Abstract
The objective of this study was to evaluate the morphological and immunohistochemical alterations of tissue removed from the upper third of anterior vaginal wall in a sample group of the female population presenting homogenous risk factors associated with pelvic organ prolapse (POP). The case study consisted of 14 patients with POP and there were 10 patients in the control group. Patient selection was carried on the basis of specific criteria and all of the patients involved in the study presented one or more of the recognized POP risk factors. Samples were taken from POP patients during vaginal plastic surgery following colpohysterectomy, and from control patients during closure of the posterior fornix following hysterectomy. Samples were processed for histological and immunohistochemical analyses for Collagen I and Collagen III, α-Smooth Muscle Actin (α-SMA), Platelet-Derived-Growth-Factor (PDGF), matrix metalloproteinase 3 (MMP3), tissue inhibitors metalloproteinase 1 (TIMP1), Caspase3. Immunofluorescence analyses for Collagen I and III and PDGF were also carried out. In prolapsed specimens our results show a disorganization of smooth muscle cells that appeared to have been displaced by an increased collagen III deposition resulting in rearrangement of the muscularis propria architecture. These findings suggest that the increase in the expression of collagen fibers in muscularis could probably be due to a phenotypic switch resulting in the dedifferentiation of smooth muscle cells into myofibroblasts. These alterations could be responsible for the compromising of the dynamic functionality of the pelvic floor.
Key words: Pelvic organ prolapse, immunohistochemistry, smooth muscle cells, collagen, PDGF
Introduction
Genital prolapse is a highly invalidating condition for women with considerable repercussions not only on their health but also on their quality of life and social well-being. Epidemiological estimates of pelvic descensus reveal a high prevalence in menopausal women.1,2 The causes of the pelvic organ prolapse (POP) are multifactorial. Literature confirms that the main cause is childbirth,3-5 however this needs to be considered in light of acquired and congenital risk factors. Various research groups have analyzed a range of risk factors for POP including childbirth, birth trauma, menopause, body mass index (BMI) and chronic obstructive pulmonary disease (COPD).6,7 With regards to a genetical predisposition to pelvic floor dysfunction, epidemiological studies carried out on monozygotic twins have highlighted an association between genital prolapse and varicose veins, hiatal and inguinal hernias and hypermobile joint syndrome but an absence of association with environmental factors.8,9
Pelvic floor muscles, uterosacral ligaments and endopelvic fascia represent the anatomical support of female pelvic organs and several studies have investigated histological changes in the pelvic tissue and in the composition of the vaginal wall demonstrating that these alterations are involved in POP.10-13 However, the use of different evaluation methods and sample tissue sites makes the comparison between studies difficult and at times, the results appear conflicting. Nevertheless, all studies would appear to agree on the presence of qualitative alterations of the connective tissue resulting from a disequilibrium between collagen type I and collagen type III. Together with the increase of type III collagen, an increase in the activity of the metalloproteinases MMP-2, MMP-3 and MMP-9 and a decrease in the activity of their inhibitors has been observed; this is probably linked to a stress mechanism activated by the overstretching of the pelvic connective tissue with consequent tissue remodeling and adaptation.11,14-16 There is also a reduction in the concentration of elastin and a rise in elastinolitic activity in the endopelvic fascia and vaginal wall of women suffering from genital prolapse.12,17
These biochemical alterations result in a failure in the resistance of the pelvic connective tissue as well as an increase in its extensibility with progressive loss of tissue integrity and consequently the slow and inexorable onset of genital prolapse.18-20 These data are in agreement with studies carried out on patients suffering from hereditary connective tissue diseases such as the Marfan and Cutis Laxa syndromes.21 Overall the information acquired allows the outlining of a possible damage mechanism at the basis of pelvic floor disorders that are responsible for a series of structural alterations of the connective tissue.
The contribution of smooth muscle cells (SMCs) of muscularis of anterior vaginal wall, that appears to be involved in early stages of POP, has been less extensively studied. Previous studies have demonstrated a marked difference in structural features of smooth muscle cells in women with POP where collagen fibers were loosely dispersed among the poorly organized muscle bundles, compared to women without POP.22,23 In particular conditions, these cell type, driven by Platelet Derived Growth Factor β (PDGF β), can lead to a process known as phenotypic modulation, consisting in the transition from a quiescent contractile phenotype to a myofibroblast-like proliferative synthetic state. During transition to the synthetic phenotype, activated myofibroblasts synthesize extracellular matrix proteins (ECM) causing tissue remodeling.24,25
Hence, systematic studies need to be carried out to evaluate the mechanisms that cause these changes in the vaginal wall in patients suffering from genital prolapse in order to provide a targeted therapy and if possible preventive strategies. With this in mind, the aim of our study was to analyze the morphological and immunohistochemical alterations of muscularis in tissue removed from the upper third of the anterior vaginal wall in a sample group of the female population presenting homogenous risk factors associated with POP.
Materials and Methods
The study consisted of two sample population groups that met the criteria for a casecontrol study. The case group consisted of 14 patients suffering from stage III genital prolapse undergoing colpohysterectomy and anterior and posterior plastic vaginal surgery. The control group was made of 10 patients undergoing laparoscopic hysterectomy for uterine fibromatosis. Patient selection was carried on the basis of specific criteria. The average age in both sample groups was 55 (SD=5.38) and none of the patients had undergone or were undergoing hormone replacement therapy (HRT). Patients over the age of 65, patients with case histories of neoplastic vulvar, cervical and vaginal pathologies, connective diseases, dystrophic vulvar and vaginal lesions, incontinence or patients who had undergone gynecological surgery were excluded from the study. The selection of samples was carried out after careful, pre-operative clinical and case history evaluations. All of the patients in the study presented several of the acquired risk factors for pelvic descensus (Table 1). None of the patients had a case history of BPCO, constipation, neurodegenerative pathologies, hiatal and inguinal hernias. Half of the case study group presented varicose veins in their lower legs. Six of the fourteen patients suffering from POP had a family history of stage 1 POP. The case histories of all patients included 2 spontaneous births, episiotomy and/or perineal lacerations. None of the patients had undergone operative vaginal birth, the Kristeller manoeuvre or fetal macrosomia. During the clinical evaluation, all of the patients had a BMI between 25 and 28, an anovulvar distance between 2 and 4 cm, episiotomy scarring, hypotonic central fibrous nucleus, a negative PC test and reduced and non-selective pelvic-perineal contraction endurance. Biopsy samples were taken from POP case patients during vaginal plastic surgery following colpohysterectomy, and from control patients during closure of the posterior fornix following laparoscopic hysterectomy. All patients have provided the consent to the processing of personal data and this study protocol was approved by Institutional Ethic Committee (n. 36944).
Table 1.
Risk factors for pelvic organ prolapse.
| Samples | Controls | |||
|---|---|---|---|---|
| Mean age of 65 years | 14 | 100% | 10 | 100% |
| HRT | - | - | - | - |
| COPD | - | - | - | - |
| Constipation | - | - | - | - |
| Neurodegenerative pathologies | - | - | - | - |
| Varicose veins* | 7 | 50% | - | - |
| History of 2 spontaneous delivery | 14 | 100% | 10 | 100% |
| Episiotomy | 10 | 71.4% | 9 | 90% |
| Perineal tears | 4 | 28.5% | 1 | 10% |
| Operative vaginal delivery | - | - | - | - |
| Kristeller maneuver | - | - | - | - |
| Fetal macrosomia | - | - | - | - |
| 25<BMI<28 | 14 | 100% | 10 | 100% |
| Ano-vulvae distance >3 | 14 | 100% | 10 | 100% |
HRT, hormone replacement therapy; COPD, chronic obstructive pulmonary disease;
*a significant association between POP and varicose veins; BMI, body mass index.
Tissue acquisition and preparation
Samples collected during surgery were immediately divided into two parts. The tissues were immersed in 10% buffered formalin in phosphate buffer saline (PBS, pH 7.4) for 12 h at room temperature, then dehydrated in graded ethanol and embedded in low-temperature-fusion paraffin for histological and immunohistochemistry studies.
Histology, immunohistochemistry and immunofluorescence
Serial 3 µm sections were stained with Hematoxylin and Eosin (H&E) in order to check that the collected samples represented the vaginal wall containing the mucosa, submucosa and muscularis. Connective tissue and elastic fibers were evaluated using Masson’s Trichrome and Weigert Van Gieson staining, respectively. Stained sections were observed using an Olympus BX51 Light Microscope (Olympus, Optical Co. Ltd, Tokyo, Japan). For immunohistochemical (IHC) analyses, sections were incubated for 40 min in methanol and 3% hydrogen peroxidase solution and then rinsed in PBS. Thereafter, sections were incubated overnight at 4°C with polyclonal antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) to Collagen I (sc-8784), Collagen III (sc-8781), α-Smooth Muscle Actin (α-SMA, sc-32251), Platelet-Derived-Growth-Factor (PDGF-A, sc-128), Matrix Metalloproteinase 3 (MMP3, sc-6839), Tissue Inhibitors for Metalloproteinase (TIMP1, sc-6834) and Caspase 3 used at dilutions of 1:400, 1:400, 1:400, 1:500, 1:400, 1:100, 1:100 respectively. Samples were then rinsed with PBS for 5 min and incubated with a labeled streptavidin-biotin-peroxidase conjugate kit (Dako LSAB plus, cod. K0675, Dako Cytomation, Milan, Italy). After rinsing in PBS for 10 min the sections were incubated with 3,3-diaminobenzidine-tetrahydrochloride (DAB, Sigma-Aldrich, St. Louis, MO, USA) for 1-3 min. Lastly, the samples were counter-stained with Mayer’s Hematoxylin and observed under a photomicroscope Olympus BX51 Light Microscope (Olympus).
For immunofluorescence (IF) staining, non specific protein binding was blocked with 5% Bovine Serum Albumin (BSA) in PBS for 1 h at room temperature. Sections were incubated overnight at 4°C with anti collagen I (sc-8784) anti-collagen III (sc-8781) and anti PDGF (sc-128) (Santa Cruz Biotechnology) used at dilutions of 1:400. After rinsing in PBS for 10 min for three times, section were incubated with anti-goat IgG-FITC (sc-2024) (Santa Cruz Biotechnology) for collagen I and collagen III and with anti-rabbit IgG-FITC (sc-2012) (Santa Cruz Biotechnology). All secondary Abs were diluted (1:200) and incubated at room temperature for 30 min. Sections were examined with Zeiss Axio Imager 2 equipped with DFC250 videocam (Carl Zeiss Microscopy LLC, Thornwood, NY, USA).
To demonstrate the specifity of immunoreaction, negative and positive controls were performed for all immunoreactions. For negative controls the primary antibody was replaced (same dilution) with normal serum from the same species. For positive controls, the following tissue were tested: human colon carcinoma was used for PDGF while α-SMA, collagen I and III and MMP3 are intrinsic in the samples.
Observations were processed with an image analysis system (IAS, Delta system, Rome, Italy) and were independently performed by two pathologists (AV, RS) in a blinded fashion.
Quantitative digital image analysis of immunohistochemical staining and statistical analyses
Quantitative comparison of immunohistochemical staining was measured by digital image analysis ImageJ public domain software (W.S., Rasband, Image J, U.S. National Institute of Health, Bethesda, MD, USA; imagej.nih.gov/ij/). IHC stained samples were implemented using the open source image processing software Image J (imagej.en.softonic.com), after installing the IHC profiler plug-in. Microscopic fields with a homogenous morphology were selected from control and POP groups and photographed at the same magnification. The microphotographs were saved as digital images, each of them having the same amount of pixels. This procedure was carried out in order to get images with comparable digital features and repeated for each tested antibody. Then the amount of immunostaining was analyzed through the IHC profiler software. The immunopositivity was expressed as a percentage in the total software-classified areas and the data obtained were plotted on histograms.
Results were expressed as means ±SD; a P value <0.05 was considered statistically significant.
Results
Whole mount tissue histology
H&E staining (not shown) revealed that all layers were present and there was no inflammatory infiltration of the lamina propria or sub epithelium in any of the samples. The examination of prolapsed fragments of the anterior vaginal wall with Masson’s Thricrome showed the presence of disorganization in the muscularis layer and distortion of muscle tissue architecture with a high degree of collagen deposition within smooth muscle cells, together with a reduction in elastic fibers. In the control samples, smooth muscle cells (SMCs) were more tightly packed, organized in orientated fibers, with a more regular distribution of elastic fibers (Figure 1).
Figure 1.
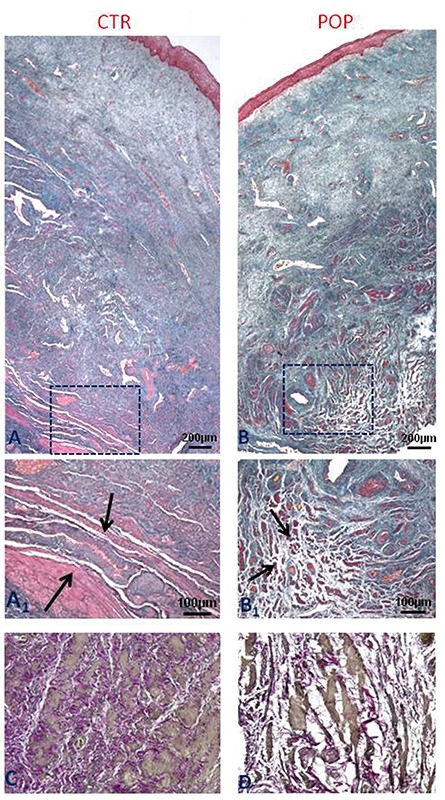
Histological features of anterior vaginal wall. Masson Trichrome staining (original magnification, 5X, 10X). A) Control specimens show the typical architecture of the anterior vaginal wall with SMCs well organized in fibers (A1) within the muscularis propria. B) In women with POP, the muscularis layer shows poorly organized SMCs with a distortion of muscular architecture (B1); Weigert Van Gieson staining (original magnification, 20X). C) In control specimens elastic fibers of muscularis showing typical wavelike aspect are continuous and well organized compared to POP samples (D). Scale bars: A,B) 200 µm; A1, B1) 100 µm; C,D) 50 µm.
Whole mount tissue immunohistochemistry and immunofluorescence
Immunohistochemistry and immunofluorescence analyses showed that type I and III collagen were expressed in both groups however significant differences existed immunoreactions. The immunostaining in IHC and IF of type I collagen was predominant in the control tissue compared to the prolapsed tissue, while there was a tendency towards an increase of expression of type III collagen in the lamina propria and muscularis layer in the prolapsed specimens (Figure 2). Immuno histochemistry and immunofluorescence analyses showed that the expression of PDGF was significantly higher in prolapsed specimens throughout the muscularis propria compared to controls where immunostaining was mild or absent (Figure 2). MMP3 and TIMP1 immunopositivity were also higher in the muscle cells of the muscle layer of prolapsed fragments compared to controls (Figure 3).
Figure 2.
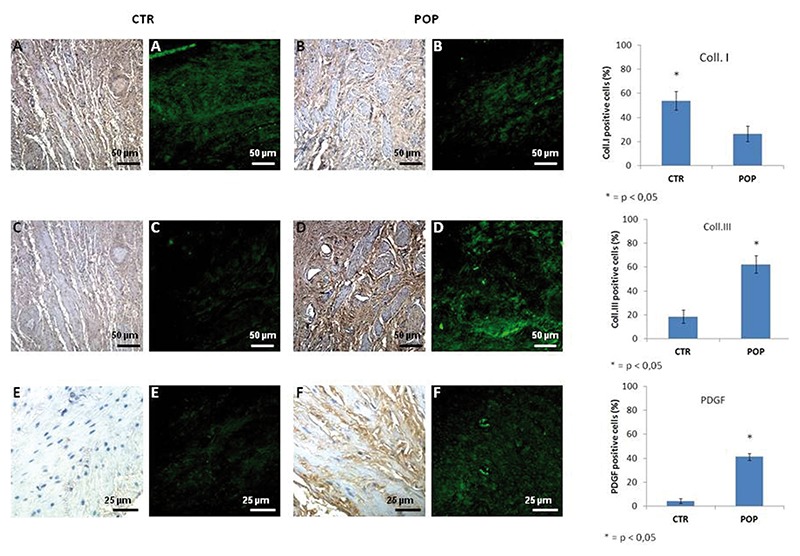
Collagen I-III and PDGF expression in muscularis layer. IHC (original magnification, 20X). A) IHC and IF expression for collagen I was prevalent in normal tissue (A) compared to prolapsed tissue (B), on the contrary there was a tendency of more type III collagen in the prolapsed specimens (D) with respect to controls (C). Scale bars: 50 µm. IHC (original magnification, 20X). IIC and IF expression for PDGF was significantly higher in prolapsed specimens throughout the muscularis propria (F) compared to controls where immunostaining was absent (E). Scale bars: 25 µm.
Figure 3.
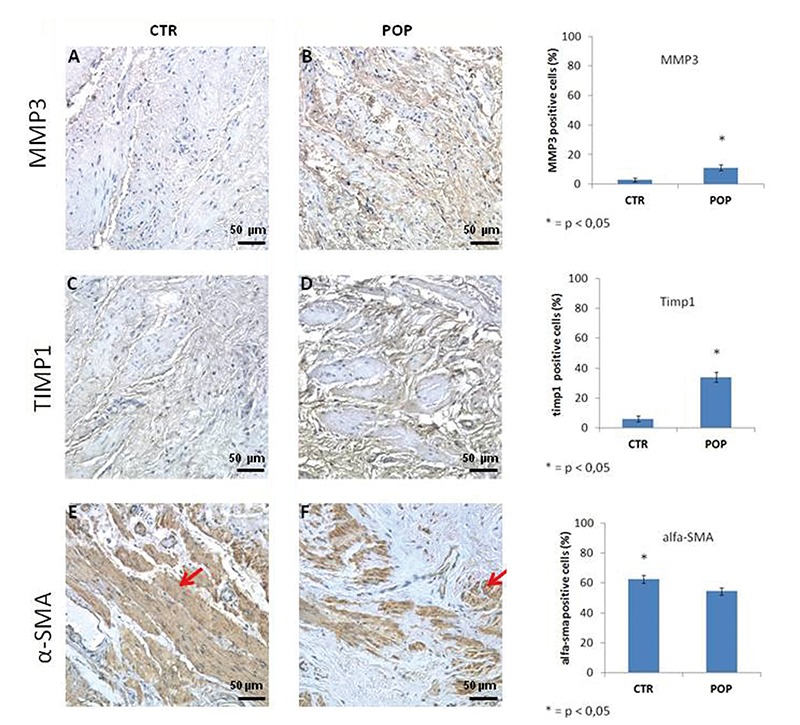
MMP3, TIMP1 and α-SMA expression in muscularis layer. IHC (original magnification, 20X). MMP3 and TIMP1 immunopositivity was increased within the muscle cells of the muscle layer of prolapsed fragments (B-D) respect to controls (A-C). In control fragments α-SMA immunoreactivity was localized in typical sites with a regular distribution of SMCs in the muscularis (E) while in prolapsed fragments smooth muscle cells appeared displaced by an increased presence of connective tissue (F). Scale bars: 50 µm.
In control fragments, α-SMA immunoreactivity of smooth muscle cells was localized in typical sites showing a regular distribution within the longitudinal and circular muscle layers of the muscularis propria while in the prolapsed fragments, α-SMA expression revealed disorganization of smooth muscle cells that appear to have been displaced by an increased presence of connective tissue resulting in rearrangement of the muscularis propria architecture (Figure 3). Caspase 3 immunostaining was absent both in control and prolapsed specimen in the muscularis layer (data not shown).
Discussion
In our study we demonstrate that, independently of acquired risk factors, women suffering from genital prolapse present morphological alterations of the anterior vaginal wall, characterized by disorganization of the architecture of the muscularis. Immunohistochemical evaluation of pathological samples highlighted a general disequilibrium in the expression of type I and type III collagen, which is in agreement with other recent reports.2,11,20 In the muscularis vaginal wall of POP patients, we found an increase in the expression of type III collagen, which is made of thinner fibers and a reduced expression of type I collagen, characterized by thicker more robust fibers, when compared to controls.
In accordance with previous observations,12,22,23 smooth muscle cells appeared to be more disorganized and disrupted in POP patient, when compared to controls. We observed collagen III deposition and rare elastic fibers, distinguished by a typical wave-like aspect, interspersed among muscular fiber bundles, resulting in alteration of the normal architecture of the muscularis propria. A phenotypic smooth muscle cells to myofibroblasts differentiation switch may be the underlying cause of these structural modifications. Indeed, smooth muscle cells undergo an aberrant switch from a contractile to a synthetic extracellular matrix producing phenotype consequent to their trans-differentiation into myofibroblasts.16,25,26 Platelet Derived Growth Factor (PDGF), which was also overexpressed in POP samples, may be involved in this metaplastic smooth muscle cell to myofibroblast trans-differentiation.
PDGF is expressed primarily by megakaryocytes but also by macrophages, endothelial cells, fibroblasts, glial cells and a variety of tumor cell lines. Its expression is low under normal conditions but is increased in several pathological conditions.27-30 Mechanical and physical stress activates the PDGF signaling pathway in smooth muscle cells, resulting in muscle remodeling.30 Furthermore, PDGF promotes fibroblasts migration and is responsible for the excessive deposition of ECM leading to aberrant organ fibrosis with significant local and systemic consequences.25,28 Some studies have proposed the intriguing hypothesis that hypoxia and trauma may cause the apoptosis of SMC within the uterosacral ligament, inducing fibroblasts migration and proliferation, resulting in abnormal collagen III synthesis and deposition.14 Bearing this in mind, we assessed whether an apoptotic mechanism was active in the anterior vaginal wall and whether this could be involved in the replacement muscle with connective tissue. IHC performed on anterior vaginal wall sections, demonstrated that SMC were negative for Caspase3, supporting the hypothesis of transdifferentiation rather than apoptosis mechanism.
Abnormal levels of connective tissue within the muscularis of the anterior wall of the vagina in POP associate with an increased MMP3 expression, an enzyme that is involved in the degradation of extracellular matrix and may eventually be implicated in remodeling this excessive matrix.31 We also found an increase of TIMP1 immunopositivity which could explain an observation of concomitant matrix deposition and elevated MMP3 expression by way of MMP3 inhibition.
In light of our results, we hypothesize that mechanical stress during the birthing process, together with predisposing and phenotype risk factors, could result in reduced SMC contractile function in the muscularis of the anterior wall of the vagina concomitant with acquisition of a synthetic phenotype, resulting in the deposition of an excessive quantity of extracellular matrix that eventually compromises the dynamic function of the pelvic floor. Although it has been reported that mechanical stress of smooth muscle cells induces MMP activation and PDGF expression, it remains to be elucidated why this does not occur in all patients. Indeed, activation of this molecular mechanism was not detected in control patients despite their exposure to similar levels of mechanical stress in terms of both quality and quantity. On this basis, we hypothesize that late gene activation is responsible for the damage observed in POP patients.
Given that the identification and successive silencing of the gene pool responsible for this switch could be difficult to prove, clinical data is of significance, with an interesting element emerging from our study, of a significant association between varicose veins and POP. Indeed, it would appear that other muscular layers, in addition to the pelvic perineum, are also affected by this phenotypic switch, which may lead to alterations in the microcirculation, resulting in the formation of varicose veins and/or pelvic perineal dystrophy. Identification of vascular symptoms prior to the clinical manifestation of POP that may result from this phenotypic switch represents a future research objective that could permit identification high-risk patents and adoption of preventative measures, prior to the development of POP. We stress, however, that this hypothesis is based upon clinical data and must be confirmed at the molecular level.
In the meantime, we must address treatment-resistant advanced stage POP, which is treated surgically at present. Our study indicates that compromised tissue is responsible for the high rate of recurrence following fascial surgery. Indeed, culposuspension of the anatomical structure that presents histological alterations and as a consequence loss of tensile strength and resistance, results in a high rate of recurrence. Conversely, prosthetic surgery utilizing synthetic meshes to substitute for connective endopelvic structures, associates with a reduced rate of short and long term recurrence, albeit at a greater risk of complications. In the light of these recent advances, a new challenge is emerging for modern surgery, namely the use of prosthetics as an integral part and not an alternative to fascial surgery.
In summary, we demonstrate that increased type III collagen within the muscolaris layer appears to result from the phenotypic switch from SMCs to myofibroblasts, in the absence of apoptosis.
Limitation to this study includes the small number of patients and further investigations are needed to confirm whether this cellular transition from a contractile phenotype to a synthetic state is involved in the pathogenesis of POP.
References
- 1.Nygaard I, Bradley C, Brandt D. Pelvic organ prolapse in older women: prevalence and risk factors. Obstet Gynaecol 2004;104:489-97. [DOI] [PubMed] [Google Scholar]
- 2.Kerkhoff MH, Ruiz Zapata AM, Bril H, Bleeker M, Belien J, Stoop R, et al. Changes in tissue composition of the vaginal wall of premenopausal women with prolapse. Am J Obstet Gynecol 2014;210: 168.e1-9. [DOI] [PubMed] [Google Scholar]
- 3.Memon HU, Handa VL. Vaginal childbirth and pelvic floor disorders. Womens Health (Lond Engl) 2013;9:265-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berardi M, Martinez-Romero O, Elías-Zúñiga A, Rodriguez M, Ceretti E, Fiorentino A, et al. Levator ani deformation during the second stage of labour. Proc Inst Mech Eng H 2014;228:501-8. [DOI] [PubMed] [Google Scholar]
- 5.MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. Br J Obstet Gynaecol 2000;107:1460-70. [DOI] [PubMed] [Google Scholar]
- 6.Direkvand-Moghadam A, Ghazanfari Z, Sayehmiri K. Predictive factors for pelvic organ prolapse (POP) in Iranian women’s: an ordinal logistic approch. J Clin Diagn Res 2014;8:96-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kepenekci I, Keskinkilic B, Akinsu F, Cakir P, Elhal AH, Erkek AB, et al. Prevalence of pelvic floor disorders in the female population and the impact of age, mode of delivery, and parity. Dis Colon Rectum 2011;54:85-94. [DOI] [PubMed] [Google Scholar]
- 8.Knoepp LR, McDermott KC, Muñoz A, Blomquist JL, Handa VL. Joint hypermobility, obstetrical outcomes, and pelvic floor disorders. Int Urogynecol J 2013;24:735-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansell NK, Dietz H P, Treloar SA, Clarke B, Martin NG. Genetic covariation of pelvic organ and elbow mobility in twins and their sisters. Twin Res 2004;7:254-60. [DOI] [PubMed] [Google Scholar]
- 10.Vulic M, Strinic T, Tomic S, Capkun V, Jakus IA, Ivica S. Difference in expression of collagen type I and matrix metalloproteinase-1 in uterosacral ligaments of women with and without pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol 2010;155:225-8. [DOI] [PubMed] [Google Scholar]
- 11.Alarab M, Kufaishi H, Lye S, Drutz H, Shynlova O. Expression of extracellular matrix remodeling proteins is alterate in vaginal tissue of premenopausal women with severe pelvic organ prolapse. Reprod Sci 2014;21:704-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Landsheere L, Blacher S, Munaut C, Nusgens B, Robod C, Noel A, et al. Changes in elastin density in different locations of vaginal wall in women with pelvic organ prolapse. Int Urogynecol J 2014;25:1673-81. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Huang J, Hu C, Hua K. Relationship of advanced glication end products and the receptor to pelvic organ prolapse. Int J Clin Exp Pathol 2015;8:2288-99. [PMC free article] [PubMed] [Google Scholar]
- 14.Yucel N, Usta A, Guzin K, Kanter M, Bilgic E, Ozel N, et al. Immunohistochemical analysis of connective tissue in patients with pelvic organ prolapse. J Mol Histol 2013;44:97-102. [DOI] [PubMed] [Google Scholar]
- 15.Shynlova O, Bortolini MA, Alarab M. Genes responsible for vaginal extracellular matrix metabolism are modulated by women’s reproductive cycle and menopause. Int Braz J Urol 2013;39:257-67. [DOI] [PubMed] [Google Scholar]
- 16.Budatha M, Silva S, Montoya TI, Suzuki A, Shah-Simpson S, Wieslander CK, et al. Dysregulation of protease and protease inhibitors in a mouse model of human pelvic organ prolapse. PLoS One 2013;8:e56376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klutke J, Ji Q, Campeau J, Starcker B, Felix JC, Stanczyk FZ, et al. Decreased endopelvic fascia elastin content in uterine prolapse. Acta Obstet Gynecol Scand 2008; 87:111-5. [DOI] [PubMed] [Google Scholar]
- 18.Tremollieres F. Connective tissue and prolapse genesis. Gynecol Obstet Fertil 2010;38:388-93. [DOI] [PubMed] [Google Scholar]
- 19.Kannan K, McConnell A, McLeod M, Rane A. Microscopic alterations of vaginal tissue in woman with pelvic organ prolapse. J Obstet Gynecol 2011;31:250-3. [DOI] [PubMed] [Google Scholar]
- 20.Kerkhof MH, Hendriks L, Brolmann HA. Changes in connective tissue in patients with pelvic organ prolapse – a review of the current literature. J Mol Histol 2013; 44:97-102. [DOI] [PubMed] [Google Scholar]
- 21.Carley ME, Schaffer J. Urinary incontinence and pelvic organ prolapse in women with Marfan or Ehlers Danlos syndrome. Am J Obstet Gynecol 2000; 182:1021-3. [DOI] [PubMed] [Google Scholar]
- 22.Boreham MK, Wai CY, Miller RT, Schaffer JI, Word RA. Morphometric properties of posterior vaginal wall in women with pelvic organ prolapse. Am J Obstet Gynecol 2002;187:1501-9. [DOI] [PubMed] [Google Scholar]
- 23.Takacs P, Gualtieri M, Nassiri M, Candiotti K, Medina C. Vaginal smooth muscle cells apoptosis is increased in women with pelvic organ prolapse. Int Urogynecol J 2008;19:1559-64. [DOI] [PubMed] [Google Scholar]
- 24.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 2004; 84:767-801. [DOI] [PubMed] [Google Scholar]
- 25.Severi C, Sferra R, Scirocco A, Vetuschi A, Pallotta N, Pronio A, et al. Contribution of intestinal smooth muscle to Crohn’s disease fibrogenesis. Eur J Histochem 2014; 58:2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith P, Heath D, Yacoub M, Madden B, Caslin A, Gosney J. The ultrastructure of plexogenic pulmonary arteriopathy. J Pathol 1990;160:111-21. [DOI] [PubMed] [Google Scholar]
- 27.Gomez D, Owens G. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovascular Research 2012; 95:156-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostendorf T, Eitner F, Floege J. The PDGF family in renal fibrosis. Pediatr Nephrol 2012;27:1041-50. [DOI] [PubMed] [Google Scholar]
- 29.Lagna G, Ku NM, Nguyen PH, Neuman NA, Davis BN, Hata A. Control of phenotypic plasticity of smooth muscle cells by BMP signaling through the myocardin-related transcription factors. J Biol Chem 2007; 282: 37244-37255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seo KW, Lee SJ, Kim YH, Bae JU, Park SY, Bae SS, et al. Mechanical stretch increases MMP-2 production in vascular smooth muscle cells via activation of PDGFR-β/Akt signaling pathway. PLoS One 2013;8: e70437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shapiro SD. Metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol 1998. 10: 602-8. [DOI] [PubMed] [Google Scholar]


