Abstract
Lung cancer metastasising to the gastrointestinal tract normally does not occur. However, as clinicians, we must be aware that lung adenocarcinoma, as in all cancers, can and will metastasise to any part of the body. We describe a case of a patient with a presumed primary gastric adenocarcinoma who presented with shortness of breath due to pleural effusion. Pathology from the pleural effusion was positive for primary lung adenocarcinoma. Further investigation revealed that the patient's gastric mass was misdiagnosed as gastric adenocarcinoma. We correctly diagnosed the mass as metastatic lung adenocarcinoma. This was very significant because the patient was transitioning to palliative care with possible tube feeding. After the correct diagnosis, her management drastically changed and her health improved. Clinical, pathological and medical management of lung cancer metastasis to the stomach are discussed.
Background
Primary lung cancer contributes to 17% of newly diagnosed cancer cases and 23% of total cancer-related mortalities.1 Half the patients have metastatic disease at time of diagnosis, with survival rates of 20% at 1 year and 1% at 5 years.2 3 Normally, primary lung cancer metastasises to the liver, bones, adrenal glands and central nervous system. Gastric metastasis from lung cancer has a reported incidence of 0.2–0.5% at necropsy.4 5 Although rare, lung cancer metastasising to the stomach must not escape the clinician's mind. We present a case of a patient with a presumed primary gastric adenocarcinoma, which turned out to be lung adenocarcinoma that metastasised to the stomach.
Case presentation
A 77-year-old woman had been diagnosed as having primary gastric adenocarcinoma, in January 2015, at another hospital. Her pathology report from the other hospital showed a gastric body biopsy that stained cytokeratin 7 (CK7) positive, cytokeratin 20 (CK20) negative, CDX-2 negative and HER2 negative. The pathologist at the other institution had diagnosed her with poorly differentiated gastric adenocarcinoma. She decided to pursue evaluation for percutaneous endoscopic gastrostomy (PEG) tube placement for feeding and palliative care.
The patient presented to our emergency department with shortness of breath in February 2015. A chest X-ray was performed, which showed a large left-sided pleural effusion (figure 1). An ultrasound-guided thoracentesis was carried out and the pleura was positive for metastatic adenocarcinoma. However, immunohistochemistry revealed that the sample was a primary lung carcinoma because it was thyroid transcription factor 1 (TTF-1) positive, CK7 positive, CK20 negative and CDX-2 negative (figure 2).
Figure 1.
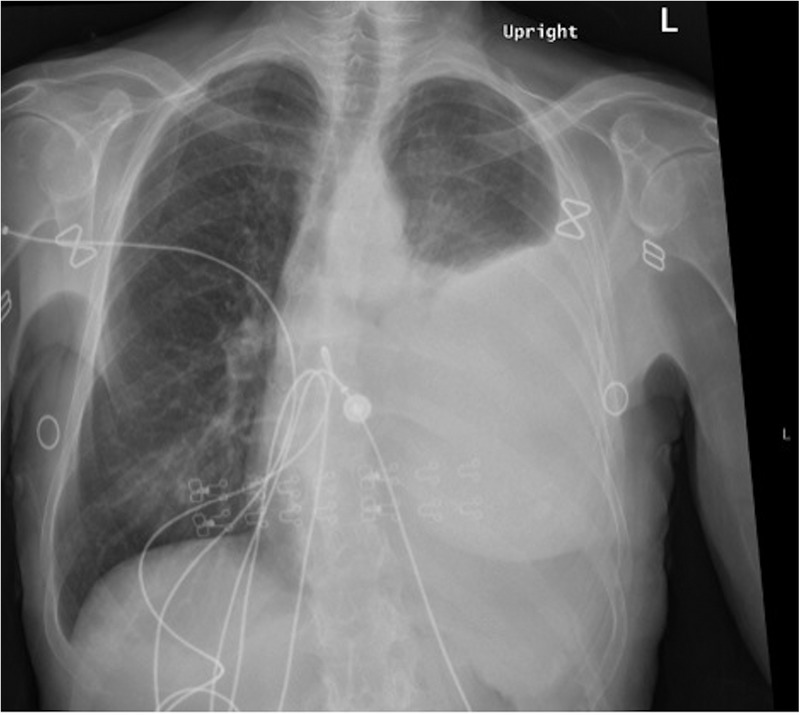
Development of large left-sided pleural effusion of indeterminate aetiology with associated compression atelectasis. The right lung is clear.
Figure 2.
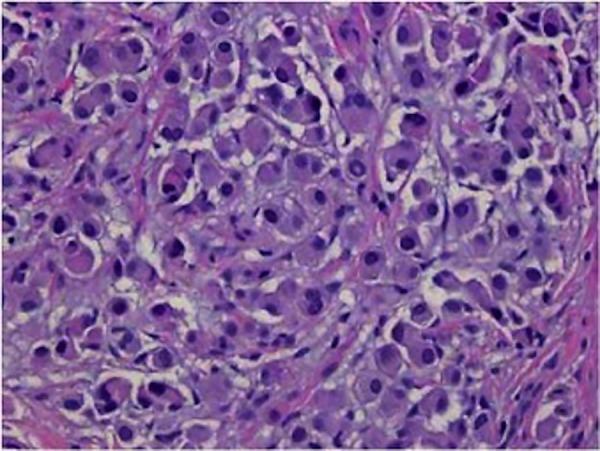
Immunohistochemistry positive for malignant cells consistent with metastatic adenocarcinoma of pulmonary origin (immunohistochemical stain not shown). Stains performed show a pattern consistent with pulmonary adenocarcinoma, specifically, the tumour is cytokeratin 7 positive, cytokeratin 20 negative, thyroid transcription factor 1 positive, napsin-A positive, villin negative, CDX2 negative and calretinin negative.
As such, there was a question as to whether or not these were two separate primary cancers, one pure metastatic gastric cancer, and one pure metastatic lung cancer.
Therefore, we endoscopically examined the patient, which demonstrated a gastric mass (figure 3). Biopsy of the gastric mass was performed: it was TTF-1 positive, CK7 positive, CK20 negative and CDX-2 negative. These results ubiquitously proved that the mass was metastatic lung adenocarcinoma. Next generation sequencing later revealed that the patient was positive for epidermal growth factor receptor (EGFR) deletion 19 and programmed death-ligand 1 (PD-L1). She was started on erlotinib and has tolerated the treatment well.
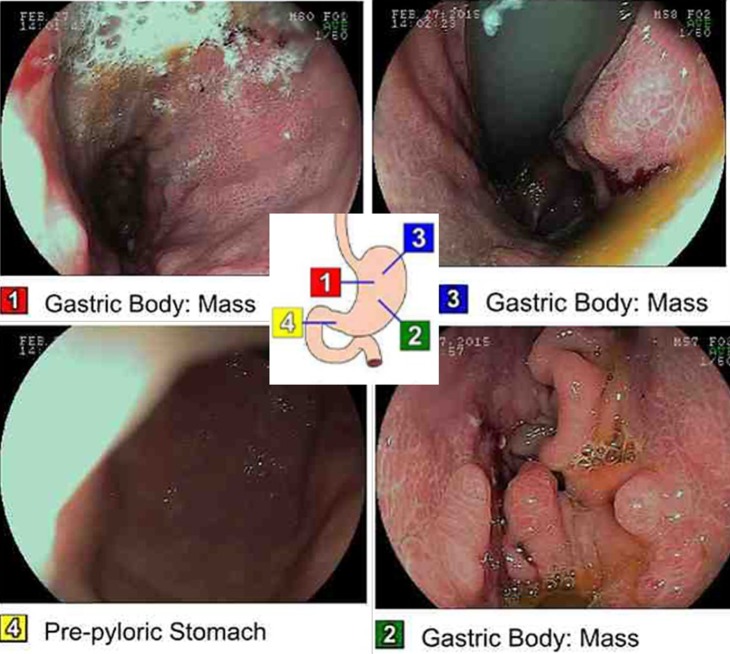
Figure 3.
Esophagogastroduodenoscopy with marked images of where the picture was taken along the tract. Findings are significant for a normal oesophagus. Also note the malignant gastric tumour in the cardia, in the gastric fundus, in the gastric body, on the anterior wall of the stomach, on the greater curvature of the stomach, on the lesser curvature of the stomach, and on the posterior wall of the stomach and at the incisura. The duodenum was normal.
Discussion
Lung cancer metastasising to the stomach is very unusual. In fact, the incidence rate of metastases to the stomach from the lung has varied from 0.2% to 0.5% at necropsy.4 5 Even more so, the diagnosis of gastric cancer is very rare in living patients.6 Luckily, it took the presentation of the primary cancer to correctly diagnose the metastasis.
Our patient came in with the reported diagnosis of primary gastric adenocarcinoma. As per record, she was discovered to have a gastric mass on endoscopy at another hospital. The pathologist there described the sections as gastric body/fundic-type mucosa with patchy infiltration by atypical epithelial cells with irregular hyperchromatic nuclei and abundant eosinophilic cytoplasm. The immunohistochemistry stained CK7 positive, CK20 negative, CDX-2 negative and HER2 negative. Although gastric cancers have the CK7−/CK20+ pattern, it can be assumed that the pathologist and other physicians at that hospital assumed gastric cancer due to the size of the mass and clinical picture. Also, it can be assumed that they did not have lung cancer on their differential, therefore they did not search for the lung cancer tumour markers, such as TTF-1.
After that diagnosis, the patient was in the process of evaluation for a feeding tube and palliative care treatment. Fortunately, she developed a malignant pleural effusion while on vacation, requiring her to come to our hospital. Further work up showed lung adenocarcinoma, which stained TTF-1 positive, CK7 positive, CK20 negative and CDX-2 negative (figure 2).
This brought up three possibilities: both of the areas could actually have been metastatic gastric cancer and our hospital's pathology department made a mistake in diagnosis; there were two separate primary cancers (gastric and lung); or the patient had lung cancer at both sites and her previous diagnosis of gastric adenocarcinoma was wrong.
Therefore, we endoscopically examined the patient's stomach, and the gastric mass looked impressive (figure 3). Although it appeared large, confirmatory diagnosis had to be made by biopsy and histopathological examination. There is a risk of misdiagnosis with gastric metastases, because they can manifest prior to the discovery of the primary malignancy (in this case lung adenocarcinoma).
In order to truly differentiate gastric cancer from lung metastases to the stomach, we used TTF-1, CK7 and CK20 to distinguish the mass as a lung adenocarcinoma metastasis. TTF-1 is highly specific for adenocarcinomas of pulmonary origin exhibiting a positive predictive value of 100%.7 In addition, primary lung carcinomas usually express the immunophenotype of CK7+/CK20−, whereas gastrointestinal carcinomas have the CK7−/CK20+ pattern.7 Our patient was TTF-1 positive, CK7+/CK20−.
The goal of treatment of advanced non-small cell lung cancer is to prolong and maintain quality of life. It is important for the physician to recognise the palliative intent of treatment. Therefore, as in our patient, the tumour tissue must be assessed for the presence of a driver mutation that stimulates tumour growth.8 Our patient was EGFR positive, and meta-analysis has shown that EGFR tyrosine kinase inhibitors (TKI) significantly prolong progression-free survival in patients with advanced non-small cell lung cancer as compared with platinum-based chemotherapy.9
Primary lung cancer metastasising to the stomach may not be seen frequently. However, the astute clinician must be aware of its possibility. Comprehensive initial history must always question the patient-reported disease. Visible inspection combined with advanced laboratory techniques will aid in the final diagnosis and management. EGFR TKI therapy is the treatment of choice for EGFR mutation-positive lung adenocarcinoma. Furthermore, the patient is a candidate for PD-1/PD-L1 therapy in the future.
Learning points.
Primary lung cancer metastasising to the stomach may not be seen frequently, but still must be considered.
Visible inspection combined with advanced laboratory techniques will aid in the final diagnosis and management.
Thyroid transcription factor 1 is highly specific for adenocarcinomas of pulmonary origin.
Primary lung carcinomas usually express the immunophenotype of cytokeratin 7 (CK7) positive/cytokeratin 20 (CK20) negative, whereas gastrointestinal carcinomas have the CK7−/CK20+ pattern.
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor therapy is the treatment of choice for EGFR mutation-positive lung adenocarcinoma.
Footnotes
Contributors: MDR compiled the data, researched and reviewed the articles, and wrote the manuscript. HT saw and diagnosed the patient after careful evaluation and efficient testing. HT also carefully reviewed and revised the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Jemal A, Bray F, Center MM et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2.Yang CJ, Hwang JJ, Kang WY et al. Gastro-intestinal metastasis of primary lung carcinoma: clinical presentations and outcome. Lung Cancer 2006;54: 319–23. 10.1016/j.lungcan.2006.08.007 [DOI] [PubMed] [Google Scholar]
- 3.Greenlee RT, Hill-Harmon MB, Murray T et al. Cancer statistics, 2001. CA Cancer J Clin 2001;51:15–36. 10.3322/canjclin.51.1.15 [DOI] [PubMed] [Google Scholar]
- 4.Antler AS, Ough Y, Pitchumoni CS et al. Gastrointestinal metastases from malignant tumors of the lung. Cancer 1982;49:170–2. [DOI] [PubMed] [Google Scholar]
- 5.Green LK. Hematogenous metastases to the stomach. A review of 67 cases. Cancer 1990;65:1596–600. [DOI] [PubMed] [Google Scholar]
- 6.Suzaki N, Hiraki A, Ueoka H et al. Gastric perforation due to metastasis from adenocarcinoma of the lung. Anticancer Res 2002;22:1209–12. [PubMed] [Google Scholar]
- 7.Rossi G, Marchioni A, Romagnani E et al. Primary lung cancer presenting with gastrointestinal tract involvement: clinicopathologic and immunohistochemical features in a series of 18 consecutive cases. J Thorac Oncol 2007;2:115–20. 10.1016/S1556-0864(15)30037-X [DOI] [PubMed] [Google Scholar]
- 8.Kerr KM, Bubendorf L, Edelman MJ et al. Second ESMO consensus conference on lung cancer: pathology and molecular biomarkers for non-small-cell lung cancer. Ann Oncol 2014;25:1681 10.1093/annonc/mdu145 [DOI] [PubMed] [Google Scholar]
- 9.Lee CK, Brown C, Gralla RJ et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst 2013;105:595 10.1093/jnci/djt072 [DOI] [PubMed] [Google Scholar]