Abstract
In patients with left ventricular heart failure (HF), the development of pulmonary hypertension (PH) and right ventricular (RV) dysfunction are frequent and have important impact on disease progression, morbidity, and mortality, and therefore warrant clinical attention. Pulmonary hypertension related to left heart disease (LHD) by far represents the most common form of PH, accounting for 65–80% of cases. The proper distinction between pulmonary arterial hypertension and PH-LHD may be challenging, yet it has direct therapeutic consequences. Despite recent advances in the pathophysiological understanding and clinical assessment, and adjustments in the haemodynamic definitions and classification of PH-LHD, the haemodynamic interrelations in combined post- and pre-capillary PH are complex, definitions and prognostic significance of haemodynamic variables characterizing the degree of pre-capillary PH in LHD remain suboptimal, and there are currently no evidence-based recommendations for the management of PH-LHD. Here, we highlight the prevalence and significance of PH and RV dysfunction in patients with both HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF), and provide insights into the complex pathophysiology of cardiopulmonary interaction in LHD, which may lead to the evolution from a ‘left ventricular phenotype’ to a ‘right ventricular phenotype’ across the natural history of HF. Furthermore, we propose to better define the individual phenotype of PH by integrating the clinical context, non-invasive assessment, and invasive haemodynamic variables in a structured diagnostic work-up. Finally, we challenge current definitions and diagnostic short falls, and discuss gaps in evidence, therapeutic options and the necessity for future developments in this context.
Keywords: Pulmonary hypertension, Heart failure, Pre-capillary, Post-capillary
Introduction
Pulmonary hypertension (PH) is a frequent condition, which may occur as a consequence of pulmonary vascular disease, chronic left heart or lung disease, pulmonary embolism, or other aetiologies.1–3 Among the various PH groups, PH associated with left heart failure (HF) represents by far the most common form of PH. In fact, left heart diseases (LHD) account for 65–80% of PH cases. Whereas targeted therapies are available for pulmonary arterial hypertension (PAH),3,4 these treatments have not been adequately evaluated or are not indicated and may even be harmful in patients with PH related to LHD. It is therefore of key importance to establish a precise diagnosis and classification of PH before treatment decisions are made. However, in clinical practice, there are significant inconsistencies, which are a matter of concern: on one hand, patients with PH and occult LHD are frequently misclassified as PAH and treated with PAH-specific drugs, or patients with PH of unclear origin receive targeted therapy without proper classification. On the other hand, HF is often associated with PH and RV dysfunction, which have an important impact on disease progression, morbidity, and mortality, and this tends to be underestimated in the field of HF.5 Nevertheless, the haemodynamic interrelations when a pre-capillary component is present in post-capillary PH are complex, and the efficacy and safety of targeted PH therapies remain unproven. Here, we aim to highlight the prevalence and significance of PH and RV dysfunction in patients with left ventricular (LV) HF, provide insights into the complex pathophysiology of cardiopulmonary interaction in LHD, challenge current definitions and diagnostic short falls, and to discuss therapeutic options and the necessity for future developments in this context.
Haemodynamic definitions and classification of pulmonary hypertension related to left heart disease
Regardless of the origin, PH is defined by a mean pulmonary artery pressure (PAP) ≥25 mmHg.1,6 Based on the left-sided filling pressure [determined either as LV end-diastolic pressure (LVEDP), left atrial pressure (LAP), or pulmonary arterial wedge pressure (PAWP)], the haemodynamic definition further distinguishes pre- (≤15 mmHg) and post-capillary PH (>15 mmHg). In post-capillary PH, the elevation of PAWP leads to a proportionate increase of the mean PAP, maintaining a normal transpulmonary pressure gradient (TPG = mPAP – PAWP) <12 mmHg, and low pulmonary vascular resistance (PVR) <3 Wood units (WU) or <240 dynes s cm−5. However, chronic elevation of the left-sided filling pressure associated with neurohormonal and other mediator activation as well as neurogenic effects may cause excess vasoconstriction with or without vascular remodelling leading to a ‘disproportionate’ increase of the PAP, and thus resulting in an elevated TPG and PVR, which has been described as ‘reactive’, ‘out-of-proportion’, or ‘mixed’ PH.1,7 While the TPG is influenced by volume load and cardiac function, and does not prognosticate outcome in PH-LHD,8 the diastolic pressure gradient (DPG)—defined by the difference between diastolic PAP and PAWP—is assumed to be less dependent of stroke volume and loading conditions,9 and was shown to correlate with pulmonary vascular remodelling in PH-LHD.10 These findings led to the current terminology and classification of post-capillary PH as either isolated post-capillary PH (Ipc-PH), if the DPG is <7 mmHg and/or PVR ≤3 WU, or combined post- and pre-capillary PH (Cpc-PH), if the DPG is ≥7 mmHg and/ or PVR >3 WU.11,12
Prevalence and significance of pulmonary hypertension in left ventricular heart failure
The presence and extent of PH and RV dysfunction—which are both common in LV HF—are associated with disease progression, decreased exercise tolerance, and an unfavourable outcome. Data on the prevalence of PH in patients with HF yield variable numbers and depend on the method of PAP measurement, the definition of PH, and the populations studied. Although both HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF) belong to the clinical syndrome of ‘heart failure’, they constitute distinct entities with regard to pathophysiology, clinical characteristics, haemodynamics, cardiopulmonary interaction, and response to therapy, and should thus be viewed separately. In HFrEF, the prevalence of PH as assessed by RHC was reported to be between 40 and 75%.8,13,14 In patients with HFpEF, recent studies utilizing either echocardiography or RHC indicated a PH prevalence in a range between 36 and 83%.15–17
A community-based study in patients with HF demonstrated that pulmonary artery systolic pressure (PASP) estimated by echocardiography strongly predicted all-cause and cardiovascular mortality independently of known predictors of outcome.18 While numerous studies have consistently shown an inverse correlation between PH and survival, a combination of elevated PAP and reduced RV systolic function was particularly associated with an unfavourable outcome in HFrEF.13 In HFpEF, studies demonstrated that the presence of PH was also strongly associated with mortality.15–17 Furthermore, HFpEF patients commonly display RV dysfunction, which is associated with elevated PAP, occurs at more advanced stages, and represents a strong predictor of death.19,20
Haemodynamic parameters that are predictive of poor survival in HF include an increased PAWP, mean PAP, and PVR, and a reduced PA compliance/capacitance.8,14,21–23 Significant efforts were made to better define PH-LHD and harmonize definitions. However, there is still a debate on the best haemodynamic predictor of outcome. Recent studies on the prognostic value of the DPG in PH-LHD have yielded conflicting results.10,14,22–24 This may be explained, at least in part, by the populations studied and by methodological limitations including inconsistencies in data due to the presence of negative DPG and elevated DPG in patients without PH. The nature of the controversial value of the DPG in HF patients needs to be further addressed in future studies. Recent data indicate that—depending on the consideration of the DPG and/or PVR—the prevalence of Cpc-PH in patients with HF is 12–38%.10,14 The above data collectively indicate that PH and RV dysfunction are frequent and associated with a poor outcome in patients with LV HF.
Pathobiology of pulmonary hypertension related to left heart disease
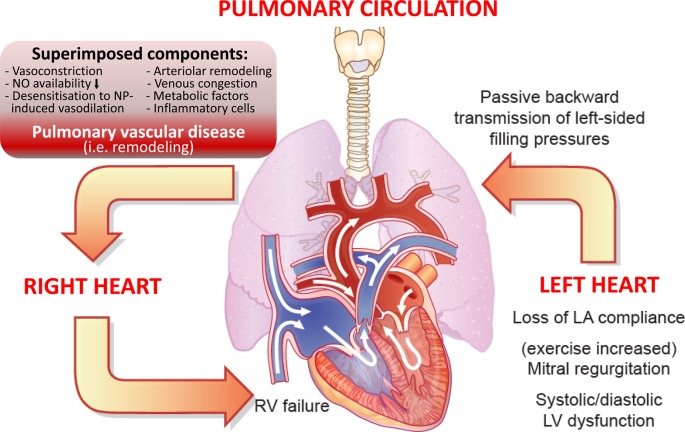
The pathobiology of PH in left HF is complex and highly heterogeneous, and remains incompletely understood. Pulmonary hypertension primarily results from the passive backward transmission of elevated left-sided filling pressures, which occur as a consequence of systolic or diastolic LV dysfunction (Figure 1).11 Furthermore, functional mitral regurgitation (MR) will result in elevations of LAP and PAP, which usually worsen during exercise. In addition to LV remodelling and dysfunction, an increase in LA size (which is relatively load-independent and serves as a marker of morbidity and mortality in HF), interstitial fibrosis causing LA stiffness, and reduced LA compliance, as well as impaired LA contractility, contribute to pathogenic alterations in the pulmonary circuit and right heart (Figure 2). These alterations of LA diastolic and systolic properties affect cardiac filling and output, and the backward transmission of elevated left-sided filling pressures leads to an increase of pulmonary pressures, particularly during exercise.25,26 Consistently, LA dysfunction relates to symptom onset in patients with HFpEF.27 In addition to its pathophysiological significance, LA dysfunction may also determine treatment responses to targeted PH therapies in patients with LHD, since lowering of PVR and increased pulmonary blood flow may lead to increased PAWP and pulmonary oedema in patients with reduced LA compliance.
Figure 1.
Cardiopulmonary interaction and pathobiology of pulmonary hypertension (PH) in left ventricular heart failure. Shown is (i) the backward transmission of elevated left ventricular filling pressures into the pulmonary circulation (post-capillary haemodynamic profile), (ii) potential superimposed components contributing to the extent of PH (leading to a pre-capillary component),11 which may be associated with (iii) pulmonary vascular remodelling in some patients, thus leading to (iv) right ventricular strain and dysfunction over time. Right ventricular (RV) dilation and increase in wall stress/tension (internal RV afterload) result in elevated myocardial oxygen consumption, which with concomitant reduction in coronary perfusion gradient leads to RV ischaemia and progressive RV failure.
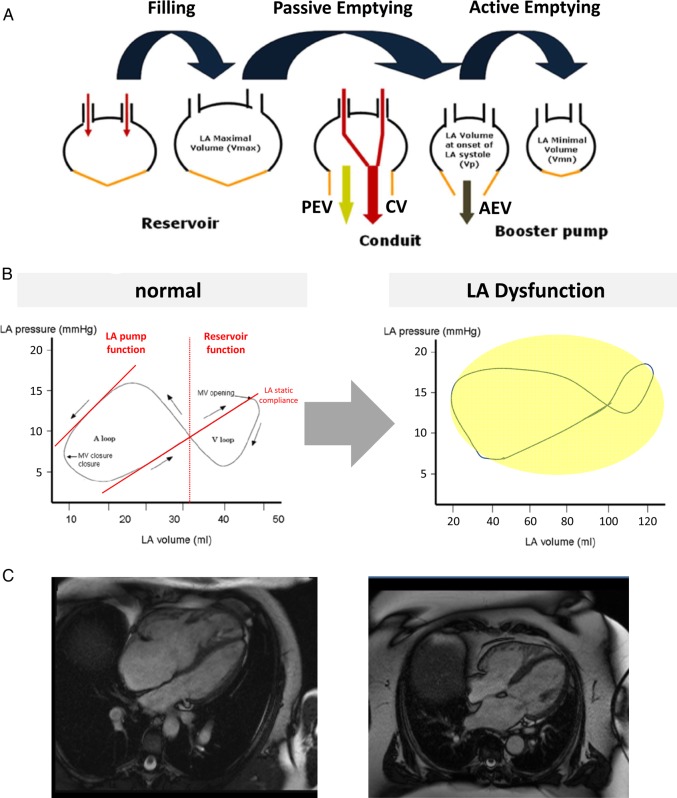
Figure 2.
Left atrial (LA) remodelling and dysfunction in heart failure. (A) Phases of LA function. PEV, passive empting volume = Vmax – Vp; CV, conduit volume = LV stroke volume – (Vmax– Vmin); AEV, active emptying volume = Vp – Vmin (modified from Rossi et al.25). (B) Left atrial pressure–volume loops in normal LA function (left) and LA dysfunction (right). (C) Left atrial imaging by cardiac magnetic resonance imaging (cMRI) in a patient with pulmonary arterial hypertension and normal LA function (left) vs. LA enlargement and dysfunction in a patient with left heart disease (right) (cMRI movies may be downloaded from the Supplementary material online).
In the pulmonary circulation, sudden increases of LAP may cause ‘alveolar-capillary stress failure’, a reversible barotrauma altering endothelial permeability and allowing leakage of erythrocytes, proteins, and fluid into the alveolar lumen, thus causing interstitial and alveolar oedema. Sustained elevation of left-sided filling pressure and pulmonary venous pressure may be accompanied by superimposed components in the pulmonary circuit, which include decreased NO availability, increased expression of endothelin-1, desensitization to natriuretic peptide-induced vasodilatation, infiltration of inflammatory cells, and neurogenic or metabolic factors.11 Furthermore, hypoxia promotes vasoconstriction and growth responses in the pulmonary circuit,28 and tachyarrhythmias, particularly atrial fibrillation, may precipitate PH in patients with LV HF. These additional components may trigger pulmonary arterial vasoconstriction, and—over time—structural remodelling of small pulmonary resistance arteries, indicative of a pre-capillary component of PH where PAP further increases in excess of PAWP elevation (Figure 1). Indeed, pulmonary arteriolar remodelling similar to PAH has been described in severe PH-LHD, particularly in patients with Cpc-PH (DPG ≥7 mmHg).10 Histopathological changes include thickening of the alveolar-capillary membrane, medial hypertrophy, intimal and adventitial fibrosis, and luminal occlusion in small pulmonary arterioles, whereas ‘plexiform lesions’ pathognomonic of PAH are not usually found.10,29,30 The fact that unloading of the LV by implantation of a LV assist device (LVAD) in patients with HFrEF and severe ‘fixed’ PH may substantially lower or even normalize PAP over time indicates that the alterations in the pulmonary circulation are partly reversible at least in some patients.31,32 In contrast to the systemic circulation, where vascular compliance is mainly determined by the aorta, arterial compliance in the lung is distributed over the entire pulmonary vascular bed, so that resistance (R) and compliance (C) are predominantly determined by the small resistance vessels. Hence, pulmonary arteriolar remodelling mainly contributes to the increase in PVR and reduced PA compliance in Cpc-PH.
Of interest, there is an inverse relation between R and C in the pulmonary circulation, and this holds true in PH-LHD.22,33 The product of R and C (RC time) determines the drop in PAP after pulmonary valve closure. It was found that in left HF with increased PAWP, the RC time under the influence of the increased PAWP is slightly decreased in comparison with PAH (Figure 3). Whether this is due to abnormal pulmonary venous remodelling, increased pulmonary vascular tone in left HF or just the consequence of the increased wedge pressure on the RC time calculations remains unknown. It has been suggested that the pressure gradient between the diastolic PAP and PAWP (DPG) is less dependent on volume load and stroke volume than the TPG, and that an elevated DPG may therefore be a better indicator of a pre-capillary component of PH.9 However, the DPG may not be an ideal parameter, as it is subject to inaccuracies related to heart rate, hypoxia, catheter whip, utilizing computer-generated measurements, and the presence of negative values or elevated DGP without PH. While in two retrospective studies, the DPG was shown to be a predictor of survival in PH-LHD,10,22 two other studies failed to demonstrate a prognostic impact of the DPG in patients with cardiomyopathy or heart transplantation and PH.14,24 Thus, its role for prognostication of survival and/or response to PH therapies must be further explored. Another implication of the constant RC is that based on the inverse relationship between compliance and resistance, PA compliance is a more sensitive marker for early disease than PVR. Indeed, a reduced PA compliance and abnormal RV/PA coupling are found even in early stages of HFpEF, and PA compliance appears to be prognostically relevant even with normal PVR.21–23,34,35
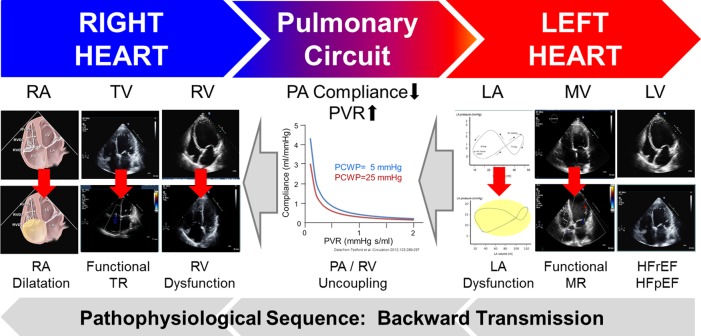
Figure 3.
Sequence of pathophysiological factors contributing to pulmonary hypertension in left ventricular heart failure. Backward transmission of left- to right-sided pathological features at the level of the ventricles, atrioventricular valves, and atria. Data derived from Tedford et al.33 showing that in left heart failure related pulmonary hypertension with increased wedge pressure the RC time is constant, but slightly decreased (leftward shift) (images containing video loops may be downloaded from the Supplementary material online).
The elevations of pulmonary pressures and PVR represent an increase of both resistive and pulsatile RV afterload, which results in dilatation and maladaptive remodelling of right heart chambers, functional tricuspid regurgitation, and ultimately RV dysfunction and failure. Pathological changes of the RV include hypertrophy, fibrosis, dilatation, and a change in RV shape from a crescent to a more spherical shape, which is associated with functional tricuspid regurgitation and elevated right atrial pressure (RAP). Right ventricular dysfunction resulting in low cardiac output is a key determinant of outcome in PH-LHD. In fact, the prognostic significance of an elevated PAP in HF increases with the degree of RV dysfunction irrespective of LVEF.13,19 However, the relationship between the severity of PH and RV dysfunction is not ‘linear’, as some patients with severe PH display normal RV function. In summary, a dysfunctional RV and clinical signs of right HF in patients with LHD occur as a consequence of pathological changes at the level of the ventricles, atrioventricular valves, and atria in both the left and right heart, and complex pathomechanisms of cardiopulmonary interaction affecting PVR and PA compliance determine the impact of LHD on the right heart (Figure 3).
Left vs. right ventricular phenotype
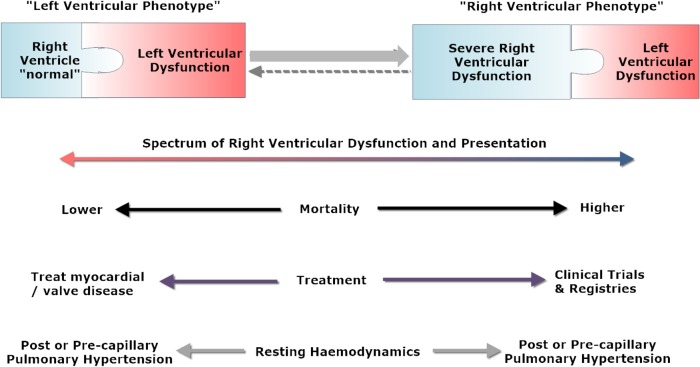
The current knowledge about the heterogeneity of PH in LV HF and the reasons, why some patients develop severe PH and RV dysfunction whereas others do not, remains limited. Two factors may be key: (i) the susceptibility for pulmonary vascular disease (due to genetic factors and/or environmental stressors and/or comorbidities) and (ii) the factor ‘time’. In many cases, only a ‘snapshot’ of a patient is considered during the course of HF development and progression. However, in HF patients who are susceptible for pulmonary vasculopathy, a potential evolution of LV disease to right ventricular (RV) dilatation and functional impairment as a result of PH may be observed. During this evolution, the heart alters progressively across a spectrum from a picture of an abnormal LV with an otherwise normal RV (‘left ventricular phenotype’) into a picture of a dilated and functionally impaired RV which may even dominate over the LV abnormality (‘right ventricular phenotype’) (Figure 4). The appearance of LV or RV phenotype depends on the point in this evolution that cardiac imaging is undertaken. This is supported by the fact that RV dysfunction in HFpEF is associated with more advanced stages of the disease.20 Examples of PH and RV phenotype developing over time—depending on patient's susceptibility—are depicted in Supplementary material online, Figure S1. Mortality increases as the RV phenotype develops. It must be emphasized that invasive haemodynamics are not always reliable in differentiating between different phenotypes across the spectrum because the use of diuretics and alterations in fluid status may influence ventricular filling pressures. While post-capillary haemodynamics indicate that LV disease is driving PH, pre-capillary haemodynamics do not exclude LV disease with certainty.
Figure 4.
‘Left ventricular phenotype’ vs. ‘right ventricular phenotype’ in pulmonary hypertension associated with left heart disease. Shown is the spectrum of right ventricular dysfunction and presentation, the impact on mortality, therapeutic implications, and resting pulmonary haemodynamics.
The dilemma of pulmonary arterial wedge pressure measurements to discriminate pre- and post-capillary pulmonary hypertension
Pulmonary arterial wedge pressure measurements are prone to significant errors which have direct implications on PH classification and treatment decisions. Limitations and uncertainties include the threshold level of PAWP that discriminates between pre- and post-capillary PH, technical difficulties to obtain correct recording of pressure tracings, as well as lack of proper standardizations of calibration (zero level), and particularly the method of PAWP reading in relation to the respiratory cycle (Table 1).
Table 1.
Potential confounders and criteria of valid measurement, reading and interpretation of pulmonary arterial wedge pressure tracings
| Measurement and reading of pressure tracings | |
| Measurement | may be challenging |
| not possible in every patient (dilated arteries) | |
| Zero point | should be mid-thoracic |
| Point of reading | end of normal expiration (during free respiration) |
| Factors preventing correct interpretation of pressure tracings | |
| Diuretic use | may artificially lower PAWP/LVEDP in patients with LHD |
| COPD | prominent respiratory swings, digital mean method may be preferred |
| Atrial fibrillation | variable/unreliable values due to interbeat variation |
| mean of five measurements should be calculated | |
| High v-wave | consider mitral regurgitation (diastolic LV dysfunction) |
| Thorax deformaties | referencing may be difficult |
| Abdominal obesity | raised intra-abdominal and intra-thoracic pressure |
| Criteria for valid PAWP measurement | |
| PAWP value | PAWP equal or lower than diastolic PAP |
| Pressure tracing | similar to atrial pressure tracing (a- and v-wave) |
| Respirophasic variations | respiratory swings visible |
| Catheter position | stationary catheter position (fluoroscopy) |
| Aspiration | free flow within the catheter (aspiration possible) |
| O2 saturation | in occluding position, aspiration of oxygen-rich blood (SO2 >94%) from the distal lumen |
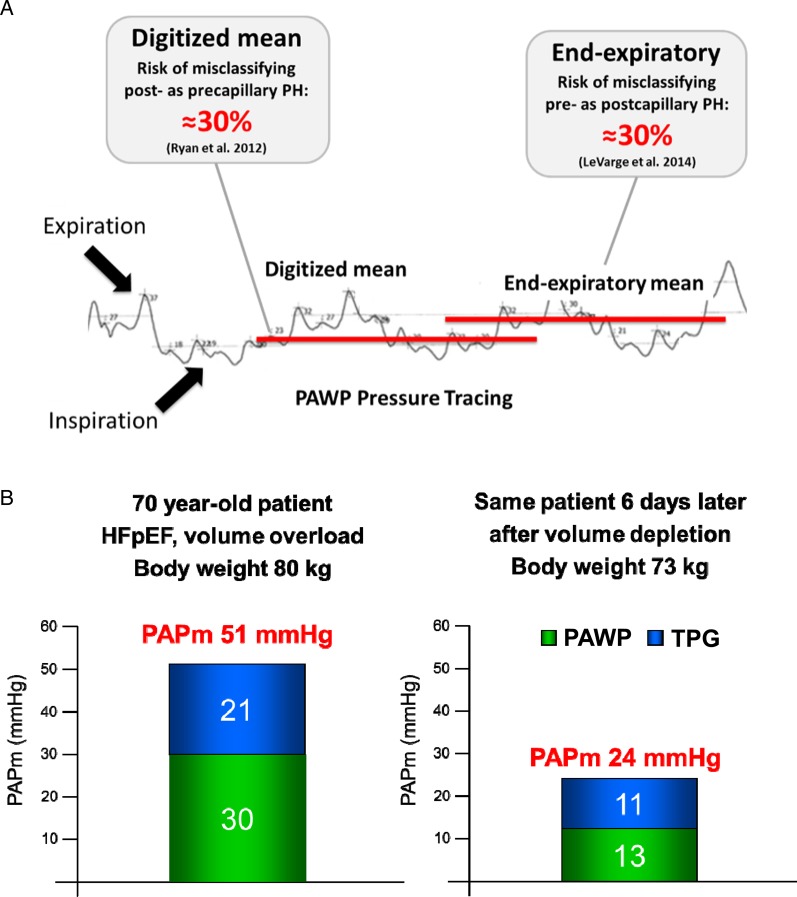
Although physicians use the same threshold level of 15 mmHg to discriminate between pre- and post-capillary PH worldwide,1,6,36 ‘expert centres’ utilize different methods of measurement, which may produce a mean difference of 4–6 mmHg.37–39 These differences are due to respirophasic variations of intra-thoracic pressures, resulting in lower values during inspiration, and higher values during expiration (Figure 5A). Additional sources of error include uncertainties about the threshold level and the impact of volume load (Figure 5B). Directly opposing studies have recently been published regarding the method of PAWP measurement and potential misclassifications between PAH and PH due to LHD (particularly HFpEF). Whereas one study indicated that the use of a digital mean value averaged across the respiratory cycle instead of the end-expiratory mean value frequently leads to misclassifications of PH-HFpEF as PAH,37 the other study revealed that reliance on end-expiratory PAWP instead of the digital mean value may lead to misclassifications of pre- as post-capillary PH38 (see Figure 5A). This dilemma highlights the high degree of variability, and the differences may in part be explained by the patient populations studied: while the end-expiratory method appears to be appropriate to distinguish between PAH and HFpEF, the digital mean value may be more appropriate in patients with COPD in whom respiratory swings are usually more pronounced.37,38,41
Figure 5.
Pulmonary arterial wedge pressure (PAWP). (A) Potential misclassifications between pre- and post-capillary pulmonary hypertension depending on the method of PAWP reading.37,38 (B) Example of a patient with pulmonary hypertension-heart failure with preserved ejection fraction before (left) and after (right) treatment with diuretics, illustrating the impact of volume load on pulmonary haemodynamics (modified from Dumitrescu et al.40).
In clinical practice, misclassifications of PH-HFpEF as PAH and inappropriate treatment of such patients with targeted PAH drugs appears much more common than vice versa. Furthermore, the end-expiratory method of PAWP reading was recently shown to correlate well with direct measurement of LVEDP, and to a pre-capillary pattern.37,42 In order to minimize the risk of misclassifying PH-LHD as PAH, at least in elderly patients and those with signs or risk factors of LHD, and in accordance with the recommendations of the 5th World Symposium on Pulmonary Hypertension 2013 in Nice,6,11 the following standardization of PAWP measurement appears most appropriate, particularly to discriminate between PAH and PH due to LHD: Nevertheless, physicians have to be aware of the limitations of PAWP measurement in general (Table 1), and of potential misclassifications when using either the digitized mean value or the end-expiratory method of PAWP reading. In patients with COPD and pronounced respiratory swings, utilization of the digitized mean value may be appropriate.
Zero level: the zero reference level should be set at the mid-thoracic position, which represents the level of the left atrium.39
Pulmonary arterial wedge pressure threshold: the threshold level to discriminate between pre- and post-capillary PH should be 15 mmHg, but suspicion for PH-HFpEF should be raised at values between 12 and 15 mmHg particularly in diuresed patients and/or risk factors for LHD.6,11
Reading of PAWP: PAWP should be measured at end-expiration, where the effects of intra-thoracic pressure swings are minimal.6 Patients should not hold their breath, and must not erroneously perform a Valsalva manoeuvre.
Direct measurement of LVEDP: should be considered if PAWP is not reliably measurable or uncertain, or if significant LHD is suspected.
Distinction between pulmonary arterial hypertension and pulmonary hypertension associated with left heart disease
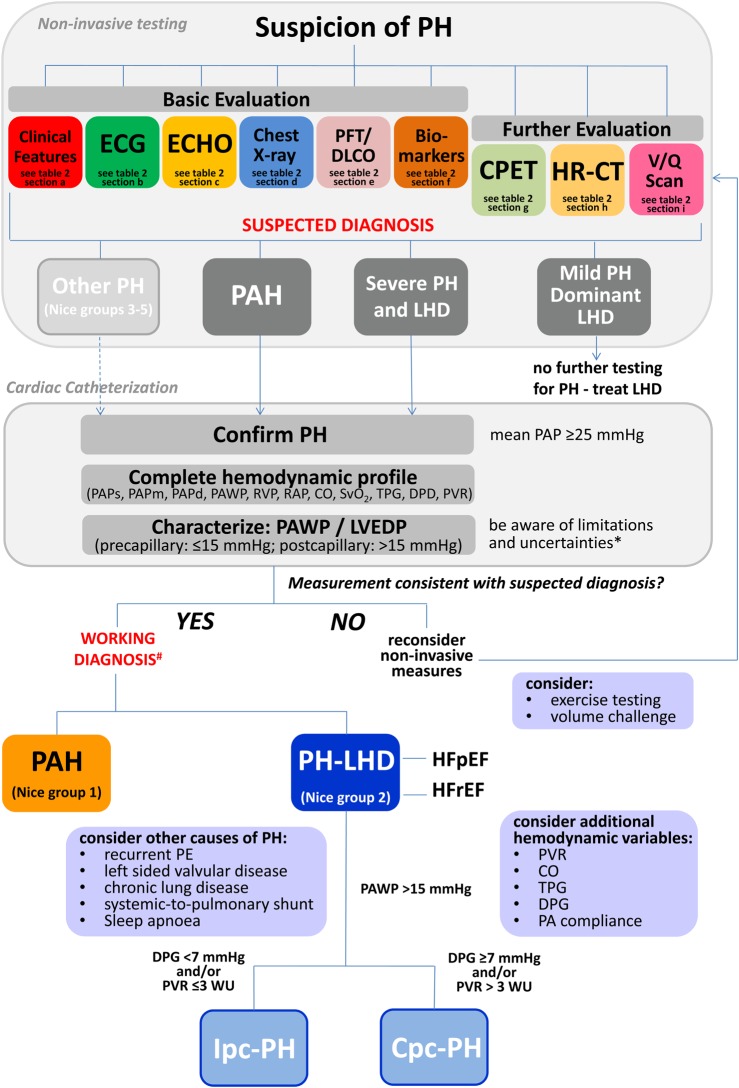
Current guidelines indicate that RHC is mandatory to confirm the diagnosis of PH, and—based on the PAWP—to allow for a precise haemodynamic classification as pre- or post-capillary PH.12 However, due to the above mentioned limitations of PAWP measurement and interpretation, and to the high degree of variability, this is not always applicable in clinical practice. While RHC is indeed required for proper diagnostic work-up in PH, invasively measured haemodynamics alone are in many cases not sufficient to establish a precise diagnosis. Instead, an integrated interpretation of invasive and non-invasive evaluations is required. Figure 6 provides a diagnostic algorithm/decision-making tree, which may help to integrate RHC and non-invasive findings, and to better elucidate the underlying cause and pathophysiology of PH in the context of LHD. Patients suspected to have PH should undergo clinical evaluation and basic non-invasive testing, and—if indicated—additional tests should be performed. A predictive model incorporating medical history, ECG, and echocardiography data helps to discriminate PAH from PH-LHD.43 Based on the results of a comprehensive non-invasive work-up, a ‘suspected diagnosis’ should be formulated. Clinical features, specific findings, and test results that may be suggestive of either PAH or PH-LHD are listed in Table 2.
Figure 6.
Differential diagnosis between pulmonary arterial hypertension (PAH, Nice Group 1) and pulmonary hypertension associated with left heart disease (PH-LHD Nice Group 2). Shown is a diagnostic algorithm, integrating non-invasive tests, and haemodynamic variables assessed by cardiac catheterization. For interpretation of non-invasive tests, see corresponding Table 2. *Limitations and uncertainties of pulmonary arterial wedge pressure/left ventricular end-diastolic pressure measurement are depicted in Table 1. Ipc-PH, isolated post-capillary PH; Cpc-PH, combined post- and pre-capillary PH.
Table 2.
Results of non-invasive diagnostic tests may be suggestive of either pulmonary arterial hypertension or pulmonary hypertension associated with left heart disease
| Suggestive of PAH (Nice group 1) | Suggestive of PH-LHD (Nice group 2) | |
|---|---|---|
| a. Clinical features | Younger age, familial cases, bendopneaa, risk factors for PAH: CTD, CHD, severe liver disease, portal hypertension, HIV | Older age, hypertension, diabetes, CAD, BMI >30, pulmonary congestion, history of pulmonary oedema, orthopnoea |
| b. ECG | RV hypertrophy, right axis, RV strain | LV hypertrophy (Sokolow–Lyon index: S in V1 + R in V6), left axis, atrial fibrillation |
| c. ECHOb | No signs of LHD, PASP elevated, RV > LV, RV hypertrophy/dysfunction (TAPSE), RVOT notchingc, small LA, dilated IVC | Enlarged LA, LV hypertrophy, signs of systolic (EF) and/ordiastolic (E/A, DT, E/E′) LV dysfunction, valvular disease |
| d. Chest X-ray | Enlarged right heart chambers, dilated PA, peripheral PA pruning | Pulmonary congestion, Kerley B lines, pleural effusions, enlargement of left heart chambers |
| e. PFT/DLCO | Normal/mild obstructive spirometry, normal or moderately decreased DLCOd, low pcCO2 (≤36 mmHg)e | Normal/obstructive spirometry, normal DLCO (may be decreased due to comorbid COPD), high pcCO2 (>36 mmHg)e |
| f. Biomarkers | BNP/NTproBNP elevated (not discriminate between Groups 1 and 2) | BNP/NTproBNP elevated (not discriminate between group 1 and 2) |
| g. CPET | Low PETCO2 at AT, decreasing during exercise; high VE/VCO2, increasing during exercise | PETCO2 at AT normal or slightly lowered, not decreasing during exercise, VE/VCO2 not increasing during exercise |
| h. HR-CT | To diagnose or rule out parenchymal lung disease (not discriminate between Groups 1 and 2) | To diagnose or rule out parenchymal lung disease (not discriminate between Groups 1 and 2) |
| i. V/Q scan | To diagnose or rule out CTEPH (not discriminate between Groups 1 and 2) | To diagnose or rule out CTEPH (not discriminate between Groups 1 and 2) |
aBendopnea is typical in PAH, but may be found in HFrEF, particularly when associated with RV dysfunction and elevated RAP as described in Ref. 44.
bA score of five echocardiographic variables may be utilized to discriminate between pre-capillary PH (RV/LV ratio ≥1.0, LVEI >1.2, E/E′ ≤10, RV forming apex, IVC >20 mm without inspiratory collapse) and post-capillary PH (RV/LV ratio <1.0, LVEI ≤1.1, E/E′ >10, RV not forming apex, IVC ≤20 mm and collapsible).53
cRVOT notching.54
dMarked hypoxemia and low DLCO may suggest pulmonary veno-occlusive disease (PVOD).
eRole of the capillary pCO2 (pcCO2) described in Ref. 45. CTEPH, chronic thromboembolic pulmonary hypertension; CAD, coronary artery disease
Since here we focus on the differentiation between PAH and PH-LHD, we do not specify other forms of PH (Nice Groups 3–5) which should certainly be diagnosed or ruled out accordingly.2,6,12 In cases of mild PH and dominant LHD, further testing for PH is usually not required. Instead, left heart catheterization may be indicated, and LHD treated. If PAH is suspected, or severe PH is present in addition to (mild) LHD, invasive assessment of pulmonary haemodynamics by RHC is usually indicated.
When performing RHC, there are three main tasks (three C’s) (Figure 6): It is of key importance to ask whether the results obtained by invasive measurement of haemodynamics are consistent with the ‘suspected diagnosis’ based on the results of non-invasive testing. If the answer is ‘yes’, then a ‘working diagnosis’ is established, and patients should be treated accordingly. If the answer is ‘no’, then non-invasive measures—particularly echocardiographic parameters—should be reconsidered, and invasive haemodynamics must be interpreted in the clinical context. Of note, as the LVEDP depends on the loading condition of the LV, unloading (e.g. by forced diuresis) can decrease LVEDP (and consecutively PAWP) to values <15 mmHg, even in patients with LHD. In cases of doubt, invasive assessment of LV filling at rest and during a stress test (either exercise or fluid challenge) may be performed. As HFpEF patients have a steep end-diastolic pressure/volume relationship, even small changes in volume are associated with a significant increase in filling pressures, and a ‘volume challenge’ (e.g. infusion of 500 mL of saline within 5–10 min) may be feasible to unmask occult pulmonary venous hypertension.46 Based on current data, a PAWP >18 mmHg may be considered as an abnormal response to fluid challenge.47 An alternative may be the integration of exercise testing into the diagnostic work-up.48 However, such tests lack proper standardization and are awaiting further evaluation and validation before their use in clinical practice can be recommended.
Confirm: the presence of PH should be confirmed by a mean PAP ≥25 mmHg.
Complete: it is crucial to obtain the complete set of pulmonary haemodynamics; this includes measurement of PAP (systolic, diastolic, and mean), RAP, cardiac output/cardiac index, mixed venous oxygen saturation, and calculation of the TPG (mean PAP – PAWP), DPG (diast PAP – PAWP), and PVR (mean PAP – PAWP/CO). Furthermore, pulmonary haemodynamics must always be interpreted in relation to systemic vascular resistance (PVR/SVR ratio).
Characterize: PH should be characterized as pre-, isolated post-capillary, or combined post- and pre-capillary, based on the assessment of LV filling pressure either measured as PAWP or LVEDP. When interpreting the pressure tracings, it is critical to be aware of the limitations and sources of error with regard to PAWP measurement (Table 1).
Non-invasive tools to differentiate between pulmonary arterial hypertension and pulmonary hypertension-heart failure with preserved ejection fraction
While systolic dysfunction in patients with PH-HFrEF is usually easy to detect, the proper distinction between PAH and PH associated with HFpEF is much more challenging. Regardless of the presence of PH, there are reliable non-invasive tools that indicate the presence of diastolic LV dysfunction (Table 2).11,49 The gold standard for the assessment of LV diastolic function is the invasive assessment of the pressure–volume relationship which usually requires an estimation of LV volume by the conductance method. It has been shown that LV stiffness is a reliable characteristic in patients with HFpEF.50,51 An alternative to the assessment of invasive haemodynamics can be comprehensive echocardiography, which—in combination with natriuretic peptides—is feasible to diagnose HFpEF as proposed by a consensus statement.49 Echocardiographic assessment for HFpEF includes estimation of LVEDP by E/E′, assessment of left atrial size, E/A ratio, and deceleration time.52 It should be noted, however, that echocardiographic parameters such as E/E′ also have their limitations in accurately diagnosing HFpEF. An integrative score of five echocardiographic variables (RV/LV ratio, left ventricular eccentricity index (LVEI), E/E′, RV forming apex, width and inspiratory collapse of IVC) as well as additional parameters such as the shape of the RV outflow tract Doppler envelope (‘RVOT notching’) may be utilized to discriminate between pre- and post-capillary PH.53,54 The presence of one or more of the above findings should prompt one to suspect PH-HFpEF instead of PAH, even if the left-sided filling pressure as assessed by PAWP or LVEDP measurement during cardiac catheterization may be <15 mmHg.
Treatment of pulmonary hypertension in left ventricular heart failure
The prognostic impact of PH and RV dysfunction in LV HF provides the rationale for targeting PH as a potential additional treatment option in both HFrEF and HFpEF. However, it is critical to precisely determine the cause and severity of PH, before any treatment decisions are made. Therefore, a comprehensive diagnostic work-up is necessary, and targeted HF treatments (medical/interventional) remain the only established therapies. Nevertheless, the treatment options in HF—particularly HFpEF—remain limited, and the classification of PH may be challenging in individual patients. In selected cases presenting with a ‘right ventricular phenotype’ in whom PH and RV dysfunction are prominent, targeted judicious PH therapy may be considered despite the presence of LHD.55
Treatment of left heart disease and lowering of left-sided filling pressure
The clinical impact of elevated PAWP/LVEDP and PAP in patients with LV HF warrants attention. Optimized treatment of the underlying LHD including medical treatments (reaching the target dosages) and interventional therapies (e.g. CRT, ICD, LVAD, MitraClip) usually helps to lower left-sided filling pressure and is always the primary aim in HF patients. Nevertheless, the consideration of pulmonary haemodynamics may be helpful, and volume depletion is usually associated with a substantial reduction of left-sided filling pressure (PAWP/LVEDP), PAP, and TPG (see Figure 5C). In this context, the CHAMPION trial has shown that consideration of the PAP (assessed by an implantable device) as an additional treatment target profoundly reduced the rate of HF-associated hospitalizations in both HFpEF and HFrEF.56,57 Of note, reductions of PAP were not achieved by targeted PAH drugs in this trial, but by optimized HF treatment including adjustment of diuretics.
Repair of mitral regurgitation in heart failure
Particularly in patients with HFrEF and dilated left-sided heart chambers, functional MR is common, may represent the main cause of PH, and leads to increased mortality.58 Even exercise-induced PH and RV dysfunction were recently shown to be associated with adverse outcome in patients with asymptomatic MR.59,60 There is good evidence in patients with LV HF and functional MR, that proper treatment of the mitral valve including catheter-based approaches such as Mitral Clipping or Cardio-Band lead to substantial improvement of pulmonary haemodynamics, including reductions of the mean PAP and PAWP (mainly via reduction of the v-wave), and a profound improvement of cardiac index.61 While the evidence remains limited and outcome trials are still lacking, percutaneous mitral valve repair was shown to improve clinical symptoms, exercise capacity, quality of life, and HF-associated hospitalizations in HF patients.62 Hence, MR has to be considered as the potential cause of PH in LV HF, and appropriate valve repair should be initiated in patients who are on optimized medical treatment.
Targeted treatment of pulmonary hypertension in patients with left ventricular heart failure
Based on the prognostic impact of PH and RV dysfunction in both HFrEF and HFpEF, treatment of PH in addition to established HF therapies may appear to be a promising approach. Targeted therapies approved for the treatment of PAH include endothelin receptor antagonists (ERAs), prostanoids, phosphodiesterase type 5 inhibitors (PDE5i), and stimulators of soluble guanylate cyclase (sGC).3,4 It should be emphasized that none of these compounds are approved for the treatment of PH-LHD. A concern with the use of targeted PAH drugs in patients with LHD is that pulmonary vasodilation and increasing pulmonary blood flow in the presence of elevated left-sided filling pressures may potentially lead to pulmonary oedema and cardiac decompensation. Despite these concerns, such compounds have been investigated in patients with HF. Randomized controlled trials (RCTs) investigating the efficacy and safety of ERAs and prostanoids have shown no benefit, or PH therapies have even proved harmful (summarized in Refs 11,55). For instance, the FIRST trial, which investigated intravenous epoprostenol in patients with PH-HFrEF, was prematurely terminated due to a trend for increased mortality in the treatment group when compared with placebo. Notably, ERAs have only been investigated in patients with HF in general without considering the presence of PH, so their impact on PH-LHD remains unknown. Few data are currently available in PH-HFpEF, and the use of ERAs may be associated with an increased risk of peripheral oedema in such patients. An ongoing Phase II trial (MELODY) currently investigates the safety and potential efficacy of the novel ERA macitentan in patients with HFpEF and Cpc-PH (Table 3).
Table 3.
Summary of recently completed and ongoing clinical trials in pulmonary hypertension associated with left heart disease
| Drug | n | Start | End | Duration | Primary endpoint | Secondary endpoints |
|---|---|---|---|---|---|---|
| HF with reduced EF | ||||||
|
Riociguat
LEPHT69 |
201 | Results available | 16 weeks | Change in mPAP from baseline | Haemodynamic and echocardiographic variables, biomarker levels, safety, pharmacokinetics | |
|
Sildenafil
Sil-HF (NCT01616381) |
210 | 9/2012 | 6/2014 | 24 weeks | Patient Global assessment 6MWD | QoL, Kansas city questionnaire, safety |
|
Tadalafil
PITCH-HF (NCT01910389) |
2102 | Study terminated Febuary 2014a |
Up to 54 months | Time to CV death or 1st HF hospitalization | Biomarkers levels, exercise capacity, QoL, safety | |
| HF with preserved EF | ||||||
|
Sildenafil
RELAX63 |
216 | Results available | 24 weeks | Change in peak VO2 from baseline | Exercise capacity, clinical status, QoL, safety | |
|
Sildenafil
Hoendermis et al.64 |
52 | Results available | 12 weeks | Change in mPAP from baseline | Change in PAWP, cardiac output, peak VO2 | |
|
Riociguat
DILATE70 |
48 | Results available | 16 weeks | Change in mPAP from baseline | Haemodynamic and echocardiographic variables, biomarker levels, safety, pharmacokinetics | |
| HF with EF >35% | ||||||
|
Macitentan
MELODY-1 (NCT02070991) |
60 | 5/2014 | 10/2015 | 12 weeks | Safety and tolerability (fluid retention) | PVR, haemodynamics, changes in TPG and DPG, echocardiographic variables (RV function) |
All studies were performed in heart failure patients with and/or without preserved ejection fraction.
mPAP, mean pulmonary artery pressure; DPG, diastolic pressure gradient; HF, heart failure; PVR, pulmonary vascular resistance; QoL, quality of life; RV, right ventricle; 6MWD, six minute walking distance; TPG, transpulmonary pressure gradient.
aThe study has been terminated by the funding agency.
More recent studies have focused on targeting the NO pathway in patients with PH-LHD (Table 3). The randomized controlled RELAX trial has shown that the PDE5i sildenafil did not improve peak oxygen consumption (primary endpoint) and exercise capacity in patients with HFpEF in the absence of PH.63 In a recent single-centre RCT, sildenafil also failed to improve haemodynamics or exercise capacity in 53 patients with HFpEF and Ipc-PH.64 On the other hand, small single-centre studies have consistently shown that sildenafil improved haemodynamic parameters and exercise capacity in patients with severe PH that was associated with either HFrEF or HFpEF.65–67 A meta-analysis of six RCTs investigating the efficacy and safety of PDE5i in patients with HFrEF—each performed in small numbers of patients—revealed that additional use of a PDE5i improved haemodynamics and exercise capacity, and reduced clinical symptoms and hospitalizations when compared with placebo.68 However, such data should be interpreted with great caution, as they were obtained in highly selected patients, and data from larger RCTs are yet lacking.
An alternative way to modulate the NO pathway is the stimulation of sGC. The sGC stimulator riociguat (which is approved for PAH and inoperable CTEPH) was recently investigated in two RCTs in PH-LHD. In patients with PH-HFrEF, the LEPHT trial failed to reach the primary endpoint of lowering PAP vs. placebo, but led to a substantial increase in cardiac index, resulting in lowering of PVR.69 Likewise, a proof-of-concept trial testing the acute effects of riociguat in patients with PH-HFpEF (DILATE) obtained similar results.70 These findings may suggest a systemic and/or cardiac effect of riociguat rather than pulmonary vasodilation. Targeting this pathway in HF awaits further investigation.
Based on the current evidence, the use of targeted PAH therapies in patients with PH related to left HF is discouraged, and selected patients with Cpc-PH and/or RV phenotype should be referred to centres with expertise in both HF and PH, and should—whenever possible—be included into clinical trials.
Clinical relevance and future considerations
In patients with LV HF, PH is a common and life-threatening complication that remains underestimated in clinical cardiology. Given the relentlessly increasing incidence and prevalence of HF, particularly HFpEF, and the poor prognosis of patients who develop PH and RV dysfunction secondary to LV HF, it is imperative to further improve our understanding of the pathophysiology of PH-LHD, and to establish an evidence-based approach for the management of HF patients developing PH. Harmonized terminology and a new classification of PH-LHD (Ipc-PH vs. Cpc-PH) have improved the clinical assessment and should help prevent the inappropriate off-label use of PAH drugs in PH-LHD. In addition, they provide better tools to identify a subset of patients with HF and PH who deserve special attention. Despite significant achievements that were made recently, important evidence gaps remain that need to be addressed in future studies.
Specific propositions
Since cardiopulmonary interaction in HF is complex and variable, the definitions and prognostic significance of haemodynamic variables characterizing the degree of pre-capillary PH in LHD need to be further improved. This may be achieved by integrated measures of pulmonary vascular and RV function.
As the differential diagnosis between pre- and post-capillary PH remains challenging, an integrative approach considering the clinical context, non-invasive assessment, and invasive haemodynamic variables is critical. The role of volume challenge and exercise stress testing to unmask occult LHD needs to be established and requires standardization and validation.
The evolution from an LV phenotype to an RV phenotype over time as a result of PH requires further investigation. We need to better understand the susceptibility for pulmonary vascular disease in HF in a subset of patients (genetic predisposition, contributing factors), and to collect data on the prevalence and timing of such an evolution, which will ideally help to predict which patients with LHD will develop significant PH and RV dysfunction.
Specifically, longitudinal studies should be performed that investigate the development of RV dysfunction in patients with LV HF over time.
Thus far, targeted PH therapies have never been investigated properly in PH-LHD. Carefully controlled randomized trials in larger populations of well characterized patients (PH-HFrEF and PH-HFpEF separately), with longer observation times, and definitive endpoints are needed. In such trials, it must be assured that patients are on optimized regimens of HF therapy and fluid balance before randomization, which may be achieved by a ‘run-in’ phase.
Proof-of-concept studies in limited numbers of haemodynamically well characterized patients, focusing on patients with Cpc-PH, together with the improvement of non-invasive variables that better characterize the degree of pre-capillary PH in PH-LHD, may better inform larger RCTs.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors' contributions
All authors contributed to literature search, figure design, and writing/reviewing the manuscript; drafted the manuscript; and made critical revision of the manuscript for key intellectual content.
Supplementary Material
Acknowledgements
The critical input of Stephan Baldus (University of Cologne, Germany), Hossein Ardeschir Ghofrani (University of Giessen, Germany, and Imperial College, London, UK), Marius M. Hoeper (Hannover Medical School, Hannover, Germany), Horst Olschewski (University of Graz, Austria), and Maria José Laureira (Almeda, Portugal) is greatly appreciated.
Conflict of interest: S.R. has received remunerations for lectures and/or consultancy from Actelion, Bayer, Gilead, GSK, Novartis, Pfizer, and United Therapeutics. His institution has received research grants from Actelion, Bayer, Novartis, Pfizer, and United Therapeutics. J.S.R.G. has received remunerations for lectures and/or consultancy from Actelion, Bayer, Gilead, GSK, Novartis, and Pfizer. R.W. has served as an investigator, consultant, or speaker for Bayer, Berlin Chemie, Boehringer Ingelheim, Boston Scientific, CVRx, Gilead, Johnson & Johnson, Medtronic, Novartis, Pfizer, Sanofi, and Servier. His institution has received research grants from Boehringer Ingelheim. T.D.M. has served as a consultant for Actelion, Gilead, and Bayer. She has received research grants from United Therapeutics, Pfizer, and Reata. A.V.-N. has received remunerations for lectures and/or consultancy from Actelion, Pfizer, Bayer, and GSK. His institution has received research grants from Actelion and GSK. J.-L.V. has received remunerations for lectures and/or consultancy from Actelion, Bayer, GSK, Lilly, and Merck. His institution has received research grants from Actelion.
References
- 1. Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G; ESC Committee for Practice Guidelines (CPG). Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30: 2493–2537. [DOI] [PubMed] [Google Scholar]
- 2. Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–D41. [DOI] [PubMed] [Google Scholar]
- 3. Rosenkranz S. Pulmonary hypertension 2015: Current definitions, terminology, and novel treatment options. Clin Res Cardiol 2015; 104: 197–207. [DOI] [PubMed] [Google Scholar]
- 4. Galiè N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, Klepetko W, McGoon MD, McLaughlin VV, Preston IR, Rubin LJ, Sandoval J, Seeger W, Keogh A. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol 2013; 62(Suppl D): D60–D72. [DOI] [PubMed] [Google Scholar]
- 5. Lüscher TF. Pulmonary embolism and pulmonary hypertension: Two issues often neglected in cardiology. Eur Heart J 2015; 36: 581–583. [DOI] [PubMed] [Google Scholar]
- 6. Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, Manes A, Satoh T, Torres F, Wilkins MR, Badesch DB. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D42–D50. [DOI] [PubMed] [Google Scholar]
- 7. Fang JC, DeMarco T, Givertz MM, Borlaug BA, Lewis GD, Rame JE, Gomberg-Maitland M, Murali S, Frantz RP, McGlothlin D, Horn EM, Benza R. World Health Organization pulmonary hypertension group 2: Pulmonary hypertension due to left heart disease in the adult – a summary statement from the pulmonary hypertension council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2012; 31: 913–933. [DOI] [PubMed] [Google Scholar]
- 8. Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction. JACC Heart Fail 2013; 1: 290–299. [DOI] [PubMed] [Google Scholar]
- 9. Naeije R, Vachiery JL, Yerly P, Vanderpool R. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J 2013; 41: 217–223. [DOI] [PubMed] [Google Scholar]
- 10. Gerges C, Gerges M, Lang MB, Zhang Y, Jakowitsch J, Probst P, Maurer G, Lang IM. Diastolic pulmonary vascular pressure gradient. A predictor of prognosis in out-of-proportion pulmonary hypertension. Chest 2013; 143: 758–766. [DOI] [PubMed] [Google Scholar]
- 11. Vachiery JL, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galiè N, Ghio S, Gibbs JS, Martinez F, Semigran M, Simonneau G, Wells A, Seeger W. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol 2013; 62(Suppl D): D100–D108. [DOI] [PubMed] [Google Scholar]
- 12. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. 2015ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2015; 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 13. Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol 2001; 37: 183–188. [DOI] [PubMed] [Google Scholar]
- 14. Tampakakis E, Leary PJ, Selby VN, De Marco T, Cappola TP, Felker GM, Russell SD, Kasper EK, Tedford RJ. The diagnostic pulmonary gradient does not predict survival in patients with pulmonary hypertension due to left heart disease. JACC Heart Fail 2015; 3: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction. A community-based study. J Am Coll Cardiol 2009; 53: 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leung CC, Moondra V, Catherwood E, Andrus BW. Prevalence and risk factors of pulmonary hypertension in patients with elevated pulmonary venous pressure and preserved ejection fraction. Am J Cardiol 2010; 106: 284–286. [DOI] [PubMed] [Google Scholar]
- 17. Shah AM, Shah SJ, Annand IS, Sweitzer NK, O'Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD; TOPCAT Investigators. Cardiac structure and function in heart failure with preserved ejection fraction: Baseline findings from the echocardiographic study of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial (TOPCAT). Circ Heart Fail 2014; 7: 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bursi F, McNallan SM, Redfield MM, Nkomo VT, Lam CS, Weston SA, Jiang R, Roger VL. Pulmonary pressures and death in heart failure. J Am Coll Cardiol 2012; 59: 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 2014; 35: 3452–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mohammed SF, Hussain I, Abou Ezzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation 2014; 130: 2310–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pellegrini P, Rossi A, Pasotti M, Raineri C, Cicoira M, Bonapace S, Dini FL, Temporelli PL, Vassanelli C, Vanderpool R, Naeije R, Ghio S. Prognostic relevance of pulmonary arterial compliance in patients with chronic heart failure. Chest 2014; 145: 1064–1070. [DOI] [PubMed] [Google Scholar]
- 22. Dragu R, Rispler S, Habib M, Sholy H, Hammerman H, Galie N, Aroson D. Pulmonary arterial capacitance in patients with with heart failure and reactive pulmonary hypertension. Eur J Heart Fail 2015; 17: 74–80. [DOI] [PubMed] [Google Scholar]
- 23. Al-Naamani N, Preston IR, Paulus JK, Hill NS, Roberts KE. Pulmonary arterial capacitance is an important predictor of mortality in heart failure with a preserved ejection fraction. JACC Heart Fail 2015; 3: 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tedford RJ, Beaty CA, Mathai SC, Kolb TM, Damico R, Hassoun PM, Leary PJ, Kass DA, Shah AS. Prognostic value of the pre-transplant diastolic pulmonary artery pressure-to-pulmonary capillary wedge pressure gradient in cardiac transplant recipients with pulmonary hypertension. J Heart Lung Transplant 2014; 33: 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rossi A, Gheorghiade M, Triposkiadis F, Solomon SD, Pieske B, Butler J. Left atrium in heart failure with preserved ejection fraction: Structure, function, and significance. Circ Heart Fail 2014; 7: 1042–1049. [DOI] [PubMed] [Google Scholar]
- 26. Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail 2015; 8: 295–303. [DOI] [PubMed] [Google Scholar]
- 27. Sanchis L, Gabrielli L, Andrea R, Falces C, Duchateau N, Perez-Villa F, Bijnens B, Sitges M. Left atrial dysfunction relates to symptom onset in patients with heart failure with preserved ejection fraction. Eur Heart J Cardiovasc Imaging 2015; 16: 62–67. [DOI] [PubMed] [Google Scholar]
- 28. Ten Freyhaus H, Dagnell M, Leuchs M, Vantler M, Berghausen E, Caglayan E, Weissmann N, Dahal BK, Schermuly RT, Östman A, Kappert K, Rosenkranz S. Hypoxia enhances PDGF signaling in the pulmonary vasculature by downregulation of protein tyrosine phosphatases. Am J Respir Crit Care Med 2011; 183: 1092–1102. [DOI] [PubMed] [Google Scholar]
- 29. Chen Y, Guo H, Xu D, Xu X, Wang H, Hu X, Lu Z, Kwak D, Xu Y, Gunther R, Huo Y, Weir EK. Left ventricular failure produces profound lung remodeling and pulmonary hypertension in mice: Heart failure causes severe lung disease. Hypertension 2012; 59: 1170–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Delgado JF. The right heart and pulmonary circulation (III): The pulmonary circulation in heart failure. Rev Esp Cardiol 2010; 63: 334–345. [DOI] [PubMed] [Google Scholar]
- 31. Kutty RS, Parameshwar J, Lewis C, Catarino PA, Sudarshan CD, Jenkins DP, Dunning JJ, Tsui SS. Use of centrifugal left ventricular assist device as a bridge to candidacy in severe heart failure with secondary pulmonary hypertension. Eur J Cardiovasc Surg 2013; 43: 1237–1242. [DOI] [PubMed] [Google Scholar]
- 32. Lundgren J, Algotsson L, Kornhall B, Radegran B. Preoperative pulmonary hypertension and its impact on survival after heart transplantation. Scand Cardiovasc J 2014; 48: 47–58. [DOI] [PubMed] [Google Scholar]
- 33. Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, Cingolani OH, Mudd JO, Borlaug BA, Redfield MM, Lederer DJ, Kass DA. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation 2012; 125: 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van Empel VP, Kaufmann BA, Bernheim AM, Goetschalckx K, Min SY, Muzzarelli S, Pfisterer ME, Kiencke S, Maeder MT, Brunner-La Rocca H-P. Interaction between pulmonary hypertension and diastolic dysfunction in an elderly heart failure population. J Cardiac Fail 2014; 20: 09–104. [DOI] [PubMed] [Google Scholar]
- 35. Andersen MJ, Hwang SJ, Kane GC, Melevonsky V, Olson TP, Fetterly K, Borlaug BA. Enhanced pulmonary vasodilator reserve and abnormal right ventricular – pulmonary artery coupling in heart failure with preserved ejection fraction. Circ Heart Fail 2015; 8: 542–550. [DOI] [PubMed] [Google Scholar]
- 36. Mc Laughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53: 1573–1619. [DOI] [PubMed] [Google Scholar]
- 37. Ryan JJ, Rich JD, Thiruvoipati T, Swamy R, Kim GH, Rich S. Current practice for determining pulmonary capillary wedge pressure predisposes to serious errors in the classification of patients with pulmonary hypertension. Am Heart J 2012; 163: 589–594. [DOI] [PubMed] [Google Scholar]
- 38. LeVarge BL, Pomerantsev E, Channick RN. Reliance on end-expiratory wedge pressure leads to misclassification of pulmonary hypertension. Eur Respir J 2014; 44: 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kovacs G, Avian A, Pienn M, Naeije R, Olschewski H. Reading pulmonary pressure tracings. How to handle the problems of zero leveling and respiratory swings. Am J Respir Crit Care Med 2014; 190: 252–257. [DOI] [PubMed] [Google Scholar]
- 40. Dumitrescu D, Gerhardt F, Viethen T, Erdmann E, Rosenkranz S. 70 year-old patient with cardiac hypertrophy and severe pulmonary hypertension: Pre- or postcapillary? Dtsch Med Wochenschr 2011; 136: 2594–2598. [DOI] [PubMed] [Google Scholar]
- 41. Boerrigter BG, Waxman AB, Westerhof N, Vonk-Noordegraaf A, Systrom DM. Measuring central pulmonary pressures during exercise in COPD: how to cope with respiratory effects. Eur Respir J 2014; 43: 1316–1325. [DOI] [PubMed] [Google Scholar]
- 42. De Oliveira RKF, Ferreira EVM, Ramos RP, Messina CMS, Kapins CEB, Silva CMC, Ota-Arakaki JS. Usefulness of pulmonary capillary wedge pressure as a correlate of left ventricular filling pressures in pulmonary arterial hypertension. J Heart Lung Transplant 2014; 33: 157–162. [DOI] [PubMed] [Google Scholar]
- 43. Jacobs W, Konings TC, Heymans MW, Boonstra A, Bogaard HJ, van Rossum AC, Vonk-Noordegraaf A. Non-invasive identification of left-sided heart failure in a population suspected of pulmonary arterial hypertension. Eur Respir J 2015; 46: 422–430. [DOI] [PubMed] [Google Scholar]
- 44. Thibodeau JT, Turer AT, Gualano SK, Ayers CR, Velez-Martinez M, Mishkin JD, Patel PC, Mammen PP, Markham DW, Levine BD, Drazner MH. Characterization of a novel symptom in patients with advanced heart failure: Bendopnea. JACC Heart Fail 2014; 2: 24–31. [DOI] [PubMed] [Google Scholar]
- 45. Olsson KM, Sommer L, Fuge J, Welte T, Hoeper MM. Capillary pCO2 helps distinguishing idiopathic pulmonary arterial hypertension from pulmonary hypertension due to heart failure with preserved ejection fraction. Respir Res 2015; 16: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Robbins IM, Hemnes AR, Pugh ME, Brittain EL, Zhao DX, Piana RN, Fong PP, Newman JH. High prevalence of occult pulmonary venous hypertension revealed by fluid challenge in pulmonary hypertension. Circ Heart Fail 2014; 7: 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fujimoto N, Borlaug BA, Lewis GD, Hastings JL, Shafer KM, Bhella PS, Carrick-Renson G, Levine BD. Hemodynamic responses to rapid saline loading: the impact of age, sex, and heart failure. Circulation 2013; 127: 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Van Empel VPM, Kaye DM. Integration of exercise evaluation into the algorithm for evaluation of patients with suspected heart failure with preserved ejection fraction. Int J Cardiol 2013; 168: 716–722. [DOI] [PubMed] [Google Scholar]
- 49. Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007; 28:2539–2550. [DOI] [PubMed] [Google Scholar]
- 50. Wachter R, Schmidt-Schweda S, Westermann D, Post H, Edelmann F, Kasner M, Lüers C, Steendijk P, Hasenfuss G, Tschöpe C, Pieske B. Blunted frequency-dependent upregulation of cardiac output is related to impaired relaxation in diastolic heart failure. Eur Heart J 2009; 30, 3027–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure - abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 2004; 350:1953–1959. [DOI] [PubMed] [Google Scholar]
- 52. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009; 10: 165–193. [DOI] [PubMed] [Google Scholar]
- 53. D'Alto M, Romeo E, Argiento P, Pavelescu A, Mélot C, D'Andrea A, Correra A, Bossone E, Calabrò R, Russo MG, Naeije R. Echocardiographic prediction of pre- versus postcapillary pulmonary hypertension. J Am Soc Echocardiogr 2015; 28: 108–115. [DOI] [PubMed] [Google Scholar]
- 54. Arkles JS, Opotowsky AR, Ojeda J, Rogers F, Liu T, Prassana V, Marzec L, Palevsky HI, Ferrari VA, Forfia PR. Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am J Respir Crit Care Med 2011; 183: 268–276. [DOI] [PubMed] [Google Scholar]
- 55. Rosenkranz S, Bondermann D, Buerke M, Felgendreher R, ten Freyhaus H, Grünig E, de Haan F, Hammerstingl C, Harreuter A, Hohenforst-Schmidt W, Kindermann I, Kindermann M, Kleber FX, Kuckeland M, Kübler WM, Mertens D, Mitrovic V, Opitz C, Schmeisser A, Schulz U, Speich R, Zeh W, Weil J. Pulmonary hypertension due to left heart disease: Updated recommendations of the Cologne Consensus Conference 2011. Int J Cardiol 2011; 154: S34–S44. [DOI] [PubMed] [Google Scholar]
- 56. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS; for the CHAMPION Trial Study Group. Wireless pulmonary artery hemodynamic monitoring in chronic heart failure: A randomized controlled trial. Lancet 2011; 377: 658–666. [DOI] [PubMed] [Google Scholar]
- 57. Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, Henderson J, Cowart P, Stevenson LW. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail 2014; 7: 935–944. [DOI] [PubMed] [Google Scholar]
- 58. Bursi F, Barbieri A, Grigioni F, Reggianini L, Zanasi V, Leuzzi C, Ricci C, Piovaccari G, Branzi A, Modena MG. Prognostic implications of functional mitral regurgitation according to the severity of the underlying chronic heart failure: a long-term outcome study. Eur J Heart Fail 2010; 12: 382–388. [DOI] [PubMed] [Google Scholar]
- 59. Kusunose K, Popović ZB, Motoki H, Marwick TH. Prognostic significance of exercise-induced right ventricular dysfunction in asymptomatic degenerative mitral regurgitation. Circ Cardiovasc Imaging 2013; 6: 167–176. [DOI] [PubMed] [Google Scholar]
- 60. Lancellotti P, Magne J, Dulgheru R, Ancion A, Martinez C, Pierard LA. Clinical significance of exercise pulmonary hypertension in secondary mitral regurgitation. Am J Cardiol 2015; 115: 1554–1561. [DOI] [PubMed] [Google Scholar]
- 61. Gaemperli O, Moccetti M, Surder D, Biaggi P, Hurlimann D, Kretschmar O, Buehler I, Bettex D, Felix C, Luscher TF, Falk V, Grunenfelder J, Corti R. Acute haemodynamic changes after percutaneous mitral valve repair: relation to mid-term outcomes. Heart 2012; 98: 126–132. [DOI] [PubMed] [Google Scholar]
- 62. Whitlow PL, Feldman T, Pedersen WR, Lim DS, Kipperman R, Smalling R, Bajwa T, Herrmann HC, Lasala J, Maddux JT, Tuzcu M, Kapadia S, Trento A, Siegel RJ, Foster E, Glower D, Mauri L, Kar S; EVEREST II Investigators. Acute and 12 month results of catheter-based mitral valve leaflet repair: EVEREST II high risk study. J Am Coll Cardiol 2012; 59: 130–139. [DOI] [PubMed] [Google Scholar]
- 63. Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013; 309: 1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hoendermis ES, Liu LCY, Hummel YM, van der Meer P, de Boer RA, van Veldhuisen DJ, Voors AA. Effects of sildenafil on invasive hemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: A randomized controlled trial. Eur Heart J 2015; 36: 2565–2573. [DOI] [PubMed] [Google Scholar]
- 65. Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Semigran MJ. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation 2007; 116: 136–144. [DOI] [PubMed] [Google Scholar]
- 66. Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: A target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 2011; 124: 164–174. [DOI] [PubMed] [Google Scholar]
- 67. Dumitrescu D, Seck C, Möhle L, Erdmann E, Rosenkranz S. Therapeutic potential of sildenafil in patients with heart failure and reactive pulmonary hypertension – results of compassionate care treatment. Int J Cardiol 2012; 154: 205–206. [DOI] [PubMed] [Google Scholar]
- 68. Wu X, Yang T, Zhou Q, Li S, Huang L. Additional use of a phophodiesterase 5 inhibitor in patients with pulmonary hypertension secondary to chronic systolic heart failure: A meta-analysis. Eur J Heart Fail 2014; 16: 444–453. [DOI] [PubMed] [Google Scholar]
- 69. Bonderman D, Ghio S, Felix SB, Ghofrani HA, Michelakis E, Mitrovic V, Oudiz RJ, Boateng F, Scalise AV, Roessig L, Semigran MJ, for the LEPHT Study Group. Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: A phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. Circulation 2013; 128: 502–511. [DOI] [PubMed] [Google Scholar]
- 70. Bonderman D, Pretsch I, Steringer-Mascherbauer R, Jansa P, Rosenkranz S, Tufaro C, Bojic A, Lam CSP, Frey R, Ocham Kilama M, Unger S, Rössig L, Lang IM. Acute hemodynamic effects of Riociguat in patients with pulmonary hypertension associated with diastolic heart failure (DILATE-1): A randomized, double-blind, placebo-controlled, single-dose study. Chest 2014; 146: 1274–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.