Abstract
Background
Iatrogenic gastrointestinal perforation is a life-threatening complication that arises very rarely in routine endoscopic procedures, with an incidence of 0.03–0.8%. It is more likely in highly complex and invasive therapeutic interventions. In certain situations, endoscopic closure of the perforation and treatment with antibiotics can obviate the need for emergency surgical repair.
Methods
This review is based on pertinent articles retrieved by a selective literature search in PubMed and on a relevant position paper.
Results
Existing clinical studies of treatment for iatrogenic gastrointestinal perforation are mainly retrospective and uncontrolled. No randomized and controlled trials have been performed to date. If the perforation is discovered soon after it arises, endoscopic treatment can be considered. Gastrointestinal perforations that are less than 30 mm in size can be closed with a clip. In the esophagus, expanding metal stents can be used as well. Clip application is successful in 80–100% of cases of gastrointestinal perforation, and the perforation remains permanently closed in 60–100% of cases. Reports on the endoscopic treatment of esophageal perforation show mixed results, with closure rates of roughly 90% and clinical success rates of roughly 80%. If endoscopic treatment is not possible, timely laparoscopic or open surgical repair is needed.
Conclusion
The endoscopic treatment of iatrogenic perforations is safe and reliable. Success depends on early detection, adequate endoscopic closure with properly mastered technique, and the early initiation of concomitant antibiotic treatment, which must be continued for a full course. Most patients who are treated in this way do not need emergency surgery.
Iatrogenic perforation of a hollow organ after diagnostic or therapeutic gastrointestinal endoscopic procedures is a rare but potentially life-threatening complication. The absolute incidence of iatrogenic perforation is assumed to increase worldwide (1, 2). The reason appears to be the more frequent use of colonoscopy screening and the more widespread use of interventional endoscopic techniques, such as endoscopic mucosal resection or endoscopic submucosal dissection. Both interventions are recommended in the guidelines to treat early neoplasia (3, 4).
In Germany, approximately 4.4 million screening colonoscopies were performed between 2003 and 2012 (5). The incidence of iatrogenic perforation during solely diagnostic endoscopic procedures is low; for diagnostic colonoscopy/sigmoidoscopy, it is in the range of 0.03 to 0.8% (1, 6, 7). While iatrogenic perforation occurs in approximately 1 in 3000 solely diagnostic colonoscopies, the risk of perforation grows with increasing extent of the intervention (8). An almost linear relationship between polyp size in the colon and risk of perforation after endoscopic resection has been reported (9). The Munich Polypectomy Study found polypectomy-related perforations in altogether 1.1% of cases; for sessile lesions in the right colon, the incidence increased to 11.7% (9). In more recent studies, perforation rates for colorectal adenomas >20 mm, which were removed by means of endoscopic mucosal resection (EMR), were considerably lower (0.003% to 1.3%) (10, 11). Likewise, the risk of complications increases with the complexity of the intervention. With the introduction of the endoscopic submucosal dissection (ESD) technique from Japan, the endoscopic treatment of mucosal cancer <2 cm was performed for the first time as an R0 resection in line with the oncological principals of tumor removal (12). As minimally invasive procedures matching the effectiveness of conventional surgical treatment, the new interventional endoscopic procedures for endoluminal tumor therapy offer significant benefits (12). However, ESD is technically demanding and even highly specialized experts in the field report colorectal perforation rates in the range of 1.9% to 12% (13– 15). Thus, the supposed advantage of endoscopic treatment must be balanced against the associated risks. Consequently, new endoscopic interventions should also provide for an effective endoscopic complication management. While colonic perforations, if they are treated in a timely and sufficient manner, are associated with a very low mortality of 0% to 0.019%, mortality increases to up to 13.2% in perforations of the esophagus which can be complicated by mediastinitis (16, 17). Based on the latest literature, this narrative review discusses the new techniques for closure of perforations against the background of the increasing use of diagnostic and, above all, interventional endoscopic procedures.
Methods
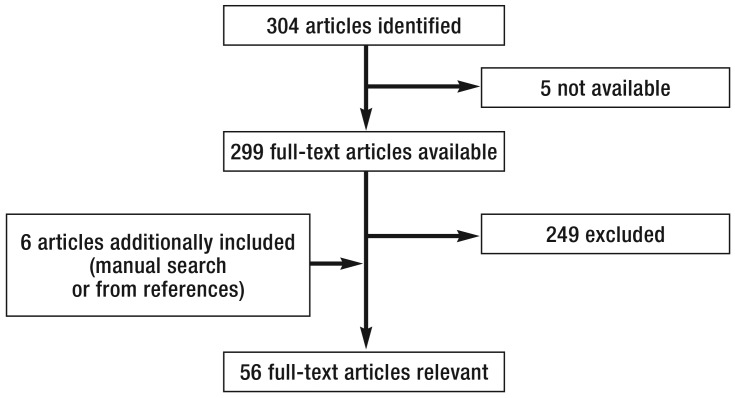
This review is based on publications retrieved by a selective literature search in PubMed for the period March 2005 to March 2015, using the search terms “gastrointestinal perforation management“, “endoclips and gastrointestinal perforation“ and “OTSC and gastrointestinal perforation“. In addition, new guidelines and reviews on this topic published in the last two years have been taken into account. The literature on which this article is based is limited to perforations related to esophagogastroduodenoscopy and colonoscopy as the most commonly performed endoscopic procedures in the gastrointestinal tract; articles on endoscopic retrograde cholangiopancreatography (ERCP)-related perforation were not included. Furthermore, relevant data from animal-experimental studies on clip closure were analyzed for this review. Case reports were not included and clinical case series were only included if they reported a minimum of 3 cases of iatrogenic perforation. Articles on fistula closure and postoperative anastomotic leak were excluded. A flow chart of the literature search is depicted in Figure 1.
Figure 1.
Flow chart of the literature search: Selection criteria are discussed in the Methods section.
Iatrogenic perforation – always surgery?
In many reviews, textbooks and lectures, immediate surgical intervention is recommended for the treatment of free perforation related to an endoscopic procedure. Current teaching does allow conservative management only in patients with small perforations, contained perforations (especially in the esophagus) and perforations with comparably mild clinical symptoms. However, a conservative approach requires intensive clinical monitoring in close consultation with the gastrointestinal surgeons in the hospital to ensure that any deterioration is identified early and can be treated surgically without delay, if needed. Thus, whilst unfortunate, not every perforation related to an endoscopic procedure represents an instance of malpractice, as long as the risk of perforation was discussed with the patient when the informed consent was obtained. However, if the perforation was not noticed or the patient did not receive treatment according to standard guidelines in time, the incident may have consequences (18). The point in time at which the perforation is discovered is crucial for further management and prognosis (8, 19). Therefore, it is important to detect any perforation ideally already during the endoscopic procedure and to document it in detail (1). Unfortunately, this is not always possible in clinical practice. In a retrospective study from 2010, only 68% of cases with perforations after colonoscopy were detected within 24 hours (20). Therefore, immediate post-interventional examination and initiation of further diagnostic investigations are vital if a patient presents with clinical signs and symptoms indicative of a perforation, including:
peritonism
severe pain in the region of the shoulder or psoas muscles
subcutaneous emphysema.
Endoscopic conservative management
General measures
Should a perforation occur, thorough documentation of location, size and time of the incident is critical (1). If the perforation is detected immediately or early, endoscopic conservative management can be attempted, subject to perforation size and type and the endoscopic expertise available. Apart from endoscopic closure of the defect, drainage of the gastrointestinal content using a nasogastric or nasoduodenal tube is recommended in patients with gastric or duodenal perforations (1). If free air and the associated increase in intraabdominal pressure results in cardiocirculatory or respiratory problems, decompression should be performed, e.g. by inserting a peripheral intravenous catheter (16– 18) into the abdominal cavity for temporary relief (1, 21, 22). Pneumothorax typically results from intrathoracic procedures, such as peroral endoscopic myotomy. If the patient develops a tension pneumothorax or respiratory compromise, insertion of a chest drain may already be required during the endoscopic procedure. For therapeutic endoscopic procedures, carbon dioxide (CO2) is the most widely used insufflation gas. However, if room air was insufflated during the procedure, it is important to immediately switch to CO2 upon detection of the perforation because CO2 is absorbed significantly more rapidly, preventing complications of tension pneumothorax and abdominal compartment syndrome (1, 23). Apart from these measures, intravenous broad-spectrum antibiotic therapy should be initiated immediately.
Endoscopic perforation closure/closure techniques
The primary aim of endoscopic perforation closure is to prevent extraluminal spillage of gastrointestinal content to avoid potentially life-threatening peritonitis or mediastinitis. Randomized animal-experimental studies showed that mediastinitis or peritonitis can be prevented by endoscopic closure of the perforation (24, 25). Furthermore, significantly less adhesions were observed after endoscopic closure of colon perforations compared with open surgery (26.1% vs. 56.5%) (24). A variety of endoscopic closure techniques are available which are mostly used regardless of the size of the perforation. Apart from animal-experimental data, most of the evidence regarding the effectiveness of the various closure techniques is based on retrospective case series and retrospective studies. So far, no randomized, controlled clinical trials evaluating these techniques have been conducted. Being rare incidents creating emergency situations, iatrogenic gastrointestinal perforations are difficult to study in a randomized controlled setting. Most data are available on the technique of endoscopic clip closure and in esophageal perforations on the use of covered stents in addition to clips. Presently, all other techniques should be considered experimental, either because of insuffienct availability or because of insufficient data.
Through-the-scope clips: Standard clips, so-called through-the-scope (TTS) clips, are applied through the working channel of the endoscope and can be used for sequential closure of gastrointestinal perforations (Figure 2). Pre-requisite for the success of the closure is sufficient tissue capture at the edges of the perforation. Here, the limiting factor is the opening width of the clip jaws. Furthermore, it has to be considered that these clips anchor on the mucosa and submucosa only, unlike the typically full-thickness surgical suture. However, this appears to be sufficient to achieve adequate wound healing, at least in animal models (26). Thus, small perforations <10 mm and those with easily “gatherable“ slit-shaped perforation edges in the stomach can generally be closed successfully using standard clips. Success rates of 98 to 99% are reported in the literature (1, 27). However, these clips are not suitable for larger gastric wall perforations (1, 28). In the colon, it is generally easier to close perforations using standard clips as the colonic wall is thinner than the wall of the esophagus or stomach. In a retrospective study by Magdeburg et al., the clinical success rate after closure of iatrogenic colon perforations using TTS clips was 83.3% (29). A recent meta-analysis reported an overall success rate of 90.2% for perforations at various locations, especially in the stomach and the colon (30).
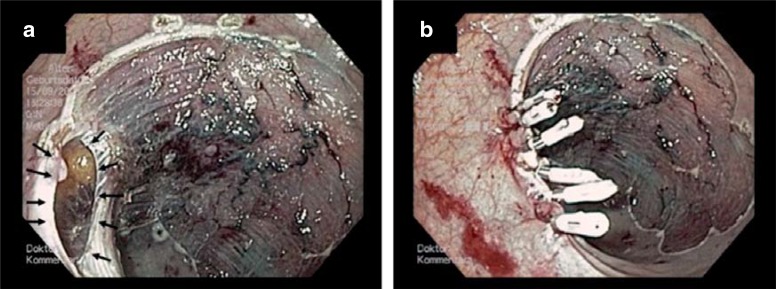
Figure 2.
Iatrogenic perforation in the upper rectum after endoscopic mucosal resection (EMR) of a flat adenoma.
a) The perforation at the lateral resection margin (arrows) was detected during the intervention.
b) The perforation was sequentially closed using through-the-scope (TTS) clips.
Over-the-scope clips: Unlike TTS clips, over-the-scope (OTS) clips are not applied through the endoscope’s working channel but with the help of an application cap mounted onto the endoscope. A grasping device can be advanced through the free working channel of the endoscope and used to grasp and adapt the perforation edges and pull them into the cap. Then, the nitinol clip is released from the cap using a string mechanism and grips the pulled-in tissue like a bear trap (Figure 3).
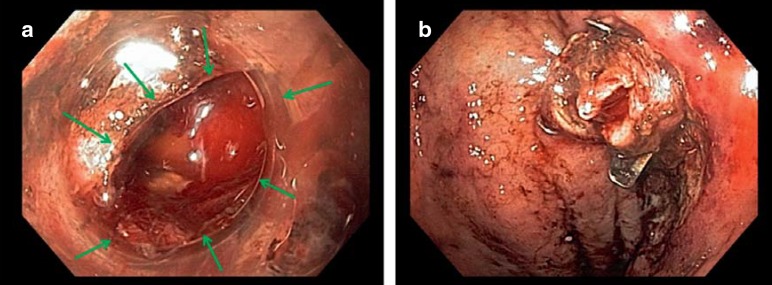
Figure 3.
Perforation closure using an over-the-scope (OTS) clip
a) The gastric iatrogenic perforation occurred during an endoscopic submucosal dissection (ESD) procedure.
b) Using an OTS clip, immediate sufficient closure was achieved.
Compared with the TTS clip, the OTS clip captures more tissue and closes the deeper tissue layers, too (31). Furthermore, the perforation can be closed in one step, translating into time savings compared with the sequential closure using standard clips (32, 33). Furthermore, OTS clips can close larger perforations (maximum 2–3 cm), if it is possible to effectively adapt the edges of the perforation (1). The closure of artificially created gastrointestinal perforations using OTS clips has been studied extensively in animal models. A survival study, using a porcine model, showed a significant advantage of OTS clips over standard clips. This was attributed to the lower rate of leakages with resulting peritonitis after closure of artificial gastric wall perforations with OTS clips (34).
In direct comparison between manually placed sutures and OTS clips, no significant differences in the burst pressures of duodenal, gastric and colonic lesions closed with the two techniques were found in animal models (35– 38). Several mostly retrospective studies evaluating OTS clips for the closure of iatrogenic gastrointestinal perforation in a clinical setting are currently available (39– 40; e1–e8) (Table). These studies included between 3 and 48 patients. The duration of the follow-up periods ranged between 1 week and 92 weeks and the technical success rates between 50% and 100%. The wide variation in the reported clinical success rates can be attributed to the significant heterogeneity of the patient populations with regard to size, location and time of diagnosis.
Table. Overview of the literature on perforation closure using over-the-scope clips.
| Author, year | Study type | Number of patients *1 | Location | Technical success | Clinical success | Follow-up (weeks) | Size of perforation | OTS clip-associated complications |
|---|---|---|---|---|---|---|---|---|
| Baron, 2012 (40) |
Retrospective case series | 5 | Esophagus (n=1) Stomach (n=2) Jejunum (n=1) Colon (n=1) |
100% | 75% | NI | NI | Clip-related obstruction of lumen of jejunum (n=1) |
| Gubler, 2012 (40) |
Prospective case series | 14 | Duodenum (n=2) Stomach (n=3) Colon (n=9) |
93% | 78% | 4–92 | 6–30mm | None |
| Hagel, 2012 (e1) |
Retrospective case series | 4 | Esophagus (n=2)) Rectum (n=2) |
50% | 0% | NI | 4–14mm | None |
| Kirschniak, 2007 (e2) |
Retrospective case series | 4 | Stomach (n=1) Colon (n=3) |
100% | 100% | 1–4 | 4–8mm | None |
| Kirschniak, 2011 (e3) |
Retrospective case series | 11 | Upper GI tract (n=7) Colon (n=4) |
100% | 100% | 1–4 | NI | None |
| Nishiyama, 2013 (e4) |
Retrospective case series | 10 | Esophagus (n=1) Stomach (n=3) Duodenum (n=2) Colon (n=2) Rectum (n=2) |
100% | 90% | 1–30 | 25–50mm | None |
| Sandmann, 2011 (e5) |
Retrospective case series | 3 | Stomach (n=1) Duodenum (n=1) Colon (n=1) |
100% | 100% | 4–32 | NI | None |
| Seebach, 2010 (e6) |
Retrospective case series | 4 | Stomach (n=1) Colon (n=3) |
75% | 50% | 10–37 | NI | None |
| Voermans 2012 (e7) |
Prospective, non-controlled study | 31 | Esophagus (n=4) Stomach (n=4) Duodenum (n=11) Colon (n=12) |
92% | 89% | 4 | Up to 30mm | One esophagus perforation related to OTS clip during insertion |
| Haito-Chavez 2014 (e8) |
Retrospective multicenter study | 48 | Esophagus (n=10)) Stomach (n=13) Duodenum/small intestine (n=12) Colorectum (n=12) |
97.5% | 90% | 30 | 4–11.5 mm | None |
| Farnik 2015 (e10) |
Retrospective study | 15 | Upper GI tract | 97.1%*2 | 71%*2 | 38 | Up to 30mm | None |
In this table, only studies with ≥ 3 patients were included.
*1Perforations only; fistulas or hemorrhages treated with OTS clips during the study were not counted.
*2based on the total number of patients OTS, over-the-scope; n, number of affected patients; n. s., non-significant; NI, no information
In the so far only prospective, multicenter study, CLIPPER, altogether 36 patients with iatrogenic gastrointestinal perforations <30 mm were included (e7). The technical and clinical success rates were 92 and 89%, respectively. A systematic review by Weiland et al. from 2013 compiled and compared data for altogether 17 clinical prospective as well as retrospective studies published between 2007 and 2013, including cases of acute anastomotic leaks (e9). The OTS clips were successful applied in 80% to 100% of cases. The rates of clinical success—defined as permanent closure of the perforation—ranged between 60% and 100%. A recently published retrospective multicenter study included 106 patients with leakages in the upper gastrointestinal tract. Of the included patients, 72 were treated with covered stents (cSEMS) and 34 with OTS clips. The group treated with OTS clips included 15 patients with iatrogenic complications after endoscopy: The technical success rate was 97.1%, the clinical success rate 71% (e10).
The authors of another retrospective multicenter study including 48 patients with iatrogenic perforation reported comparable data. Here, the long-term clinical success was even higher (90%) (e8). Based on these results, gastric and colonic closure using OTS clips was included in the recommendations of the current position statement of the European Society of Gastrointestinal Endoscopy (ESGE) (1).
Stents
Covered self-expandable metal stents (cSEMS) are an established option for the treatment of esophageal perforations which cannot be closed using clips because of their size, location or condition (1). The results of studies evaluating this technique are mixed and the available evidence primarily consists of non-controlled data from heterogeneous patient populations (e11, e12). Nonetheless, cSEMS appears to be highly effective in the treatment of esophageal perforation. According to a recent meta-analysis, the technical and clinical success rates were 91% and 81%, respectively (e13).
Endoscopic suturing techniques
Endoscopic suturing techniques currently have no defined role in perforation closure. Of all experimentally tested endoscopic suturing techniques, only instruments developed for endoscopic reflux treatment have been approved for clinical use. Retrospective studies have reported the successful use of these systems in iatrogenic gastric perforations (e14, e15). However, these devices are not everywhere available and typically expensive (single-use products). In addition, their use requires a certain level of experience of the examining endoscopist.
Endoscopic conservative versus surgical treatment
With the advent of the new endoscopic closure techniques, operative surgical treatment, both as open and laparoscopic procedures, is now less frequently performed. This makes the careful consideration of the most appropriate procedure increasingly important. Experts in the field of endoscopy publish best results with the new endoscopic closure techniques (19) when their experience puts them in the position to detect the perforation during the primary therapeutic intervention and when they have the equipment, the staff and the manual skills to immediately and competently achieve secure closure of the perforation. In this situation, it appears justified to refer to these new developments in the management of iatrogenic perforations as a paradigm shift.
Ultimately, the risk is with the patient. Therefore, it is certainly better to establish the safety of the patient with a competently performed endoscopic closure instead of a more invasive operative surgical procedure which takes more time, causes more pain and imposes more restrictions on the patient. On the other hand, it is definitely a less acceptable approach to leave the patient exposed to a high-risk situation resulting from poor endoscopic closure and to delay or complicate surgical treatment. Instead, operative surgical treatment, ideally using a minimally invasive technique (e16), should be sought. Left untreated, the tissue at the perforation site will undergo inflammatory changes and soften so that sutures can no longer be firmly anchored and the chances of a favorable outcome dwindle by the hour.
Endoscopists performing complex therapeutic procedures where the risk of perforation is high should always critically evaluate this risk. According to the ESGE’s current position statement, decision making should include a written definition of high-risk procedures and the implementation of a complication management algorithm at each center, agreed after interdisciplinary consultation (1). The local resources are critical because the quality and expertise of the endoscopic team has a strong impact on both the risk of perforation and the secure endoscopic perforation closure. Likewise, the performance and availability of the gastrointestinal surgeon who treats the perforation either primarily or after the endoscopic treatment has failed plays an important role. Another critical factor is the quality of the monitoring undertaken once the perforation has been treated, i.e. the required staff and the competent assessment of the patient’s condition in order to not miss the right point in time for operative surgical or endoscopic revision. If the 24/7 availability of all listed parameters is assured in a hospital and/or in an interdisciplinary team, the step towards the paradigm shift is justified.
Conclusion
The extension of the indication for interventional endoscopic treatment which continues to become more and more invasive goes along with an increased risk of iatrogenic perforation. Hence, advanced endoscopic closure techniques and new insights into the conservative management of hollow organ perforation have started a learning process which has found its way into the current international recommendations. The accepted basic principles of endoscopic treatment include:
early detection of perforation
adequate knowledge of interventional endoscopic procedures, especially with new techniques such as the OTS clips
endoscopy with CO2 insufflation.
Last but not least, close cooperation between the disciplines most involved— gastrointestinal endoscopy and gastrointestinal surgery—is an indispensable requirement for the use of endoscopic techniques. Open, interdisciplinary discussion of any complication that may occur is imperative to achieve the best result in the best interest of the patient. Type, extent, location, and time of the perforation should be well documented. Standard operating procedures (SOPs) for the various situations should be in place to help achieve the best possible outcome, defined in an interdisciplinary effort. Based on the current literature and the current recommendations, we propose the algorithm shown in Figure 4.
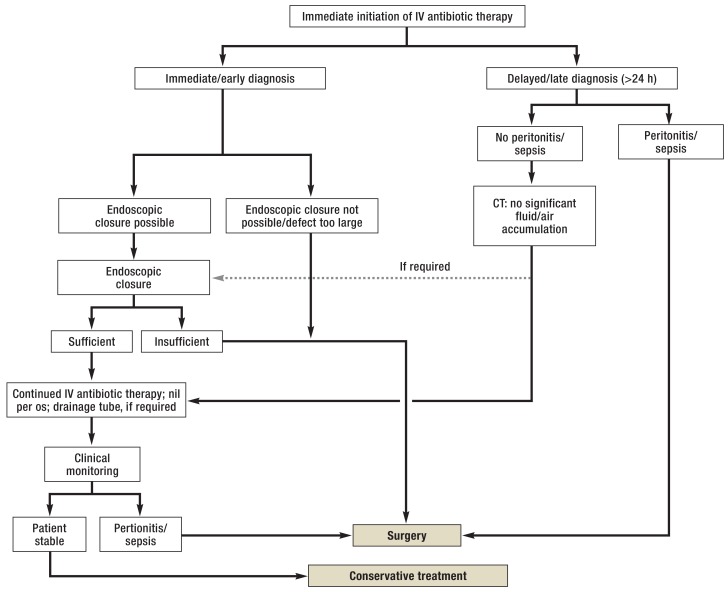
Figure 4.
Algorithm for the management of iatrogenic gastrointestinal perforation according to the position statement of the der European Society of Gastrointestinal Endoscopy (ESGE) from 2014
IV, intravenous; CT, computed tomography
Key Messages.
Increasing invasiveness and complexity of endoscopic interventions increases the risk of perforation.
New endoscopic closure technique, such as clips and metal stents, help to avoid emergency surgery.
Key to successful endoscopic management is early detection, secure endoscopic defect closure and profound knowledge of the further conservative management.
Surgical intervention is typically indicated in cases of delayed diagnosis, insufficient endoscopic closure and/or signs of peritonitis/sepsis.
Based on current international guidelines, standard operating procedures (SOPs) should be defined in an interdisciplinary effort at each center to ensure the best possible complication management.
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
Footnotes
Conflict of interest statement
Dr. Schmidt has received lecture fees and study support (third-party funding) from Ovesco Endoscopy.
Prof. Fuchs has received consultancy fees from Olympus Europa.
Prof. Caca has received lecture fees from Olympus, Cook, Boston Scientific, Ovesco, and G-Surg. He has received study support (third-party funding) from Ovesco und G-Surg.
Prof. Meining has received study support (third-party funding) from Ovesco.
Dr. Küllmer declares that no conflict of interest exists.
References
- 1.Paspatis GA, Dumonceau J, Barthet M, et al. Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2014:693–711. doi: 10.1055/s-0034-1377531. [DOI] [PubMed] [Google Scholar]
- 2.Bielawska B, Day A, Lieberman D, Hookey L. Risk factors for early colonoscopic perforation include non-gastroenterologist endoscopists: a multivariable analysis. Clin Gastroenterol Hepatol. 2014;12:85–92. doi: 10.1016/j.cgh.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Möhler M, Al-Batran SE, Andus T, et al. S3-Leitlinie Diagnostik und Therapie der Adenokarzinome des Magens und gastroösophagealen Übergangs. Z Gastroenterol. 2011;49:461–531. doi: 10.1055/s-0031-1273201. [DOI] [PubMed] [Google Scholar]
- 4.Pox C, Aretz S, Bischoff SC, et al. S3-Leitlinie Kolorektales Karzinom. Z Gastroenterol. 2013;51:753–854. doi: 10.1055/s-0033-1350264. [DOI] [PubMed] [Google Scholar]
- 5.Brenner H, Altenhofen L, Stock C, Hoffmeister M. Prevention, early detection, and overdiagnosis of colorectal cancer within 10 years of screening colonoscopy in Germany. Clin Gastroenterol Hepatol. 2015;13:717–723. doi: 10.1016/j.cgh.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 6.Stock C, Ihle P, Sieg A, Schubert I, Hoffmeister M, Brenner H. Adverse events requiring hospitalization within 30 days after outpatient screening and nonscreening colonoscopies. Gastrointest Endosc. 2013;77:419–429. doi: 10.1016/j.gie.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 7.Rabeneck L, Saskin R, Paszat LF. Onset and clinical course of bleeding and perforation after outpatient colonoscopy: a population-based study. Gastrointest Endosc. 2011;73:520–523. doi: 10.1016/j.gie.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 8.Raju GS, Saito Y, Matsuda T, Kaltenbach T, Soetikno R. Endoscopic management of colonoscopic perforations (with videos) Gastrointest Endosc. 2011;74:1380–1388. doi: 10.1016/j.gie.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Heldwein W, Dollhopf M, Meining A, et al. The Munich Polypectomy Study (MUPS): prospective analysis of complications and risk factors in 4000 colonic snare polypectomies. Endoscopy. 2005;37:1116–1122. doi: 10.1055/s-2005-870512. [DOI] [PubMed] [Google Scholar]
- 10.Moss A, Bourke MJ, Williams SJ, et al. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology. 2011;140:1908–1918. doi: 10.1053/j.gastro.2011.02.062. [DOI] [PubMed] [Google Scholar]
- 11.Buchner AM, Guarner-Argente C, Ginsberg GG. Outcomes of EMR of defiant colorectal lesions directed to an endoscopy referral center. Gastrointest Endosc. 2012;76:255–263. doi: 10.1016/j.gie.2012.02.060. [DOI] [PubMed] [Google Scholar]
- 12.Espinel J, Pinedo E, Ojeda V, Guerra M. Treatment modalities for early gastric cancer. World J Gastrointest Endosc. 2015;7:1062–1069. doi: 10.4253/wjge.v7.i12.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toyonaga T, Man-I M, East JE, et al. 1,635 endoscopic submucosal dissection cases in the esophagus, stomach, and colorectum: complication rates and long-term outcomes. Surg Endosc Other Interv Tech. 2013;27:1000–1008. doi: 10.1007/s00464-012-2555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka S, Terasaki M, Kanao H, Oka S, Chayama K. Current status and future perspectives of endoscopic submucosal dissection for colorectal tumors. Dig Endosc. 2012;24:73–79. doi: 10.1111/j.1443-1661.2012.01252.x. [DOI] [PubMed] [Google Scholar]
- 15.Repici A, Hassan C, De Paula Pessoa D, et al. Efficacy and safety of endoscopic submucosal dissection for colorectal neoplasia: a systematic review. Endoscopy. 2012;44:137–150. doi: 10.1055/s-0031-1291448. [DOI] [PubMed] [Google Scholar]
- 16.Panteris V, Haringsma J, Kuipers EJ. Colonoscopy perforation rate, mechanisms and outcome: from diagnostic to therapeutic colonoscopy. Endoscopy. 2009;41:941–951. doi: 10.1055/s-0029-1215179. [DOI] [PubMed] [Google Scholar]
- 17.Biancari F, D’Andrea V, Paone R, et al. Current treatment and outcome of esophageal perforations in adults: systematic review and meta-analysis of 75 studies. World J Surg. 2013;37:1051–1059. doi: 10.1007/s00268-013-1951-7. [DOI] [PubMed] [Google Scholar]
- 18.Rösch W, Juncker J. [What shouldn’t happen - perforation during prophylactic colonoscopy] Endo heute. 2014;27:173–175. [Google Scholar]
- 19.Baron TH, Wong Kee Song LM, Zielinski MD, Emura F, Fotoohi M, Kozarek RA. A comprehensive approach to the management of acute endoscopic perforations (with videos) Gastrointest Endosc. 2012;76:838–859. doi: 10.1016/j.gie.2012.04.476. [DOI] [PubMed] [Google Scholar]
- 20.Anderson ML, Pasha TM, Leighton JA. Endoscopic perforation of the colon: lessons from a 10-year study. Am J Gastroenterol. 2000;95:3418–3422. doi: 10.1111/j.1572-0241.2000.03356.x. [DOI] [PubMed] [Google Scholar]
- 21.Lin BW, Thanassi W. Tension pneumoperitoneum. JEM. 2010;38:57–59. doi: 10.1016/j.jemermed.2007.10.085. [DOI] [PubMed] [Google Scholar]
- 22.Fu K, Ishikawa T, Yamamoto T, Kaji Y. Paracentesis for successful treatment of tension pneumoperitoneum related to endoscopic submucosal dissection. Endoscopy. 2009;41 doi: 10.1055/s-2007-966489. [DOI] [PubMed] [Google Scholar]
- 23.Dellon ES, Hawk JS, Grimm IS, Shaheen NJ. The use of carbon dioxide for insufflation during GI endoscopy: a systematic review. Gastrointest Endosc. 2009;69:843–849. doi: 10.1016/j.gie.2008.05.067. [DOI] [PubMed] [Google Scholar]
- 24.Raju GS, Fritscher-Ravens A, Rothstein RI, et al. Endoscopic closure of colon perforation compared to surgery in a porcine model: a randomized controlled trial (with videos) Gastrointest Endosc. 2008;68:324–332. doi: 10.1016/j.gie.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Fritscher-Ravens A, Hampe J, Grange P, et al. Clip closure versus endoscopic suturing versus thoracoscopic repair of an iatrogenic esophageal perforation: a randomized, comparative, long-term survival study in a porcine model (with videos) Gastrointest Endosc. 2010;72:1020–1026. doi: 10.1016/j.gie.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 26.Dray X, Krishnamurty DM, Donatelli G, Gabrielson KL. Gastric wall healing after NOTES procedures: closure with endoscopic clips provides superior histological outcome compared with threaded tags closure setting. Gastrointest Endosc. 2010;72:343–350. doi: 10.1016/j.gie.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Mangiavillano B, Viaggi P, Masci E. Endoscopic closure of acute iatrogenic perforations during diagnostic and therapeutic endoscopy in the gastrointestinal tract using metallic clips: a literature review. J Dig Dis. 2010;11:12–18. doi: 10.1111/j.1751-2980.2009.00414.x. [DOI] [PubMed] [Google Scholar]
- 28.Minami S, Gotoda T, Ono H, Oda I, Hamanaka H. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video) Gastrointest Endosc. 2006;63:596–601. doi: 10.1016/j.gie.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 29.Magdeburg R, Collet P, Post S, Kaehler G. Endoclipping of iatrogenic colonic perforation to avoid surgery. Surg Endosc. 2008;22:1500–1504. doi: 10.1007/s00464-007-9682-1. [DOI] [PubMed] [Google Scholar]
- 30.Verlaan T, Voermans RP, Henegouwen MIVB. Endoscopic closure of acute perforations of the GI tract: a systematic review of the literature. Gastrointest Endosc. 2015;82:618–628. doi: 10.1016/j.gie.2015.03.1977. [DOI] [PubMed] [Google Scholar]
- 31.Vassiliou MC, Rothstein RI, Hampshire N. Randomized controlled trial comparing endoscopic clips and over-the-scope clips for closure of natural orifice transluminal endoscopic surgery gastrotomies. Endoscopy. 2009:1056–1061. doi: 10.1055/s-0029-1215241. [DOI] [PubMed] [Google Scholar]
- 32.Sun G, Yang Y, Zhang X, et al. Comparison of gastrotomy closure modalities for natural orifice transluminal surgery: a canine study. Gastrointest Endosc. 2013;77:774–783. doi: 10.1016/j.gie.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 33.Voermans RP, Vergouwe F, Breedveld P, Fockens P, van Berge Henegouwen MI. Comparison of endoscopic closure modalities for standardized colonic perforations in a porcine colon model. Endoscopy. 2011;43:217–222. doi: 10.1055/s-0030-1256072. [DOI] [PubMed] [Google Scholar]
- 34.von Renteln D, Vassiliou MC, Rothstein RI. Randomized controlled trial comparing endoscopic clips and over-the-scope clips for closure of natural orifice transluminal endoscopic surgery gastrotomies. Endoscopy. 2009;41:1056–1061. doi: 10.1055/s-0029-1215241. [DOI] [PubMed] [Google Scholar]
- 35.von Renteln D, Schmidt A, Vassiliou MC, Rudolph HU, Caca K. Endoscopic full-thickness resection and defect closure in the colon. Gastrointest Endosc. 2010;71:1267–1273. doi: 10.1016/j.gie.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 36.von Renteln D, Rudolph HU, Schmidt A, Vassiliou MC, Caca K. Endoscopic closure of duodenal perforations by using an over-the-scope clip: a randomized, controlled porcine study. Gastrointest Endosc. 2010;71:131–138. doi: 10.1016/j.gie.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Suhail AH, Mårvik R, Halgunset J, Kuhry E. Efficacy and safety of transgastric closure in natural orifice transluminal endoscopic surgery using the OTSC system and T-bar sutures: a survival study in a porcine model. Surg Endosc. 2012;26:2950–2954. doi: 10.1007/s00464-012-2290-8. [DOI] [PubMed] [Google Scholar]
- 38.von Renteln D, Schmidt A, Vassiliou MC, Gieselmann M, Caca K. Natural orifice transluminal endoscopic surgery gastrotomy closure with an over-the-endoscope clip: a randomized, controlled porcine study (with videos) Gastrointest Endosc. 2009;70:732–739. doi: 10.1016/j.gie.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Baron TH, Song LM, Ross A, Tokar JL, Irani S, Kozarek RA. Use of an over-the-scope clipping device: multicenter retrospective results of the first US. experience (with videos) Gastrointest Endosc. 2012;76:202–208. doi: 10.1016/j.gie.2012.03.250. [DOI] [PubMed] [Google Scholar]
- 40.Gubler C, Bauerfeind P. Endoscopic closure of iatrogenic gastrointestinal tract perforations with the over-the-scope clip. Digestion. 2012;85:302–307. doi: 10.1159/000336509. [DOI] [PubMed] [Google Scholar]
- e1.Hagel AF, Naegel A, Lindner AS, et al. Over-the-scope clip application yields a high rate of closure in gastrointestinal perforations and may reduce emergency surgery. J Gastrointest Surg. 2012;16:2132–2138. doi: 10.1007/s11605-012-1983-6. [DOI] [PubMed] [Google Scholar]
- e2.Kirschniak A, Kratt T, Stüker D, Braun A, Schurr MO, Königsrainer A. A new endoscopic over-the-scope clip system for treatment of lesions and bleeding in the GI tract: first clinical experiences. Gastrointest Endosc. 2007;66:162–167. doi: 10.1016/j.gie.2007.01.034. [DOI] [PubMed] [Google Scholar]
- e3.Kirschniak A, Subotova N, Zieker D, Königsrainer A, Kratt T. The over-the-scope clip (OTSC) for the treatment of gastrointestinal bleeding, perforations, and fistulas. Surg Endosc. 2011;25:2901–2905. doi: 10.1007/s00464-011-1640-2. [DOI] [PubMed] [Google Scholar]
- e4.Nishiyama N, Mori H, Kobara H, et al. Efficacy and safety of over-the-scope clip: including complications after endoscopic submucosal dissection. World J Gastroenterol. 2013;19:2752–2760. doi: 10.3748/wjg.v19.i18.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e5.Sandmann M, Heike M, Faehndrich M. Application of the OTSC system for the closure of fistulas, anastomosal leakages and perforations within the gastrointestinal tract. Z Gastroenterol. 2011;49:981–985. doi: 10.1055/s-0029-1245972. [DOI] [PubMed] [Google Scholar]
- e6.Seebach L, Bauerfeind P, Gubler C. “Sparing the surgeon”: clinical experience with over-the-scope clips for gastrointestinal perforation. Endoscopy. 2010;42:1108–1111. doi: 10.1055/s-0030-1255924. [DOI] [PubMed] [Google Scholar]
- e7.Voermans RP, Le Moine O, von Renteln D, et al. Efficacy of endoscopic closure of acute perforations of the gastrointestinal tract. Clin Gastroenterol Hepatol; 2012;10:603–608. doi: 10.1016/j.cgh.2012.02.005. [DOI] [PubMed] [Google Scholar]
- e8.Haito-Chavez Y, Law JK, Kratt T, et al. International multicenter experience with an over-the-scope clipping device for endoscopic management of GI defects (with video) Gastrointest Endosc. 2014;80:610–622. doi: 10.1016/j.gie.2014.03.049. [DOI] [PubMed] [Google Scholar]
- e9.Weiland T, Fehlker M, Gottwald T, Schurr MO. Performance of the OTSC system in the endoscopic closure of iatrogenic gastrointestinal perforations: a systematic review. Surg Endosc. 2013;27:2258–2274. doi: 10.1007/s00464-012-2754-x. [DOI] [PubMed] [Google Scholar]
- e10.Farnik H, Driller M, Kratt T, et al. Indication for “Over the Scope” (OTS)-Clip vs. covered self-expanding metal stent (cSEMS) is unequal in upper gastrointestinal leakage: results from a retrospective head-to-head comparison. Gastrointest Endosc. 2015;10:1–12. doi: 10.1371/journal.pone.0117483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.van Boeckel PG, Sijbring A, Vleggaar FP, Siersema PD. Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment Pharmacol Ther. 2011;33:1292–1301. doi: 10.1111/j.1365-2036.2011.04663.x. [DOI] [PubMed] [Google Scholar]
- e12.Johnsson E, Lundell L, Liedman B. Sealing of esophageal perforation or ruptures with expandable metallic stents: a prospective controlled study on treatment efficacy and limitations. Dis Esoph. 2005;18:262–266. doi: 10.1111/j.1442-2050.2005.00476.x. [DOI] [PubMed] [Google Scholar]
- e13.Dasari BVM, Neely D, Kennedy A, et al. The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann Surg. 2014;259:852–860. doi: 10.1097/SLA.0000000000000564. [DOI] [PubMed] [Google Scholar]
- e14.von Renteln D, Schmidt A, Riecken B, Caca K. Gastric full-thickness suturing during EMR and for treatment of gastric-wall defects (with video) Gastrointest Endosc. 2008;67:738–744. doi: 10.1016/j.gie.2007.10.051. [DOI] [PubMed] [Google Scholar]
- e15.Schmidt A, Bauder M, Riecken B, von Renteln D, Muehleisen H, Caca K. Endoscopic full-thickness resection of gastric subepithelial tumors: a single-center series. Endoscopy. 2015;47:154–158. doi: 10.1055/s-0034-1390786. [DOI] [PubMed] [Google Scholar]
- e16.Zhang Y, Lu W, Yao L, Qin X. Laparoscopic direct suture of perforation after diagnostic colonoscopy. Int J Colorectal Dis. 2013:1505–1509. doi: 10.1007/s00384-013-1734-5. [DOI] [PubMed] [Google Scholar]