Abstract
Proteins need to be unfolded when translocated through the pores in mitochondrial and other cellular membranes. Knotted proteins, however, might get stuck during this process, jamming the pore, since the diameter of the pore is smaller than the size of maximally tightened knot. The jamming probability dramatically increases as the magnitude of the driving force exceeds a critical value, Fc. In this numerical study, we show that for deep knots Fc lies below the force range over which molecular import motors operate, which suggest that in these cases the knots will tighten and block the pores. Next, we show how such topological traps might be prevented by using a pulling protocol of a repetitive, on-off character. Such a repetitive pulling is biologically relevant, since the mitochondrial import motor, like other molecular motors transforms chemical energy into directed motions via nucleotide-hydrolysis-mediated conformational changes, which are cyclic in character.
While struggling with tangled earphones, garden hoses, or extension cords we feel that the world would be a better place without knots. However, not all knots are useless, some are handy or even life-saving, as any sailing or rock-climbing aficionado will tell you. Since any chain long enough have a tendency to get knotted1,2,3 one finds knots also at the microscale - in DNA4, and even in proteins5,6,7. Although some of these knots might have functional importance, providing the additional stability necessary for maintaining the global fold and function of proteins under harsh conditions8,9, they can also be problematic for the cell. Knots in DNA could lead to blocking of its replication and transcription10,11, and therefore need to be quickly removed, which is accomplished by topoisomerases. In proteins, knots were hypothesized to affect the ability of the molecules to be degraded in proteasome6,12 or translocated through the intercellular membranes, e.g. during import into mitochondria13,14. It is estimated that more than 50% of the proteins produced in cells must traverse cellular membranes, thus translocation is vital for functioning of the cell15,16. In these processes, the proteins have to pass through constrictions that are too narrow to accommodate folded structures, thus translocation must be coupled to protein unfolding15,17,18,19,20. However, as shown in a number of theoretical and experimental studies, the protein knots get tightened under the tension. The radius of gyration of the tight knot, 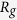 , is about 7–8 Å, whereas the diameters of the narrowest constriction of the mitochondrial pores or proteasomal openings are in the 12–15 Å range21, i.e. smaller than
, is about 7–8 Å, whereas the diameters of the narrowest constriction of the mitochondrial pores or proteasomal openings are in the 12–15 Å range21, i.e. smaller than 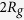 , thus knots are sterically prevented from entering the pore. The translocation would therefore be halted, unless the protein succeeds in sliding the knot off during the translocation.
, thus knots are sterically prevented from entering the pore. The translocation would therefore be halted, unless the protein succeeds in sliding the knot off during the translocation.
In this paper, through Brownian dynamics simulations of knotted protein translocation we show that knot tightening probability strongly depends on the force with which the protein is pulled into the pore. In particular, it is demonstrated that there exists a critical force, 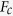 , above which the tightening becomes almost certain. For deep knots (with more than 30 aminoacids between the end of the knotted core and the free end of the protein)
, above which the tightening becomes almost certain. For deep knots (with more than 30 aminoacids between the end of the knotted core and the free end of the protein) 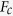 is shown to lie below the force range over which molecular import motors operate, which suggest that in these cases knots will tighten and block the pores. Next, we show how such topological traps might be prevented by using a pulling protocol of a repetitive, on-off character. Such a repetitive pulling is biologically relevant, since molecular import motors are ATP-hydrolysis driven and thus cyclic in character.
is shown to lie below the force range over which molecular import motors operate, which suggest that in these cases knots will tighten and block the pores. Next, we show how such topological traps might be prevented by using a pulling protocol of a repetitive, on-off character. Such a repetitive pulling is biologically relevant, since molecular import motors are ATP-hydrolysis driven and thus cyclic in character.
The model of the protein and the pore. Despite the rapid increase in computer power, the computational demands are still a barrier, preventing atomistically detailed simulations of the translocation process, due to the large system sizes and long timescales involved. This motivated the use of coarse-grained model of both the protein and the pore in the present study. For protein, we adopt a G -type model, in which individual amino acids are replaced by beads of uniform size placed at the locations of the Cα atoms. The effective potential of the interaction between these beads is tailored to give the lowest energy to the native state of a protein. A particular implementation of the Gō-type model followed here is by Cieplak and co-workers9,22. In short, the protein structure is represented by a chain of Cα atoms tethered along the backbone by harmonic potentials with minima at
-type model, in which individual amino acids are replaced by beads of uniform size placed at the locations of the Cα atoms. The effective potential of the interaction between these beads is tailored to give the lowest energy to the native state of a protein. A particular implementation of the Gō-type model followed here is by Cieplak and co-workers9,22. In short, the protein structure is represented by a chain of Cα atoms tethered along the backbone by harmonic potentials with minima at 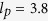 Å. Effective interactions between residues are split into native and nonnative interactions by checking for overlaps between the enlarged van der Waals surfaces of the residues23. Amino acids (i and j) that overlap are endowed with the effective Lennard-Jones potential
Å. Effective interactions between residues are split into native and nonnative interactions by checking for overlaps between the enlarged van der Waals surfaces of the residues23. Amino acids (i and j) that overlap are endowed with the effective Lennard-Jones potential 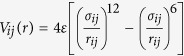 with energy scale ε and pair-by-pair distances rij. The length parameters,
with energy scale ε and pair-by-pair distances rij. The length parameters, 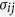 , are chosen such that the potential minima correspond pair by pair to the native state distance between the residues. Nonnative contacts are represented by hardcore repulsion to prevent entanglements. Correct chirality is imposed by the angle-dependent term in the Hamiltonian. A standard Brownian dynamics algorithm is used to move the residues, at the temperature corresponding to
, are chosen such that the potential minima correspond pair by pair to the native state distance between the residues. Nonnative contacts are represented by hardcore repulsion to prevent entanglements. Correct chirality is imposed by the angle-dependent term in the Hamiltonian. A standard Brownian dynamics algorithm is used to move the residues, at the temperature corresponding to 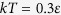 , which is the optimal folding temperature for this coarse-grained model24. The characteristic timescale
, which is the optimal folding temperature for this coarse-grained model24. The characteristic timescale 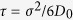 is set by the time it takes for the amino acid to diffuse the typical contact distance
is set by the time it takes for the amino acid to diffuse the typical contact distance 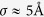 . Next, the pore is modeled as a cylindrical structure (with axis aligned with the positive z-axis) interacting with the amino acids by the potential
. Next, the pore is modeled as a cylindrical structure (with axis aligned with the positive z-axis) interacting with the amino acids by the potential 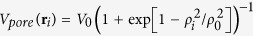 (for zi > 0), as proposed by J.M. Deutsch25. Here
(for zi > 0), as proposed by J.M. Deutsch25. Here 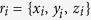 is the position of ith aminoacid, z is oriented along the pore axis and
is the position of ith aminoacid, z is oriented along the pore axis and 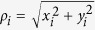 is the distance from the axis. The potential is small within the radius
is the distance from the axis. The potential is small within the radius 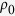 from the axis of the pore and then rises sharply. Additionally, to prevent the protein from entering the membrane except through the pore, a short range, repulsive membrane potential is introduced at its outer side with
from the axis of the pore and then rises sharply. Additionally, to prevent the protein from entering the membrane except through the pore, a short range, repulsive membrane potential is introduced at its outer side with 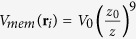 (for zi < 0,
(for zi < 0, 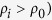 . In the simulations reported here,
. In the simulations reported here, 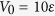 ,
, 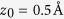 and
and 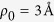 . Note that the pore potential acts on the centers of the particles. Since the van der Waals radii of amino acids are in the range of ~3.5–4.5 Å, the above value of
. Note that the pore potential acts on the centers of the particles. Since the van der Waals radii of amino acids are in the range of ~3.5–4.5 Å, the above value of 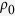 corresponds to the effective pore radius of about 6.5–7.5 Å, which is consistent with the values reported for the narrowest constriction in the mitochondrial pores21. The question of mapping the force-scales and time scales between the simulation and experiment is rather subtle, with the result depending on which experimental quantity is used to set the scale. Comparison of diffusion coefficients of the model proteins with the experimental values leads to an estimate
corresponds to the effective pore radius of about 6.5–7.5 Å, which is consistent with the values reported for the narrowest constriction in the mitochondrial pores21. The question of mapping the force-scales and time scales between the simulation and experiment is rather subtle, with the result depending on which experimental quantity is used to set the scale. Comparison of diffusion coefficients of the model proteins with the experimental values leads to an estimate 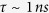 26. On the other hand the comparison of the protein folding times in the model and in vivo leads to
26. On the other hand the comparison of the protein folding times in the model and in vivo leads to 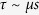 (e.g. ubiqutin in silico folds on the timescale of 103
τ27 whereas in vivo - it is in tens of millisecond range28). Such a discrepancy is connected with a relatively small frustration of the Gō-type protein models29. As for the forces, the conversion between experimental and numerical values is provided by the correlation of the maximum resistance force in constant velocity pulling. The comparison of the forces measured in AFM experiments with those recorded in the numerical simulations for 28 different proteins have shown a good correlation (with Pearson correlation coefficient of 0.89) provided that the force unit in the model, ε/Å, is translated into about 70pN24,30.
(e.g. ubiqutin in silico folds on the timescale of 103
τ27 whereas in vivo - it is in tens of millisecond range28). Such a discrepancy is connected with a relatively small frustration of the Gō-type protein models29. As for the forces, the conversion between experimental and numerical values is provided by the correlation of the maximum resistance force in constant velocity pulling. The comparison of the forces measured in AFM experiments with those recorded in the numerical simulations for 28 different proteins have shown a good correlation (with Pearson correlation coefficient of 0.89) provided that the force unit in the model, ε/Å, is translated into about 70pN24,30.
Results and Discussion
Translocation simulations
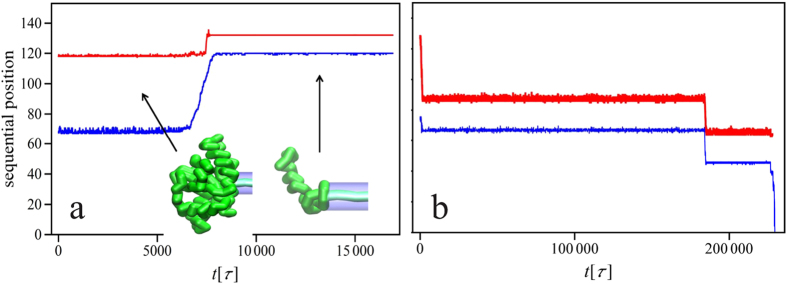
A schematic illustration of the simulation setup is presented in Fig. 1. At the beginning of the simulations the protein in its native conformation is placed at the outer side of the membrane near the pore entrance. In the cell, the transport of proteins into mitochondria is usually mediated by a loosely folded presequence, which is modeled here as a loose piece of a peptide chain (10 amino acid long). One end of the presequence is attached to the protein terminus (N or C), while the other end is pulled with the force 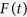 along the pore axis. The force is either constant in time or switched on and off with the period T. Several knotted proteins were studied, as summarized in Table 1. During the simulation we not only record the conformation of the protein but also track the position of the knotted core, i.e. the smallest region that will remain knotted when the residues are successively deleted from both ends8. Thereby we obtain the trajectories of knot’s ends in the sequential space, such as those shown in Fig. 2.
along the pore axis. The force is either constant in time or switched on and off with the period T. Several knotted proteins were studied, as summarized in Table 1. During the simulation we not only record the conformation of the protein but also track the position of the knotted core, i.e. the smallest region that will remain knotted when the residues are successively deleted from both ends8. Thereby we obtain the trajectories of knot’s ends in the sequential space, such as those shown in Fig. 2.
Figure 1. The simulation setup: the protein (here 1j85) and the pore (blue).
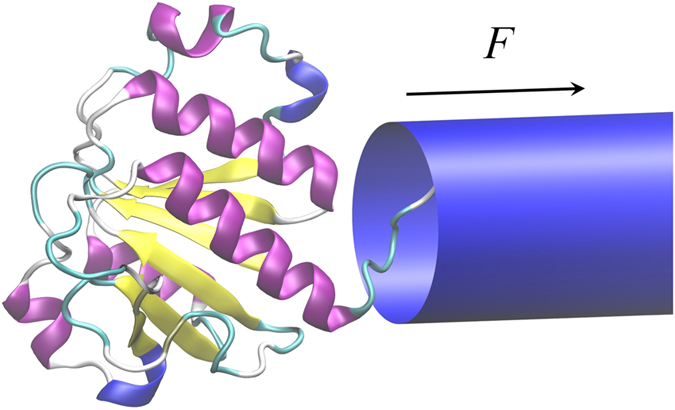
Table 1. Proteins considered and the characteristics of their knots as well as critical forces, Fc, at which they jam the pores (cf. Eq. 1); N and C stand for pulling by the N and C terminus respectively; free means that the protein always translocates.
| Protein | pdb | length | knotted core | knot type | Fc(N)[ε/Å] | Fc(C)[ε/Å] |
|---|---|---|---|---|---|---|
| YbeA from E. coli | 1ns5 | 1–153 | 67–121 | trefoil | 0.57 ± 0.04 | 0.15 ± 0.05 |
| zinc-finger motif | 2k0a | −1–107 | 21–73 | trefoil | 2.65 ± 0.03 | free |
| YibK methyltransferase | 1j85 | 1–156 | 75–120 | trefoil | free | 0.2 ± 0.05 |
| YbeA-like (T.maritima) | 1o6d | 1–147 | 65–118 | trefoil | 1.64 ± 0.03 | 0.16 ± 0.04 |
| Ribbon-helix-helix protein | 2efv | 6–87 | 13–80 | trefoil | free | free |
| transcarbamylase (X.campestris) | 1yh1 | 3–336 | 172–254 | trefoil | 0.05 ± 0.05 | 0.1 ± 0.05 |
| FLIN2 chimaeric protein | 1j2o(14) | 1–114 | 42–95 | figure-of-eight | free | 1.95 ± 0.02 |
Figure 2. The movement of knotted core during the translocation of the protein 1ns5 pulled into the pore by the N terminus with F = 2.2ε/Å (a) and with the force F = 1.4ε/Å by the C terminus (b).
The colors mark the two ends of the knot as they move along the chain. In (a) the knot gets tightened and blocks the pore, whereas in (b) - it slides off the chain. The insets of (a) show the conformations of the protein backbone at the beginning of the translocation and at jamming. The final position of the knot is between the aminoacids 119 and 134. Only a portion of a longer trajectory (up to 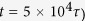 trajectory is shown in panel (a), however no further changes in the knot position were observed beyond
trajectory is shown in panel (a), however no further changes in the knot position were observed beyond 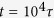 .
.
The simulation setup is similar in spirit to some of the previous models of protein translocation13,14,31,32,33,34,35,36,37,38. In particular, West et al. have studied differences and similarities between the AFM-induced protein stretching and the translocation-induced unfolding, finding that in the latter process the protein structures near the rim of the pore are responsible for the overall mechanical stability of a molecule. In an important series of papers31,33,39,40, Makarov and co-workers have explored the energy landscape associated with translocation and found it to be significantly different from that characterizing a simple AFM stretching.
These results were complemented by the studies of Cecconi et al.36,37,41 who have related the free-energy landscape of protein translocation to the mean translocation time, identifying the bottlenecks of the transport. Importantly, Huang and Makarov have also studied the translocation of the knotted polypeptide chains34, which was shown to proceed through multiple slippage events suggesting a rugged energy landscape with multiple metastable minima. The presence of a knot was found to considerably increase the translocation time, an effect increasing with knot complexity. However, in contrast to the present study and the simulations reported in13,14 the parameters of the pore allowed the knotted chain to enter inside it, thus jamming was not possible. Nevertheless, as mentioned above, careful experimental studies on the size of the tight protein knots12 supported by both all-atom42 as well as coarse-grained MD simulations9 put the size of the smallest proteinic knots at 7.2 Å, i.e. too large to enter either the mitochondrial pore or proteasome opening. Thus, as it was demonstrated in13,14 two outcomes are eventually possible: either the knot moves towards the end of the chain and simply slides away, or it gets tightened and jams the opening. Representative examples of the trajectories of the knot’s ends in these two cases are presented in Fig. 2, with the left panel (as well as movie S1 in the SM) illustrating the jamming of protein 1ns5 pulled into the pore by the N terminus. Contrastingly, the right panel presents the situation in which the same protein is pulled by the C terminus and the knot slides off the chain. The most striking feature of the motion of the knot under the force is that it is a collection of successive jumps over multiple energy barriers, interspersed with waiting periods in metastable states. As elucidated in9 this is because one or both of the knot’s ends get pinned on sharp turns of a protein backbone. During successive jumps the knot invariably shrinks in size until it gets fully tightened, reaching the size of 12–15 aminoacids (for a trefoil knot), or slides off the chain. Once the knot gets fully tightened, we never observe it to loosen up again, at least in the force range studied.
Macroscopic analogy
A macroscopic analogy can be helpful here - let us tie a loose knot on a piece of rope and pull it through a cylindrical hole (cf. Fig. 3). If pulled sharply, the knot invariably tightens; however if tugged slowly - it might be able to slide towards the free end of the rope and then get untied. There are several things to be learned from such a macroscale rope experiment. First of all, an important factor in knot self-tightening is friction43: if the friction coefficient is large enough, then the knot, once tightened, will hold no matter what force is applied: tugging at the rope will only increase the normal forces squeezing the two pieces of the rope together which results in larger frictional forces preventing the slippage. Moreover, the forms of the knot tightened around the pore opening are similar at macro- and micro-scale (cf. Figs 2 and 3) - they both involve a fastened loop around the entrance of the pore. And yet the rope analogy holds only to a limited degree, mostly because it neglects the role of thermal fluctuations: whereas the rope, once tightened, stays tight, a protein knot might be spontaneously loosened due to the Brownian motion. Additionally, proteins - in contrast to ropes - are highly nonhomogeneous, with an intricate network of contacts between different amino acids. This inhomogeneity was shown to be important for the knot dynamics, even in the absence of the pore: as the knotted core shrinks in the process of tightening, one or both of the knot’s ends get pinned on sharp turns of a protein backbone9. This effect is even more dramatic during the translocation, with sharp turns acting as potential pinning centers which can block the backbone on its way through the pore opening leading to the tightening of the knot and blocking of the pore14. This is why deep knots (with more than 30 aminoacids between the end of the knotted core and the free end of the protein) can easily jam the pores. We note that in this context whether the knot can be called “shallow” or “deep” depends on the pulling direction - if the protein is pulled e.g. by the N terminus, then the distance between the end of the knot and the C terminus determines its behaviour.
Figure 3. Pulling the knotted rope through a model pore.
Trefoil (upper left) and figure-of-eight knot (lower left) on the climbing rope is tugged sharply into the spool opening, which results in tightening of the knot (right panels).
Jamming probability is force-dependent
As illustrated in Fig. 4, the probability of knot tightening during the translocation (marked  in the inset) rapidly increases with the force. The sigmoidal shape of
in the inset) rapidly increases with the force. The sigmoidal shape of 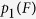 dependence is consistent with the presence of two alternative pathways, one leading to the tightening
dependence is consistent with the presence of two alternative pathways, one leading to the tightening  , the other - to the translocation
, the other - to the translocation  . Such pathways have been observed previously in the knot tightening dynamics9, and - at least for moderate forces - they were shown to follow the Bell’s model44, with the relative probability of tightening given by
. Such pathways have been observed previously in the knot tightening dynamics9, and - at least for moderate forces - they were shown to follow the Bell’s model44, with the relative probability of tightening given by 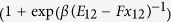 , where
, where 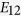 and
and 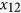 is the difference in energy barrier heights and locations of transition states, respectively. However, we observe that in the large force limit the probability of jamming of the pore asymptotes to a constant different from 1. We hypothesize that this behaviour is connected with the form of the underlying energy landscape. Namely, at high forces the kinetic barriers disappear and both states (B and C) find themselves lower than A on the energy landscape, forming so-called valley-ridge inflection point45,46. In such a situation - where a pathway forks into two downhill routes with a dynamically unstable ridge separating them, the relative probability of ending up in a particular state depends on the tiny details of the initial conformation, which are largely determined by thermal fluctuations and not by the force. A simplest model taking this asymptotics into account would give the jamming probability of the form
is the difference in energy barrier heights and locations of transition states, respectively. However, we observe that in the large force limit the probability of jamming of the pore asymptotes to a constant different from 1. We hypothesize that this behaviour is connected with the form of the underlying energy landscape. Namely, at high forces the kinetic barriers disappear and both states (B and C) find themselves lower than A on the energy landscape, forming so-called valley-ridge inflection point45,46. In such a situation - where a pathway forks into two downhill routes with a dynamically unstable ridge separating them, the relative probability of ending up in a particular state depends on the tiny details of the initial conformation, which are largely determined by thermal fluctuations and not by the force. A simplest model taking this asymptotics into account would give the jamming probability of the form
Figure 4. Jamming probability as a function of force for the protein 2k0a pulled by the N terminus.
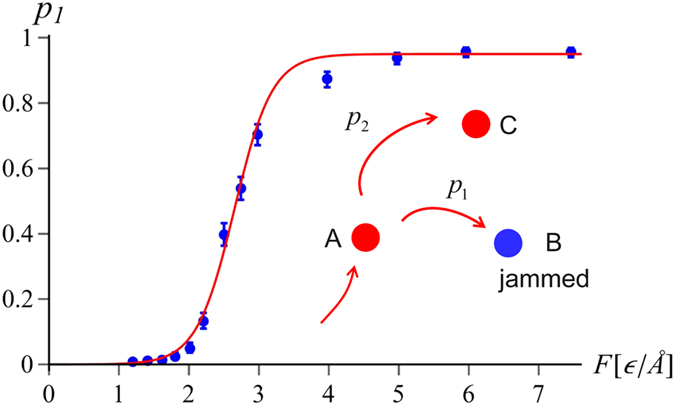
The data points in this and other figures have been obtained based on the average over 100 simulations runs. The red line represents the fit to the two-pathway model (1) with 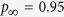 ,
, 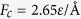 and
and 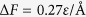 . The inset shows schematically the kinetic partitioning between the translocation
. The inset shows schematically the kinetic partitioning between the translocation  and knot tightening
and knot tightening  . Error bars mark 68% Wilson confidence intervals73.
. Error bars mark 68% Wilson confidence intervals73.
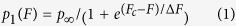 |
where 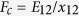 is a critical force at which the jamming transition occurs whereas
is a critical force at which the jamming transition occurs whereas 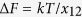 measures the width of the transition region. As illustrated in Fig. 4 the simulation data are well-fitted by Eq. (1), which confirms the applicability of the Bell’s model in the description of the tightening dynamics. However, within the studied proteins, in three cases only
measures the width of the transition region. As illustrated in Fig. 4 the simulation data are well-fitted by Eq. (1), which confirms the applicability of the Bell’s model in the description of the tightening dynamics. However, within the studied proteins, in three cases only 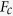 was found to lie within the numerically accessible force regime (F > 1ε/Å). This takes place for relatively shallow knots. For deep knots, critical forces are lower and the simulations are probing large-F tail of the distribution only. Unfortunately, collecting statistically meaningful data in low force regime (F < 1ε/Å) is difficult due to long translocation times involved. On the other hand, molecular motors importing the proteins act with the forces of the order of 30pN47, which corresponds to
was found to lie within the numerically accessible force regime (F > 1ε/Å). This takes place for relatively shallow knots. For deep knots, critical forces are lower and the simulations are probing large-F tail of the distribution only. Unfortunately, collecting statistically meaningful data in low force regime (F < 1ε/Å) is difficult due to long translocation times involved. On the other hand, molecular motors importing the proteins act with the forces of the order of 30pN47, which corresponds to 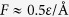 in the present context. By fitting (1) to the simulation data we can nevertheless estimate the critical forces
in the present context. By fitting (1) to the simulation data we can nevertheless estimate the critical forces 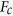 also in these cases, as quoted in Table 1. Relatively large errors of these values are related to the difficulty in estimating the parameters of Eq. (1) from the tail of the distribution. Nevertheless, this data suggests that there is a group of proteins for which the critical jamming forces are in 3–10 pN range, significantly below the forces relevant for the import motors. Importantly, the decrease of the pulling force beyond 20–30pN is not a viable option for the import machinery, since lower forces will not be able to denature protein structures over biological time scales. Shallow knots have much larger
also in these cases, as quoted in Table 1. Relatively large errors of these values are related to the difficulty in estimating the parameters of Eq. (1) from the tail of the distribution. Nevertheless, this data suggests that there is a group of proteins for which the critical jamming forces are in 3–10 pN range, significantly below the forces relevant for the import motors. Importantly, the decrease of the pulling force beyond 20–30pN is not a viable option for the import machinery, since lower forces will not be able to denature protein structures over biological time scales. Shallow knots have much larger 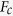 and they will translocate easily when pulled by molecular motors. There is also a group of very shallow knots which never get stuck - in these cases the stretch of the protein backbone between the knot’s end and the terminus does not contain any sharp turns.
and they will translocate easily when pulled by molecular motors. There is also a group of very shallow knots which never get stuck - in these cases the stretch of the protein backbone between the knot’s end and the terminus does not contain any sharp turns.
Jamming of the translocating knotted polymers at high pulling forces has first been reported by Rosa et al. in the simulations of polyelectrolyte chains48. The differences between the knot tightening in homo- and heteropolymers are elucidated in49, where it is in particular shown that only in the latter case a complete self-tightening of the knot can be achieved. This is confirmed by the results of this study, where we find that once the knot tightens it becomes completely immobilized, until the force is relaxed. Conversely, Rosa et al. report that even at the highest forces considered there, the effective mobility of the chain remains nonzero, and the chain still translocates, albeit at a much decreased rate.
Interestingly, no jamming is observed in the passive ejection of DNA out of a spherical cavity, e.g. virial capsid50,51. In the most recent study of these systems Marenduzzo et al.52 emphasize the importance of cholesteric interactions in a DNA ejection problems. The ordering effect of such interactions leads to the prevalence of torus knots in the capsid, which unravel gradually by simplifying their topology in a stepwise fashion. Although occasionally the stochastic movement of the chain inside the capsid leads to a spontaneous formation of more complicated knots which can stop the ejection, in most cases the knotted chain is able to exit the capsid without getting jammed.
Periodic forces trigger knot untying during translocation of knotted proteins
How does nature gets around the topological traps in the protein translocation? Our numerical results seem to suggest that a very efficient way of avoiding pore jamming is by using the forces of cyclic, on-off character. In fact, molecular motors never operate in a continuous way, since they are fed by the ATP hydrolysis. To perform a net work during a hydrolysis cycle, a motor undergoes a conformational change (a power stroke) while bound to the substrate, followed by a recovery stroke while detached53,54. Since the force exerted during the power-stroke is nearly constant55, the simplest model of the motor action involves a force acting in an off-on manner: for the first half of the period it is 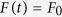 , whereas for the second
, whereas for the second 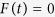 . The effect of such a cyclic force on the translocation is shown in Fig. 5 and Movie S2. At first, when the force is on, the knot tightens in analogy with Fig. 2a. However, as the force is switched off, the protein relaxes and the knot swells in a process similar to “knotted core breathing” mechanism described first in56 - local length fluctuations in exchange with the knot’s immediate vicinity slightly change its size, allowing diffusive motion of the knot along the chain. After 103–104
τ the knot relaxes from the tightened state and finds itself in a vicinity of a previous intermediate state (A). In the next force cycle, the protein again attempts to translocate through the pore. A mechanism by which the repetitive pulling allows to avoid the topological traps is thus similar in spirit to that reported by Tian and Andricioaei in the context of avoiding long-lived intermediates during co-translocational unfolding57: since the probability of getting trapped in each of n successive tries is
. The effect of such a cyclic force on the translocation is shown in Fig. 5 and Movie S2. At first, when the force is on, the knot tightens in analogy with Fig. 2a. However, as the force is switched off, the protein relaxes and the knot swells in a process similar to “knotted core breathing” mechanism described first in56 - local length fluctuations in exchange with the knot’s immediate vicinity slightly change its size, allowing diffusive motion of the knot along the chain. After 103–104
τ the knot relaxes from the tightened state and finds itself in a vicinity of a previous intermediate state (A). In the next force cycle, the protein again attempts to translocate through the pore. A mechanism by which the repetitive pulling allows to avoid the topological traps is thus similar in spirit to that reported by Tian and Andricioaei in the context of avoiding long-lived intermediates during co-translocational unfolding57: since the probability of getting trapped in each of n successive tries is 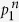 , which rapidly decreases with n, repetitive trying always leads to a final success.
, which rapidly decreases with n, repetitive trying always leads to a final success.
Figure 5. The movement of knotted core during the translocation of the protein 1ns5 pulled into the pore by the N terminus with an on-off cycle of 4500τ when the force is switched between F = 4ε/Å in on-state and F = 0 in off-state.
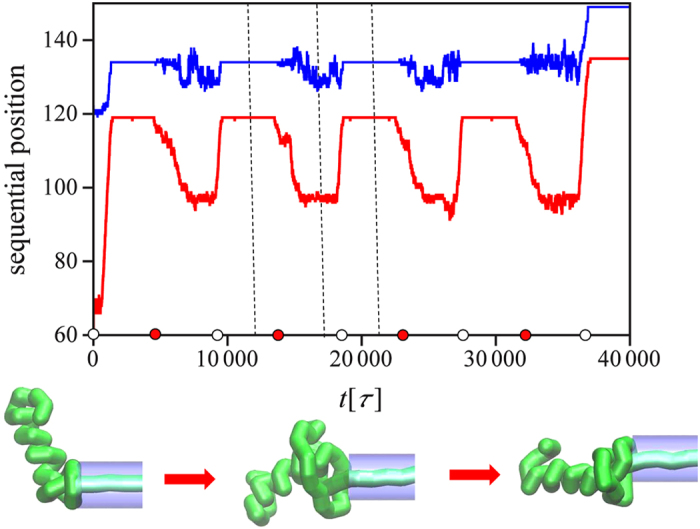
The colors (red and blue) mark the two ends of the knot as they move along the chain. The circles on the time axis represent the moments of switching the force on (white) and off (red). The conformations below correspond to the time moments marked by the dashed lines.
Dependence of the translocation time on the force period
The dependence of the translocation time 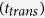 on the period of the force (Fig. 6) shows a clear minimum corresponding to an optimal switching period. This can be rationalized by noting that a successful translocation after a jamming event, needs i) loosening of the knot during the off-force period, i.e. the insertion of some stored length into the knotted core, which allows it to escape from a tightened configuration (B) to the previous metastable state (A), and ii) a successful translocation to state C avoiding the trap in the tightened state (cf. the inset of. Fig. 6). The translocation time will then be of the form
on the period of the force (Fig. 6) shows a clear minimum corresponding to an optimal switching period. This can be rationalized by noting that a successful translocation after a jamming event, needs i) loosening of the knot during the off-force period, i.e. the insertion of some stored length into the knotted core, which allows it to escape from a tightened configuration (B) to the previous metastable state (A), and ii) a successful translocation to state C avoiding the trap in the tightened state (cf. the inset of. Fig. 6). The translocation time will then be of the form
Figure 6. Mean translocation time as a function of the force period for a knotted protein (1ns5) pulled by the N terminus.
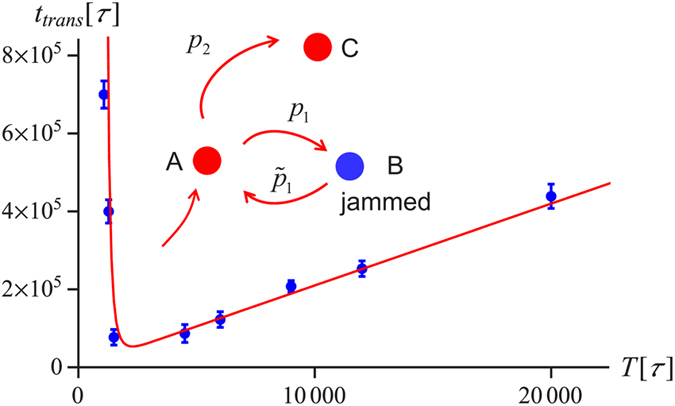
The amplitude of the force is F0 = 3ε/Å. The curve represents Eq. (2), with 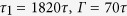 and
and 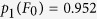 , estimated as described in the text. The error bars mark the standard deviation from the mean.
, estimated as described in the text. The error bars mark the standard deviation from the mean.
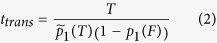 |
where 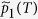 and
and 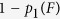 are the probabilities of (i) and (ii) respectively. The former depends on T since too short a period makes it impossible for the knot to escape from a basin of attraction of the tightened state. The latter is a function of the force, as given by Eq. (1). Performing a series of simulations starting from the tightened state, we have obtained the distribution of first passage times of the knot on
are the probabilities of (i) and (ii) respectively. The former depends on T since too short a period makes it impossible for the knot to escape from a basin of attraction of the tightened state. The latter is a function of the force, as given by Eq. (1). Performing a series of simulations starting from the tightened state, we have obtained the distribution of first passage times of the knot on  trajectory and found it follow an inverse Gaussian distribution58, as shown in Fig. 7. This suggests that the swelling of the knot during the off-force state can be treated as one-dimensional biased diffusion along the coordinate N, corresponding to the size of the knotted core.
trajectory and found it follow an inverse Gaussian distribution58, as shown in Fig. 7. This suggests that the swelling of the knot during the off-force state can be treated as one-dimensional biased diffusion along the coordinate N, corresponding to the size of the knotted core.
Figure 7. The distribution of knot loosening times for the protein 1ns5 after the force is relaxed.
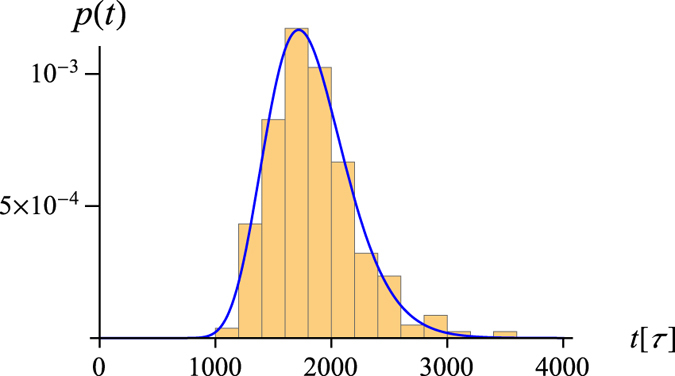
Initially the knot is fully tightened with the knotted core spanning 15 aminoacids (corresponding to the plateaus in Fig. 5) and the final state corresponds to the knotted core spanning ~30 aminoacids (corresponding to the minima of the red curve in Fig. 5). The blue curve is a fit to the inverse Gaussian distribution 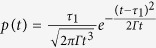 with
with 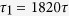 and
and 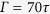 .
.
The bias here comes from the contact interactions between the amino acids, which are trying to refold the protein and is absent in the case of homogeneous chains, where the movement of the knot was shown to be purely diffusive59. Treating state A as the absorbing boundary, we get the probability that A is reached within time T as60,
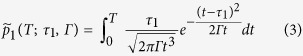 |
where 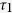 is the mean first passage time and
is the mean first passage time and 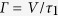 with V being the variance. The theoretical estimate of
with V being the variance. The theoretical estimate of 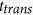 according to (2) with the above-given
according to (2) with the above-given 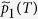 is shown as a solid curve in Fig. 6. Note that the curve is not a direct fit to the data, since
is shown as a solid curve in Fig. 6. Note that the curve is not a direct fit to the data, since 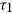 and V has been obtained by an independent analysis of the first passage time distribution, whereas
and V has been obtained by an independent analysis of the first passage time distribution, whereas 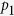 has been obtained by performing a series of translocation attempts starting from A.
has been obtained by performing a series of translocation attempts starting from A.
Dependence of the translocation time on the force magnitude
Finally, let us analyze the dependence of the translocation time on the magnitude of the pulling force. As illustrated in Fig. 8, there is an exponential increase of 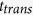 at small forces which is not related to the presence of the knot, but simply to the presence of kinetic barriers in protein unfolding. On the other hand, the increase of
at small forces which is not related to the presence of the knot, but simply to the presence of kinetic barriers in protein unfolding. On the other hand, the increase of 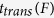 at larger forces and the subsequent plateau visible in the upper panel of Fig. 8 are closely connected with the force-dependent jamming probability (1). Altogether, the
at larger forces and the subsequent plateau visible in the upper panel of Fig. 8 are closely connected with the force-dependent jamming probability (1). Altogether, the 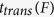 dependence at fixed force period, T0, is well described by the formula
dependence at fixed force period, T0, is well described by the formula
Figure 8. Mean translocation time as a function of the magnitude of the pulling force for repetitive protocol for the protein 2k0a pulled by the N terminus (top) and 1ns5 pulled by the C terminus (bottom).
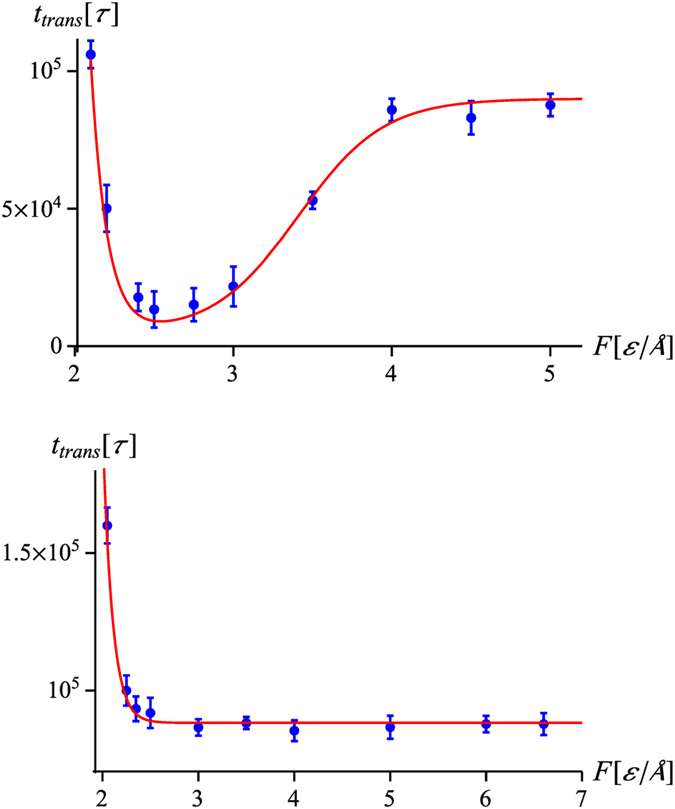
The period of the force in both cases is equal to 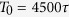 . The red curve in the top panel represents the formula
. The red curve in the top panel represents the formula 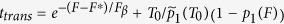 , as given by Eq. (4) . The values of the parameters are
, as given by Eq. (4) . The values of the parameters are 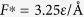 ,
, 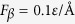 ,
, 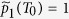 , whereas
, whereas 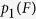 is given by Eq. (1) with
is given by Eq. (1) with 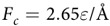 and
and 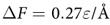 . In the bottom panel, the red curve is given by
. In the bottom panel, the red curve is given by 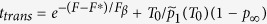 , since the forces involved are much larger than Fc for this protein (cf. Table 1). The remaining parameters are
, since the forces involved are much larger than Fc for this protein (cf. Table 1). The remaining parameters are 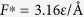 ,
, 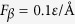 ,
, 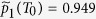 and
and 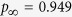 . The error bars mark the standard deviation from the mean.
. The error bars mark the standard deviation from the mean.
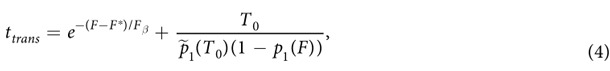 |
shown by a red line in Fig. 8. Again, this is only a partial fit, since 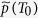 and
and 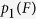 are assessed independently. In the above,
are assessed independently. In the above, 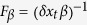 is a characteristic thermal force associated with the main kinetic barrier61, with
is a characteristic thermal force associated with the main kinetic barrier61, with 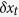 being the position of the respective transition state along the reaction coordinate. Next, F * is a threshold force at which the barrier disappears60,62,63. If F * < Fc, then the
being the position of the respective transition state along the reaction coordinate. Next, F * is a threshold force at which the barrier disappears60,62,63. If F * < Fc, then the 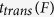 has a form analogous to that in the upper panel of Fig. 8. If, on the other hand, F * > Fc, then the effects of the first, exponential term in
has a form analogous to that in the upper panel of Fig. 8. If, on the other hand, F * > Fc, then the effects of the first, exponential term in 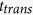 dominate those of the second term and the translocation times monotonically decrease with the force, as illustrated in the lower panel of Fig. 8.
dominate those of the second term and the translocation times monotonically decrease with the force, as illustrated in the lower panel of Fig. 8.
Summary
To summarize, Brownian dynamics simulations of protein translocation suggest that the pore jamming by tight protein knots can be avoided by the use of a pulling protocol of a repetitive, on-off character. This mechanism is remarkably simple and yet very robust - all of the proteins considered here, no matter how large and complex, will eventually make it through the pore, becoming untied in the process. Importantly, such a repetitive pulling is biologically relevant, since the mitochondrial import motors are cyclic in character.
Experimental verification of these conclusions should be possible. One option would be to fuse a mitochondrial targeting sequence to one of the knotted proteins64 and subject it to in vitro import into isolated mitochondria, analogously to the experiments on other proteins65,66. Another possibility is to use modern artificial nanopore technology. Engineered protein nanopores have been successfully used for the detection of DNA chains67,68, including the knotted ones69. Recently, it was demonstrated that they can also be used for the detection of proteins70,71,72. These techniques should provide a means for experimental analysis of the impact of periodic forces on knotted protein translocation.
Additional Information
How to cite this article: Szymczak, P. Periodic forces trigger knot untying during translocation of knotted proteins. Sci. Rep. 6, 21702; doi: 10.1038/srep21702 (2016).
Supplementary Material
Acknowledgments
This work was supported by the Polish Ministry of Science and Higher Education under research Grant No. N N202 055440. The author benefited from discussions with Marek Cieplak, Joanna Sułkowska and Piotr Sułkowski. The Brownian dynamics code used in the simulations was based on the protein-modeling package by Marek Cieplak.
Footnotes
Author Contributions P.S. designed research, performed research, analyzed data and wrote the paper.
References
- Frank-Kamenetskii M. D., Lukashin A. V. & Vologodskii A. V. Statistical mechanics and topology of polymer chains. Nature 258, 398–402 (1975). [DOI] [PubMed] [Google Scholar]
- Raymer D. M. & Smith D. E. Spontaneous knotting of an agitated string. Proc. Natl. Acad. Sci. USA 104, 16432–16437 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumners D. & Whittington S. G. Knots in self-avoiding walks. J. Phys. A: Math. Gen. 21, 1689 (1988). [Google Scholar]
- Bates A. D., Maxwell A. DNA Topology (Oxford University Press, 2005). [Google Scholar]
- Taylor W. R. & Lin K. Protein knots: a tangled problem. Nature 421, 25–25 (2003). [DOI] [PubMed] [Google Scholar]
- Virnau P., Mirny L. & Kardar M. Intricate knots in proteins: Function and evolution. PLoS Comput. Biol. 2, e122 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millett K. C., Rawdon E. J., Stasiak A. & Sułkowska J. I. Identifying knots in proteins. Biochem. Soc. Trans 41, 533–537 (2013). [DOI] [PubMed] [Google Scholar]
- Taylor W. R. A deeply knotted protein structure and how it might fold. Nature 406, 916–919 (2000). [DOI] [PubMed] [Google Scholar]
- Sulkowska J., Sulkowski P., Szymczak P. & Cieplak M. Tightening of knots in proteins. Phys. Rev. Lett. 100, 058106 (2008). [DOI] [PubMed] [Google Scholar]
- Portugal J. & Rodrguez-Campos A. T7 RNA polymerase cannot transcribe through a highly knotted DNA template. Nucleic Acids Res. 24, 4890–4894 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deibler R. W., Mann J. K., Sumners D. W. & Zechiedrich L. Hin-mediated DNA knotting and recombining promote replicon dysfunction and mutation. BMC Mol. Biol. 8, 44 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornschlögl T. et al. Tightening the knot in phytochrome by single-molecule atomic force microscopy. Biophys. J. 96, 1508–14 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymczak P. Tight knots in proteins: can they block the mitochondrial pores? Biochem. Soc. Trans. 41, 620–624 (2013). [DOI] [PubMed] [Google Scholar]
- Szymczak P. Translocation of knotted proteins through a pore. Eur. Phys. J. Special Topics 223, 1805–1821 (2014). [Google Scholar]
- Panja D., Barkema G. T. & Kolomeisky A. B. Through the eye of the needle: recent advances in understanding biopolymer translocation. J. Phys.: Condens. Matt. 25, 413101 (2013). [DOI] [PubMed] [Google Scholar]
- Muthukumar M. Polymer Translocation (CRC Press, 2011). [Google Scholar]
- Pfanner N. & Meijer M. Pulling in the proteins. Curr. Biol 5, 132–135 (1995). [DOI] [PubMed] [Google Scholar]
- Matouschek A., Pfanner N. & Voos W. Protein unfolding by mitochondria. EMBO Rep. 1, 404–410 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokranjac D. & Neupert W. Protein import into mitochondria. Biochem. Soc. Trans. 33 1019–1023 (2005). [DOI] [PubMed] [Google Scholar]
- Muthukumar M. Macromolecular mechanisms of protein translocation. Protein Pept. Lett. 21, 209–216 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehling P., Brandner K. & Pfanner N. Mitochondrial import and the twin-pore translocase. Nat. Rev. Mol. Cell Biol. 5, 519–30 (2004). [DOI] [PubMed] [Google Scholar]
- Cieplak M., Hoang T. X. & Robbins M. O. Thermal effects in stretching of Go-like models of titin and secondary structures. Proteins: Struct. Funct. Bio. 56, 285 (2003). [DOI] [PubMed] [Google Scholar]
- Tsai J., Taylor R., Chotchia C. & Gerstein M. The packing density in proteins: Standard radii and volumes. J. Mol. Biol. 290, 253 (1999). [DOI] [PubMed] [Google Scholar]
- Sułkowska J. I. & Cieplak M. Selection of optimal variants of Go-like models of proteins through studies of stretching. Biophys. J 95, 3174–3191 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch J. M. Biophysics software for interdisciplinary education and research. Amer. J. Phys. 82, 442–450 (2014). [Google Scholar]
- Szymczak P. & Cieplak M. Stretching of proteins in a uniform flow. J. Chem. Phys. 125, 164903 (2006). [DOI] [PubMed] [Google Scholar]
- Cieplak M. & Szymczak P. Protein folding in a force clamp. J. Chem. Phys. 124, 194901 (2006). [DOI] [PubMed] [Google Scholar]
- Briggs M. S. & Roder H. Early hydrogen-bonding events in the folding reaction of ubiquitin. Proc. Natl. Acad. Sci. USA 89, 2017–2021 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nymeyer H., Garca A. E. & Onuchic J. N. Folding funnels and frustration in off-lattice minimalist protein landscapes. Proc. Natl. Acad. Sci. USA 95, 5921–5928 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulkowska J. I. & Cieplak M. Mechanical stretching of proteins - a theoretical survey of the Protein Data Bank. J. Phys.: Condens. Matt 19, 283201 (2007). [Google Scholar]
- Huang L., Kirmizialtin S. & Makarov D. Computer simulations of the translocation and unfolding of a protein pulled mechanically through a pore. J. Chem. Phys. 123, 124903 (2005). [DOI] [PubMed] [Google Scholar]
- West D. K., Brockwell D. J. & Paci E. Prediction of the translocation kinetics of a protein from its mechanical properties. Biophys. J 91, L51–3 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmizialtin S., Huang L. & Makarov D. E. Computer simulations of protein translocation. Phys. Status Solidi B 243, 2038–2047 (2006). [Google Scholar]
- Huang L. & Makarov D. E. Translocation of a knotted polypeptide through a pore. J. Chem. Phys. 129, 121107 (2008). [DOI] [PubMed] [Google Scholar]
- Ammenti A., Cecconi F., Marconi U. M. B. & Vulpiani A. A statistical model for translocation of structured polypeptide chains through nanopores. J. Phys. Chem. B 113, 10348–10356 (2009). [DOI] [PubMed] [Google Scholar]
- Chinappi M., Cecconi F. & Casciola C. M. Computational analysis of maltose binding protein translocation. Philos. Mag. 91, 2034–2048 (2011). [Google Scholar]
- Bacci M., Chinappi M., Casciola C. M. & Cecconi F. Protein translocation in narrow pores: Inferring bottlenecks from native structure topology. Phys. Rev. E 88, 022712 (2013). [DOI] [PubMed] [Google Scholar]
- Wojciechowski M., Szymczak P., Carrión-Vázquez M. & Cieplak M. Protein unfolding by biological unfoldases: Insights from modeling. Biophys. J 107, 1661–1668 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarov D. E. Computer simulations and theory of protein translocation. Acc. Chem. Res. 42, 281–289 (2008). [DOI] [PubMed] [Google Scholar]
- Makarov D. E. Computational and theoretical insights into protein and peptide translocation. Protein Pept. Lett. 21, 217–226 (2014). [DOI] [PubMed] [Google Scholar]
- Cecconi F., Bacci M. & Chinappi M. Protein transport across nanopores: A statistical mechanical perspective from coarse-grained modeling and approaches. Protein Pept Lett. 21, 227–234 (2014). [DOI] [PubMed] [Google Scholar]
- Dzubiella J. Sequence-specific size, structure, and stability of tight protein knots. Biophys. J 96, 831–9 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddocks J. H. & Keller J. B. Ropes in equilibrium. SIAM J. Appl. Math. 47, 1185–1200 (1987). [Google Scholar]
- Bell G. I. Models for the specific adhesion of cells to cells. Science 200, 618–627 (1978). [DOI] [PubMed] [Google Scholar]
- Hornsby C. E. & Paton R. S. It is all downhill from here. Nat. Chem. 6, 88–89 (2014). [DOI] [PubMed] [Google Scholar]
- Ess D. H. et al. Bifurcations on potential energy surfaces of organic reactions. Angew. Chem. Int. Ed. 47, 7592–7601 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder N. N. & Theg S. M. Energy use by biological protein transport pathways. Trends Biochem. Sci. 28, 442–451 (2003). [DOI] [PubMed] [Google Scholar]
- Rosa A., Di Ventra M. & Micheletti C. Topological jamming of spontaneously knotted polyelectrolyte chains driven through a nanopore. Phys. Rev. Lett. 109, 118301 (2012). [DOI] [PubMed] [Google Scholar]
- Kirmizialtin S. & Makarov D. E. Simulations of the untying of molecular friction knots between individual polymer strands. J. Chem. Phys. 128, 094901 (2008). [DOI] [PubMed] [Google Scholar]
- Matthews R., Louis A. & Yeomans J. Knot-controlled ejection of a polymer from a virus capsid. Phys. Rev. Lett. 102, 088101 (2009). [DOI] [PubMed] [Google Scholar]
- Marenduzzo D. et al. DNA-DNA interactions in bacteriophage capsids are responsible for the observed DNA knotting. Proc. Natl. Acad. Sci. USA 106, 22269–22274 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenduzzo D., Micheletti C., Orlandini E. & Sumners D. W. Topological friction strongly affects viral DNA ejection. Proc. Natl. Acad. Sci. USA 110, 20081–20086 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas N., Imafuku Y. & Tawada K. Molecular motors: thermodynamics and the random walk. Proc. R. Soc. Lond. Ser. B. Biol. Sci. 268, 2113–2122 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. & Oster G. Ratchets, power strokes, and molecular motors. Appl. Phys. A 75, 315–323 (2002). [Google Scholar]
- Oster G. & Wang H. Reverse engineering a protein: the mechanochemistry of ATP synthase. Biochim. Biophys. Acta, Bioenerg 1458, 482–510 (2000). [DOI] [PubMed] [Google Scholar]
- Metzler R. et al. Diffusion mechanisms of localised knots along a polymer. Europhys. Lett. 76, 696–702 (2006). [Google Scholar]
- Tian P. & Andricioaei I. Repetitive pulling catalyzes co-translocational unfolding of barnase during import through a mitochondrial pore. J. Mol. Biol. 350, 1017–34 (2005). [DOI] [PubMed] [Google Scholar]
- Redner S. A guide to first-passage processes (Cambridge University Press, 2001). [Google Scholar]
- Ben-Naim E., Daya Z., Vorobieff P. & Ecke R. E. Knots and random walks in vibrated granular chains. Phys. Rev. Lett. 86, 1414 (2001). [DOI] [PubMed] [Google Scholar]
- Luccioli S., Imparato A., Mitternacht S., Irbäck A. & Torcini A. Unfolding times for proteins in a force clamp. Phys, Rev. E 81, 010902 (2010). [DOI] [PubMed] [Google Scholar]
- Evans E. & Ritchie K. Dynamic strength of molecular adhesion bonds. Biophys. J. 72, 1541–1555 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymczak P. & Cieplak M. Stretching of proteins in a force-clamp. J. Phys. Condens. Matt. 18, L21 (2006). [Google Scholar]
- West D. K., Brockwell D. J., Olmsted P. D., Radford S. E. & Paci E. Mechanical resistance of proteins explained using simple molecular models. Biophys. J 90, 287–297 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C. & van Loon A. P. How proteins find mitochondria and intramitochondrial compartments. Trends Biochem. Sci. 11, 204–207 (1986). [Google Scholar]
- Wilcox A. J., Choy J., Bustamante C. & Matouschek A. Effect of protein structure on mitochondrial import. Proc. Natl. Acad. Sci. USA 102, 15435–15440 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Esaki M., Fernandez J. M. & Endo T. Comparison of the protein-unfolding pathways between mitochondrial protein import and atomic-force microscopy measurements. Proc. Natl. Acad. Sci. USA 102, 17999–8004 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G. F. & Dekker C. DNA sequencing with nanopores. Nat. Biotechnol. 30, 326–328 (2012). [DOI] [PubMed] [Google Scholar]
- Bayley H. Nanopore sequencing: From imagination to reality. Clin. Chem. 61, 25–31 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesa C. et al. Observation of DNA knots using solid-state nanopores. Biophys. J 108, 166a (2015). [Google Scholar]
- Li W. et al. Single protein molecule detection by glass nanopores. ACS nano 7, 4129–4134 (2013). [DOI] [PubMed] [Google Scholar]
- Plesa C. et al. Fast translocation of proteins through solid state nanopores. Nano Lett. 13, 658–663 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. B., Rodriguez-Larrea D. & Bayley H. Single-molecule site-specific detection of protein phosphorylation with a nanopore. Nat. Biotechnol. 32, 179–181 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar R. B. Maximum likelihood estimation and inference: with examples in R, SAS and ADMB (John Wiley & Sons, 2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.