Abstract
This systematic review and meta-analysis evaluated anti–programmed cell death (PD)-1 immunotherapy (nivolumab or pembrolizumab) for overall efficacy, safety, and effective dose relative to standard chemotherapy or other conventional drugs in the treatment of malignant tumors. We searched the following databases, PubMed, Medline, Embase, Cochrane, Wangfang Data, Weipu, and China National Knowledge Infrastructure, and the reference lists of the selected articles for randomized controlled trials (RCTs) of anti–PD-1 therapies in humans. The outcome measures were overall survival, treatment response, and adverse events. Only four randomized controlled trials met our inclusion criteria. Three of these evaluated responses to nivolumab, whereas one tested pembrolizumab. The result of our analysis suggested that nivolumab may improve the overall response rate in treating melanoma relative to chemotherapy and has few associated adverse events. Similarly, in metastatic melanoma patients, nivolumab had a significant advantage over dacarbazine in terms of 1-year survival, progression-free survival, and objective response rate. Regarding dose levels of nivolumab for patients with metastatic renal cell carcinoma, the outcomes in response to 2 and 10 mg/kg were similar, but both had significant advantages over 0.3 mg/kg. In addition, pembrolizumab showed similar outcomes in response to 2- and 10-mg/kg treatment. Anti–PD-1 immunotherapy appears to be safe and effective for patients with melanoma or metastatic renal cell carcinoma. Our meta-analysis is limited, but additional clinical trials are warranted to verify this preliminary evidence of positive outcomes and before anti–PD-1 therapy can be recommended for routine clinical use.
Introduction
Tumor malignancy remains the leading cause of death worldwide. Based on the World Cancer Report of 2014 published by the World Health Organization [1], in 2012, there were approximately 14 million new cases and 8.2 million cancer-related deaths. Moreover, the burden of cancer was predicted to increase by 70% over the next 2 decades [1]. Annual cancer cases were expected to rise from 14 million in 2012 to 22 million within the next 2 decades.
Most patients with malignant disease that is advanced cannot be cured by standard cancer therapies such as surgery, radiation, or chemotherapy, and therefore additional options with few adverse effects are being investigated. Cancer cells often acquire unlimited replication potential and insensitivity to antigrowth signals. Other characteristics include resisting cell death, sustained angiogenesis, metastasis, avoiding immune detection, deregulating cellular energetics, genome instability, and acquiring genetic mutations. Scientists have focused on the signaling pathways that directly or indirectly underlie and regulate these characteristics. Successful treatment of cancers by targeting specific signaling pathways has been limited by factors such as drug resistance and tumor reoccurrence.
Recently, the focus of research has shifted toward the immune system, which has important roles in both tumor progression and its elimination [2]. Consequently, immunotherapy has become a therapeutic option in many cancer treatment regimens [3] that promises to increase clinical efficacy for these patients [4]. Among the many immune targets, programmed cell death-1 (PD-1) has shown promising results in the treatment of melanoma and some other malignant tumors.
The PD-1 immunoinhibitory receptor is a type-1 transmembrane protein that belongs to the cluster of differentiation 28 (CD28) family and is usually expressed by activated immune cells like CD4 + and CD8 + T cells. It mediates the immune response through immunosuppression. In the context of tumors, it constitutes an adaptive immune response, whereby dendritic cells detect the antigen on tumor cells and eventually present the tumor antigen to T and B cells. Subsequently, the T lymphocytes are activated when the T cell receptor binds to the tumor antigen along with MHC proteins and other co-stimulatory/inhibitory molecules and undergoes clonal expansion. These T lymphocytes then migrate to the tumor sites and generate a tumor suppressive response. Therefore, these co-stimulatory and co-inhibitory molecules act as checkpoints to ensure the appropriate response against foreign tumor antigens [5].
The PD-1 receptor has two ligands: PD-L1 and PD-L2. In the tumor microenvironment, when PD-L1 that is expressed on tumor cells binds to PD-1 on T cells, this results in recruitment of SHP-2 phosphatase and inactivation of the P13K pathway. In addition, production and secretion of cytotoxic mediators are inhibited, and thus finally, the T cell effector response that is required for killing tumor cells is attenuated. Blocking this pathway was found to enhance the antitumor immune response in cancer [6].
Anti–PD-1 antibodies can block the PD-1 pathway and prevent suppression of the antitumor response [7], [8], [9]. The most common antibodies presently used to block PD-1 are nivolumab and pembrolizumab. Recently, the focus of anti–PD-1 therapy has been in melanoma, but its therapeutic efficacy has also been tried in other cancers such as renal cell carcinomas.
Although research into anti–PD-1 therapy is in the early stages and few clinical trials have been undertaken, we have conducted a systematic evaluation of this therapy to gauge its overall effectiveness. We also seek to identify any additional parameters that may be included in future clinical trials. Therefore, the present study is a systematic review and meta-analysis undertaken to evaluate the positive benefit and effective dose of anti–PD-1 therapy for the treatment of malignant tumors. Specifically, we investigated the overall survival (OS) and effective rates, effective dose, associated adverse events, and cancer recurrence rates.
Material and Methods
Identification of Studies
We searched the following databases electronically for all the anti–PD-1 therapy-related studies, published or unpublished, ongoing or pending, from January 1980 to December 2014: the Cochrane central register of controlled trials in the Cochrane library, PubMed, Medline, Embase, Ovid, National Institutes of Health, Chinese Biomedical Literature Database, China National Knowledge Infrastructure, Chinese Medical Current Contents, Wangfang Data, and Weipu. In addition, to identify ongoing studies, we searched the following clinical trial registries: International Standard Randomized Controlled Trial Number, ClinicalTrials.gov, Chinese Clinical Trial Register (www.chictr.org), WHO International Clinical Trial Registration Platform search portal (www.who.int/trialsearch), and Australian New Zealand Clinical Trials Registry (www.anzctr.org.au/). Moreover, we found information concerning ongoing studies through two annual meetings of the European Society of Medical Oncology.
Study Selection Criteria
Studies selected for the meta-analysis were all randomized controlled trials (RCTs) involving patients with confirmed malignant tumors, regardless of origin, tumor stage, age, or gender. All the studies compared anti–PD-1 treatment with eligible control treatment regimens. The compared, eligible control could be a placebo or no intervention, conventional therapies (such as surgery, chemotherapy, or radiotherapy), or other complementary or alternative medicine. In addition, the studies contained survival rate data for 1, 2, 3, or 5 years. Finally, the studies contained data for complete response, partial response, stable disease, and progressive disease.
The studies were excluded if they were 1) non-RCTs; 2) animal studies or in vitro studies; 3) without any information about the dose of PD-1 antibody or the method of treatment; or 4) not reporting either of the outcomes mentioned above.
Data Collection and Analysis
Two authors were independently assigned to peruse the relevant articles found from the search of the databases and other sources, and to identify trials that conformed to the inclusion criteria. First, they read the title and abstract to screen out irrelevant trials or duplicate studies. Next, they reviewed the full-text articles to extract the relevant information based on the inclusion criteria. Cases of conflicting opinion were resolved through consultation with the third author and discussion.
A data collection form was used to summarize the characteristics and information from each RCT. The data fell into the following categories: General information included publishing status, language, authors, title, and date of publication. Information concerning methods consisted of end points, intervention model, masking, and the primary purpose of the study. Participant data included the total number and the number in each comparison group, baseline characteristics, age, gender, inclusion and exclusion criteria, and the study setting. Intervention data consisted of the drug preparation, dose, regimen, and follow-up schedule. Outcomes included primary, secondary, and other outcomes and adverse events associated with treatment. Finally, information gathered from each study included the data analyzed and the statistical methods used for data analysis.
Assessment of Risk Bias
We assessed the risk of bias in the included studies using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions [10].
Data Synthesis
We performed the meta-analysis using Review Manager Version 5.2 software [10]. Summary measures of efficacy and response rate and relative risk (RR) for dichotomous variables, with 95% confidence intervals (95% CIs), were calculated using a fixed-effects inverse variance model.
Results
Based on the search criteria, we initially identified 3996 studies from our database search (Figure 1). In addition, we found one other study through an annual meeting of the European Society of Medical Oncology. Among these, 3785 studies were excluded because they did not meet our selection criteria. Of the remaining 212 studies, only 4 passed the inclusion criteria, and the rest were excluded because of repetitive or insufficient information. After reading the full texts of these four studies, we found that all of them met our inclusion criteria and hence were included for further analysis.
Figure 1.
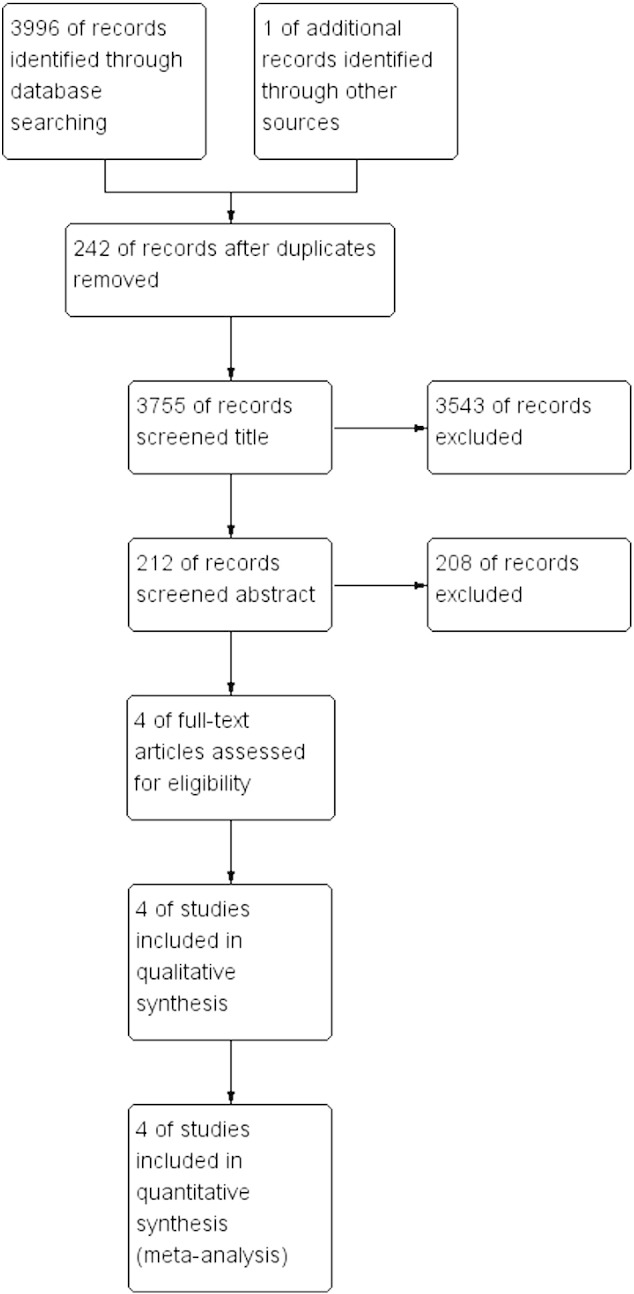
Flowchart representing the systematic identification of studies.
All of the 4 included studies evaluated the effectiveness of anti–PD-1 therapy, with data from a combined total of 1294 patients. Among the four studies, three [11], [12], [13] contained data from melanoma patients, and one study concerned metastatic renal cell carcinoma [14].
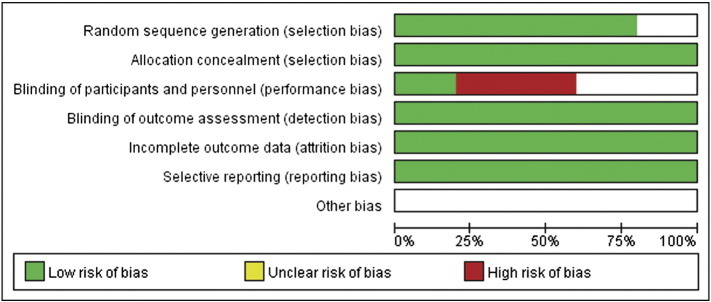
We assessed the quality of each study (i.e., risk of bias) independently based on the RCT quality evaluation standards of the Cochrane review manual (Figure 2). Information regarding random sequence generation, allocation concealment, blinding method, incomplete outcome data, and selective reporting was gathered from each study. If there was information for all the parameters, then the study was assigned as low bias. If there was no information at all, the study was described as high bias. The risk of bias in studies with partial or unclear information was defined as unclear. We could only obtain the information about the method of allocation from all the studies, and they were found to be randomized. Only one study had information regarding blinding [14].
Figure 2.
Assessment of risk bias.
Comparison of Anti–PD-1 Interventions
Nivolumab cf. chemotherapy
Overall response rate
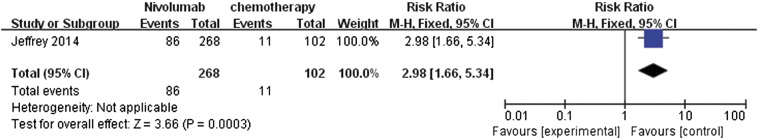
Among the four RCTs, only one study [12] contained data for the overall response rate after comparing nivolumab treatment relative to chemotherapy. The data were reported as dichotomous. After meta-analysis, it was represented as a forest plot (Figure 3). The overall response rate was statistically significant (RR 2.98, 95% CI 1.66 to 5.43, P = .0003) in favor of nivolumab. It was suggested that patients were more likely to respond to anti–PD-1 treatment and that nivolumab can improve the overall response rate.
Figure 3.
Forest plot depicting the overall response rate of nivolumab compared with chemotherapy.
Adverse events
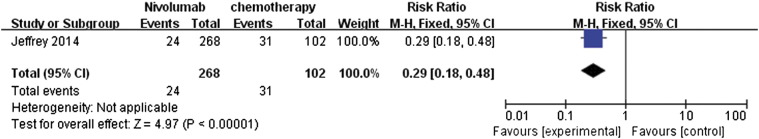
The same study by Weber et al. (2014) also provided data comparing adverse events between nivolumab and chemotherapy (Figure 4) [12]. The meta-analysis of drug-related adverse events showed that nivolumab had fewer associated adverse events. The RR ratio was 0.29 (95% CI 0.18 to 0.48) and was highly significant (P < .000001).
Figure 4.
Forest plot depicting the comparison of adverse events associated with nivolumab and chemotherapy.
Nivolumab cf. dacarbazine
One year-OS, progression-free survival (PFS), and objective response rate
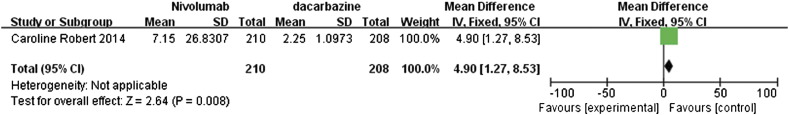
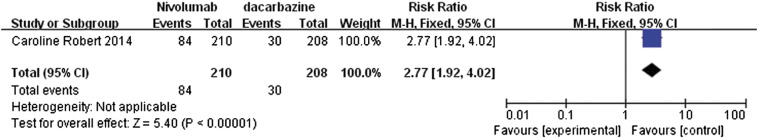
The study by Caroline et al. [13] compared the effects of nivolumab and dacarbazine in the treatment of metastatic melanoma patients without BRAF (B-Raf proto-oncogene, serine/threonine kinase) mutation. The data retrieved from this study were pooled and presented in forest plots for 1-year OS, median PFS, and objective response rate (Figure 5, Figure 6, Figure 7).
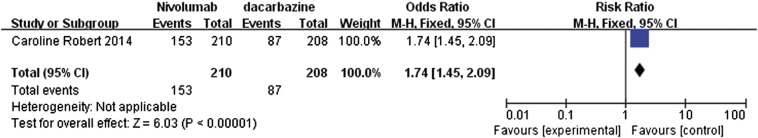
Figure 5.
Forest plot depicting the 1-year OS rates of nivolumab and dacarbazine.
Figure 6.
Forest plot depicting the PFS rates associated with nivolumab and dacarbazine.
Figure 7.
Forest plot depicting the objective response rates associated with nivolumab and dacarbazine.
The 1-year rate for OS with RR value of 1.74 (95% CI 1.45 to 2.09) suggested that nivolumab had a clear significant advantage over dacarbazine (P < .00001; Figure 5). Similarly, the median PFS also showed significant advantage for nivolumab with a mean difference (MD) of 4.90 (95% CI 1.27 to 8.53, P = .008, Figure 6). In addition, the data regarding the objective response rates to these two treatments were analyzed and represented as a forest plot; this analysis also highlighted the significant advantage of nivolumab over dacarbazine, with RR ratio of 2.77 (95% CI 1.92 to 4.02, P < .00001, Figure 7).
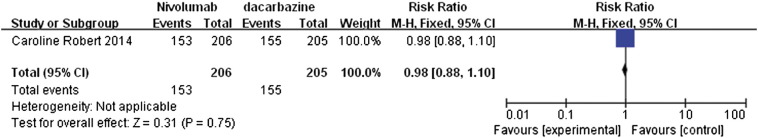
Adverse events
The analysis of adverse events comparing nivolumab with dacarbazine in melanoma patients suggested that nivolumab was associated with slightly fewer adverse events than dacarbazine (Figure 8), although the difference was not statistically significant. The observed RR value was 0.98 (95% CI 0.88 to 1.10, P = .75).
Figure 8.
Forest plot depicting the comparison of adverse events associated with nivolumab and dacarbazine.
Assessment of Effective Anti–PD-1 Doses
Pembrolizumab (2 mg cf. 10 mg)
Overall response rate, PFS, and 1-year survival rate
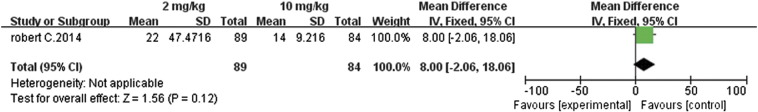
The study by Robert et al. [11] compared the efficacy and safety of pembrolizumab, an anti–PD-1 antibody, in patients who had ipilimumab-refractory advanced melanoma. The two different doses, 2 and 10 mg/kg, were administered every 3 weeks to these patients. The meta-analysis of this dichotomous data revealed that the overall response rates from both these concentrations were not significantly different, with an RR value of 0.99 (95% CI 0.60 to 1.63, P = .96, Figure 9). This suggested that increasing the dose from 2 to 10 mg/kg did not enhance the overall response rate.
Figure 9.
Forest plot depicting the overall response rate with different doses (2 and 10 mg/kg) of pembrolizumab.
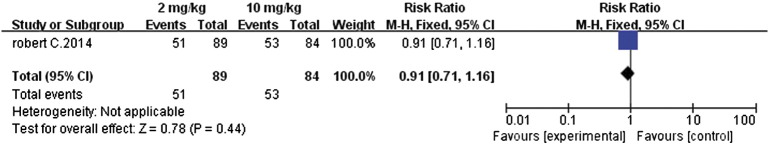
Similarly, further analysis of PFS (MD 8.0, 95% CI − 2.06 to 18.06, P = .12, Figure 10) and the 1-year survival rates (RR 0.91, 95% CI 0.71 to 1.16, P = .44, Figure 11) also revealed that there were insignificant differences between the 2- and 10-mg/kg doses. The 10-mg/kg dose had no advantage in improving PFS or the 1-year survival rate.
Figure 10.
Forest plot depicting the PFS rates associated with different doses (2 and 10 mg/kg) of pembrolizumab.
Figure 11.
Forest plot depicting 1-year survival rates associated with different doses (2 and 10 mg/kg) of pembrolizumab.
Nivolumab (0.3 mg cf. 2 mg)
Overall response rate, PFS, and OS
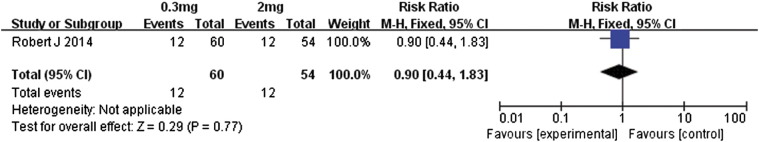
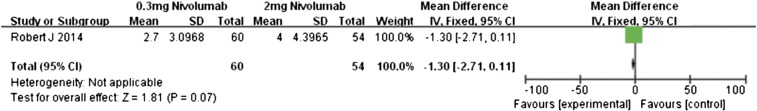
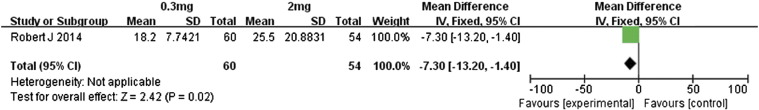
Motzer et al. [14] conducted a comparison of different doses of nivolumab in patients with metastatic renal cell carcinoma. Our meta-analysis of the data was performed for patients who received either 0.3 or 2 mg/kg of nivolumab (Figure 12). The overall response rate was almost similar between these two doses (RR 0.90, 95% CI 0.44 to 1.83, P = .77). In contrast, the patients receiving 2 mg/kg had significantly better PFS than did those administered 0.3 mg/kg (MD − 1.30, 95% CI − 2.71 to 0.11, P = .07, Figure 13). Those in the 2-mg/kg group also had significantly better OS (MD − 7.30, 95% CI − 13.20 to − 1.40, P = .02, Figure 14). This suggests that 2-mg/kg nivolumab therapy was associated with longer PFS and OS relative to that of 0.3 mg/kg, whereas the overall response rates were similar.
Figure 12.
Forest plot depicting overall response rates of different doses (0.3 and 2 mg/kg) of nivolumab.
Figure 13.
Forest plot depicting the PFS rates associated with different doses (0.3 and 2 mg/kg) of nivolumab.
Figure 14.
Forest plot depicting the OS rates associated with different doses (0.3 and 2 mg/kg) of nivolumab.
Nivolumab (0.3 mg cf. 10 mg)
Overall response rate, PFS, and OS
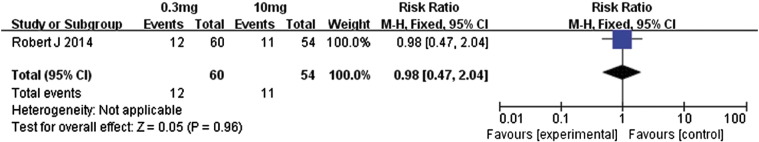
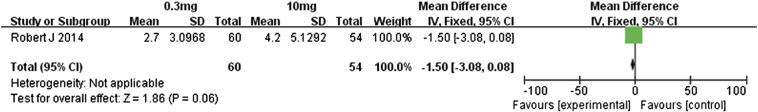
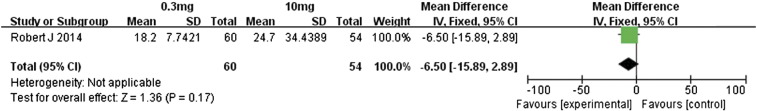
Motzer et al. [14] compared the efficacies associated with 0.3- and 10-mg/kg doses of nivolumab in metastatic renal cell carcinoma patients. Regarding the overall response rates, the 10-mg/kg dose had no significant advantage over the 0.3-mg/kg dose (RR 0.98, 95% CI 0.47 to 2.04, P = .96, Figure 15). However, the higher dose was associated with significantly better PFS and OS (Figure 16, Figure 17): for PFS, the MD value was − 1.50 (95% CI − 3.08 to 0.08, P = .06), and for OS, the MD value was − 6.50 (95% CI − 5.89 to 2.89, P = .17). Therefore, the 10-mg/kg dose of nivolumab was associated with significantly longer PFS and OS relative to the 0.3-mg/kg dose. However, the overall response rate did not differ much between these two doses.
Figure 15.
Forest plot depicting the overall response rates associated with different doses (0.3 and 10 mg/kg) of nivolumab.
Figure 16.
Forest plot depicting PFS rates associated with different doses (0.3 and 10 mg/kg) of nivolumab.
Figure 17.
Forest plot depicting the OS rates associated with different doses (0.3 and 10 mg/kg) of nivolumab.
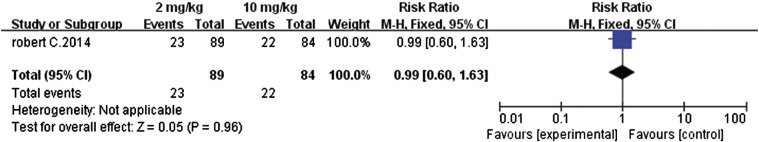
Nivolumab (2 mg cf. 10 mg)
Overall response rate, PFS, and OS
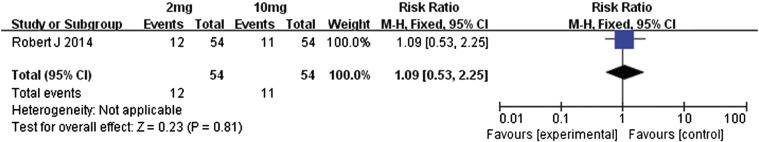
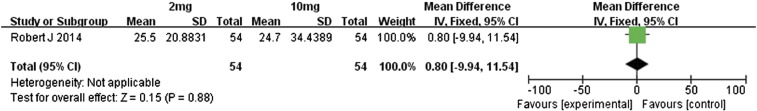
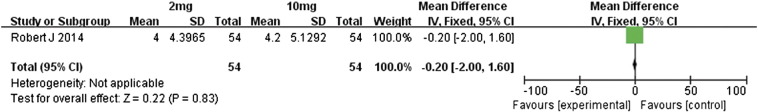
We also performed a meta-analysis of the differences in outcomes between the 2- and 10-mg/kg doses of nivolumab in metastatic renal cell carcinoma patients [14]. With regard to overall response rates, OS, and PFS, the two treatment groups were similar in outcomes (Figure 18, Figure 19, Figure 20, respectively); the RR value for overall response rates was 1.09 (95% CI 0.53 to 2.25, P = .81), MD value for OS was–0.80 (95% CI − 9.94 to 11.54, P = .88), and MD value for PFS was − 0.20 (95% CI − 2.00 to 1.60, P = .83).
Figure 18.
Forest plot depicting the overall response rates associated with different doses (2 and 10 mg/kg) of nivolumab.
Figure 19.
Forest plot depicting the OS rates associated with different doses (2 and 10 mg/kg) of nivolumab.
Figure 20.
Forest plot depicting the PFS rates associated with different doses (2 and 10 mg/kg) of nivolumab.
Discussion
Promising clinical data regarding antibodies that block PD-1 and PD-L1 in different malignancies have recently begun to emerge. Among cancer studies, the major focus of anti–PD-1 therapy has been in melanoma, but preliminary reports have suggested positive outcomes in other cancers as well. The initial evidence is that anti–PD-1 antibodies display strong and significant antitumor activity by targeting the PD-1/PD-L1 pathway in cancers such as melanoma and lymphoma [15], [16], [17], leukemia [7], [18], neuroblastoma [19], brain and central nervous system cancers [20], [21], retinoblastoma [22], Wilm’s tumor [23], soft-tissue sarcoma [24], and osteosarcoma [25]. The open-label, randomized, phase 3 study by Weber et al. [12] showed that anti–PD-1 therapy was associated with a higher rate of objective response than chemotherapy.
In the current study, we undertook a comprehensive systematic review by meta-analysis of RCTs to assess the efficacy, safety, and effective dose of anti–PD-1 therapy in tumor treatments. An effort was made to pool the available data from individual trials and produce an overall summary of OS, PFS, 1-year survival, adverse events, and effective dose concentrations. Our first analysis comparing nivolumab with chemotherapy in advanced melanoma patients revealed a significantly higher overall response rate associated with nivolumab compared with chemotherapy. In addition, nivolumab treatment resulted in fewer adverse events. The analysis comparing nivolumab with dacarbazine in metastatic melanoma patients again revealed that nivolumab had a significant advantage with regard to 1-year survival, PFS, and objective response rate. Nivolumab also had fewer associated adverse events than dacarbazine, but the difference was not significant. It can be deduced that analysis of more studies might reveal a more significant advantage. We also compared different doses of pembrolizumab or nivolumab. For pembrolizumab, all of the effects analyzed at the tested doses (2 and 10 mg/kg) were statistically similar. In contrast, for nivolumab, the analyses for 0.3, 2, and 10 mg/kg suggested that the 2 and 10 mg/kg resulted in significantly longer PFS and OS relative to the 0.3-mg/kg treatment, whereas no differences were found in overall response rates.
Our meta-analysis is limited in that most of the results were based on a single RCT, and the sample size of each study was small. Both of these limitations resulted in low statistical power in assessing the overall efficacy of anti–PD-1 therapy. However, as anti–PD-1 immunotherapy is a relatively new approach in the treatment of malignant tumors, there are little published data; most RCT studies are currently still under way. Because of the limited number of studies for each analysis, we could not confidently assess publication bias or conduct a sensitivity analysis. Another limitation concerns the effective dose concentrations of these treatments. Effective doses have not been standardized, but we observed that, past a certain dose, no further improvement was observed in patient outcomes. Therefore, effective and minimum doses need to be determined so that patients can achieve maximum therapeutic benefit with minimum adverse effects.
Our analysis indicated that anti–PD-1 therapy is associated with fewer adverse events than either chemotherapy or dacarbazine treatments, which is in accord with previous reports. The most common adverse events observed with anti–PD-1 therapy are fatigue, pruritus, nausea, diarrhea, rash, vitiligo, constipation, asthenia, and vomiting. Therefore, when standardizing an anti–PD-1 therapy regimen for malignant tumors, additional combinations of drugs to prevent or treat these adverse events should be considered.
Conclusions
In summary, our meta-analysis results suggest that anti–PD-1 therapy is indeed a promising approach for the treatment of malignant tumors, with significant associated improvements in PFS, OS, and 1-year survival rates relative to chemotherapy and other treatments, and fewer adverse events. However, because our analyses were based on a single RCT study with a small sample size, caution must be considered. In addition, the outcomes in these studies were not blinded. To improve the credibility of future anti PD-1 therapy studies requires multicenter RCTs with large sample size, analysis of a wide range of doses, and explicit blinding of the outcome assessors, and finally, the cost-utility of the therapy should be addressed.
Footnotes
This study was supported by the International S&T Cooperation Program of China (2014DFA30580 S2012ZR0128), the BaGui Scholars Program Foundation, the Key S&T Program of Guangxi Chinese Medicine University (14124004-2-2, 1355006-7, GZKZ-Z1103), and New Century Excellent Talents (NCET-13-0745) project manager.
References
- 1.Stewart BW, Wild CP. IARC Nonserial Publication; 2014. World Cancer Report. [Google Scholar]
- 2.Bryan LJ, Gordon LI. Blocking tumor escape in hematologic malignancies: the anti–PD-1 strategy. Blood Rev. 2015;29:25–32. doi: 10.1016/j.blre.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Li R, Wang C, Liu L, Du C, Cao S, Yu J, Wang SE, Hao X, Ren X, Li H. Autologous cytokine-induced killer cell immunotherapy in lung cancer: a phase II clinical study. Cancer Immunol Immunother. 2012;61:2125–2133. doi: 10.1007/s00262-012-1260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hontscha C, Borck Y, Zhou H, Messmer D, Schmidt-Wolf IG. Clinical trials on CIK cells: first report of the international registry on CIK cells (IRCC) J Cancer Res Clin Oncol. 2011;137:305–310. doi: 10.1007/s00432-010-0887-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015;37:764–782. doi: 10.1016/j.clinthera.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang PA, Heng DY. Programmed death 1 pathway inhibition in metastatic renal cell cancer and prostate cancer. Curr Oncol Rep. 2013;15:98–104. doi: 10.1007/s11912-012-0284-2. [DOI] [PubMed] [Google Scholar]
- 7.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Quezada SA, Peggs KS. Exploiting CTLA-4, PD-1 and PD-L1 to reactivate the host immune response against cancer. Br J Cancer. 2013;108:1560–1565. doi: 10.1038/bjc.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JPT, Green S. The Cochrane Collaboration. 2011. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.2.0. [Google Scholar]
- 11.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 12.Weber JS, Minor DR, D'Angelo S, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Grob J. European Society for Medical Oncology 2014 Congress. 2014. A phase 3 randomized, open-label study of nivolumab (anti–PD-1; BMS-936558; ONO-4538) versus investigator's choice chemotherapy (ICC) in patients with advanced melanoma with prior anti-CTLA-4 therapy. [Madrid] [Google Scholar]
- 13.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, Vaishampayan UN, Drabkin HA, George S, Logan TF. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015;33:1430–1437. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blank C, Kuball J, Voelkl S, Wiendl H, Becker B, Walter B, Majdic O, Gajewski TF, Theobald M, Andreesen R. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int J Cancer. 2006;119:317–327. doi: 10.1002/ijc.21775. [DOI] [PubMed] [Google Scholar]
- 16.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 17.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 18.Afreen S, Dermime S. The immunoinhibitory B7-H1 molecule as a potential target in cancer: killing many birds with one stone. Hematol Oncol Stem Cell Ther. 2014;7:1–17. doi: 10.1016/j.hemonc.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 20.Yao Y, Tao R, Wang X, Wang Y, Mao Y, Zhou LF. B7-H1 is correlated with malignancy-grade gliomas but is not expressed exclusively on tumor stem-like cells. Neuro Oncol. 2009;11:757–766. doi: 10.1215/15228517-2009-014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, Weller M, Wiendl H. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462–7467. [PubMed] [Google Scholar]
- 22.Usui Y, Okunuki Y, Hattori T, Takeuchi M, Kezuka T, Goto H, Usui M. Expression of costimulatory molecules on human retinoblastoma cells Y-79: functional expression of CD40 and B7H1. Invest Ophthalmol Vis Sci. 2006;47:4607–4613. doi: 10.1167/iovs.06-0181. [DOI] [PubMed] [Google Scholar]
- 23.Routh JC, Ashley RA, Sebo TJ, Lohse CM, Husmann DA, Kramer SA, Kwon ED. B7-H1 expression in Wilms tumor: correlation with tumor biology and disease recurrence. J Urol. 2008;179:1954–1959. doi: 10.1016/j.juro.2008.01.056. [discussion 1959–1960] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S, Kim KM, Park HS, Lee H, Moon WS, Chung MJ. Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PLoS One. 2013;8:e82870. doi: 10.1371/journal.pone.0082870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lussier DM, Nieves LM, McAfee MS, Hyunh TP, Holechek S. Society for Immunotherapy of Cancer 28th Annual Meeting 2013. 2013. Enhancement of T cell immunity to osteosarcoma through modulation of PD-1/PD-L1 interactions. [Google Scholar]