Abstract
Current techniques for autologous auricular reconstruction produce substandard ear morphologies with high levels of donor-site morbidity, whereas alloplastic implants demonstrate poor biocompatibility. Tissue engineering, in combination with noninvasive digital photogrammetry and computer-assisted design/computer-aided manufacturing technology, offers an alternative method of auricular reconstruction. Using this method, patient-specific ears composed of collagen scaffolds and auricular chondrocytes have generated auricular cartilage with great fidelity following 3 months of subcutaneous implantation, however, this short time frame may not portend long-term tissue stability. We hypothesized that constructs developed using this technique would undergo continued auricular cartilage maturation without degradation during long-term (6 month) implantation. Full-sized, juvenile human ear constructs were injection molded from high-density collagen hydrogels encapsulating juvenile bovine auricular chondrocytes and implanted subcutaneously on the backs of nude rats for 6 months. Upon explantation, constructs retained overall patient morphology and displayed no evidence of tissue necrosis. Limited contraction occurred in vivo, however, no significant change in size was observed beyond 1 month. Constructs at 6 months showed distinct auricular cartilage microstructure, featuring a self-assembled perichondrial layer, a proteoglycan-rich bulk, and rounded cellular lacunae. Verhoeff's staining also revealed a developing elastin network comparable to native tissue. Biochemical measurements for DNA, glycosaminoglycan, and hydroxyproline content and mechanical properties of aggregate modulus and hydraulic permeability showed engineered tissue to be similar to native cartilage at 6 months. Patient-specific auricular constructs demonstrated long-term stability and increased cartilage tissue development during extended implantation, and offer a potential tissue-engineered solution for the future of auricular reconstructions.
Introduction
Deformity of the auricle, or external ear, can result from congenital defects, oncologic resection, or traumatic injury.1,2 Current clinical practice utilizes autologous costal cartilage to fabricate auricular facsimiles with long-term stability3,4; however, this method is limited by donor-site morbidity,3–6 a complex surgical sculpting process,3–5,7 and differing mechanical properties compared to elastic auricular cartilage.4,5 Alternatively, alloplastic implants produce predictable shapes and eliminate donor-site surgery,3,8 but suffer from poor biocompatibility and high rates of infection and extrusion.3,6 A tissue engineering approach to auricular reconstructions would overcome the limitations of both autologous and alloplastic contemporary treatments.
Toward this end, several tissue engineering methods have been applied to fabricate full-scale auricular constructs. Several synthetic scaffolds, such as polyglycolic acid,3,5,6,9–11 polylactic acid,12 polycaprolactone,3,12 or copolymer combinations, have been applied with varying success. However, the degradation of these polymers has been reported to cause inflammatory reactions.3,4 Natural polymer scaffolds and hydrogels, such as alginate13 and fibrin,14 are easily molded and have high biocompatibility,4 but are limited by low stiffness, making them difficult to handle.15 Chondrocytes encapsulated in alginate and then implanted also become fibrochondrogenic.16 In addition to scaffolds, choice of cell source is important. While articular or costal chondrocytes may be more abundant, these cells do not produce elastin fibers when implanted.17 Mesenchymal stem cells (MSCs) are also a potential source, but these require in vitro chondrogenic induction before implantation.18
Despite the extent of auricular cartilage engineering, limitations still exist. Few groups have worked with methods that can apply patient-specific morphologies to engineered ear constructs,19,20 resulting in generic ears that must be surgically manipulated to reproduce particular aesthetics. Additionally, the maintenance of ear morphology following implantation often requires mechanical assistance, either through external stenting over the skin14 or internal plastic21 or metal10,22–24 support that is included with the implant. Our group has previously applied digital photogrammetry and computer-assisted design/computer-aided manufacturing (CAD/CAM) techniques to fabricate molds replicating juvenile patient ears, a novel technique to recreate specific ear morphology.25 Ear constructs were then produced by injection molding of auricular chondrocytes encapsulated within high-density type I collagen hydrogels, which were implanted in vivo without external stenting or internal synthetic components. Following 3 months in vivo, these constructs maintained detailed morphological characteristics and developed structural features and mechanical properties similar to native auricular cartilage.25
Although our previous study demonstrated the potential of utilizing photogrammetric and CAD/CAM methods to produce patient-specific molds and constructs, the long-term stability (greater than 3 months) of the developing tissue in vivo has not been verified. In general, few studies utilizing complete human ear geometries have exceeded this time point,3,6,10,12,23,26 and none of these featured a collagen scaffold. Additionally, the biochemical composition of these constructs must be characterized. To further validate our tissue engineering approach for clinical translation, an extended implantation interval must be explored. In this study, the long-term stability of patient-specific engineered ear constructs in vivo was evaluated and the microstructural, biochemical, and mechanical properties were assessed following 6 months of implantation.
Materials and Methods
Ethics statement
All animal care and experimental procedures were in compliance with the Guide for the Care and Use of Laboratory Animals27 and were approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee (Protocol No. 2011-0036).
Isolation of chondrocytes
Bovine auricular chondrocytes were isolated as previously described.28 Briefly, ears were obtained from freshly slaughtered 1- to 3-day-old calves (Gold Medal Packing, Oriskany, NY). Auricular cartilage was dissected from the surrounding skin and perichondrium under sterile conditions. Cartilage was diced into 1 mm3 pieces and digested overnight in 0.3% collagenase (Worthington Biochemicals Corp., Lakewood, NJ), 100 μg/mL penicillin, and 100 μg/mL streptomycin in Dulbecco's modified Eagle's medium (DMEM; Mediatech, Inc., Manassas, VA). Cells were filtered, washed, and counted the following day.
Construct design and mold fabrication
Molds for the generation of ear constructs were designed and fabricated as previously described.25 Briefly, high-resolution images of the ear of a 5-year-old female with informed consent were obtained using a Cyberware Rapid 3D Digitizer (3030 Digitizer, Monterey, CA). Images were processed using PlyEdit software (Cyberware, Inc., Monterey, CA), converted to stereolithography files, and imported into SolidWorks (Dassault Systems Corp., Waltham, MA) where the continuous three-dimensional ear surface was embedded into a virtual block. This was used to design seven-part molds, which were printed out of acrylonitrile butadiene styrene plastic using a Stratasys FDM 2000 3D printer (Eden Prairie, MN). Molds were sterilized by washing with Lysol® (Parsippany, NJ) followed by a 1-h soak in 70% ethanol and 30 min of drying in a sterile biological safety cabinet before use.
Implant fabrication
Collagen was extracted and reconstituted as previously described.29,30 At the time of fabrication, stock collagen solution was returned to pH 7.0 and maintained at 300 mOsm by mixing with appropriate volumes of 1 N NaOH (Mallinckrodt Baker, Inc., Phillipsburg, NJ), 10× phosphate-buffered saline (PBS; Mediatech, Inc.), and 1× PBS (Mediatech, Inc.) as previously described.30,31 This collagen solution was immediately mixed with cells suspended in media and injected into ear molds as previously described.25 Briefly, an 8 mL collagen–cell mixture, with a final collagen concentration of 10 mg/mL and a final cell concentration of 25 × 106 cells/mL,13 was injected into the molds and allowed to gel for 50 min at 37°C. After 50 min, the ear constructs were removed from the molds and cultured for 3–5 days in media composed of DMEM, 10% fetal bovine serum (Gemini Bio Products, Sacramento, CA), 100 μg/mL penicillin (Mediatech, Inc.), 100 μg/mL streptomycin (Mediatech, Inc.), 0.1 mM nonessential amino acids (Gibco, Grand Island, NY), 50 μg/mL ascorbic acid (Sigma-Aldrich, St. Louis, MO), and 0.4 mM l-proline (Sigma-Aldrich) before implantation. A total of six cell-seeded samples were generated for the 6-month time point in this study. Those implanted constructs that were not complicated by wound infection or seroma formation were included in the analysis.
In vivo implantation and explantation
Ear constructs were implanted as previously described.25 Briefly, 10-week-old male athymic nude rats (RNU; Charles River, Wilmington, MA) were anesthetized through intraperitoneal injection of ketamine (80 mg/kg) and xylazine (8 mg/kg). The surgical sites were shaved and prepped and all animals received a subcutaneous injection of buprenorphine (0.1 mg/kg) and an intraperitoneal injection of cefazolin (11 mg/kg) before surgical manipulation. A small subcutaneous pocket overlying the dorsum was dissected and a construct was inserted and oriented such that the anterior surface was directed toward the overlying skin. Incisions were closed with metallic wound clips and a sterile occlusive dressing was placed before recovery from anesthesia.
Animals were sacrificed through CO2 asphyxiation followed by bilateral thoracotomy after 6 months. Constructs were harvested, weighed, and imaged. Construct length was measured along the lobule–helix axis and construct width was defined as the largest dimension measured along an axis perpendicular to the lobule–helix axis. Half of each specimen was snap frozen in liquid nitrogen for biochemical and biomechanical analyses, whereas the remainder was fixed in 10% neutral buffered formalin for 48 h and transferred to 70% ethanol before histologic analyses.
Histologic analyses
The fixed portions of samples were dehydrated by sequential washes in ethanol, embedded in paraffin, cut into 5 μm sections, and stained with Safranin O/Fast Green to assess proteoglycan distribution, Picrosirius Red to assess collagen organization, and Verhoeff's/Van Gieson to assess the presence of elastin fibers. Images were taken in brightfield at 100× and 200× using a Nikon Eclipse TE2000-S microscope (Nikon Instruments, Melville, NY) fitted with a SPOT RT camera (Diagnostic Instruments, Sterling Heights, MI).
Biochemical analyses
Biochemical analyses were performed as previously described.32 Briefly, three samples were collected from each of the frozen 6-month ears, previously studied 1- and 3-month ears,25 and native juvenile bovine ears. Samples were weighed, refrozen, lyophilized, and weighed again. Samples were then digested with 1.25 mg/mL papain (Sigma-Aldrich) solution overnight at 60°C and analyzed for DNA content through the Hoechst DNA assay,33 sulfated glycosaminoglycan (GAG) content through a modified 1,9-dimethylmethylene blue assay,34 and collagen through a hydroxyproline assay.35 Biochemical properties are reported normalized to the dry weight of the samples.
Mechanical analysis
Six millimeter diameter by 1 mm height disks were cut from the central portion of each frozen ear, as well as from native juvenile bovine ears, using dermal biopsy punches. Confined compression testing was performed as previously described.13,25 Briefly, samples were thawed in PBS containing protease inhibitors (Roche Diagnostics, Indianapolis, IN) and placed in a cylindrical confining chamber mounted in an ELF 3200 test frame (EnduraTec, Eden Prairie, MN). Samples were compressed to 50% of their original height in 10 steps of 50 μm each, with 5 min between steps to allow for full stress relaxation. Resultant stresses were recorded at 1 Hz and the temporal profiles of stress were fit to a poroelastic model of tissue behavior using custom MATLAB (MathWorks, Natick, MA) code to calculate the equilibrium modulus and hydraulic permeability.32
Statistics
All size, biochemical, and mechanical data were analyzed by Kruskal–Wallis one-way ANOVA by ranks with Dunn's method for post hoc analysis, with the exception of the hydraulic permeability, which underwent a logarithmic transform and one-way ANOVA using Tukey's t-test for post hoc analysis. ANOVA by ranks was used for data that failed a normality or equal variance test. A t-test was applied to compare the sizes of implanted and nonimplanted constructs. A value of p < 0.05 was used as a threshold for statistical significance. All data are expressed as mean plus one standard deviation.
Results
Ex vivo gross analyses
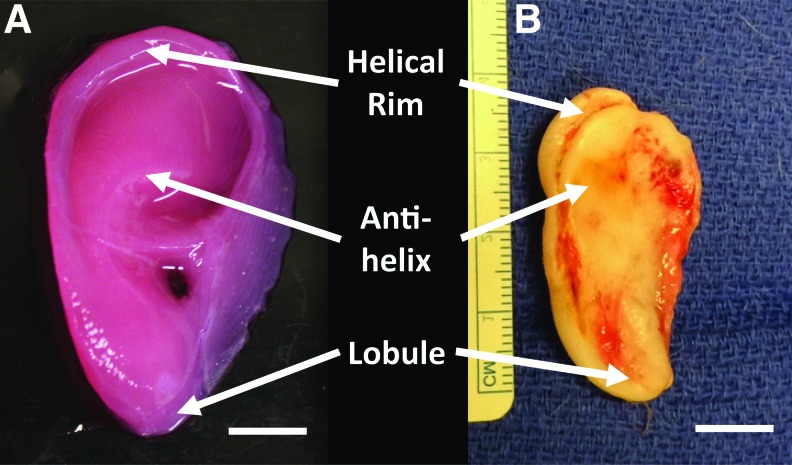
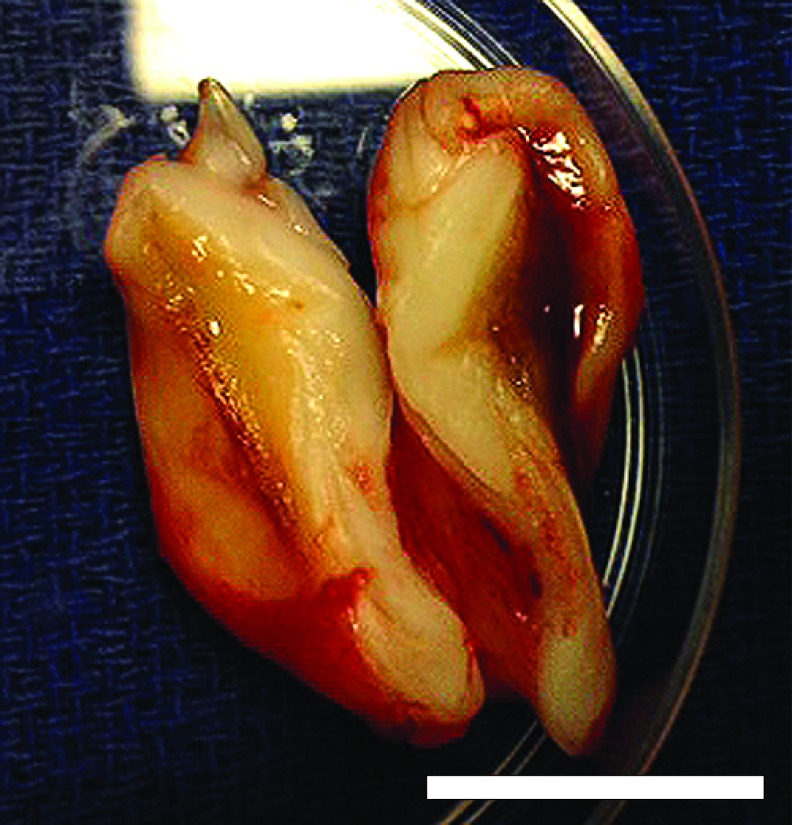
Constructs maintained patient-specific geometry and ear-specific external features following long-term implantation. After 6 months, constructs maintained a visible projection through the dorsal skin of the rats and retained general shape definition. This was confirmed upon ex vivo inspection, which demonstrated that ear constructs maintained anatomic shape in comparison to the preimplant constructs (Fig. 1). At 6 months, constructs maintained definition of the lobule and helical rim structures, with the antihelix represented to a slightly lesser degree. Ear constructs showed no signs of necrotic cores and displayed healthy tissue through full thickness of the ear when inspected in cross-section (Fig. 2).
FIG. 1.
Ex vivo gross analysis of injection molded ear before implantation (A) and explanted following 6 months in vivo (B) displaying maintained anatomic fidelity. Scale bar = 1 cm. Color images available online at www.liebertpub.com/tea
FIG. 2.
Ex vivo horizontal cross-section through the center of an ear construct 6 months after implantation displaying healthy, living tissue through full thickness. Scale bar = 1 cm. Color images available online at www.liebertpub.com/tea
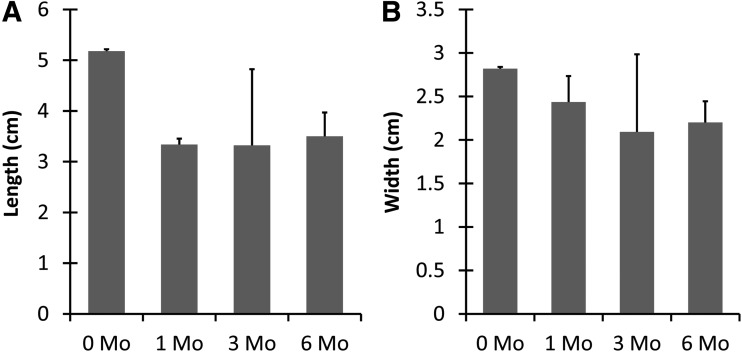
The sizes of the postharvest constructs were consistent for the 1-, 3-, and 6-month constructs. Neither the lengths nor widths at these time points displayed significant differences, with the mean lengths ranging from 3.32 to 3.50 cm and the widths from 2.01 to 2.44 cm (p < 0.05) (Fig. 3). In contrast, when all implanted constructs were compared to the construct before implantation, the implanted ears significantly contracted to 65% of the preimplant ear length of 5.18 cm (p < 0.01), whereas the widths contracted to 78% of the initial 2.82 cm width.
FIG. 3.
Analysis of explanted construct length and width. Both the length (A) and width (B) of the postharvest constructs displayed no change following 1 month in vivo, but the dimensions of all explanted constructs decreased compared to preimplantation 0-month constructs n = 3–5, p < 0.05.
Histological analyses
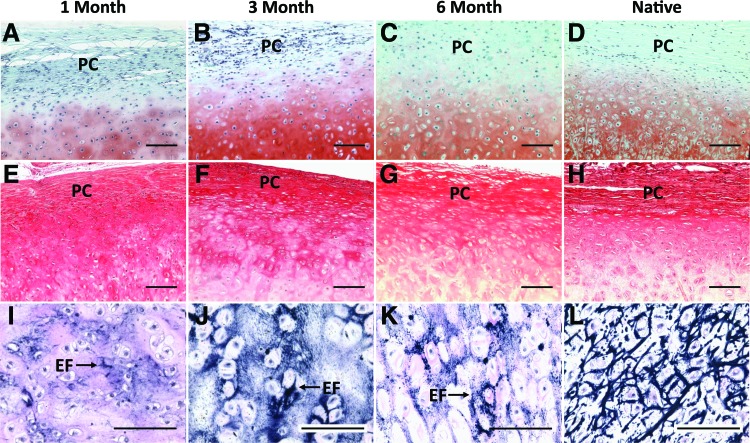
Safranin O and Picrosirius Red stains indicated the formation of distinct auricular cartilage microstructure. Staining with Safranin O revealed a profuse and consistent proteoglycan matrix surrounding rounded cell lacunae that was unchanged between 3 and 6 months after implantation (Fig. 4A–C). By 6 months, the tissue stained for proteoglycans was almost indistinguishable from native auricular cartilage (Fig. 4D). Picrosirius Red staining showed that the outermost tissue layer was composed of organized collagen bundles and that the cells contained in this region were smaller and more fibroblast-like than the chondrocytes contained in the proteoglycan bulk (Fig. 4E–G). Picrosirius Red staining demonstrated a decreasing collagen composition within the deeper layers of the cartilage over time. The outer layering of collagen containing small, tightly packed cells closely resembles the perichondrial layer observed in the native bovine tissue (Fig. 4H).
FIG. 4.
Histological staining of constructs harvested after 1 (A, E, I), 3 (B, F, J) and 6 months (C, G, K) compared to native bovine auricular cartilage (D, H, L). Safranin O staining (with Fast Green counterstain) (A–D) displayed increasing proteoglycan deposition and development of cellular lacunae. Picrosirius Red staining (E–H) displayed the formation of bundled collagen fibers forming a PC layer. Verhoeff's stain (I–L) indicates increasing development of EF in the tissue bulk. Microstructure of both 3- and 6-month constructs appears similar to native tissue (scale bar = 100 μm). EF, elastin fibers; PC, perichondrial. Color images available online at www.liebertpub.com/tea
The Verhoeff's stain demonstrated a progressive increase in the deposition of elastin fibers over time. While staining was limited to small areas after 1 month in vivo, the intensity of the staining increased by the 3-month time point (Fig. 4I, J). After 6 months, the constructs maintained this dense staining for elastin fibers and displayed a more widespread and organized elastin matrix that approaches the highly developed structure seen in native tissue (Fig. 4K, L).
Biochemical analyses
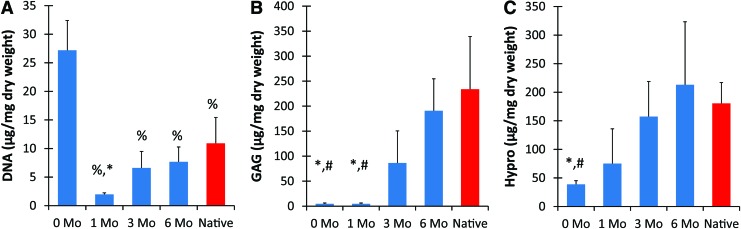
The preimplant constructs, which contained only cells and collagen, had a significantly higher dry weight DNA content when compared to implanted constructs (p < 0.001) (Fig. 5A). The DNA content decreased during the first month in vivo, following which monotonic increases were observed. There was no significant difference between the engineered tissues at 3 and 6 months and the native bovine ear, with the 6-month ears attaining ∼70% of the DNA content of native.
FIG. 5.
Biochemical analysis of constructs and native auricular cartilage normalized to dry weight. DNA (A) rapidly decreased after 1 month in vivo, but steadily rose to approach native amounts. Both GAG (B) and hydroxyproline (C) composition increased in vivo, reaching similar values to native cartilage content by 3 months (n = 4–6). %, Significant difference from 0 month; #, significant difference from 6 month; *, significant difference from native (p < 0.05). GAG, glycosaminoglycan. Color images available online at www.liebertpub.com/tea
Ear constructs at 6 months displayed an almost 40-fold increase in GAG content as compared to the preimplant constructs (p < 0.05) and contain over 80% of the total for native tissue (Fig. 5B). Both preimplant and 1-month constructs had significantly less (p < 0.05) proteoglycan deposition than 6-month and native tissue, while monotonic increases in GAG content were observed from 1 to 6 months of implantation. No observable GAG deposition occurred during the first month in vivo.
The hydroxyproline contents of all implanted constructs were not statistically different from native cartilage, with a monotonic increase observed through all time points (Fig. 5C). After 6 months, engineered tissue had significantly higher content when compared to preimplanted constructs (p < 0.05) and was within 15% of the native hydroxyproline amounts.
Mechanical analysis
The mechanical properties of the engineered ears improved from the soft, preimplant constructs following implantation. Although freshly molded constructs were durable enough for placement during subcutaneous surgery, they would tear under excessive force. Constructs explanted at 6 months, however, demonstrated the ability to deform significantly and elastically return to the original shape (Supplementary Movie S1 and S2; Supplementary Data are available online at www.liebertpub.com/tea).
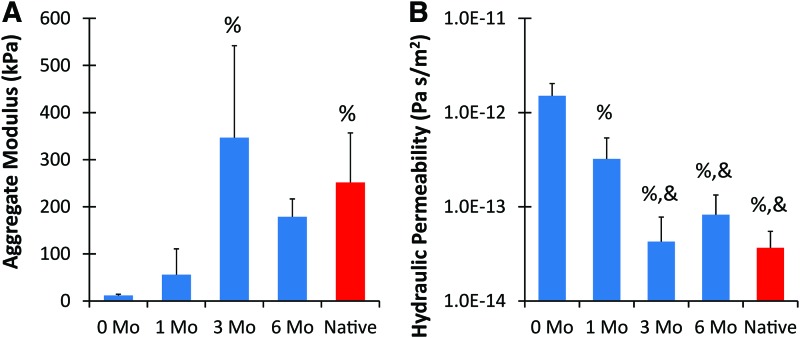
Mechanical analysis demonstrated that the 3- and 6-month constructs matched the equilibrium modulus and hydraulic permeability of native cartilage (Fig. 6). The development of the tissue in vivo resulted in a significantly higher equilibrium modulus at 3 months compared to the preimplant constructs, and all engineered tissues became stiffer with time in vivo. After 6 months, the engineered tissue constructs possessed 15× greater modulus than the preimplant constructs and reached 71% of the value for native tissue (p < 0.05). All in vivo tissues demonstrated lowered hydraulic permeability when compared to preimplant constructs, with constructs after 3 and 6 months featuring a significantly lower permeability than after 1 month (p < 0.05). No significant difference existed between the 3-month, 6-month, and native cartilage values.
FIG. 6.
Mechanical properties of ear constructs and native auricular cartilage. The aggregate modulus of implanted ears were significantly higher after 3 months than the preimplanted constructs and no difference existed between 3 months, 6 months, and native tissue (A). The hydraulic permeability decreased significantly after both 1 and 3 months in vivo (B) (n = 4–6). %, Significant difference from 0 month; &, significant difference from 1 month (p < 0.05). Color images available online at www.liebertpub.com/tea
Discussion
The purpose of this study was to verify the long-term stability of patient-specific ear constructs engineered using high-density collagen scaffolds without stenting or support of synthetic materials. The results of this study demonstrated that the engineered ear constructs retained a high degree of morphologic fidelity, developed auricular cartilage microstructure, and maintained or improved biochemical and mechanical properties when compared to constructs explanted at earlier time points. Following 6 months in vivo, the ear constructs also displayed structural organization and properties similar to native auricular cartilage tissue.
For a tissue-engineered reconstructive implant such as the auricle, it is critical that the construct shape remains stable over a long interval in vivo and that degradation, inflammation, or invasion of other tissue types does not occur. Additionally, the continued development or maintenance of key auricular cartilage properties must be observed. While some studies found significant loss of gross morphology beyond 3 months in vivo,3,6 our constructs maintained overall geometry and aesthetics at 6 months, and the amount of contraction observed was comparable to other long-term implantations.12 Additionally, this was accomplished without the use of additional metal implants, which have been used in other studies to provide mechanical support for implantations beyond 3 months.10,23
Constructs in this study displayed key indicators of long-term auricular cartilage development. Proteoglycan deposition, appearance of cellular lacunae, and formation of the perichondrial layer were consistent with previous research,3,6,10,12,23 and a continuous elastin network was found similar to only one other tissue-engineered ear study at a comparable time point.23 In contrast to other long-term studies, auricular constructs at 6 months displayed no signs of osteophyte development,6,10 necrosis,10 fibrocartilage formation,10 or degradation at the periphery,26 despite the production of thicker cartilage tissue.23 Beyond the microstructure, biochemical and mechanical properties of native cartilage were also either reached or maintained through 6 months and were comparable to previously reported values.23 The initial observed reduction in DNA content per dry weight at 1 month was likely a result of both decreasing cell number as the tissue approaches native cellular density and increasing matrix content as the encapsulated chondrocytes remodeled the collagen scaffold and deposited proteoglycans and elastin. While observed hydroxyproline values appear elevated, this is possibly due to cross-reaction of the assay to hydroxyproline present in both collagen and elastin.36,37
Although this study displayed the generation of auricular cartilage, certain limitations in our technique must still be addressed. While the engineered constructs did not contract further after 1 month in vivo, some contraction occurred shortly following implantation, and may have contributed to the loss of fine details of the patient-specific auricular morphology. Contraction is commonly observed in cell-seeded collagen hydrogels,38,39 and a potential solution could be combined riboflavin/ultraviolet A crosslinking, which has been demonstrated to significantly reduce collagen hydrogel contraction without loss of cell viability.39 Since the constructs seen here maintained overall ear geometry, an alternative option is enlarging the initial constructs to account for the contraction. Another area of improvement is in the initial mechanical properties. Before implantation, the constructs were durable enough for the surgical procedure, but were significantly less stiff than native cartilage and could potentially be damaged if exposed to high loads. We note that implantations in the murine model are performed under loose skin, while clinical implantation under human skin may apply increased forces on the early constructs. Again, riboflavin crosslinking can increase the stiffness of the preimplant hydrogel and reduce the risk of damage before tissue remodeling can occur.40,41
A final hurdle for clinical translation of this technique is cell source and testing within immunocompetent hosts. This study utilized primary neonatal bovine auricular chondrocytes, which can be isolated in large enough numbers to produce full-sized ears requiring ∼200 × 106 cells. However, for clinical use, cells from a more mature, human source would be required. Further studies can apply human auricular chondrocytes, which can be acquired from a patient's microtic ear remnant or a biopsy of the healthy contralateral ear, to the described scaffold and manufacture technique, however, the in vitro expansion of chondrocytes can result in loss of chondrogenic capacity.42 Additionally, autologous MSCs offer a potential cell source that can undergo chondrogenic differentiation43 and can be obtained with minimally invasive surgery from the patient undergoing reconstruction.42 Improvements in chondrocyte expansion techniques and/or supplementation with MSCs can increase the number of autologous cells available.
The success of the long-term implantation of these patient-specific auricular constructs has demonstrated the potential of this technique for tissue engineering auricular cartilage implants. The use of a noninvasive imaging modality allows for the application of this technique to a wide array of patients, and the type I collagen hydrogel scaffold promotes cartilage deposition without the potential of harmful degradation products or additional synthetic implant materials. The tissue-engineered auricles formed in this study offer a potentially superior clinical option to current autologous and alloplastic ear reconstruction procedures.
Supplementary Material
Acknowledgments
Funding for this research was provided by NIH Grant 5T35EB006732, NYSTAR, and 3D BioCorp. This work was presented in part at the Northeastern Society of Plastic Surgeons 28th Annual Meeting in Amelia Island, FL, the Plastic Surgery Research Council 57th and 60th Annual Meetings in Ann Arbor, MI, and Seattle, WA, respectively, the American Society of Plastic Surgeons 2012 Annual Meeting in New Orleans, LA, the American Society for Reconstructive Microsurgery 2013 and 2015 Annual Meetings in Naples, FL, and Paradise Island, BS, and the Biomedical Engineering Society 2014 Annual Meeting in San Antonio, TX.
Disclosure Statement
Professor Bonassar is a co-founder and equity holder in 3D BioCorp. No competing financial interests exist for other authors.
References
- 1.Luquetti D.V., Heike C.L., Hing A. V, Cunningham M.L., and Cox T.C. Microtia: epidemiology and genetics. Am J Med Genet A 158A, 124, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandes R. Ear Reconstruction. Local and Regional Flaps Head Neck Reconstruction: A Practical Approach. First. Hoboken, NJ: John Wiley & Sons, Inc., 2015, pp. 170–185 [Google Scholar]
- 3.Shieh S., Terada S., and Vacanti J.P. Tissue engineering auricular reconstruction: in vitro and in vivo studies. Biomaterials 25, 1545, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Bichara D.A., O'Sullivan N.-A., Pomerantseva I., Zhao X., Sundback C.A., Vacanti J.P., and Randolph M.A. The tissue-engineered auricle: past, present, and future. Tissue Eng Part B Rev 18, 51, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Angela R., Cao Y.L., Clemente I., Pap S., Vacanti M., Eavey R.D., and Vacanti C.A. Characteristics of cartilage engineered from human pediatric auricular cartilage. Plast Reconstr Surg 103, 1111, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Britt J.C., and Park S.S. Autogenous tissue-engineered cartilage. Arch Otolaryngol Head Neck Surg 124, 671, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Brent B. Microtia repair with rib cartilage grafts. A review of personal experience with 1000 cases. Clin Plast Surg 29, 257, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Romo T., and Reitzen S.D. Aesthetic microtia reconstruction with Medpor. Facial Plast Surg 24, 120, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Cao Y., Vacanti J.P., Paige K.T., Upton J., and Vacanti C.A. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast Reconstr Surg 100, 297, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Kamil S.H., Vacanti M.P., Aminuddin B.S., Jackson M.J., Vacanti C.A., and Eavey R.D. Tissue engineering of a human sized and shaped auricle using a mold. Laryngoscope 114, 867, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Zhang L., He A., Yin Z., Yu Z., Luo X., Liu W., Zhang W., Cao Y., Liu Y., and Zhou G. Biomaterials regeneration of human-ear-shaped cartilage by co-culturing human microtia chondrocytes with BMSCs. Biomaterials 35, 4878, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Isogai N., Asamura S., Higashi T., Ikada Y., Morita S., Hillyer J., Jacquet R., and Landis W.J. Tissue engineering of an auricular cartilage model utilizing cultured chondrocyte-poly(L-lactide-e-caprolactone) scaffolds. Tissue Eng 10, 673, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Chang S.C.N., Rowley J.A., Tobias G., Genes N.G., Roy A.K., Mooney D.J., Vacanti C.A., and Bonassar L.J. Injection molding of chondrocyte/alginate constructs in the shape of facial implants. J Biomed Mater Res 55, 503, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Xu J.-W., Zaporojan V., Peretti G.M., Roses R.E., Morse K.R., Roy A.K., Mesa J.M., Randolph M.A., Bonassar L.J., and Yaremchuk M.J. Injectable tissue-engineered cartilage with different chondrocyte sources. Plast Reconstr Surg 113, 1361, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Awad H.A., Wickham M.Q., Leddy H.A., Gimble J.M., and Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials 25, 3211, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Cao Y., Angela R., Vacanti M., Ibarra C., Arevalo C., and Vacanti C.A. Comparative study of the use of poly(glycolic acid), calcium alginate and pluronics in the engineering of autologous porcine cartilage. J Biomater Sci Polym Ed 9, 475, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Isogai N., Kusuhara H., Ikada Y., Ohtani H., Jacquet R., Hillyer J., Lowder E., and Landis W.J. Comparison of different chondrocytes for use in tissue engineering of cartilage model structures. Tissue Eng 12, 691, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Pelttari K., Winter A., Steck E., Goetzke K., Hennig T., Ochs B.G., Aigner T., and Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum 54, 3254, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Liu Y., Zhang L., Zhou G., Li Q., Liu W., Yu Z., Luo X., Jiang T., Zhang W., and Cao Y. In vitro engineering of human ear-shaped cartilage assisted with CAD/CAM technology. Biomaterials 31, 2176, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Sterodimas A., and de Faria J. Human auricular tissue engineering in an immunocompetent animal model. Aesthet Surg J 33, 283, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Lee S.J., Broda C., Atala A., and Yoo J.J. Engineered cartilage covered ear implants for auricular cartilage reconstruction. Biomacromolecules 12, 306, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Zhou L., Pomerantseva I., Bassett E.K., Bowley C.M., Zhao X., Bichara D.A., Kulig K.M., Vacanti J.P., Randolph M.A., and Sundback C.A. Engineering ear constructs with a composite scaffold to maintain dimensions. Tissue Eng Part A 17, 1573, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Liao H.T., Zheng R., Liu W., Zhang W.J., Cao Y., and Zhou G. Prefabricated, ear-shaped cartilage tissue engineering by scaffold-free porcine chondrocyte membrane. Plast Reconstr Surg 135, 313, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Xue J., Feng B., Zheng R., Lu Y., Zhou G., Liu W., Cao Y., Zhang Y., and Zhang W.J. Engineering ear-shaped cartilage using electrospun fibrous membranes of gelatin/polycaprolactone. Biomaterials 34, 2624, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Reiffel A.J., Kafka C., Hernandez K.A., Popa S., Perez J.L., Zhou S., Pramanik S., Brown B.N., Ryu W.S., Bonassar L.J., and Spector J.A. High-fidelity tissue engineering of patient-specific auricles for reconstruction of pediatric microtia and other auricular deformities. PLoS One 8, e56506, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Von Bomhard A., Veit J., Bermueller C., Rotter N., Staudenmaier R., Storck K., and The, H.N. Prefabrication of 3D cartilage contructs: towards a tissue engineered auricle—a model tested in rabbits. PLoS One 8, e71667, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Institute of Laboratory Animal Research Committee. Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington DC: The National Academies Press, 2011, p. 118 [Google Scholar]
- 28.Chang S.C.N., Tobias G., Roy A.K., Vacanti C.A., and Bonassar L.J. Tissue engineering of autologous cartilage for craniofacial reconstruction by injection molding. Plast Reconstr Surg 112, 793, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Elsdale T., and Bard J. Collagen substrata for studies on cell behavior. J Cell Biol 54, 626, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowles R.D., Williams R.M., Zipfel W.R., and Bonassar L.J. Self-assembly of aligned tissue-engineered annulus fibrosus and intervertebral disc composite via collagen gel contraction. Tissue Eng Part A 16, 1339, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cross V.L., Zheng Y., Won Choi N., Verbridge S.S., Sutermaster B.A., Bonassar L.J., Fischbach C., and Stroock A.D. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials 31, 8596, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ballyns J.J., Gleghorn J.P., Niebrzydowski V., Rawlinson J.J., Potter H.G., Maher S.A., Wright T.M., and Bonassar L.J. Image-guided tissue engineering of anatomically shaped implants via MRI and micro-CT using injection molding. Tissue Eng Part A 14, 1195, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Kim Y.J., Sah R.L., Doong J.Y., and Grodzinsky A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem 174, 168, 1988 [DOI] [PubMed] [Google Scholar]
- 34.Enobakhare B.O., Bader D.L., and Lee D.A. Quantification of sulfated glycosaminoglycans in chondrocyte/alginate cultures, by use of 1,9-dimethylmethylene blue. Anal Biochem 243, 189, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Neuman R.E., and Logan M.A. The determination of hydroxyproline. J Biol Chem 184, 299, 1950 [PubMed] [Google Scholar]
- 36.Neuman R.E., and Logan M.A. The determination of collagen and elastin in tissues. J Biol Chem 186, 549, 1950 [PubMed] [Google Scholar]
- 37.Bentley J.P., and Hanson A.N. The hydroxyproline of elastin. Biochim Biophys Acta 175, 339, 1969 [DOI] [PubMed] [Google Scholar]
- 38.Puetzer J.L., and Bonassar L.J. High density type I collagen gels for tissue engineering of whole menisci. Acta Biomater Acta Mater 9, 7787, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Ibusuki S., Halbesma G.J., Randolph M.A, Redmond R.W., Kochevar I.E., and Gill T.J. Photochemically cross-linked collagen gels as three-dimensional scaffolds for tissue engineering. Tissue Eng 13, 1995, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Wollensak G., Spoerl E., and Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol 135, 620, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Ahearne M., Yang Y., Then K.Y., and Liu K.-K. Mechanical characterisation of UVA-riboflavin crosslinked collagen hydrogels. Br J Ophthalmol 92, 268, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Meretoja V.V, Dahlin R.L., Kasper F.K., and Mikos A.G. Enhanced chondrogenesis in co-cultures with articular chondrocytes and mesenchymal stem cells. Biomaterials 33, 6362, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., and Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.