Abstract
Background
Adiponectin levels display ethnic disparities, and are inversely associated with the risk of type 2 diabetes (T2DM). However, the association of adiponectin with prediabetes risk in diverse populations has not been well-studied. Here, we assessed baseline adiponectin levels in relation to incident prediabetes in a longitudinal biracial cohort.
Research design and methods
The Pathobiology of Prediabetes in A Biracial Cohort study followed non-diabetic offspring of parents with T2DM for the occurrence of prediabetes, defined as impaired fasting glucose and/or impaired glucose tolerance. Assessments at enrollment and during follow-up included a 75 g oral glucose tolerance test, anthropometry, biochemistries (including fasting insulin and adiponectin levels), insulin sensitivity and insulin secretion. Logistic regression was used to evaluate the contribution of adiponectin to risk of progression to prediabetes.
Results
Among the 333 study participants (mean (SD) age 44.2 (10.6) year), 151(45.3%) were white and 182 (54.8%) were black. During approximately 5.5 (mean 2.62) years of follow-up, 110 participants (33%) progressed to prediabetes (N=100) or T2DM (N=10), and 223 participants (67%) were non-progressors. The mean cohort adiponectin level was 9.41+5.30 μg/mL (range 3.1–45.8 μg/mL); values were higher in women than men (10.3+5.67 μg/mL vs 7.27+3.41 μg/mL, p<0.0001) and in white than black offspring (10.7+5.44 μg/mL vs 8.34+4.95 μg/mL, p<0.0001). Adiponectin levels correlated inversely with adiposity and glycemia, and positively with insulin sensitivity and high-density lipoprotein cholesterol levels. Baseline adiponectin strongly predicted incident prediabetes: the HR for prediabetes per 1 SD (approximately 5 μg/mL) higher baseline adiponectin was 0.48 (95% CI 0.27 to 0.86, p=0.013).
Conclusions
Among healthy white and black adults with parental history of T2DM, adiponectin level is a powerful risk marker of incident prediabetes. Thus, the well-known association of adiponectin with diabetes risk is evident at a much earlier stage in pathogenesis, during transition from normoglycemia to prediabetes.
Keywords: Adiponectin, Insulin Sensitivity, Impaired Fasting Glucose, Impaired Glucose Tolerance
Key messages.
In a large sample of healthy African-American and Caucasian offspring of parents with type 2 diabetes, higher adiponectin levels are associated with a favorable cardiometabolic profile, characterized by lower measures of adiposity and glycemia, lower triglyceride levels, and higher high-density lipoprotein cholesterol levels.
Using the rigorous methodologies of hyperinsulinemic euglycemic clamp and frequently sampled intravenous glucose tolerance test, this study demonstrates that plasma adiponectin levels are correlated directly with insulin sensitivity and inversely with insulin secretion in healthy African-Americans and Caucasians.
This study is the first to demonstrate that baseline plasma adiponectin levels predict progression from normoglycemia to prediabetes in African-Americans and Caucasians, despite the known ethnic differences in adiponectin levels.
Introduction
Adiponectin, the most abundant secreted product of adipocytes, is strongly correlated with cardiometabolic risk.1–4 Several previous studies have shown that adiponectin levels are positively associated with insulin sensitivity, and inversely associated with the development of diabetes and progression from prediabetes to type 2 diabetes (T2DM).1 5–11 The development of T2DM in individuals at genetic risk is punctuated by a variable interlude of prediabetes, defined as impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT).12–16 Persons with IGT or IFG develop T2DM at an annual rate of approximately 10%.17 Although the evidence linking adiponectin to diabetes risk is strong,1–11 the chronology of the association between adiponectin status and glucose homeostasis has not been fully determined. Specifically, the potential role of adiponectin in modulating early glucose abnormalities during transition from normoglycemia to prediabetes is a subject of significant interest. Since both the prevalence of T2DM and the distribution of plasma levels of adiponectin display significant racial/ethnic disparities,18–22 it is important to assess the fidelity of the interaction between adiponectin and dysglycemia in a diverse population.
The Pathobiology of Prediabetes in A Biracial Cohort (POP-ABC) study is a prospective, natural history study of incident prediabetes in African-Americans and European Americans who have one or both biological parents with T2DM.23–25 Subjects who qualified for this study were by definition at increased risk of future diabetes, on the basis of their parental history.26 Recently, we reported that the incidence of prediabetes among initially normoglycemic participants enrolled in the POP-ABC study was approximately 11%/year.27 However, it remains to be determined whether the association observed between adiponectin levels and the risk of T2DM extends to prediabetes as well. Here, we have tested the hypothesis that higher adiponectin levels at baseline are associated with decreased rates of progression to prediabetes in our biracial cohort.
Research design and methods
Study subjects
The study subjects were participants in the POP-ABC study.23–25 Eligibility criteria for the POP-ABC study included age 18–65 years; self-reported non-Hispanic white (European American) or non-Hispanic black (African-American) race/ethnicity status; one or both biological parents with T2DM; no evidence of diabetes; normal fasting plasma glucose (FPG) (<100 mg/dL(5.6 mmol/L)) and/or normal glucose tolerance (NGT) (2 h plasma glucose (2 hPG) <140 mg/dL (7.8 mmol/L) during a 75 g oral glucose tolerance test (OGTT)); and good overall health, as previously described.23–25
The exclusion criteria were prevalent diagnosis of diabetes; use of antidiabetes medications or medications known to alter blood glucose; enrollment in behavioral, pharmacological, or other active weight loss program; history of liposuction or bariatric surgery; current pregnancy or being within 12 months postpartum; and any recent hospitalization (within 6 weeks of the screening visit).23–25
Individuals self-reported their race/ethnicity, based on the 1990 U.S. Census questionnaire.28 Parental history of T2DM was documented using a diabetes-focused questionnaire that captured information on the number of affected biological parents, parent's gender and age at diagnosis, use of antidiabetes medications, diabetes complications and contact information of the parents’ physicians.23–25 27 The study protocol was approved by the Institutional Review Board; all participants gave written informed consent before initiation of the study, which was conducted at the University of Tennessee General Clinical Research Center (GCRC).
Assessments
Enrolled participants arrived at the GCRC after an overnight fast for baseline assessments, which included a structured medical interview and a general physical examination; measurement of weight, height, waist circumference and blood pressure; and a standard 75 g OGTT.23 25 27 The body-mass index (BMI) was calculated as the weight in kilogram divided by the height in meter squared. The OGTT was preceded by written instructions to consume a usual diet with adequate carbohydrates, refrain from strenuous exercise and alcohol consumption for 24 h, and avoid smoking in the morning of the test. The test was initiated between 07:00 and 11:00 in participants who had been fasting for approximately 10–14 h: venous blood specimens for glucose measurement were obtained before (0 min) and at 30 min and 120 min after ingestion of 75 g flavored glucose (Trutol 75; Custom Laboratories, Baltimore, Maryland, USA). Additional baseline measurements included glycated haemoglobin (HbA1c), fasting lipid profile, insulin and adiponectin levels. The homeostasis model of insulin resistance (HOMA-IR) was derived from fasting glucose and insulin values.29 Following the baseline visit, FPG was measured quarterly and OGTT annually, in addition to other prespecified assessments (including anthropometry, clinical examination, biochemistries, body composition, insulin secretion and insulin sensitivity by hyperinsulinemic euglycemic clamp), as previously described.23 25 27
Insulin sensitivity and insulin secretion
In brief, the hyperinsulinemic euglycemic clamp procedure was performed in participants who had fasted overnight for approximately 12 h. A primed, continuous intravenous infusion of regular insulin (2 mU/kg/min;14.4 pmol/kg/min) was administered for 180 min while blood glucose concentration was maintained at approximately 100 mg/dL (5.6 mmol/L) with a variable rate dextrose (20%) infusion. Arterialized blood specimens for measurement of glucose and insulin levels were obtained every 10 min. The rate of total insulin-stimulated glucose disposal (M) was calculated for the past 60 min of insulin infusion, and corrected for steady-state plasma insulin levels, to derive the insulin sensitivity index (Si-clamp).30 31
The frequently sampled intravenous glucose tolerance test (FSIVGTT) was used for the direct assessment of insulin secretion.13 27 After an overnight fast, participants received an intravenous bolus of dextrose (25 g). Arterialized blood samples for the measurement of glucose and insulin levels were collected 30 min before and at 2, 3, 4, 5, 7 and 10 min following the intravenous dextrose bolus.13 27 The acute insulin response (AIR) was computed as the mean incremental insulin concentration from 3 to 5 min after the dextrose bolus.13 27
Biochemical measurements
Plasma glucose was measured with a glucose oxidase method (Yellow Spring Instruments Co, Inc, Yellow Spring, Ohio, USA). Plasma levels of insulin and adiponectin were measured immunochemically in our Endocrine Research Laboratory, using commercial ELISA kits. The total adiponectin assay (ELISA kit EZHADP-61K, Millipore, St. Charles, Missouri, USA) captured all the multimeric forms of circulating adiponectin, with a sensitivity of 0.78 ng/mL and within-batch and between-batch coefficients of variation of 1.8% and 6.2%, respectively. HbA1c and fasting plasma lipid profiles were measured in a contract clinical laboratory.
Definition of outcome measures
The primary outcome was the occurrence of prediabetes (IFG and/or IGT) or diabetes.27 For all partiicipants, any occurrence of diabetes, as indicated by an FPG value of 126 mg/dL (7.0 mmol/L) or higher or 2 hPG of 200 mg/dL (11.1 mmol/L) or higher, or prescription of a diabetes medication, was an end point. Of the enrollees, 75% met the dual criteria of having normal FPG and NGT, and 25% had either normal FPG or NGT at baseline. For participants enrolled with normal FPG and NGT, the occurrence of IFG and/or IGT constituted an end point. For those enrolled with normal FPG (and isolated IGT), progression to IFG constituted an end point. For those enrolled with NGT (and isolated IFG), progression to IGT was an end point.27 A confirmatory test was performed within 6 weeks for each end point occurrence. The 75 g OGTT was the method of confirmation. All end points were independently adjudicated by the Institutional Data and Safety Officer (Murray Heimberg, MD, PhD).
Statistical analysis
Data were reported as means±SD. Differences between defined groups were analyzed using unpaired t tests for continuous variables and χ2 test for categorical variables. General linear regression models were used to compare anthropometric and metabolic characteristics between African-Americans and Caucasians and between participants who experienced glycemic progression and no-progressors. The relationship between log-transformed adiponectin concentrations (to achieve a more normal distribution) and anthropometric and metabolic variables was analyzed using Pearson correlation coefficients. Logistic regression models were used to analyze baseline adiponectin values as predictors of incident diabetes/prediabetes, after adjustments for age, sex and BMI. All statistical analyses were performed with the use of SAS statistical software, V.9.3 (SAS Institute Inc, Cary, North Carolina, USA).
Results
Cohort description
A total of 376 offspring of parents with T2DM (217 black, 159 white), who met all eligibility criteria, were enrolled in the main POP-ABC study.24 25 The 333 study offspring who had complete adiponectin values and evaluable follow-up data are included in the present report. Of the 333 participants, 151(45.3%) were white and 182 (54.8%) were black; their mean age was 44.2+10.6 years and BMI was 30.2+7.23 kg/m2. Baseline adiponectin levels were widely distributed among POP-ABC study participants; values ranged from 3.14 to 45.8 μg/mL, in a skewed distribution. The median adiponectin value was 7.96 μg/mL, and the mean value was 9.41 μg/mL. The previously reported gender and ethnic differences in adiponectin levels 20–22 were evident in our cohort: values were higher in women than men (10.3+5.67 μg/mL vs 7.27+3.41μg/mL, p<0.0001) and in white offspring than black offspring (10.7+5.44 μg/mL vs 8.34+4.95 μg/mL, p<0.0001). At enrollment, the black participants had higher BMI and HbA1c values, and lower age and FPG levels, compared to white participants (table 1).
Table 1.
Baseline characteristics and adiponectin levels in 333 offspring of parents with type 2 diabetes
| Characteristic | White | Black | p Value |
|---|---|---|---|
| Number | 151 | 182 | |
| Age (years) | 46.5±10.5 | 42.5±10.3 | 0.0003 |
| BMI (kg/m2) | 28.8±6.8 | 31.2±7.4 | 0.0011 |
| Waist circumference (cm) | 92±17 | 95±16 | 0.09 |
| Female | 90±16 | 95±15 | 0.02 |
| Male | 99±11 | 97±18 | 0.73 |
| Fasting plasma glucose (mg/dL) | 92.1±7.58 | 90.0±7.74 | 0.0067 |
| 2 h plasma glucose (mg/dL) | 125±23.4 | 124±28.6 | 0.66 |
| Adiponectin (μg/mL) | 10.7±5.44 | 8.34±4.95 | <0.0001 |
Plus–minus values are means±SD. To convert the values for glucose to millimoles per liter, multiply by 0.0555. To convert adiponectin concentrations to micromoles per L, divide by 30.
BMI, body-mass index.
Metabolic correlates of adiponectin
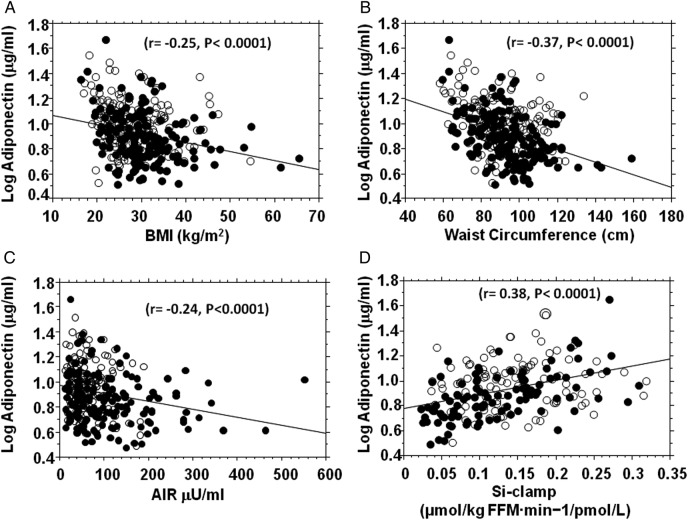
We observed significant univariate relationships between adiponectin and several metabolic variables in our biracial cohort. Plasma adiponectin concentration was negatively correlated with BMI, waist circumference, blood pressure, FPG, triglycerides, fasting insulin, HOMA-IR and insulin secretion, and positively correlated with age, HDL cholesterol and insulin sensitivity (table 2 and figure 1). These associations were nominally and directionally consistent, though of variable strengths, in black and white participants (table 2 and figure 1). The strongest associations that were concordant in both ethnic groups were between baseline adiponectin and waist circumference (Black r=−0.45, p<0.0001; White r=−0.28, p=0.0006), FPG (Black: r=−0.34, p=<0.0001; White r=−0.20, p=0.015), HDL cholesterol (Black r=0.53, p<0.0001; White r=0.50, p<0.0001), triglycerides (Black r=−0.37, p<0.0001; White r=−0.35, p<0.0001) and insulin secretion (AIR) (Black r=−0.17; p=0.024; White r=−0.21, p=0.011; table 2). The association of adiponectin levels with age was much stronger in white participants than black participants, whereas the association with measures of insulin sensitivity (fasting insulin, HOMA-IR and Si-clamp) was much stronger in African-American participants than European American participants (table 2).
Table 2.
Univariate associations of adiponectin with age and metabolic variables
| All participants | Black Offspring | White Offspring | ||||
|---|---|---|---|---|---|---|
| Variable | r | p Value | r | p Value | r | p Value |
| Age (years) | 0.18 | 0.0013 | 0.09 | 0.24 | 0.20 | 0.016 |
| BMI (kg/m2) | −0.25 | <0.0001 | 0.28 | 0.0001 | 0.12 | 0.14 |
| Waist (cm) | −0.37 | <0.0001 | 0.45 | <0.0001 | 0.28 | 0.0006 |
| Systolic BP (mm Hg) | −0.12 | 0.033 | −0.14 | 0.06 | −0.06 | 0.46 |
| Diastolic BP (mm Hg) | −0.20 | 0.0003 | −0.29 | 0.0003 | −0.10 | 0.17 |
| FPG (mg/dL) | −0.21 | 0.0002 | −0.34 | <0.0001 | −0.20 | 0.015 |
| HDL cholesterol (mg/dL) | 0.49 | <0.0001 | 0.53 | <0.0001 | 0.50 | <0.0001 |
| Triglycerides (mg/dL) | −0.24 | <0.0001 | −0.37 | <0.0001 | −0.35 | <0.0001 |
| Fasting insulin (µU/mL) | −0.25 | <0.0001 | 0.41 | <0.0001 | −0.14 | 0.11 |
| AIR (µU/mL) | −0.24 | <0.0001 | −0.17 | 0.024 | −0.21 | 0.011 |
| HOMA-IR | −0.23 | <0.0001 | −0.36 | <0.0001 | −0.11 | 0.21 |
| Si-clamp | 0.38 | <0.0001 | 0.56 | <0.0001 | 0.13 | 0.19 |
AIR, acute insulin response; BMI, body mass index; BP, blood pressure; FPG, fasting plasma glucose; HDL chol, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model of insulin resistance; Si-clamp, insulin sensitivity by euglycemic clamp (N=203(103 black, 100 white)).
Figure 1.
Fasting plasma adiponectin concentration in relation to body mass index (BMI) (A), waist circumference (B), acute insulin response (AIR) (C) and insulin sensitivity (Si-clamp) (D) in non-diabetic black (closed circles) and white (open circles) offspring of parents with type 2 diabetes. Acute insulin secretory response was assessed using the frequently sampled intravenous glucose tolerance test (N=300 (163 black, 137 white) and insulin sensitivity was measured by hyperinsulinemic euglycemic clamp (N=203(103 black, 100 white)).
Adiponectin and progression to prediabetes
During approximately 5.5 years of follow-up (mean 2.62 years), 110 participants (33%, ‘Progressors’) developed prediabetes (N=100) or diabetes (N=10), and 223 participants (67%) remained free of incident prediabetes/diabetes (‘Non-progressors’). In our previous report, it was noted that POP-ABC participants who progressed to prediabetes/diabetes had higher age, BMI and FPG at baseline, and were more likely to be male, but self-reported race/ethnicity was not a predictor of glycemic progression.27 In the present report, participants who progressed to prediabetes/diabetes had a lower mean plasma adiponectin level at baseline, compared with non-progressors, (8.37+4.02 μg/mL vs 9.92+5.77 μg/mL, p=0.012). The median value for adiponectin was 7.13 μg/mL (range 3.14–23.1) in progressors and 8.54 μg/mL (range 3.18–45.8) in non-progressors. To account for the gender and ethnic differences in adiponectin levels, we examined the relationship between adiponectin and glycemic progression status separately in men and women, and in black and white participants. Our analysis showed that the pattern of lower plasma adiponectin levels in progressors compared to non-progressors was consistent across ethnicity and gender (figure 2). In logistic regression models, adiponectin levels (adjusted for age and sex) were inversely associated with progression to prediabetes/diabetes: the OR (95% CI) was 0.48 (95% CI 0.27 to 0.86), p=0.013 (table 3). After additional adjustment for BMI, the OR was 0. 61(95% CI 0.32 to 1.14), p=0.12 (table 3). Thus, a 1-SD (approximately 5 μg/mL) higher baseline adiponectin level was associated with approximately 40% lower rate of progression to prediabetes.
Figure 2.
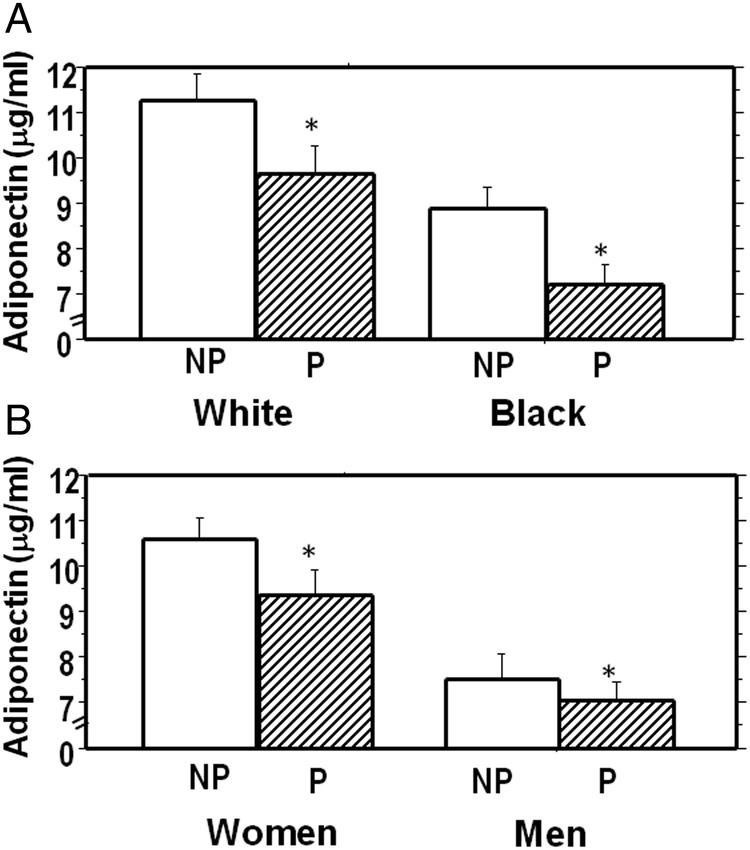
Plasma adiponectin levels by race/ethnicity (1A) and gender (1B) among study participants who developed prediabetes/diabetes (Progressors, P) compared to those who remained free of incident prediabetes (Non-Progressors, NP) *p=0.012, Progressor versus Non-progressor groups.
Table 3.
Logistic regression models of adiponectin as a predictor of prediabetes*
| OR | 95% CI | p Value | |
|---|---|---|---|
| All participants | |||
| Adiponectin (per 1SD) | 0.48 | 0.27 to 0.86 | 0.013 |
| Black offspring | |||
| Adiponectin (per 1SD) | 0.44 | 0.20 to 0.95 | 0.033 |
| White offspring | |||
| Adiponectin (per 1SD) | 0.48 | 0.20 to 1.13 | 0.093 |
*Adjusted for age and sex. 1 SD, one SD (approximately 5 μg/mL) from mean adiponectin level of the cohort's distribution.
Discussion
In our biracial cohort study of healthy offspring of patients with diabetes, we found an inverse association between adiponectinemia and the risk of incident prediabetes/diabetes during longitudinal follow-up. Among initially normoglycemic persons, an approximately 5 μg/dL higher baseline plasma adiponectin level corresponded to approximately 40% lower rate of progression to prediabetes/diabetes during a mean follow-up period of 2.6 years. Among the 110 participants who reached an end point, only 10 participants progressed directly to T2DM, whereas the vast majority (100 participants) progressed to prediabetes. Thus, our findings are particularly germane to the pathobiology of prediabetes. Despite the well-known ethnic and gender differences in plasma adiponectin levels,20–22 which were evident in the present study, the association of adiponectin status with incident prediabetes was quite consistent across gender and ethnicity in our cohort of African-Americans and European Americans with parental history of T2DM. The point estimates of decreased risk for prediabetes were of the same order of magnitude in black and white offspring (0.44 and 0.48, respectively), although the 95% CI was wider among the latter. Notably, adjustment for BMI increased the adiponectin-associated OR for prediabetes from 0.48 to 0.61, with a wider 95% CI. However, the observation of a nominal reduction (approximately 40%) in the risk of incident prediabetes, despite adjustment for BMI, suggests that the decreased prediabetes risk associated with higher baseline adiponectin levels is partly but not entirely mediated by adiposity-related mechanisms.
Several previous cross-sectional and longitudinal studies have established that low adiponectin levels are associated with increased risk of T2DM.5–11 In the prospective Diabetes Prevention Programme (DPP), which enrolled participants with prediabetes, an approximately 3 μg/mL higher baseline adiponectin level predicted a 20–40% lower risk of progression to T2DM during approximately 3 years of follow-up.11 In the present report, we found that an approximately 5 μg/mL (1 SD) higher baseline adiponectin levels predicted a 40% lower risk of progression from normoglycemia to prediabetes. The latter finding indicates that the association of adiponectin with diabetes risk is evident at a much earlier stage in the pathogenesis of dysglycemia. Thus, the ability to maintain high adiponectin production might be protective of dysglycemia in persons at high risk for T2DM, whereas hypoadiponectinemia could be a risk factor for the initiation of early glucose abnormalities leading to prediabetes and diabetes.
These notions are consistent with the favorable cardiometabolic profile of adiponectin that has emerged since its discovery.1–11 32 33 The mechanisms whereby adiponectin exerts its favorable cardiometabolic effects are not fully understood, but could involve improvement in insulin action, decreased inflammatory tone, interaction with fibroblast growth factor (FGF)-21 and amelioration of lipotoxicity, among others.34–39 In rodent models, overexpression of adiponectin is associated with several beneficial effects, including improved metabolic flexibility, decreased inflammatory markers, decreased steatosis, improved insulin sensitivity, decreased apoptosis and preservation of β cell function.35 36 An interaction between adiponectin and FGF21, a potent regulator of metabolism and energy utilization, has also been reported.38 39 The administration of FGF-21 decreases blood glucose levels and increases insulin sensitivity in mouse models of obesity and insulin resistance, through upregulation of adiponectin expression.39 Furthermore, FGF-21 is involved in the clearance of toxic ceramides in obese animals, and adiponectin-knockout mice appear to be refractory to the ceramide-lowering effects of FGF-21.39 These data indicate that adiponectin may be a critical mediator of the favorable metabolic and insulin-sensitizing effects FGF-21.39 40 At the clinical level, the improvement in insulin sensitivity following treatment with PPAR-γ agonists has been shown to be mediated, at least in part, by upregulation of adiponectin expression.41 42
From the foregoing, interventions that increase adiponectin levels would be attractive candidates for clinical trials, with the goal of diabetes prevention and improvement in cardiometabolic health. Interventions that have been reported to increase adiponectin levels include exercise,43 weight loss,44 smoking cessation45 and treatment with PPAR-γ agonists,41 42 46 47 PPAR-α agonists,48 ACE inhibitors 49 and angiotensin II receptor blockers.50 In clinical trials, many of the aforementioned interventions have been associated with favorable glucoregulatory outcomes. Indeed, prediabetic participants randomized to the lifestyle intervention arm in the DPP experienced an increase in circulating adiponectin levels, and showed a 58% relative risk reduction in incident diabetes, compared to the placebo group.11
Cross-sectional and longitudinal studies have previously reported a consistent association between low adiponectin levels and prevalent T2DM, impaired glucose homeostasis and an unfavorable cardiometabolic profile.2–11 The present report has several strengths, by extending the previous observations in important directions. First, we demonstrate in a prospective study that the previously reported association of lower adiponectin levels with increased risk of T2DM manifests at a more proximal stage, and is evident during transition from normoglycemia to prediabetes. Second, our findings suggest that the putative mechanisms whereby adiponectinemia interacts with glucose homeostasis to confer protection against dysglycemia are operative even in persons who are among the highest risk groups for diabetes, namely, offspring of parents with T2DM. Third, our findings were obtained from a biracial cohort and were consistent in men and women, indicating that the known gender and ethnic differences in adiponectin expression do not abrogate the interaction between adiponectinemia and glucose homeostasis. Finally, we have used rigorous methods (including hyperinsulinemic euglycemic clamp and FSIVGTT) to acquire novel biracial data on the associations between adiponectinemia and insulin action, insulin secretion and dyslipidemia in otherwise healthy African-Americans and European Americans. Our data indicate that the association of adiponectin with insulin sensitivity is particularly robust among African-Americans (table 2), which suggests that interventions that increase adiponectin secretion may have enhanced insulin-sensitizing potency in African-Americans.
The present report has some limitations related to the population studied: our findings were obtained from offspring of parents with T2DM. As these participants represent a selected group at high risk for diabetes, the strong association between baseline adiponectin levels and incident prediabetes may not be exactly applicable to individuals without a family history of diabetes, or the general population. Also, our analyses focused on baseline adiponectin levels; thus, our assessment did not include the potential effects of changes in adiponectin secretion that may have occurred during the course of the POP-ABC study.
In conclusion, among healthy African Americans and European Americans with parental history of T2DM enrolled in the POP-ABC study, baseline adiponectin levels were inversely related to the risk of incident prediabetes. This predictive relationship was evident, despite gender and ethnic differences in baseline adiponectin levels. Based on our finding, it can be predicted that interventions that boost adiponectin levels may offer protection against the risk of dysglycemia, regardless of gender or ethnicity.
Acknowledgments
The POP-ABC study was supported by Grants R01 DK067269 and R01 DK067269-04S1 from the National Institutes of Health and Grant 7-07-MN-13 from the American Diabetes Association. The authors are indebted to the participants who volunteered for this study.
Footnotes
Collaborators: Current Samuel Dagogo-Jack, MD (Principal Investigator), Ann Ammons, BS, Fatoumatta Ceesay, BS, Sotonte Ebenibo, MBBS, MPH, Ibiye Owei, MBBS, MPH, Nkiru Umekwe, MBBS, Jim Wan, PhD. Past members: Emmanuel Chapp-Jumbo, MBBS (2009–2011), Chimaroke Edeoga, MBBS, MPH (2007–2013), Ruben Cuervo, MD (2006–2007), Nonso Egbuonu, MBBS (2007–2010), Nicoleta Ionica, MD (2007–2008), Dorota Malinowski, MD (2007–2008). Consultant Steven Haffner, MD; Data and Safety Officer: Murray Heimberg, MD, PhD.
Contributors: SE collected data, reviewed and revised the manuscript. YJ and IO collected data, reviewed and revised the manuscript. JW performed statistical analysis, reviewed and revised the manuscript. SD-J is the guarantor.
Funding: The funding sources (National Institutes of Health, American Diabetes Association) had no role in the design and execution of the POP-ABC study, or analysis and publication of the data obtained from the study.
Competing interests: None declared.
Ethics approval: University of Tennessee Institutional Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
Contributor Information
Collaborators: Ann Ammons, Fatoumatta Ceesay, Ibiye Owei, Nkiru Umekwe, Emmanuel Chapp-Jumbo, Chimaroke Edeoga, Ruben Cuervo, Nonso Egbuonu, Nicoleta Ionica, Dorota Malinowski, Steven Haffner, and Murray Heimberg
References
- 1.Scherer PE, Williams S, Fogliano M et al. . A novel serum protein similar to c1q produced exclusively in adipocytes. J Biol Chem 1995;270:26746–9. 10.1074/jbc.270.45.26746 [DOI] [PubMed] [Google Scholar]
- 2.Snehalatha C, Mukesh B, Simon M et al. . Plasma adiponectin is an independent predictor of type 2 diabetes in Asian Indians. Diabetes Care 2003;26:3226–9. [DOI] [PubMed] [Google Scholar]
- 3.Halperin F, Beckman JA, Patti ME et al. . The role of total and high molecular-weight complex of adiponectin in vascular function in offspring whose parents both had type 2 diabetes. Diabetologia 2005;48:2147–54. [DOI] [PubMed] [Google Scholar]
- 4.Kadowaki T, Yamauchi T, Kubota N et al. . Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 2006;116:1784–92. 10.1172/JCI29126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein BJ, Scalia R. Adipokines and vascular disease in diabetes. Curr Diab Rep 2007;7:25–33. 10.1007/s11892-007-0006-6 [DOI] [PubMed] [Google Scholar]
- 6.Lindsay RS, Funahashi T, Hanson RL et al. . Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 2002;360:57–8. 10.1016/S0140-6736(02)09335-2 [DOI] [PubMed] [Google Scholar]
- 7.Duncan BB, Schmidt MI, Pankow JS et al. . Adiponectin and the development of type 2 diabetes: the Atherosclerosis Risk in Communities Study. Diabetes 2004;53:2473–8. [DOI] [PubMed] [Google Scholar]
- 8.Spranger J, Kroke A, Mohlig M et al. . Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003;52:812–7. 10.2337/diabetes.52.3.812 [DOI] [PubMed] [Google Scholar]
- 9.Knobler H, Benderly M, Boyko V et al. . Adiponectin and the development of diabetes in patients with coronary artery disease and impaired fasting glucose. Eur J Endocrinol 2006;154:87–92. 10.1530/eje.1.02054 [DOI] [PubMed] [Google Scholar]
- 10.Choi KM, Lee J, Lee KW et al. . Serum adiponectin concentrations predict the developments of type 2 diabetes and the metabolic syndrome in elderly Koreans. Clin Endocrinol (Oxf) 2004;61:75–80. 10.1111/j.1365-2265.2004.02063.x [DOI] [PubMed] [Google Scholar]
- 11.Mather KJ, Funahashi T, Matsuzawa Y et al. . Adiponectin, change in adiponectin, and progression to diabetes in the Diabetes Prevention Program. Diabetes 2008;57:980–6. 10.2337/db07-1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edelstein SL, Knowler WC, Bain RP et al. . Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 1997;46:701–10. 10.2337/diab.46.4.701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weyer C, Bogardus C, Mott DM et al. . The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999;104:787–94. 10.1172/JCI7231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genuth S, Alberti KG, Bennett P et al. . The expert committee on the diagnosis and classification of diabetes mellitus: 2003 follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–7. 10.2337/diacare.26.12.3331 [DOI] [PubMed] [Google Scholar]
- 15.Meigs JB, Muller DC, Nathan DM et al. . Baltimore Longitudinal Study of Aging. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes 2003;52:1475–84. 10.2337/diabetes.52.6.1475 [DOI] [PubMed] [Google Scholar]
- 16.Nyenwe EA, Dagogo-Jack S. Metabolic syndrome, prediabetes and the science of primary prevention. Minerva Endocrinol 2011;36:129–45. [PubMed] [Google Scholar]
- 17.Knowler WC, Barrett-Connor E, Fowler SE et al. . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brancati FL, Kao WH, Folsom AR et al. . Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA 2000;283:2253–9. 10.1001/jama.283.17.2253 [DOI] [PubMed] [Google Scholar]
- 19.Selvin E, Parrinello CM, Sacks DB et al. . Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med 2014;160:517–25. 10.7326/M13-2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardener H, Crisby M, Sjoberg C et al. . Serum adiponectin in relation to race-ethnicity and vascular risk factors in the Northern Manhattan Study. Metab Syndr Relat Disord 2013;11:46–55. 10.1089/met.2012.0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schutte AE, Huisman HW, Schutte R et al. . Differences and similarities regarding adiponectin investigated in African and Caucasian women. Eur J Endocrinol 2007;157:181–8. 10.1530/EJE-07-0044 [DOI] [PubMed] [Google Scholar]
- 22.Hulver MW, Saleh O, MacDonald KG et al. . Ethnic differences in adiponectin levels. Metabolism 2004;53:1–3. 10.1016/j.metabol.2003.07.002 [DOI] [PubMed] [Google Scholar]
- 23.Dagogo-Jack S, Edeoga C, Nyenwe E et al. . Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC): design and methods. Ethn Dis 2011;21:33–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Ebenibo S, Edeoga C, Ammons A et al. . Recruitment strategies and yields for the Pathobiology of Prediabetes in a Biracial Cohort: a prospective natural history study of incident dysglycemia. BMC Med Res Methodol 2013;13:64 10.1186/1471-2288-13-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dagogo-Jack S, Edeoga C, Ebenibo S et al. . Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC) study: baseline characteristics of enrolled subjects. J Clin Endocrinol Metab 2013;98:120–8. 10.1210/jc.2012-2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes 2000;49:2201–7. 10.2337/diabetes.49.12.2201 [DOI] [PubMed] [Google Scholar]
- 27.Dagogo-Jack S, Edeoga C, Ebenibo S et al. , Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC) Research Group. Lack of racial disparity in incident prediabetes and glycemic progression among black and white offspring of parents with type 2 diabetes: the Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC) Study. J Clin Endocrinol Metab 2014;99:E1078–87. 10.1210/jc.2014-1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bureau of the Census. Census of the population. Washington DC, U.S. Government Printing Office, 1990. [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS et al. . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 30.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–223. [DOI] [PubMed] [Google Scholar]
- 31.Ebenibo S, Edeoga C, Wan J et al. . Glucoregulatory function among African Americans and European Americans with normal or pre-diabetic hemoglobin A1c levels. Metab Clin Exp 2014;63:767–72. 10.1016/j.metabol.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arita Y, Kihara S, Ouchi N et al. . Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999;257:79–83. 10.1006/bbrc.1999.0255 [DOI] [PubMed] [Google Scholar]
- 33.Weyer C, Funahashi T, Tanaka S et al. . Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 2001;86: 1930–5. 10.1210/jcem.86.5.7463 [DOI] [PubMed] [Google Scholar]
- 34.Yamauchi T, Kamon J, Waki H et al. . The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001;7:941–6. 10.1038/90984 [DOI] [PubMed] [Google Scholar]
- 35.Kusminski CM, Holland WL, Sun K et al. . MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med 2012;18:1539–49. 10.1038/nm.2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holland WL, Miller RA, Wang ZV et al. . Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 2011;17:55–63. 10.1038/nm.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willarreal-Molina MT, Antuna-Puente B. Adiponectin: anti-inflammatory and cardioprotective effect. Biochimie 2012;94:2143–9. 10.1016/j.biochi.2012.06.030 [DOI] [PubMed] [Google Scholar]
- 38.Goetz R. Metabolism: adiponectin-a mediator of specific metabolic actions of FGF21. Nat Rev Endocrinol 2013;9:506–8. 10.1038/nrendo.2013.146 [DOI] [PubMed] [Google Scholar]
- 39.Holland WL, Adams AC, Brozinick JT et al. . An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab 2013;17:790–7. 10.1016/j.cmet.2013.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez X, Goldfine AB, Holland WL et al. . Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. J Pediatr Endocrinol Metab 2013;26:995–8. 10.1515/jpem-2012-0407 [DOI] [PubMed] [Google Scholar]
- 41.Pajvani UB, Hawkins M, Combs TP et al. . Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 2004;279:12152–62. [DOI] [PubMed] [Google Scholar]
- 42.Kubota N, Terauchi Y, Kubota T et al. . Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and -independent pathways. J Biol Chem 2006;281:8748–55. 10.1074/jbc.M505649200 [DOI] [PubMed] [Google Scholar]
- 43.Kriketos AD, Gan SK, Poynten AM et al. . Exercise increases adiponectin levels and insulin sensitivity in humans. Diabetes Care 2004;27:629–30. [DOI] [PubMed] [Google Scholar]
- 44.Yang WS, Lee WJ, Funahashi T et al. . Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab 2001;86:3815–19. 10.1210/jcem.86.8.7741 [DOI] [PubMed] [Google Scholar]
- 45.Kotani K, Hazama A, Hagimoto A et al. . Adiponectin and smoking status: a systematic review. J Atheroscler Thromb 2012;19:787–94. 10.5551/jat.11833 [DOI] [PubMed] [Google Scholar]
- 46.Yu JG, Javorschi S, Hevener AL et al. . The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes 2002;51:2968–74. [DOI] [PubMed] [Google Scholar]
- 47.Esteghamati A, Azizi R, Ebadi M et al. . The comparative effect of pioglitazone and metformin on serum osteoprotegerin, adiponectin and intercellular adhesion molecule concentrations in patients with newly diagnosed type 2 diabetes: a randomized clinical trial. Exp Clin Endocrinol Diabetes 2015;123:289–95. 10.1055/s-0034-1396864 [DOI] [PubMed] [Google Scholar]
- 48.Sahebkar A. Head-to-head comparison of fibrates versus statins for elevation of circulating adiponectin concentrations: a systematic review and meta-analysis. Metab Clin Exp 2013;62:1876–85. 10.1016/j.metabol.2013.08.017 [DOI] [PubMed] [Google Scholar]
- 49.Fontana V, de Faria AP, Oliveira-Paula GH et al. . Effects of angiotensin-converting enzyme inhibition on leptin and adiponectin levels in essential Hypertension. Basic Clin Pharmacol Toxicol 2014;114:472–5. 10.1111/bcpt.12195 [DOI] [PubMed] [Google Scholar]
- 50.Hass A, Oz H, Mashavi M et al. . Role of RAAS and adipokines in cardiovascular protection: effect of different doses of angiotensin II receptor blocker on adipokines level in hypertensive patients. J Am Soc Hypertens 2014;8:709–14. 10.1016/j.jash.2014.07.033 [DOI] [PubMed] [Google Scholar]