Abstract
Background
Invasive infections with non-typhoidal Salmonella (NTS) lead to bacteremia in children and adults and are an important cause of illness in Africa; however, few data on the burden of NTS bacteremia are available. We sought to determine the burden of invasive NTS disease in a rural and urban setting in Kenya.
Methods
We conducted the study in a population-based surveillance platform in a rural setting in western Kenya (Lwak), and an informal urban settlement in Nairobi (Kibera) from 2009 to 2014. We obtained blood culture specimens from participants presenting with acute lower respiratory tract illness, or acute febrile illness to a designated outpatient facility in each site, or any hospital admission for a potentially infectious cause (rural site only). Incidence was calculated using a defined catchment population and adjusting for specimen collection and healthcare seeking practices.
Results
A total of 12,683 and 9,524 blood cultures were analyzed from Lwak and Kibera, respectively. Of these, 428 (3.4%) and 533 (5.6%) grew a pathogen; among those 208 (48.6%) and 70 (13.1%) were positive for NTS in Lwak and Kibera, respectively. Overall, the adjusted incidence of invasive NTS disease was higher in Lwak (839.4 per 100,000 person-years observation [pyo]) compared with Kibera (202.5 per 100,000 pyo). The highest adjusted incidences were observed in children <5 years of age (Lwak 3,914.3 per 100,000 pyo and Kibera 997.9 per 100,000 pyo). In Lwak the highest adjusted annual incidence was 1,927.3 per 100,000 pyo (in 2010) and in Kibera 220.5 per 100,000 pyo (in 2011); the lowest incidences were 303.3 and 62.5 per 100,000 pyo respectively (in 2012). In both sites, invasive NTS disease incidence generally declined over the study period.
Conclusion
We observed an extremely high burden of invasive NTS disease in a rural area of Kenya and a lesser, but still substantial, burden in an urban slum. Although the incidences in both sites declined during the study period, invasive NTS infections remain an important cause of morbidity in these settings, particularly among children < 5 years old.
Introduction
Non-typhoidal Salmonella (NTS, or Salmonella enterica serotypes other than Typhi, Paratyphi A, B [tartrate-negative] and C), is a leading cause of bloodstream infection with a reported high burden of disease in low and middle income countries, particularly in Africa [1–6]. Data from a study conducted in Kenya from 2006 to 2009 showed a large burden of invasive NTS disease in rural western Kenya, with the highest incidence observed in children < 5 years old (2,085 per 100,000 person-years) and in human immunodeficiency virus (HIV)-infected adults (1,555 per 100,000 person-years) [2]. This same study revealed a much lower incidence of invasive NTS infections in urban Kenya (57 per 100,000 person-years overall and 260 per 100,000 person-years among children <5 years old). Invasive NTS disease rates have been found in Kenya [7, 8] and elsewhere [9] to correlate with peaks in malaria transmission, suggesting that current or recent malaria infection may be a risk factor for invasive NTS infection [8]. Other factors associated with invasive NTS disease include HIV infection and malnutrition [8, 10].
Understanding the burden of invasive NTS disease is important for guiding efforts to reduce transmission, prevent new infections, and for the development of vaccines against NTS [11]. In recent years in western Kenya, programmatic efforts have been undertaken to reduce the burden of malaria [12], HIV [13], and malnutrition [14]. Changes in the prevalence of important underlying risk factors for invasive NTS infections may alter the relative burden of disease over time and across age groups and geographic regions. We examined data from an ongoing population-based surveillance platform to describe trends in invasive NTS disease from January 2009 through December 2014 that updates the previously reported data from 2006 through 2009 on the burden from the same rural and urban settings in Kenya[2].
Methods
Study site and population
The Kenya Medical Research Institute (KEMRI), in collaboration with the US Centers for Disease Control and Prevention (CDC), has implemented population-based infectious disease surveillance (PBIDS) in rural and urban areas since late 2006. The PBIDS methods have been described in detail elsewhere [15]. Briefly, PBIDS operates in two sites; one is located in Lwak, a rural location in Siaya County in western Kenya along Lake Victoria, and the other in Kibera, an urban informal settlement in Nairobi. The Lwak surveillance area is 100 km2 with an average population of 25,000 (250 people/km2), and is holo-endemic for malaria. In Kibera, the average population of the surveillance area is 28,500 in a 0.37 km2 area (77,000 people/km2). Kibera residents live in crowded conditions and have inadequate access to water and sanitation facilities; human and animal waste drain into open sewers. Malaria is not endemic but does occur primarily due to rural-urban migration. Surveillance participants include all people living within the surveillance areas for a minimum of 4 months.
Trained community interviewers visit PBIDS participants in their households every two weeks, collecting data on illnesses, births, and deaths since the prior visit. When participants report illness, they are further questioned about symptoms, health care seeking, and treatment. Abbreviated physical examinations were carried out on ill persons present during the visit, including axillary temperature, one minute respiratory rate, evaluation for lower chest wall indrawing and stridor in ill children, and observation for signs of dehydration. If currently ill, participants are encouraged to seek care at the study clinics (Lwak Mission Hospital in Lwak or Tabitha Clinic in Kibera) where they receive free medical care for most acute conditions. The study clinics are located within five km and one km of all PBIDS residences in Lwak and Kibera, respectively.
Participants who present to the study clinics are assessed by a clinical officer and data are gathered on symptoms, physical examination findings, diagnosis, treatment, and outcome. Blood samples for culture are collected from individuals meeting surveillance case definitions for acute lower respiratory tract infection (ALRI) or acute febrile illness (AFI) as well as all individuals hospitalized at Lwak Mission Hospital for conditions unrelated to injury or obstetrics; Tabitha Clinic does not have an inpatient service. ALRI is defined in persons ≥5 years old as cough, difficulty breathing, chest pain, or sore throat, plus either temperature ≥38.0°C, oxygen saturation <90%, or admitted with respiratory illness. For children <5 years old, ALRI criteria are cough or difficulty breathing, and at least one of the following: chest indrawing, stridor, oxygen saturation <90%, unable to breastfeed or drink, vomiting everything, convulsions, lethargy, unconsciousness, or admitted with respiratory illness. AFI is defined as an axillary temperature ≥38.0°C (Kibera temperature changed to ≥37.5°C from January 2012 onwards). Cases meeting both ALRI and AFI criteria are classified as ALRI. In Lwak, only the first five AFI cases <5 years old and the first case ≥5 years old per day have blood cultures performed. There were no limits on the number of blood cultures performed for ALRI cases or AFI cases in Kibera.
Sample collection and laboratory testing
For eligible participants a 1–3 mL of whole blood sample is collected from children <5 years, and 7–10 mL from individuals ≥5 years. The blood sample is inoculated into a commercially produced broth bottle (BACTECTM Plus Aerobic/F and Peds PlusTM/F culture vials, Becton Dickinson, Belgium) and sent to the KEMRI laboratory in Kisian, Kisumu or Kibera, Nairobi. The inoculated blood culture bottles are incubated in a continuously monitored BACTEC 9050 instrument at 35°C for 1–5 days. Bottles with growth are removed from the incubator; a smear prepared on a slide, stained using Gram’s stain method and examined under the microscope for morphological appearance and Gram’s reaction. The broth is sub-cultured on blood agar, chocolate agar, and MacConkey agar media and incubated for 18–24 hours at 37°C in a 5% CO2 incubator. The isolated colonies on the plates are identified morphologically, microscopically as above and biochemically. The colonies suspicious of being Salmonella from the results of the biochemical tests are confirmed serologically by slide agglutination technique using group antisera (Denka Seiken, Tokyo, Japan). In addition, routine Giemsa blood smears for malaria are performed and read by trained microscopists in the clinics. HIV home based counseling and testing was offered in 2008 to adults (aged ≥18 years), minors (aged 13–17 years), and children (aged <13years) after providing consent or assent [16]. Pre-test counseling, testing and post-test counseling followed Kenyan Ministry of Health (MOH) guidelines and recommendations, and was provided by a trained and certified Voluntary Counseling and Testing (VCT) counselor. HIV testing is also available at the surveillance clinics.
Data Analysis
We conducted analysis on data from 1 January 2009, through 31 December 2014 across both sites. Crude incidences were calculated as the number of cases of invasive NTS disease per 100,000 person-years of observation (pyo). Participating individuals contributed person-time according to their time of residence within the surveillance area during the study period. We applied two adjustments to the crude incidences. First, a multiplier was included for the percentage of persons visiting the surveillance clinics or admitted to the inpatient service (Lwak only) that met criteria for blood culture and did not have a culture done. A second adjustment was made based on the percentage of persons identified during the biweekly household visits with ALRI or AFI who visited clinics other than Lwak or Tabitha for that illness. For household visits, slightly modified case definitions were used. We assumed that those who met criteria for blood culture at the surveillance clinic but did not have culture performed and those that visited a non-surveillance clinic for ALRI or AFI had a similar spectrum of etiologies to those that visited Lwak or Tabitha and were tested. 95% confidence intervals were calculated for crude incidences using the delta method [17]. Trends in incidence were assessed using Poisson regression with year as an independent variable. Deaths during acute invasive NTS illness and within 30 days of illness were recorded and used to estimate case fatality proportions. Malnutrition among children <5 years was assessed using the World Health Organization growth standards; we calculated the prevalence of height-for-age and weight-for-length z scores <−2 [18].
Ethical review
Informed consent was obtained from all the participants who met the case definitions for recruitment after screening. Parents, next of kin, or guardians gave written informed consent on behalf of children aged <15 years. Children aged 7–14 years provided written assent for their participation The protocol and written informed consent forms for surveillance and home-based HIV testing were reviewed and approved by the Ethical Review Board of KEMRI (SSC #1899) and the Institutional Review Board of Center for Disease Control, Atlanta (IRB #4566).
Results
Among 12,683 blood cultures analyzed in Lwak, 870 (6.9%) grew a likely contaminant, while 428 (3.4 %) grew a pathogen; among the pathogens, 222 (51.9%) were Salmonella, including 208 (48.6%) NTS (Table 1). In Kibera, from 9,524 blood cultures grew 288 likely contaminants and 533 (5.6%) pathogens, of which 295 (55.3%) were Salmonella and 70 (13.1%) were NTS (Table 1). NTS was the most commonly detected pathogen in Lwak and the third most common (after S. enterica serovar Typhi and Streptococcus pneumoniae) in Kibera. The age group with the highest proportion of NTS among all isolated pathogens was 0–4 years old in both Lwak (66.2%) and Kibera (21.6%).
Table 1.
Results of blood culture among patients with acute febrile illness and/or acute lower respiratory illness, Lwak and Kibera Kenya, 2009–2014.
| Site | Age in years | Number of patients (n) | Number of blood cultures, n (%) | Likely contaminants n (%) | Pathogens isolated n (%) | NTS recovered n (%) | NTS cases with concurrent malaria n (%) | NTS cases with recent malaria n (%) | HIV-infected/number tested n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Lwak | 0–4 | 34554 | 5895 (17.1) | 433 (7.3) | 148 (2.5) | 98 (66.2) | 28 (28.6) | 18 (18.4) | 4/14 (28.6) |
| 5–9 | 19345 | 2941 (15.2) | 208 (7.1) | 66 (2.2) | 24 (36.4) | 10 (41.7) | 0 (0.0) | 0/0 (0.0) | |
| 10–17 | 21814 | 1560 (7.2) | 93 (6.0) | 40 (2.6) | 19 (47.5) | 6 (31.6) | 2 (10.5) | 0/6 (0.0) | |
| 18–49 | 27249 | 1713 (6.3) | 100 (5.8) | 133 (7.8) | 54 (40.6) | 3 (5.6) | 0 (0.0) | 28/35 (80.0) | |
| >50 | 11290 | 574 (5.1) | 36 (6.3) | 41 (7.1) | 13 (31.7) | 0 (0.0) | 0 (0.0) | 4/10 (40.0) | |
| Total | 114252 | 12683 (11.1) | 870 (6.9) | 428 (3.4) | 208 (48.6) | 47 (22.6) | 20 (9.6) | 36/65 (55.4) | |
|
| |||||||||
| Kibera | 0–4 | 48028 | 4822 (10.0) | 146 (3.0) | 222 (4.6) | 48 (21.6) | 4 (8.3) | 2 (4.2) | n/a |
| 5–9 | 16588 | 1852 (11.2) | 50 (2.7) | 122 (6.6) | 7 (5.7) | 1 (14.3) | 0(0.0) | n/a | |
| 10–17 | 14804 | 1090 (7.4) | 48 (4.4) | 69 (6.3) | 2 (2.9) | 0 (0) | 0(0.0) | n/a | |
| 18–49 | 40164 | 1676 (4.2) | 39 (2.3) | 113 (6.7) | 13 (11.5) | 1 (7.7) | 0(0.0) | n/a | |
| >50 | 3989 | 84 (2.1) | 5 (6.0) | 7 (8.3) | 0 (0) | 0 (0) | 0(0.0) | n/a | |
| Total | 123573 | 9524 (7.7) | 288 (3.0) | 533 (5.6) | 70 (13.1) | 6 (8.6) | 2 (2.9) | n/a | |
Among 65 invasive NTS cases with available HIV status (all from Lwak), 36 (55.4%) were HIV-infected. Among 35 patients in the 18–49 year age group with available HIV status, 28 (80.0%) were infected. Concurrent malaria infection was detected in 47 (22.0%) cases of invasive NTS disease in Lwak and 6 cases (8.6 %) in Kibera. An additional 20 (9.6%) in Lwak and 2 (2.9%) in Kibera had been diagnosed with malaria during the preceding 30 days. Heights and weight were available for 123 (84.2%) of children <5 years; among those, the prevalence of a z score <−2 for height/length-for-age was 37.4% (95%CI 28.4, 46.4) and for weight-for-height/length was 13.8% (95%CI 7.3, 20.3). The frequency of low height/length for age was more common in Kibera (18/32, 56.3% [95%CI 37.5, 75.0]) than in Lwak (28/91 30.8% [95%CI 20.7, 40.8]), although the difference was not significant. The prevalence of low weight-for-age was similar across sites (4/32, 12.5% in Kibera and 13/91, 14.3% in Lwak)
Among the 278 case-patients with invasive NTS infection, 2 died during 1 week following the date of sample collection and an additional 5 died 7–30 days following the sample collection, yielding an overall case fatality proportion of 2.5%. The case fatality proportion was higher in Lwak (6/208, 2.9%) compared with Kibera (1/70, 1.4%), although the difference was not significant (p=0.510).
The crude incidence of invasive NTS disease was 153.5 cases per 100,000 pyo in Lwak and 67.0 per 100,000 pyo in Kibera. The adjusted incidences were 839.4 per 100,000 pyo in Lwak and 202.5 per 100,000 pyo in Kibera (Table 2). Across all age strata, the adjusted incidence in Lwak was at least 4 times greater than that of Kibera.
Table 2.
Crude and adjusted incidence of invasive NTS disease in Lwak and Kibera Kenya by age group, January 2009 through December 2014.
| Age in years | Site | Invasive NTS | pyo | Crude Rate per 100,000 pyo (95% CI) | Rate Extrapolation 1 ** per 100,000 pyo (95% CI) | Rate Extrapolation 2 ** per 100,000 pyo (95% CI) |
|---|---|---|---|---|---|---|
| 0–4 | LWAK | 98 | 19,531.5 | 501.8 (411.6–611.6) | 2305.6 (2102.2–2528.7) | 3914.3 (3646.5–4201.9) |
| KIBERA | 48 | 18,833.4 | 254.9 (192.1–338.2) | 735.2 (622.4–868.5) | 997.9 (864.9–1151.3) | |
| 5–9 | LWAK | 24 | 20,282.1 | 118.3 (79.3–176.5) | 210.9 (156.3–284.6) | 374.5 (299.1–468.9) |
| KIBERA | 7 | 16,740.8 | 41.8 (19.9–87.7) | 64.1 (35.2–116.6) | 82.5 (48.7–139.8) | |
| 10–17 | LWAK | 19 | 30,237.8 | 62.8 (40.1–98.5) | 115.2 (82.6–160.6) | 216.1 (169.6–275.4) |
| KIBERA | 2 | 19,055.4 | 10.5 (2.6–42.0) | 17.2 (5.8–50.7) | 21.3 (8.1–56.4) | |
| 18–49 | LWAK | 54 | 46,685.7 | 115.7 (88.6–151) | 186.4 (151.1–230.0) | 325.7 (277.9–381.9) |
| KIBERA | 13 | 46,499.4 | 28.0 (16.2–48.1) | 44 (28.5–67.8) | 62.2 (43.2–89.6) | |
| >50 | LWAK | 13 | 18,780.8 | 69.2 (40.2–119.2) | 126.1 (84.3–188.7) | 249.5 (187.4–332.3) |
| KIBERA | 0 | 3,395.0 | - | - | - | |
| OVERALL | LWAK | 208 | 135,517.9 | 153.5 (134–175.8) | 471.9 (436.7–510) | 839.4 (792–889.6) |
| KIBERA | 70 | 104,524.0 | 67.0 (53–84.6) | 149.8 (128–175.2) | 202.5 (177–231.7) |
Extrapolated for patients meeting indication for blood culture who were not cultured in the clinic.
Extrapolated for patients meeting indication for blood culture who were not cultured in the clinic and those with same illness syndromes at the home visit who sought care at area clinics besides Lwak and Tabitha clinics.
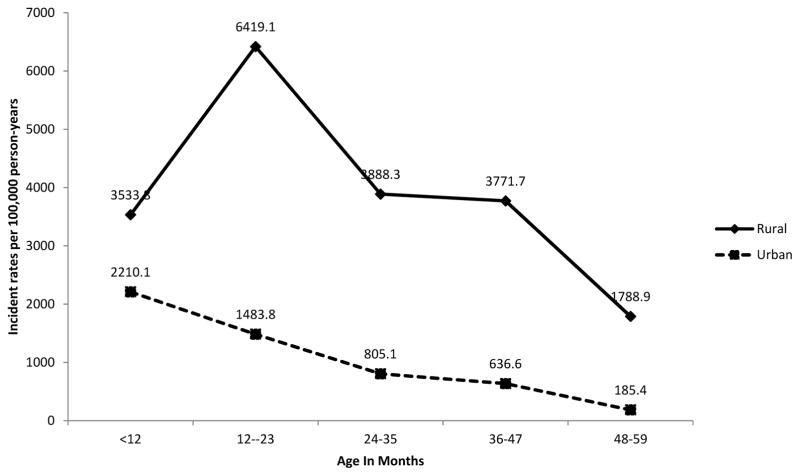
In both sites, the highest incidence of invasive NTS disease was observed among children <5 years of age; adjusted incidences in this age group were 3,914.3 and 997.9 per 100,000 pyo in Lwak and Kibera, respectively. Within the <5 year age group, adjusted incidence peaked in the 12–23 month olds in Lwak (6,419.1 per 100,000 pyo) while in Kibera children <12 months had the highest incidence (2210.1 per 100,000 pyo) (Figure 1). Among the <5 year olds, those aged 48 to 59 months had the lowest incidence across both sites; however those adjusted incidences (1,788.9 and 185.4 per 100,000 pyo in Lwak and Kibera, respectively) were still higher than the incidence observed among older children and adults in each site.
Figure 1.
Invasive NTS disease adjusted incidence among children <5 years in Lwak and Kibera, January 2009 through December 2014
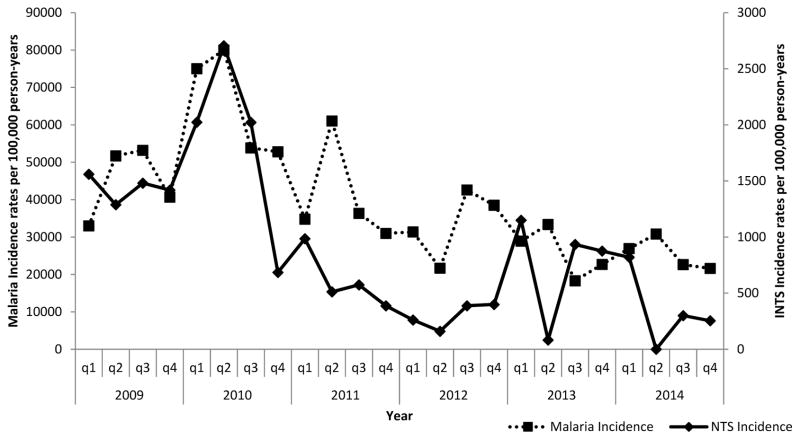
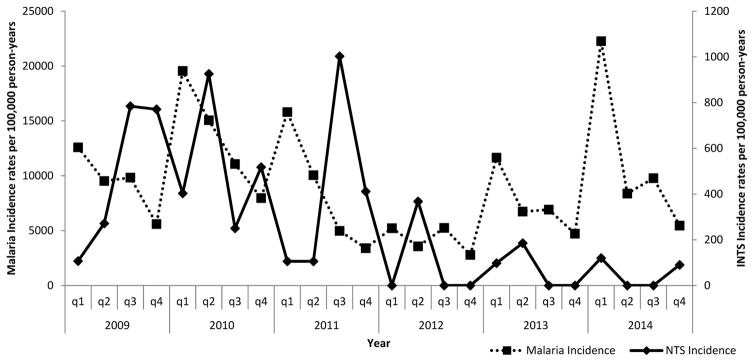
While adjusted malaria incidence seemed to peak in the second or third quarter of each year in Lwak (a malaria endemic area), there was no clear seasonal pattern for malaria in Kibera (a non-endemic area) nor for invasive NTS infections in either setting (Figures 2 and 3). Nonetheless, over the study period, invasive NTS disease incidence correlated with that of malaria infection in Lwak (Spearman’s correlation, rho=0.5435 p=0.0061). There was no significant correlation between malaria and invasive NTS infections in Kibera (Spearman’s correlation, rho=0.1743 p=0.4153). The highest adjusted annual incidence in Lwak was observed in 2010 (1927.3 per 100,000 pyo) and in Kibera in 2011 (220.5 per 100,000 pyo). The lowest adjusted incidence at both sites occurred in 2012 (303.3 and 62.5 per per 100,000 pyo in Lwak and Kibera, respectively). Both sites experienced a significant decline in adjusted incidence over the study period (p<0.0001).
Figure 2.
Quarterly invasive NTS disease and malaria incidence in Lwak, January 2009 through December 2014.
Figure 3.
Quarterly invasive NTS disease and malaria incidence in Kibera, January 2009 through December 2014.
Discussion
In this updated assessment of the burden of invasive NTS disease in a rural setting and an urban informal settlement in Kenya, we show that invasive infections with NTS continue to be an important public health problem and that the burden is dynamic and affects certain groups disproportionately. As has been noted previously in data from these same sites [2], the age group most heavily affected was children <5 years old and the burden of invasive NTS disease was found to be much higher in the rural area compared with an urban setting. However, a closer examination of invasive NTS disease incidence among children <5 years old showed heterogeneity across sites in the specific age group at greatest risk, and additional years of data revealed variability in incidence over time. Despite some degree of year-to-year variability in each site, the overall trend during the study period was towards declining incidence across both sites, raising questions about the factors that affect invasive NTS disease incidence and the most appropriate public health interventions to reduce the burden.
In Lwak, a malaria-endemic area, the decline in invasive NTS infections seems to have coincided with a downward trend in malaria incidence. Efforts have been made scale up malaria control interventions in western Kenya in recent years [12]. Studies from other African settings, including the Gambia [19], Tanzania [9], and Kenya [7] have reported temporally associated declines in malaria and invasive NTS infections. The association between malaria (particularly sever malaria) and invasive NTS disease has been extensively described [20]. While the physiologic basis for the association is not entirely clear, increasing evidence suggests that interventions to reduce malaria may provide the added benefit of driving down invasive NTS disease burden [20]. As has been noted previously [2], malaria is likely a primary factor shaping the differences in invasive NTS disease incidence we observed between the rural (malaria holo-endemic) area and the urban area (malaria non-endemic).
In addition to malaria infection, well recognized risk factors for invasive NTS infections include HIV infection and malnutrition (in children) [21]. Although we did not conduct an assessment of risk factors using a control group, the high frequency of HIV infection among those cases with available HIV results suggests that HIV is an important risk factor. Home-based counseling and testing conducted in Western Kenya in 2008 found the HIV prevalence among first time testers to be 10.9% overall, and 22.2% among the 35–49 year old age group [16]. In contrast, more than half of invasive NTS disease cases with available results were HIV-infected and nearly 80% of those cases aged 18–49 years were infected. On the other hand, the overall prevalence of malnutrition among invasive NTS disease cases aged <5 years was generally similar to what has previously been reported [22, 23]. It is interesting to note, however, that the prevalence of stunting in Kibera was nearly double that in Lwak. While the difference was not statistically significant, it seems plausible that in an urban environment with relatively little exposure to malaria, malnutrition may play a more important role as a predisposing risk factor for invasive NTS infections. While our study was not designed to test hypotheses about factors contributing to the observed decline in invasive NTS disease, it is possible that strengthening HIV prevention and care and nutritional interventions may have played a role.
Despite the decline observed in invasive NTS disease from 2009 to 2014, even the lowest incidences observed in Lwak were relatively high compared to other African settings with population-based surveillance. Incidence estimates from Kilifi, Kenya (88 per 100,000 among <5 year olds)[5], Mozambique (120 per 100,000 among <5 year olds)[3], the Gambia (262 to 300 among children aged 2 to 29 months per 100,000)[24] are all remarkably lower than the burden we report among children <5 years old. Even the highest incidence among them, from the Gambia, is less than a third of the incidence we found in Kibera and less than one tenth of the incidence we observed in Lwak among children <5 years. It is likely that surveillance methodology plays a role in the differences in disease burden estimates, since most surveillance is conducted at hospitals among inpatients and our surveillance includes both inpatients and outpatients. One study in Kenya found that extending surveillance, including blood culture, to a hospital outpatient department more than doubled the previous estimate of bacteremia incidence that had been based on inpatient data alone[25]; while the study did not address NTS bacteremia specifically, presumably the effect on invasive NTS would be similar. The low case fatality we report (2.5%) is also certainly influenced by the predominance of out-patient cases captured by our surveillance system. Globally the case fatality among patients with invasive NTS infections has been estimated to be 20% [6].
The invasive NTS disease incidence estimates we present are higher than those reported from the same sites by Tabu et. al. for the periods of October 2006 to September 2009 for Lwak (580 per 100,000) and March 2007 to February 2009 for Kibera (57 per 100,000 pyo) [2]. While the surveillance methods have remained stable over time, we found year to year variability in the invasive NTS disease burden in both sites. It is possible that the overall decline in invasive NTD infections we observed during the study is a reflection of that variability and not necessarily a sustained downward trend. These results highlight the importance of long-term population based surveillance in order to monitor the burden of invasive NTS infections and the impact of interventions such as malaria control programs, improved HIV prevention and care, nutrition programs, improved water and sanitation, and other future potential interventions such as an NTS vaccine [1, 11].
An important limitation to this study is the assumptions made in estimating incidence. We assume that the frequency of NTS we found in tested individuals is the same among those with the same clinical syndrome who did not have a specimen collected or those who sought care at a non-surveillance clinic. Such assumptions may be inaccurate and can lead to distortions in adjusted incidence estimates. Prior antimicrobial use is relatively common in the surveillance setting and may have led to an underestimate of the true burden of invasive NTS disease. Sparse HIV testing results limited our ability to make inferences regarding HIV and invasive NTS infections in this population. We also lack data on NTS serotype and subtype throughout the study period, so could not monitor trend in particular subtypes of interest, such as S. enterica serovar Typhimurium ST313.
Despite these limitations, we clearly show an important burden of invasive NTS infections in Kenya, particularly in the rural setting and among children <5 years old. Although invasive NTS disease incidence declined across both sites during the study time period, the remaining burden is still high and highlights the need for reducing exposure to NTS through improved water and food safety, as well as additional NTS prevention tools such as a vaccine. Further research is needed to better understand factors that may have contributed to the observed decline, and subtyping of isolates is necessary to fully understand trends in the burden of illness caused by NTS and to guide prevention interventions. Our results also demonstrate the dynamic nature of invasive NTS disease over time, across geographic regions and age groups, and emphasize the importance of continued surveillance to fully understand the disease burden.
Supplementary Material
Table 3.
Crude and adjusted incidence of invasive NTS in disease in Lwak and Kibera Kenya by year, January 2009 through December 2014
| Study Year | Site | Invasive NTS | pyo | Crude Rate per 100,000 pyo (95% CI) | Rate Extrapolation 1 ** per 100,000 pyo (95% CI) | Rate Extrapolation 2 ** per 100,000 pyo (95% CI) |
|---|---|---|---|---|---|---|
| 2009 | LWAK | 45 | 19,574.6 | 229.9 (171.6–307.9) | 850.6 (730.7–990.1) | 1428.7 (1270.7–1606.4) |
| KIBERA | 22 | 20,569.7 | 107 (70.4–162.4) | 140.1 (97.3–201.9) | 185.5 (135.0–254.7) | |
| 2010 | LWAK | 76 | 20,556.6 | 369.7 (295.3–462.9) | 1167.5 (1028.7–1324.9) | 1927.3 (1746.6–2126.8) |
| KIBERA | 24 | 19,075.7 | 125.8 (84.3–187.7) | 160.5 (112.6–228.7) | 218.5 (161.3–296.1) | |
| 2011 | LWAK | 23 | 21,862.2 | 105.2 (69.9–158.3) | 349.3 (279.1–437.2) | 608.5 (513.4–721.2) |
| KIBERA | 15 | 17,762.9 | 84.4 (50.9–140.1) | 154.0 (105.9–224.1) | 220.5 (161.2–301.5) | |
| 2012 | LWAK | 15 | 23,563.7 | 63.7 (38.4–105.6) | 185.0 (137.5–249) | 303.3 (240.6–382.5) |
| KIBERA | 4 | 16,844.4 | 23.7 (8.9–63.3) | 45.9 (22.7–92.9) | 62.5 (34.1–114.3) | |
| 2013 | LWAK | 34 | 24,880.0 | 136.7 (97.6–191.3) | 376.8 (307.8–461.4) | 745.5 (645.5–860.8) |
| KIBERA | 3 | 15,785.1 | 19.0 (6.1–58.9) | 73.2 (41.1–130.2) | 93.4 (56.1–155.6) | |
| 2014 | LWAK | 15 | 25,080.7 | 59.8 (36.1–99.2) | 161.0 (118.2–219.1) | 337.8 (273.0–418.0) |
| KIBERA | 2 | 14,486.2 | 13.8 (3.5–55.2) | 69.6 (37.5–129) | 87.2 (50.2–151.3) | |
| OVERALL | LWAK | 208 | 135,517.9 | 153.5 (134.0–175.8) | 471.9 (436.7–510) | 839.4 (792.0–889.7) |
| KIBERA | 70 | 104,524.0 | 67.0 (53.0–84.6) | 149.8 (128–175.2) | 202.5 (177.0–231.7) |
Extrapolated for patients meeting indication for blood culture who were not cultured in the clinic.
Extrapolated for patients meeting indication for blood culture who were not cultured in the clinic and those with same illness syndromes at the home visit who sought care at area clinics besides Lwak and Tabitha clinics.
Acknowledgments
We thank the people of Asembo and Kibera for their continued support and cooperation with KEMRI/CDC. We send our gratitude to Alfred Musekiwa for providing statistical support and Dorothy L. Southern and Susan Jack for providing guidance in scientific writing and critically reviewing this manuscript. The population-based infectious disease surveillance project from which these data were collected and analyzed is approved by the KEMRI Ethical Review Committee (ERC) and the CDC Institutional Review Board. This work was supported by the Centers for Disease Control and Prevention (CDC US). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This publication was made possible by a grant of the Bill & Melinda Gates Foundation (OPP1125993).
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC).
References
- 1.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. The Lancet. 2012;379(9835):2489–99. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabu C, Breiman RF, Ochieng B, et al. Differing Burden and Epidemiology of Non-Typhi Salmonella Bacteremia in Rural and Urban Kenya, 2006–2009. PLoS ONE. 2012;7(2):e31237. doi: 10.1371/journal.pone.0031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigaúque B, Roca A, Mandomando I, et al. Community-Acquired Bacteremia Among Children Admitted to a Rural Hospital in Mozambique. The Pediatric Infectious Disease Journal. 2009;28(2):108–13. doi: 10.1097/INF.0b013e318187a87d. [DOI] [PubMed] [Google Scholar]
- 4.Adejuyigbe EA, Ako-Nai AK, Adisa B. Bacterial Isolates in the Sick Young Infant in Ile-Ife, Nigeria. Journal of Tropical Pediatrics. 2004;50(6):323–7. doi: 10.1093/tropej/50.6.323. [DOI] [PubMed] [Google Scholar]
- 5.Berkley JA, Lowe BS, Mwangi I, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 6.Ao TT, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA. Global burden of invasive nontyphoidal Salmonella disease, 2010(1) Emerging infectious diseases. 2015;21(6) doi: 10.3201/eid2106.140999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott JAG, Berkley JA, Mwangi I, et al. Relation between falciparum malaria and bacteraemia in Kenyan children: a population-based, case-control study and a longitudinal study. The Lancet. 2011;378(9799):1316–23. doi: 10.1016/S0140-6736(11)60888-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brent AJ, Oundo JO, Mwangi I, Ochola L, Lowe B, Berkley JA. Salmonella Bacteremia in Kenyan Children. The Pediatric Infectious Disease Journal. 2006;25(3):230–6. doi: 10.1097/01.inf.0000202066.02212.ff. [DOI] [PubMed] [Google Scholar]
- 9.Mtove G, Amos B, Nadjm B, et al. Decreasing incidence of severe malaria and community-acquired bacteraemia among hospitalized children in Muheza, north-eastern Tanzania, 2006–2010. Malaria Journal. 2011;10(1):320. doi: 10.1186/1475-2875-10-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bronzan RN, Taylor TE, Mwenechanya J, et al. Bacteremia in Malawian Children with Severe Malaria: Prevalence, Etiology, HIV Coinfection, and Outcome. Journal of Infectious Diseases. 2007;195(6):895–904. doi: 10.1086/511437. [DOI] [PubMed] [Google Scholar]
- 11.Martin LB. Vaccines for typhoid fever and other salmonelloses. Current Opinion in Infectious Diseases. 2012;25(5):489. doi: 10.1097/QCO.0b013e328356ffeb. [DOI] [PubMed] [Google Scholar]
- 12.Desai M, Buff AM, Khagayi S, et al. Age-specific malaria mortality rates in the KEMRI/CDC health and demographic surveillance system in western Kenya, 2003–2010. PLoS ONE. 2014;9(9):e106197. doi: 10.1371/journal.pone.0106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maina WK, Kim AA, Rutherford GW, et al. Kenya AIDS Indicator Surveys 2007 and 2012: implications for public health policies for HIV prevention and treatment. Journal of acquired immune deficiency syndromes. 2014;66(Suppl 1):S130–7. doi: 10.1097/QAI.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minstry of Public Health and Sanitation. National Nutirion Action Plan 2012–2017. 2013 available at http://scalingupnutrition.org/wp-content/uploads/2013/10/Kenya-National-Nutrition-Action-Plan-2012-2017-final.pdf.
- 15.Feikin DR, Audi A, Olack B, et al. Evaluation of the optimal recall period for disease symptoms in home-based morbidity surveillance in rural and urban Kenya. International Journal of Epidemiology. 2009;39(2):450–8. doi: 10.1093/ije/dyp374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bigogo G, Amolloh M, Laserson K, et al. The impact of home-based HIV counseling and testing on care-seeking and incidence of common infectious disease syndromes in rural western Kenya. BMC Infectious Diseases. 2014;14(1):376. doi: 10.1186/1471-2334-14-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oehlert G. A note on the delta method. American Statistician. 1992;(46):26–9. [Google Scholar]
- 18.World Health Organization. [Accessed 15 June];WHO Anthro (version 3.2.2) and macros. Available at: http://www.who.int/childgrowth/software/en/
- 19.Mackenzie G, Ceesay SJ, Hill PC, et al. A Decline in the Incidence of Invasive Non-Typhoidal Salmonella Infection in the Gambia Temporally Associated with a Decline in Malaria Infection. PLoS ONE. 2010;5(5):e10568. doi: 10.1371/journal.pone.0010568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takem E, Roca A, Cunnington A. The association between malaria and non-typhoid Salmonella bacteraemia in children in sub-Saharan Africa: a literature review. Malaria Journal. 2014;13(1):400. doi: 10.1186/1475-2875-13-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham SM. Nontyphoidal salmonellosis in Africa. Curr Opin Infect Dis. 2010;23(5):409–14. doi: 10.1097/QCO.0b013e32833dd25d. [DOI] [PubMed] [Google Scholar]
- 22.Bloss E, Wainaina F, Bailey RC. Prevalence and predictors of underweight, stunting, and wasting among children aged 5 and under in western Kenya. J Trop Pediatr. 2004;50(5):260–70. doi: 10.1093/tropej/50.5.260. [DOI] [PubMed] [Google Scholar]
- 23.Olack B, Burke H, Cosmas L, et al. Nutritional status of under-five children living in an informal urban settlement in Nairobi, Kenya. Journal of health, population, and nutrition. 2011;29(4):357–63. doi: 10.3329/jhpn.v29i4.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enwere G, Biney E, Cheung YB, et al. Epidemiologic and clinical characteristics of community-acquired invasive bacterial infections in children aged 2–29 months in The Gambia. Pediatr Infect Dis J. 2006;25(8):700–5. doi: 10.1097/01.inf.0000226839.30925.a5. [DOI] [PubMed] [Google Scholar]
- 25.Brent AJ, Ahmed I, Ndiritu M, et al. Incidence of clinically significant bacteraemia in children who present to hospital in Kenya: community-based observational study. The Lancet. 2006;367(9509):482–8. doi: 10.1016/S0140-6736(06)68180-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.