Abstract
A 68-year-old woman presented to the emergency department owing to exertional dyspnoea and bilateral leg oedema for 3 weeks. Her vital signs included the following: heart rate of 95 bpm, respiratory rate of 24 breaths/min, oxygen saturation of 73% on room air and a blood pressure of 184/108 mm Hg. Physical examination revealed tachypnoea with clear lungs to auscultation, elevated jugular veins, cyanosis and bilateral pitting oedema. A chest X-ray demonstrated cardiomegaly without obvious pulmonary oedema. A CT of the chest was negative for pulmonary embolus; however, the scan did reveal a large right ventricular (RV) mass. An echocardiogram with bubble study confirmed a patent foramen ovale with significant right-to-left shunting and a large RV mass that significantly obstructed the pulmonary outflow tract. A cardiac biopsy revealed a low-grade neuroendocrine tumour. The patient underwent successful debridement and adjuvant chemotherapy. She improved greatly and was asymptomatic at a 9-month follow-up visit.
Background
Cardiac tumours are classified as either primary or metastatic. Primary cardiac tumours are uncommon with an incidence ranging from 0.0017% to 0.19%.1 Autopsy studies in patients with metastatic cancer revealed cardiac metastases in up to 12% of individuals. The main cancer types that can metastasise to the heart include melanoma, breast cancer, lymphoma, leukaemia or sarcoma.2 A neuroendocrine tumour (NET) of the heart is rare and generally secondary to metastatic disease from a gastrointestinal source.3 We report a case of a patient with a primary NET of the heart that was managed with palliative debridement and adjuvant chemotherapy.
Case presentation
A 68-year-old woman with a history of ovarian adenocarcinoma successfully treated 12 years prior with surgery and chemotherapy presented to the emergency department, with exertional dyspnoea and bilateral leg oedema for 3 weeks. Her vital signs included the following: heart rate of 95 bpm, respiratory rate of 24 breaths/min, peripheral oxygen saturation of 73% on room air and a blood pressure of 184/108 mm Hg. Physical examination revealed tachypnoea and tachycardia with clear lungs to auscultation, elevated jugular veins and bilateral pitting oedema. Her central cyanosis did not respond to the administration of 100% oxygen. After obtaining a chest X-ray, which demonstrated cardiomegaly, transthoracic echocardiogram (TTE) revealed a large right ventricular (RV) mass with an enlarged right atrium (RA). CT of the chest with intravenous contrast did not show any pulmonary embolus, but did reveal a large RV mass (figure 1). A transoesophageal echocardiogram with bubble study confirmed a patent foramen ovale (PFO) with significant right-to-left shunting, severe tricuspid regurgitation and a large RV mass that significantly obstructed the pulmonary outflow tract of the RV. The proposed mechanism of the right-to-left shunt was thought to be caused by the large RV mass leading to RV outflow tract obstruction and subsequent RA hypertension. This mechanism was confirmed via right heart catheterisation, which showed the RA mean pressure to be 25 mm Hg, the RV pressure to be 52/25 mm Hg (systolic/diastolic) and an elevated mean pulmonary artery pressure of 38 mm Hg. The PFO was not crossed during the procedure and therefore a left atrium pressure was not obtained. A cardiac biopsy performed during the procedure revealed a low-grade NET. Although abdomen and pelvis CT showed moderate ascites (figure 2), an octreotide scan confirmed no evidence of metastatic disease, indicating primary cardiac NET (figure 3). Cardiac MRI was limited due to contrast allergy. The patient agreed to surgical tumour resection and PFO closure. Following sternotomy and pericardectomy, surgical inspection of the heart showed that the tumour had eroded through the free wall of the RV, which had not been apparent using any of the preoperative cardiac imaging techniques (figure 4). The patient underwent tumour debulking of the RV outflow tract and PFO closure. Administration of inotropic agents facilitated weaning from cardiopulmonary bypass. On postoperative day 2, the patient was extubated and weaned from vasoactive agents. However, her oxygenation was marginal and she required 100% oxygen via positive pressure ventilation to maintain oxygen saturation of 90%. Her oxygenation status took 1 week to improve and she was completely weaned off oxygen. A repeat TTE showed reduction in size of the RV mass. Surgical pathology revealed a more aggressive grade-2 NET with lymphovascular invasion and Ki-67 proliferation index of 15%. The patient completed 2 days of inpatient chemotherapy with etoposide (VP16) and was discharged to rehabilitation 27 days after admission. She underwent further outpatient chemotherapy with etoposide and carboplatin. A repeat cardiac MRI after three cycles of chemotherapy showed 50% decrease in tumour size (figure 5). The patient's chromogranin A levels dropped from 1604 to 374 ng/mL. Her cardiac function improved remarkably. She was asymptomatic at 9-month follow-up and demonstrated an exercise capacity of walking 4–5 blocks without tachypnoea.
Figure 1.
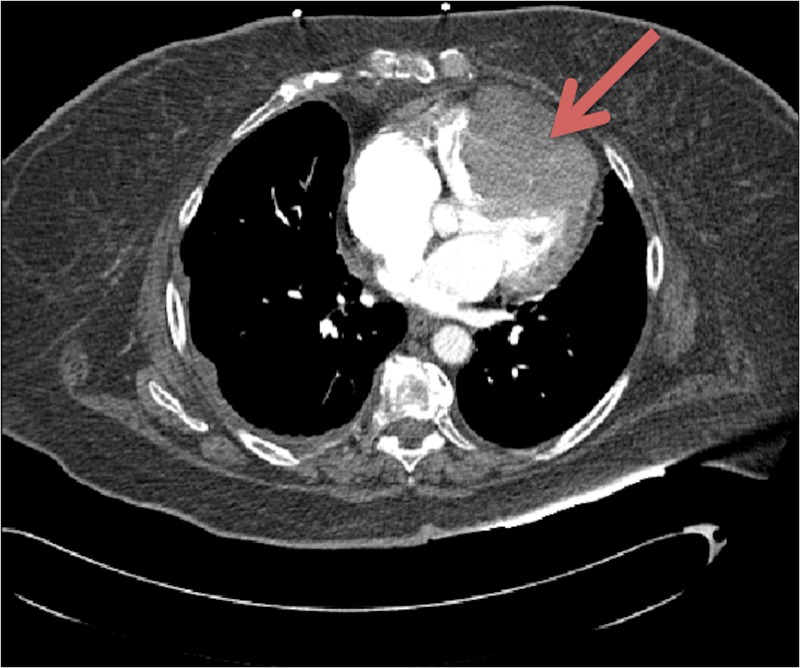
CT of the chest with intravenous contrast revealing a large right ventricular (RV) mass showing enhancement on venous phase consistent with malignancy.
Figure 2.
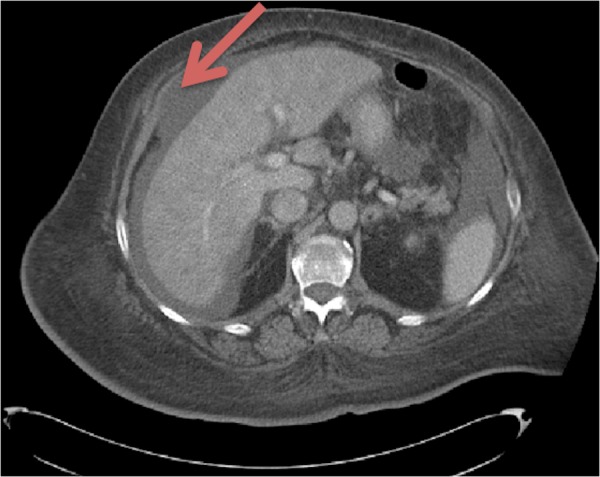
CT of the abdomen showing presence of a moderate amount of ascites and abnormal liver enhancement, suggestive of congestion.
Figure 3.
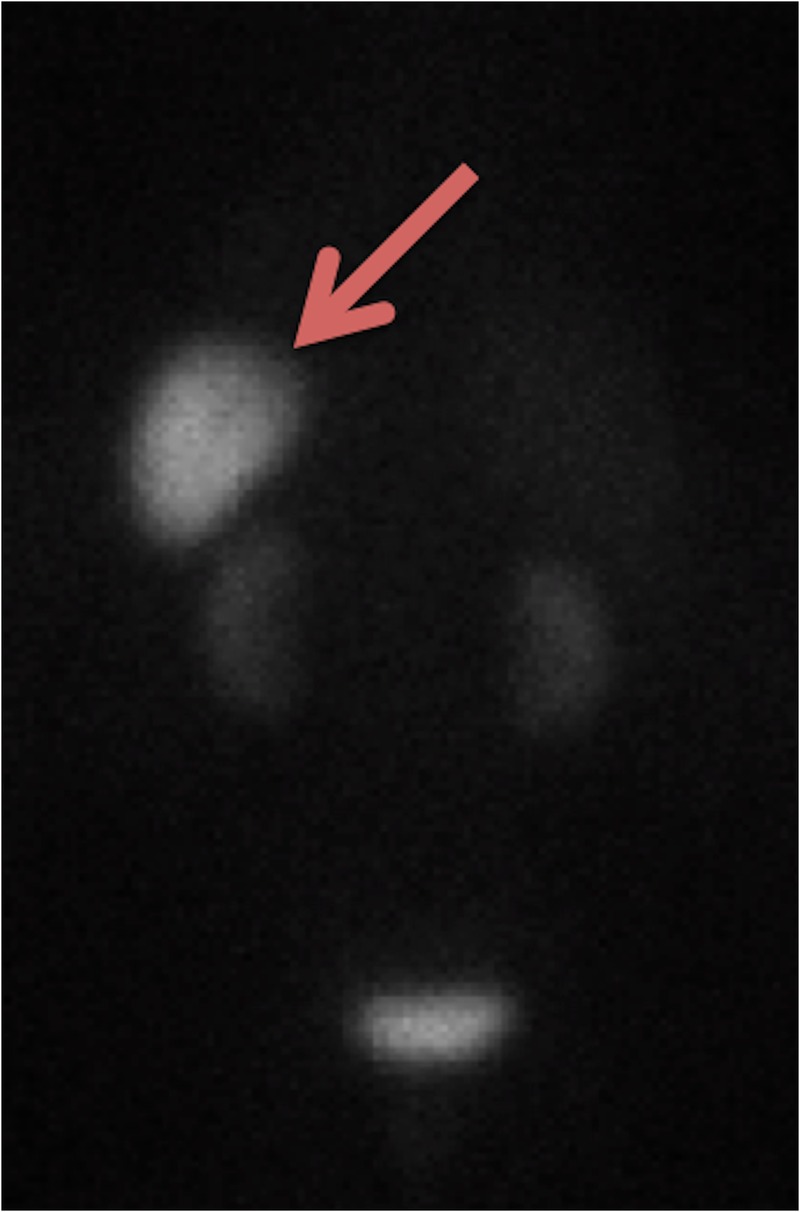
Octreotide scan revealing tumour present only in the heart, there was no evidence of metastatic disease.
Figure 4.
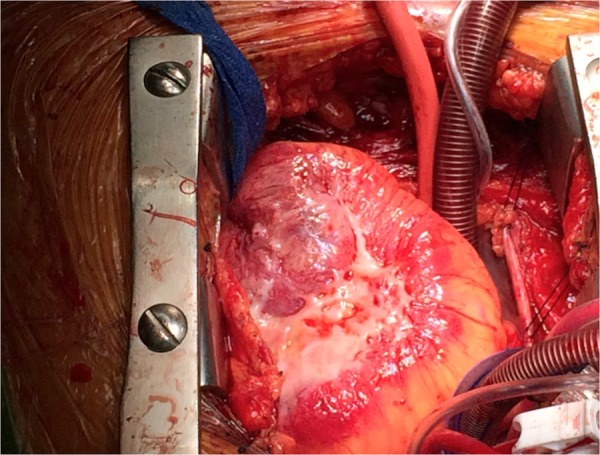
Intraoperative image revealing necrotic tumour invasion of the right ventricular free wall.
Figure 5.
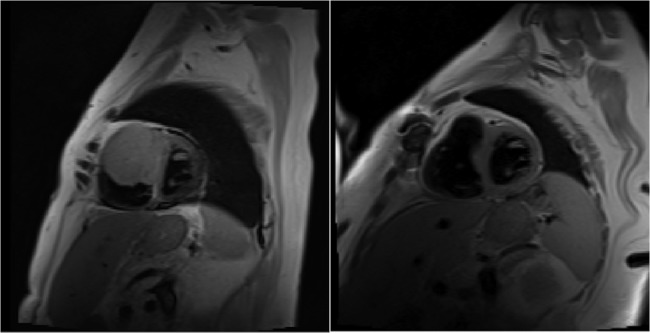
Cardiac MRI before (left) and after (right) surgical resection and chemotherapy. There is a 50% decrease in the tumour size.
Outcome and follow-up
The patient was asymptomatic at 9-month follow-up and demonstrates an exercise capacity of walking 4–5 blocks without tachypnoea.
Discussion
Carcinoid tumours are rare neuroendocrine malignancies that usually arise from the gastrointestinal tract (67.5%) or the bronchopulmonary system (25.3%), with an incidence of 1 in 100 000 individuals.3 These tumours grow slowly and do not cause symptoms until after metastasising to the liver. Carcinoid malignancies have a 5-year survival of 67.2%.3 Carcinoid syndrome results from the tumour releasing 5-hydroxytryptamine, tachykinins and bradykinins.4 Patients experience flushing, diarrhoea and bronchospasm. Echocardiographic studies have shown that more than half of patients with this syndrome have cardiac involvement, usually with the presence of tricuspid valve regurgitation.5 Those patients with cardiac involvement have a mortality benefit from undergoing surgical resection, however, a complete cure is rare.6
This case demonstrates challenging features including symptomatic right-sided heart failure and hypoxic respiratory failure. This patient had significant shunting across her PFO, because of significant RV outflow tract obstruction. A primary cardiac NET is extremely rare and its management is not well described. There has only been one reported case in the medical literature, which was managed by palliative resection and chemotherapy.7 Since these tumours are slow growing, every effort must be made to perform surgical tumour resection or debridement. Postoperative adjuvant chemotherapy with etoposide and carboplatin is generally well tolerated. Patients can be followed up with serial chromogranin A levels to monitor the response to treatment.
Learning points.
Cardiac tumours are classified as either primary or metastatic.
Primary cardiac tumours are much rarer than metastatic cardiac tumours.
The main cancer types that can metastasise to the heart include melanoma, breast cancer, lymphoma, leukaemia and sarcoma.
A neuroendocrine tumour of the heart is rare and generally secondary to metastatic disease from a gastrointestinal source.
Since these tumours are generally slow growing, they can be managed with surgical resection and adjuvant chemotherapy, with a relatively good prognosis.
Footnotes
Contributors: SYN wrote the case report. DH, SF and HH edited the case report and were the active physicians involved in the decision-making process regarding the patient's care.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bakaeen FG, Reardon MJ, Coselli JS et al. . Surgical outcome in 85 patients with primary cardiac tumors. Am J Surg 2003;186:641–7; discussion 47 10.1016/j.amjsurg.2003.08.004 [DOI] [PubMed] [Google Scholar]
- 2.Abraham KP, Reddy V, Gattuso P. Neoplasms metastatic to the heart: review of 3314 consecutive autopsies. Am J Cardiovasc Pathol 1990;3:195–8. [PubMed] [Google Scholar]
- 3.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934–59. 10.1002/cncr.11105 [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharyya S, Davar J, Dreyfus G et al. . Carcinoid heart disease. Circulation 2007;116:2860–5. 10.1161/CIRCULATIONAHA.107.701367 [DOI] [PubMed] [Google Scholar]
- 5.Pellikka PA, Tajik AJ, Khandheria BK et al. . Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Circulation 1993;87:1188–96. 10.1161/01.CIR.87.4.1188 [DOI] [PubMed] [Google Scholar]
- 6.Møller JE, Pellikka PA, Bernheim AM et al. . Prognosis of carcinoid heart disease: analysis of 200 cases over two decades. Circulation 2005;112:3320–7. 10.1161/CIRCULATIONAHA.105.553750 [DOI] [PubMed] [Google Scholar]
- 7.Guajardo-Salinas GE, Anaya-Ayala JE, Rice DC et al. . Primary high-grade neuroendocrine carcinoma of the heart. Tex Heart Inst J 2013;40:71–4. [PMC free article] [PubMed] [Google Scholar]


