Abstract
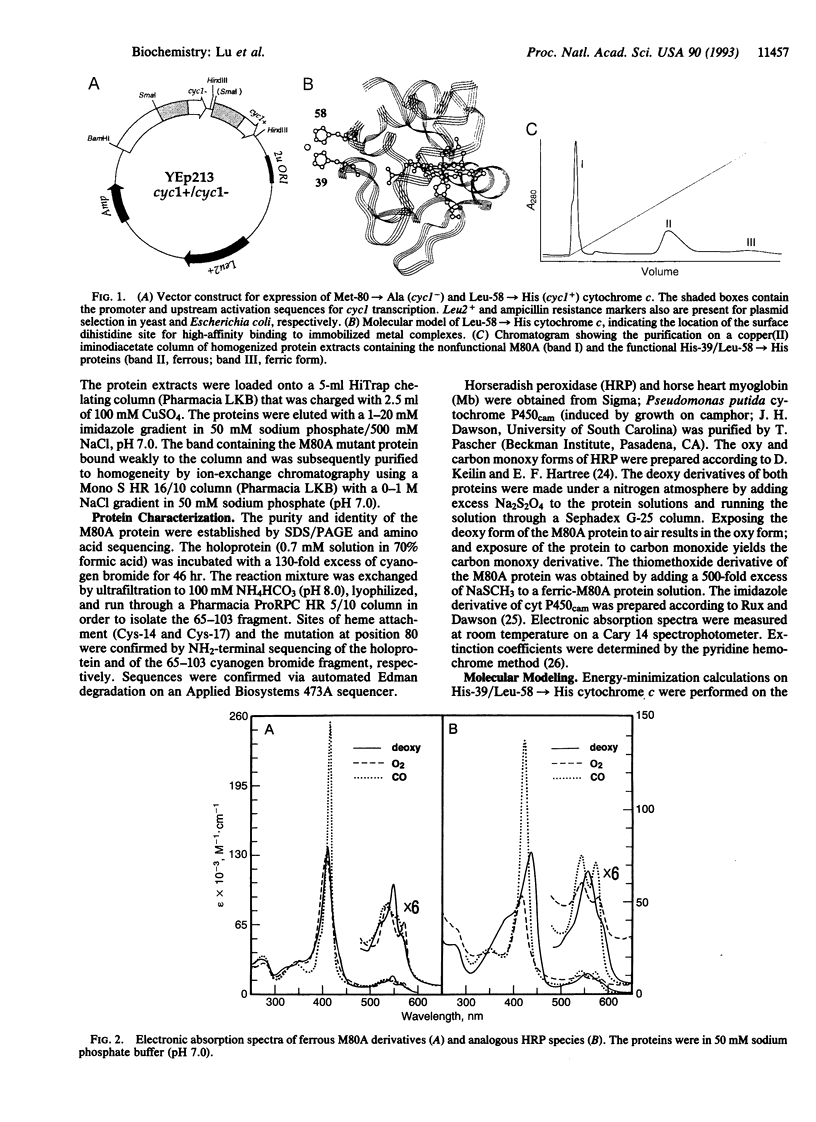
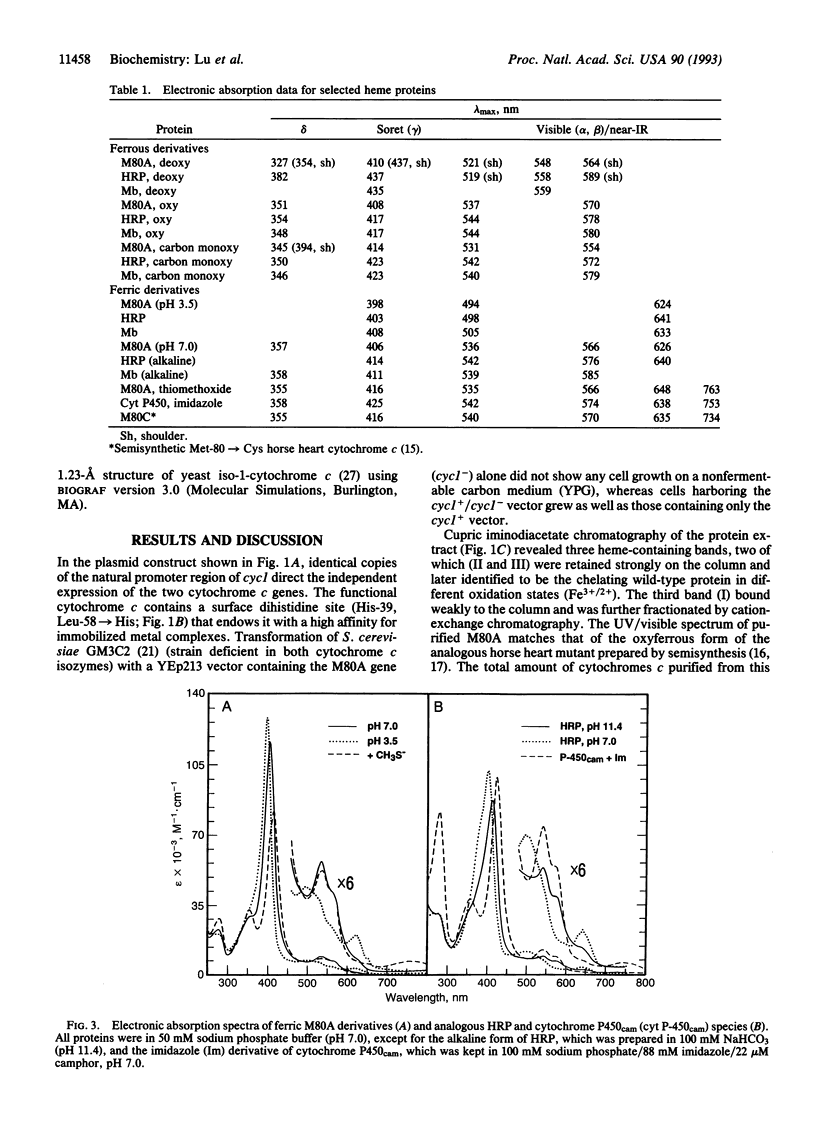
A strategy has been developed to express and purify a recombinant, nonfunctional axial-ligand mutant of iso-1-cytochrome c (Met-80-->Ala) in Saccharomyces cerevisiae in quantities necessary for extensive biophysical characterization. It involves coexpressing in the same plasmid (YEp213) the nonfunctional gene with a functional gene copy for complementation in a selective medium. The functional gene encodes a product with an engineered metal-chelating dihistidine site (His-39 and Leu-58-->His) that enables efficient separation of the two isoforms by immobilized metal-affinity chromatography. The purified Met-80-->Ala protein possesses a binding site for dioxygen and other exogenous ligands. Absorption spectra of several derivatives of this mutant show striking similarities to those of corresponding derivatives of horseradish peroxidase, myoglobin, and cytochrome P450. The use of a dual-gene vector for cytochrome c expression together with metal-affinity separation opens the way for the engineering of variants with dramatically altered structural and catalytic properties.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold F. H., Haymore B. L. Engineered metal-binding proteins: purification to protein folding. Science. 1991 Jun 28;252(5014):1796–1797. doi: 10.1126/science.1648261. [DOI] [PubMed] [Google Scholar]
- Becker D. M., Guarente L. High-efficiency transformation of yeast by electroporation. Methods Enzymol. 1991;194:182–187. doi: 10.1016/0076-6879(91)94015-5. [DOI] [PubMed] [Google Scholar]
- Berry E. A., Trumpower B. L. Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal Biochem. 1987 Feb 15;161(1):1–15. doi: 10.1016/0003-2697(87)90643-9. [DOI] [PubMed] [Google Scholar]
- Cutler R. L., Pielak G. J., Mauk A. G., Smith M. Replacement of cysteine-107 of Saccharomyces cerevisiae iso-1-cytochrome c with threonine: improved stability of the mutant protein. Protein Eng. 1987 Feb-Mar;1(2):95–99. doi: 10.1093/protein/1.2.95. [DOI] [PubMed] [Google Scholar]
- Dumont M. E., Ernst J. F., Sherman F. Coupling of heme attachment to import of cytochrome c into yeast mitochondria. Studies with heme lyase-deficient mitochondria and altered apocytochromes c. J Biol Chem. 1988 Nov 5;263(31):15928–15937. [PubMed] [Google Scholar]
- Faye G., Leung D. W., Tatchell K., Hall B. D., Smith M. Deletion mapping of sequences essential for in vivo transcription of the iso-1-cytochrome c gene. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2258–2262. doi: 10.1073/pnas.78.4.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampsey D. M., Das G., Sherman F. Yeast iso-1-cytochrome c: genetic analysis of structural requirements. FEBS Lett. 1988 Apr 25;231(2):275–283. doi: 10.1016/0014-5793(88)80834-2. [DOI] [PubMed] [Google Scholar]
- Hilgen S. E., Pielak G. J. The function of the Saccharomyces cerevisiae iso-1-cytochrome c gene is independent of the codon at invariant residue Phe82 when the gene is present on a low-copy-number vector. Protein Eng. 1991 Jun;4(5):575–578. doi: 10.1093/protein/4.5.575. [DOI] [PubMed] [Google Scholar]
- Holzschu D., Principio L., Conklin K. T., Hickey D. R., Short J., Rao R., McLendon G., Sherman F. Replacement of the invariant lysine 77 by arginine in yeast iso-1-cytochrome c results in enhanced and normal activities in vitro and in vivo. J Biol Chem. 1987 May 25;262(15):7125–7131. [PubMed] [Google Scholar]
- KEILIN D., HARTREE E. F. Purification of horse-radish peroxidase and comparison of its properties with those of catalase and methaemoglobin. Biochem J. 1951 Jun;49(1):88–104. doi: 10.1042/bj0490088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie G. V., Brayer G. D. High-resolution refinement of yeast iso-1-cytochrome c and comparisons with other eukaryotic cytochromes c. J Mol Biol. 1990 Jul 20;214(2):527–555. doi: 10.1016/0022-2836(90)90197-T. [DOI] [PubMed] [Google Scholar]
- Pielak G. J., Mauk A. G., Smith M. Site-directed mutagenesis of cytochrome c shows that an invariant Phe is not essential for function. Nature. 1985 Jan 10;313(5998):152–154. doi: 10.1038/313152a0. [DOI] [PubMed] [Google Scholar]
- Rux J. J., Dawson J. H. Magnetic circular dichroism spectroscopy as a probe of axial heme ligand replacement in semisynthetic mutants of cytochrome c. FEBS Lett. 1991 Sep 23;290(1-2):49–51. doi: 10.1016/0014-5793(91)81222-t. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F. Studies of yeast cytochrome c: how and why they started and why they continued. Genetics. 1990 May;125(1):9–12. doi: 10.1093/genetics/125.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace C. J., Clark-Lewis I. Functional role of heme ligation in cytochrome c. Effects of replacement of methionine 80 with natural and non-natural residues by semisynthesis. J Biol Chem. 1992 Feb 25;267(6):3852–3861. [PubMed] [Google Scholar]
- Wuttke D. S., Bjerrum M. J., Winkler J. R., Gray H. B. Electron-tunneling pathways in cytochrome C. Science. 1992 May 15;256(5059):1007–1009. doi: 10.1126/science.256.5059.1007. [DOI] [PubMed] [Google Scholar]



