator owns stock in Merck & Co., Inc., Bayer AG, and Johsnon & Johnson; Kenneth Emancipator’s spouse is an employee of and owns stock in Celgene. Steffen Filskov Sørensen, Jeanette Baehr Georgsen, Peter Meldgaard, and Henrik Hager report nothing to disclose.
Contributor Information
Steffen Filskov Sorensen, Email: stefsoer@rm.dk.
Wei Zhou, Email: wei.zhou2@merck.com.
Marisa Dolled-Filhart, Email: marisa.dolled-filhart@merck.com.
Jeanette Baehr Georgsen, Email: jeanette.georgsen@aarhus.rm.dk.
Zhen Wang, Email: zhen.wang4@merck.com.
Kenneth Emancipator, Email: kenneth.emancipator@merck.com.
Dianna Wu, Email: luna.immuno@gmail.com.
Michael Busch-Sørensen, Email: michael.buschsorensen@merck.com.
Peter Meldgaard, Email: petemeld@rm.dk.
Henrik Hager, Email: henrikhager@me.com.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. http://dx.doi.org/10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Chan BA, Hughes BG. Targeted therapy for non–small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res. 2015;4(1):36–54. doi: 10.3978/j.issn.2218-6751.2014.05.01. http://dx.doi.org/10.3978/j.issn.2218-6751.2014.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garon EB, Rizvi NA, Leighl N, Balmanoukian AS, Eder JP, Patnaik A. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. http://dx.doi.org/10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 4.Gettinger SN, Shepherd FA, Antonia SJ, Brahmer JR, Man Chow LQ, Juergens RA. First-line nivolumab (anti-PD-1; BMS-936558, ONO-4538) monotherapy in advanced NSCLC: safety, efficacy, and correlation of outcomes with PD-L1 status. J Clin Oncol. 2014;32(5 Suppl.) [abstract 8024] [Google Scholar]
- 5.Soria JC, Cruz C, Bahleda R, Delord J, Horn L, Herbst R. Presented at the European Cancer Congress (ECC) 2013; September 27–October 3, 2013; Amsterdam, The Netherlands. 2013. Clinical activity, safety and biomarkers of PD-L1 blockade in non-small cell lung cancer (NSCLC): additional analyses from a clinical study of the engineered antibody MPDL3280A (anti-PD-L1) [abstract 3408] [Google Scholar]
- 6.Mahoney KM, Atkins MB. Prognostic and predictive markers for the new immunotherapies. Oncology (Williston Park) 2014;28(Suppl. 3):39–48. [PubMed] [Google Scholar]
- 7.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J. Comparison of four chemotherapy regimens for advanced non–small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 8.Ardizzoni A, Boni L, Tiseo M, Fossella FV, Schiller JH, Paesmans M. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non–small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst. 2007;99(11):847–857. doi: 10.1093/jnci/djk196. [DOI] [PubMed] [Google Scholar]
- 9.Coate LE, John T, Tsao MS, Shepherd FA. Molecular predictive and prognostic markers in non–small-cell lung cancer. Lancet Oncol. 2009;10(10):1001–1010. doi: 10.1016/S1470-2045(09)70155-X. [DOI] [PubMed] [Google Scholar]
- 10.Philips GK, Atkins M. Therapeutic uses of anti–PD-1 and anti–PD-L1 antibodies. Int Immunol. 2015;27(1):39–46. doi: 10.1093/intimm/dxu095. [DOI] [PubMed] [Google Scholar]
- 11.Domingues D, Turner A, Silva MD, Marques DS, Mellidez JC, Wannesson L. Immunotherapy and lung cancer: current developments and novel targeted therapies. Immunotherapy. 2014;6(11):1221–1235. doi: 10.2217/imt.14.82. [DOI] [PubMed] [Google Scholar]
- 12.Casaluce F, Sgambato A, Sacco PC, Palazzolo G, Maione P, Rossi A. Emerging drugs targeting PD-1 and PD-L1: reality or hope? Expert Opin Emerg Drugs. 2014;19(4):557–569. doi: 10.1517/14728214.2014.964682. [DOI] [PubMed] [Google Scholar]
- 13.Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. The presence of programmed death 1 (PD-1)–positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139(3):667–676. doi: 10.1007/s10549-013-2581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Wang L, Li Y, Pan Y, Wang R, Hu H. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Oncol Targets Ther. 2014;7:567–573. doi: 10.2147/OTT.S59959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muenst S, Schaerli AR, Gao F, Daster S, Trella E, Droeser RA. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146(1):15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishii H, Azuma K, Kawahara A, Yamada K, Imamura Y, Tokito T. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol. 2015;10(3):426–430. doi: 10.1097/JTO.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 17.Massi D, Brusa D, Merelli B, Ciano M, Audrito V, Serra S. PD-L1 marks a subset of melanomas with a shorter overall survival and distinct genetic and morphological characteristics. Ann Oncol. 2014;25(12):2433–2442. doi: 10.1093/annonc/mdu452. [DOI] [PubMed] [Google Scholar]
- 18.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 19.Choueiri TK, Fay AP, Gray KP, Callea M, Ho TH, Albiges L. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann Oncol. 2014;25(11):2178–2184. doi: 10.1093/annonc/mdu445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28(3):682–688. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- 21.Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non–small cell lung cancer: a 5-year-follow-up study. Tumori. 2012;98(6):751–755. doi: 10.1177/030089161209800612. [DOI] [PubMed] [Google Scholar]
- 22.Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M. Programmed death ligand-1 expression in non–small cell lung cancer. Lab Invest. 2014;94(1):107–116. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang CY, Lin MW, Chang YL, Wu CT, Yang PC. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer. 2014;50(7):1361–1369. doi: 10.1016/j.ejca.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Kang S, Shen J, He J, Jiang L, Wang W. Prognostic significance of programmed cell death 1 (PD-1) or PD-1 ligand 1 (PD-L1) expression in epithelial-originated cancer: a meta-analysis. Medicine (Baltimore) 2015;94(6):e515. doi: 10.1097/MD.0000000000000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou ZJ, Zhan P, Song Y. PD-L1 over-expression and survival in patients with non–small cell lung cancer: a meta-analysis. Transl Lung Cancer Res. 2015;4(2):203–208. doi: 10.3978/j.issn.2218-6751.2015.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang A, Wang HY, Liu Y, Zhao MC, Zhang HJ, Lu ZY. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol. 2015 doi: 10.1016/j.ejso.2015.01.020. [Epub Jan 31] [DOI] [PubMed] [Google Scholar]
- 27.Sun J-M, Zhou W, Choi Y-L, Choi S-J, Kim SE, Wang Z. PD-L1 expression and survival in patients with non–small cell lung cancer (NSCLC) in Korea. J Clin Oncol. 2014;32(5 Suppl.) [abstract 8066] [Google Scholar]
- 28.Garon EB, Leighl NB, Rizvi NA, Blumenschein GR, Balmanoukian AS, Eder JP. Safety and clinical activity of MK-3475 in previously treated patients (pts) with non–small cell lung cancer (NSCLC) J Clin Oncol. 2014;32(5 Suppl.) [abstract 8020] [Google Scholar]
- 29.D'Incecco A, Andreozzi M, Ludovini V, Rossi E, Capodanno A, Landi L. PD-1 and PD-L1 expression in molecularly selected non–small-cell lung cancer patients. Br J Cancer. 2015;112(1):95–102. doi: 10.1038/bjc.2014.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14(4):847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]