Abstract
Limited research exists regarding the most aggressive forms of hepatoblastoma. Cell lines of the rare subtypes of hepatoblastoma with poor prognosis are not only difficult to attain but also challenging to characterize histologically. A community-driven approach to educating parents and families, regarding the need for donated tissue, is necessary for scientists to have access to resources for murine models and drug discovery. Herein, we describe the currently available resources, existing gaps in research, and the path to move forward for uniform cure of hepatoblastoma.
Keywords: hepatoblastoma, roadmap, xenograft models, cell lines, tissue procurement
Introduction
Hepatoblastoma is the most common primary liver tumor diagnosed in childhood (1), with approximately 100 cases in the U.S. annually (2). Despite a high cure rate for those children whose tumor is resectable, there remains a group of children for whom a cure is out of reach.
The disease predominantly occurs in young children, from birth to 5 years of age (1). The histological subtypes of hepatoblastoma are fetal, embryonal, mixed epithelial–mesenchymal, and small cell undifferentiated (3). However, there is currently a lack of understanding regarding the origins and pathophysiology of these different subtypes of hepatoblastoma.
Clinically, the empirically driven advancements in postoperative chemotherapy and surgery, including the multidisciplinary approach set forth through the Pretreatment Extent of Disease guidelines (1), has improved outcomes for hepatoblastoma. These guidelines rely on a standardized staging system using imaging for detecting amount of tumor involvement (4). Despite these clinical advancements, the more aggressive forms of hepatoblastoma remain difficult to treat. Current treatments for aggressive forms of hepatoblastoma include doxorubicin, irinotecan (clinical trials), hepatic artery chemoembolization in addition to chemotherapy agents, as well as liver transplantation or partial resection with neoadjuvant chemotherapy (5).
Scientists and clinicians are now seeking non-chemotherapeutic treatments for patients with unresectable or metastatic tumor – treatments that directly target the molecular underpinnings of hepatoblastoma progression. For example, clinical trials regarding cixutumumab and pazopanib, monoclonal antibodies, and alisertib, a kinase inhibitor, have all been completed or are actively being investigated in phase 2 clinical trails for the treatment of refractory hepatoblastoma (6).
In order to find other targeted therapies, researchers need hepatoblastoma tissues and cell lines. There is an absence of diversity in hepatoblastoma cell lines for scientists and clinicians to use to better understand the disease. In this paper, we describe the need for more cell lines and murine models to advance the discovery of therapeutic targets for the more aggressive subtypes of hepatoblastoma.
Methods
An extensive literature review of hepatoblastoma via PubMed was conducted to obtain information on research with unique hepatoblastoma cell lines and murine models. First, the authors found the number of distinct hepatoblastoma cell lines published in the literature. The search terms used were “‘hepatoblastoma’ [All Fields] and ‘cell line’ [MeSH Terms].” The search provided approximately 450 publications, all reviewed by the authors of this paper. Any publication that had a focus on hepatoblastoma was mentioned in Table 1. For each unique cell line, the authors attempted to find the primary article characterizing the cell line. Secondary publications using hepatoblastoma cell lines were also included in Table 1.
Table 1.
Literature review of hepatoblastoma cell lines and mouse models.
| Cell lines | Name/ID | Public availability | Age | Gender (m/f) | Year made | Histological subtype | Mutations | Primary reference | Secondary reference |
|---|---|---|---|---|---|---|---|---|---|
| True hepatoblastoma | |||||||||
| Hep G2 | ATCC | 15 years | m | 1975 | Epithelial | CTNNB1; Δ116 aa, 25–140, exon 3 and 4 | (7, 8) | (9) | |
| HUH6 | JCRB | 12 months | m | 1985 | Mixed; predominant embryonal | CTNNB1; T41A | (10) | (9, 11) | |
| HepT1 (DZ25) | Dr. Steven Warmann (Germany) | 34 months | f | Embryonal, poorly differentiated | CTNNB1; Δ76 aa, 5–80 exon 3 | (12) | (9) | ||
| HepT3 (tumor D204) | 9 months | m | Fetal and embryonal | CTNNB1; T41A | (9) | (13) | |||
| Hep293TT | 5 years | f | Mixed; predominant embryonal | CTNNB1; Δ117aa | (14) | ||||
| HepT8 | (15) | ||||||||
| HepT4 | (15) | ||||||||
| HepT5 (tumor D717) | Epithelial | CTNNB1; Δ76 amino acids, exon 3 | (15) | ||||||
| HepT2 (tumor D166) | 48 months | m | Epithelial | CTNNB1 | (15) | ||||
| HepU1 | 53 months | m | Fetal and embryonal | (16) | |||||
| HepU2 | 58 months | m | Fetal and embryonal | (16) | |||||
| OHR | 4 months | m | Anaplastic (no alpha fetal protein) | TP53; R281H | (17) | (18) | |||
| USM | 11 months | f | (18) | ||||||
| HB1 | 6 months | m | Mixed fetal and mesenchymal | (19) | |||||
| c-HB3 | 1 years | m | 1979 | Well-differentiated fetal | (20) | ||||
| Unsure hepatoblastoma | |||||||||
| SMMC7721 | (21) | ||||||||
| Hep 3b | (8) | (22) | |||||||
| HUH-7 | (23) | (11) | |||||||
| WRL-68 | (24, 25) | ||||||||
| COG-H-430 (unpublished) | COG | Unpublished | |||||||
| Hepatoblastoma-derived fibroblasts | |||||||||
| GM08206 (unpublished) | Coriell Institute | 7 months | m | Fibroblast | Unpublished | ||||
| Mouse models | Metastatic (y/n) | Comments | |||||||
| Chemically induced | |||||||||
| B6C3F1; diethylnitrosamine (DEN) and sodium phenobarbital (PB) | Embryonal or small cell type | 62% hepatoblastoma penetrance; HCA and HCC occurred in 54% | (26) | ||||||
| Transgenic | |||||||||
| Cited1-CreERTM-GFP; Ctnnb1þ/ex3(fl) | 8 weeks | y | Embryonal undifferentiated, pure fetal hepatoblastomas | 38% hepatoblastoma penetrance; HCC occurred as well | (27) | ||||
| ApoE-LIN28B | 6 months | Fetal and cholangioblastic pattern | 100 hepatoblastoma penetrance; HCC occurred as well | (28) | |||||
| LAP-MYC | Mixed embryonal and fetal, predominant embryonal | (28) | |||||||
| Alb-MYC | Mixed embryonal and fetal, predominant embryonal | (28) | |||||||
| Cell line-derived xenografts | |||||||||
| Subcutaneous | |||||||||
| Nu/nu Balb/c mice w/2 × 107 HuH6 cells | Mixed; predominant embryonal | (29–31) | |||||||
| NMRI-Foxn1nu w/2–3 × 106 HuH6 cells | f | Paravertebral areas | (32) | ||||||
| NOD/LtSz-scid IL2Rγnull 2 × 106 HuH6 cells | Paravertebral areas | (33) | |||||||
| Athymic nude mice w/1 × 107 HepG2 cells | f | Left flank | (34) | ||||||
| NOD/SCID immunodeficient w/5 × 105 HuH6 cells | m | (35) | |||||||
| NOD/SCIDw/1 × 105 HepG2 cells | 14 days | (36) | |||||||
| Nude mice w/USM cells | (18) | ||||||||
| Atyhmic nude mice w/2 × 106 HepG2 cells | 3 weeks | m | Left thigh | (37) | |||||
| Nude mice (BALB/c, nu/nu) w/2 mm HB3 tissue cubes | 14.5 days | f | Well differentiated fetal | Back | (20) | ||||
| NOD/LtSz-scid IL2Rγnull mice w/2 × 106 HUH6 cells | 4 weeks | Paravertebral areas | (38) | ||||||
| Orthotopic | |||||||||
| NOD/LtSz-scid IL2Rγnull w/1 × 106 HuH6 cells | 5 weeks | n | 83% hepatoblastoma penetrance – injected intrasplenically, no tumor growth via intravenous or intraperitoneal injection | (13) | |||||
| NOD/LtSz-scid IL2Rγnull w/1 × 106 HepT1 cells | 5 weeks | n | 50% hepatoblastoma penetrance – injected intrasplenically, no tumor growth via intravenous or intraperitoneal injection | (13) | |||||
| NOD.Cg-Prkdcscid-IL2rgtmWjl/Sz w/1 × 106 HuH6 cells | 4 weeks | y | Embryonal | 82% penetrance – injected intrasplenically | (39) | ||||
| Patient-derived xenografts | |||||||||
| HB-213 | XenTech | 19 months | f | y | (40) | ||||
| HB-214 | XenTech | 30 months | f | y | Small cell undifferentiated | (40) | |||
| HB-217 | XenTech | 24 months | m | n | (40) | ||||
| HB-229 | XenTech | 54 months | m | y | (40) | ||||
| HB-232 | XenTech | 6 months | m | n | (40) | ||||
| HB-233 | XenTech | 16 months | m | n | Small cell undifferentiated | (40) | |||
| HB-236 | XenTech | 8 months | f | n | (40) | ||||
| HB-238 | XenTech | 110 months | f | n | (40) | ||||
| HB-239 | XenTech | 113 months | m | n | Small cell undifferentiated | (40) | |||
| HB-243 | XenTech | 52 months | m | n | (40) | ||||
| HB-244 | XenTech | 114 months | m | n | (40) | ||||
| HB-252 | XenTech | 14 months | f | n | (40) |
Manuscripts that had differing characterizations of certain cell lines were included in the “Unsure Hepatoblastoma” portion of Table 1.
Next, the authors reviewed literature via PubMed to find murine models of hepatoblastoma. The search terms used were “hepatoblastoma murine models.” This provided approximately 50 publications that the authors reviewed. All publications that studied murine models of hepatoblastoma were included in Table 1. The models were classified as chemically induced, transgenic, and cell-derived xenografts. Furthermore, the xenograft studies were sorted based on subcutaneous or orthotopic models.
Finally, the authors searched for patient-derived xenografts via PubMed and found no manuscript publications. The authors then searched the European Journal of Cancer using the term “hepatoblastoma xenograft” and found a published abstract using patient-derived xenografts, which is included in Table 1.
Results
Data regarding histological characterization and experimental murine models from only a few hepatoblastoma cell lines exist. These cell lines tend to have a favorable histology, leading to an underrepresentation of the high-risk subtypes (41).
Cell Lines
Fifteen hepatoblastoma cell lines are described in current literature (Table 1). Additionally, there are four cell lines that are potentially hepatoblastoma, but significant inconsistencies in the literature render the data obtained from these lines unreliable. Even among the confirmed hepatoblastoma cell lines, however, there are many documented instances in which cell lines were mistaken for hepatocellular carcinoma (7). A fibroblast cell line harvested from the liver of a Beckwith–Wiedemann syndrome patient with hepatoblastoma is described (Table 1).
Most confirmed cell lines are of the mixed histology subtype. However, there are no cell lines of the small cell undifferentiated subtype, which carries the worst prognosis (7).
Murine Models
Only one chemically induced murine model of hepatoblastoma has been reported (Table 1). Although four different transgenic murine models are described in the literature, these murine models were not specifically developed for the purpose of modeling hepatoblastoma. The transgenic murine models phenotypically express both hepatocellular carcinoma and hepatoblastoma (Table 1).
Ten unique cell line-derived subcutaneous xenografts and three cell line-derived orthotopic murine models of human hepatoblastoma exist (Table 1). These models primarily utilize the Hep G2 and HuH6 cells lines. Twelve unique patient-derived xenografts exist (Table 1).
Potential Genetic Targets for Aggressive Hepatoblastoma
Many studies have noted genetic mutations specific to histological subtypes of hepatoblastoma (42). Hepatoblastoma cells have shown gain of 2q, 1q, Xp, and Xq; loss of 4q, 2q, and 1q; and loss of heterozygosity of insulin growth factor 2 (5). Subtypes with increased Notch expression are of the fetal subtype and tend to have a better prognosis. Those with overexpression of the Wingless-type MMTV Integration Site Family pathway are of the small cell undifferentiated subtype and carry a less favorable prognosis (3). Additionally, the more aggressive forms of hepatoblastoma have telomerase reverse transcriptase promoter mutations (43). Blocking the Wingless-type MMTV Integration Site Family pathway using NK1R antagonists has been shown to slow the progression of hepatoblastoma cell growth in vitro (44). Hepatoblastoma cells show an increase in activity of the hedgehog pathway, and abnormal signaling has been linked to more malignant potential (45). Forkhead Box G1 is overexpressed in hepatoblastoma, specifically the more aggressive subtypes, when compared to the fetal subtype (46).
Discussion
In order to find targeted therapeutic options for hepatoblastoma, basic science studies need to be conducted. The few cell lines characterized and the inconsistencies in the literature on certain cell lines provide a major hurdle toward this goal. In addition, the availably of the cell lines is limited, which explains the narrow spectrum of cell lines used to derive xenografts from the already few hepatoblastoma cell lines. Additionally, diversity of histological subtypes is needed in order to find better treatment modalities for the more aggressive forms of hepatoblastoma. Interestingly, expression of fibroblasts enhances the growth of hepatoblastoma (47), which is why the hepatoblastoma-derived fibroblast cell line, GM08206 (Table 1), carries potential for more advanced studies. It is of important note that the majority of liver cells are aneuploid, which has been thought to protect the liver from chronic injury (48). Culturing surrounding normal liver tissue in addition to the tumor would provide insight into premalignant tissue field effect at the site of the tumor (46).
Certain repositories for hepatoblastoma are in the early stages of developing around the world, providing optimism for advancements in basic science research, and potentially leading to clinical trials for hepatoblastoma. The Children’s Oncology Group developed a Rare Tumor Committee that has lead to promising clinical trials for rare pediatric cancer (49), including a current clinical trial involving combination chemotherapy for different stages of hepatoblastoma. Although histological analysis is not used in the staging process, this trial presents the opportunity to provide awareness of hepatoblastoma and an opportunity to increase tissue donation. Currently, the Children’s Oncology group has one hepatoblastoma cell line (COG-H-430), not available on the open distribution list, but can be obtained with a materials transfer agreement (personal communication).
In addition to the Children’s Oncology Group, the Japanese Collection of Research Biosources hosts a cell bank that provided the cell lines for the majority of hepatoblastoma manuscripts in the literature review (9). However, currently only HUH6 is available for public distribution. Many published hepatoblastoma cell lines found in the literature review were not within the last decade, which could explain the difficulty in obtaining certain cell lines today.
Most importantly, many international groups, such as Childhood Liver Tumors Strategy Group and the Society for Pediatric Oncology and Hematology, have collaborated with Children’s Oncology Group and the Japanese Collection of Research Biosources, which initially led to the Pretreatment Extent of Disease guidelines (50). It is this type of collaboration that can result in an increase in cell lines and tissue-banking repositories. One example is the Childhood Liver Tumors Strategy Group, which runs a tissue bank for childhood liver tumors (51).
Recently, further collaboration has allowed for the Children’s Hepatic tumors International Collaboration, to obtain data on 1,605 hepatoblastoma patients, aimed at creating a database to identify prognostic factors for this rare pediatric cancer (52). One limitation to the database, mentioned by the authors, was the exclusion of histology due to the lack of international consensus in characterizing subtypes (52).
As more interaction among family members is made, newer registries, in addition to those previously mentioned, will continue to grow. The Macy Easom Foundation has committed to funding development of the Hepatoblastoma Registry, as well as the expense of administration, data compilation, and analysis (18).
Despite the current development of repositories, an increase in cell lines and murine models available for research purposes cannot progress unless methods are in place to increase awareness for tissue donation in hepatoblastoma. Both parents and treating physicians must be made aware of the need for hepatoblastoma tissue and the opportunity to support research via autopsy tissue donations. The decision, whether to make an autopsy tissue donation, is difficult, intensely personal, and unique for each family. The authors recognize the delicate balance between making parents aware of the need and opportunity while taking care to respect every family’s response and perspective.
A parent who wishes to arrange for an autopsy donation should not be burdened with making the arrangements. With parents’ consent, volunteers and professionals must be in place to make the necessary contacts and establish logistics of the donation. These arrangements may include contacting the treating physician, speaking with the local pathologist who will perform the autopsy, connecting the researcher who will receive the donated tissue with the pathologist, and arranging for transport of the body from the child’s home to the hospital (and return to the funeral home).
Grassroots communication and interaction among family members, caregivers, and others affected by a particular diagnosis has significantly influenced progress in some areas of pediatric cancer research. As an example, interaction among families affected by diffuse intrinsic pontine glioma (DIPG) in an online discussion group is considered by some to be the first step in raising a tide turning awareness in that community. The result was a promising therapeutic drug, panobinostat, for treatment (53).
In summary, the greatest potential for the development of targeted therapy for aggressive forms of hepatoblastoma will come when scientists have access to hepatoblastoma cells lines and tissues with histological subtype diversity (Figure 1).
Figure 1.
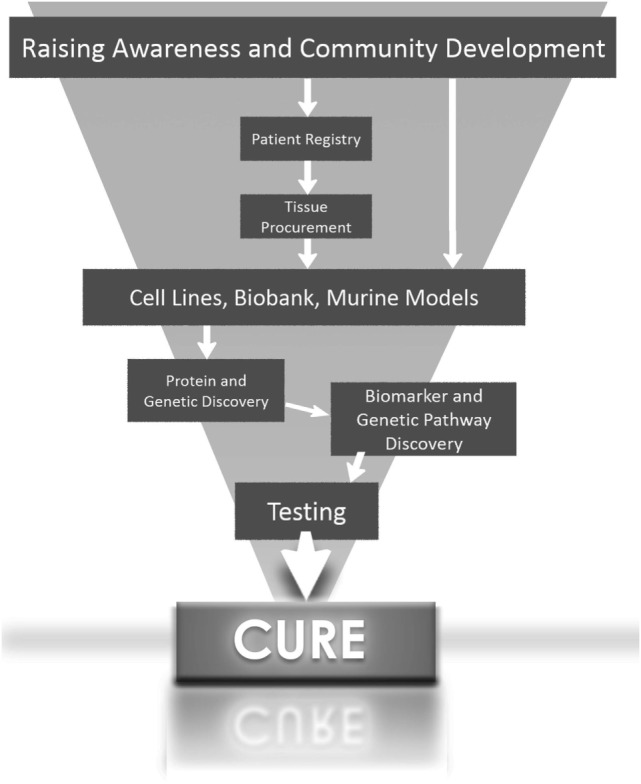
Roadmap to finding a cure for hepatoblastoma.
Author Contributions
RR, KS, RH, MSB, MB, and LH: contributed significantly to the acquisition, intellectual content, and final approval and are in agreement with all aspects of the work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the 2012 Nanocourse Group focused on hepatoblastoma and who provided a jump-start for this project: Deb Fuller, Zaahid Kaahn, Lisa Petke, and Jonathan Winegarden. We also appreciate the insight and analysis of Matthew Randolph, D.V.M. Finally, we are especially thankful to Charles Keller, M.D., for his commitment to reproducible, tangible research and his dedication to improving the outcomes for children diagnosed with hepatoblastoma.
References
- 1.Khaderi S, Guiteau J, Cotton RT, O’Mahony C, Rana A, Goss JA. Role of liver transplantation in the management of hepatoblastoma in the pediatric population. World J Transplant (2014) 4(4):294–8. 10.5500/wjt.v4.i4.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson KJ, Williams KS, Ross JA, Krailo MD, Tomlinson GE, Malogolowkin MH, et al. Parental tobacco and alcohol use and risk of hepatoblastoma in offspring: a report from the children’s oncology group. Cancer Epidemiol Biomarkers Prev (2013) 22(10):1837–43. 10.1158/1055-9965.EPI-13-0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cairo S, Armengol C, De Reynies A, Wei Y, Thomas E, Renard CA, et al. Hepatic stem-like phenotype and interplay of Wnt/beta-catenin and Myc signaling in aggressive childhood liver cancer. Cancer Cell (2008) 14(6):471–84. 10.1016/j.ccr.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 4.Pappo AS, Furman WL, Schultz KA, Ferrari A, Helman L, Krailo MD. Rare tumors in children: progress through collaboration. J Clin Oncol (2015) 33(27):3047–54. 10.1200/JCO.2014.59.3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkatramani R, Furman WL, Fuchs J, Warmann SW, Malogolowkin MH. Current and future management strategies for relapsed or progressive hepatoblastoma. Paediatr Drugs (2012) 14(4):221–32. 10.2165/11597740-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 6.Cixutumumab in Treating Patients with Relapsed or Refractory Solid Tumors clinicaltrials.gov (2015). Available from: https://clinicaltrials.gov/ct2/show/study/NCT00831844?term=hepatoblastoma&rank=9§=X70156
- 7.Lopez-Terrada D, Cheung SW, Finegold MJ, Knowles BB. Hep G2 is a hepatoblastoma-derived cell line. Hum Pathol (2009) 40(10):1512–5. 10.1016/j.humpath.2009.07.003 [DOI] [PubMed] [Google Scholar]
- 8.Zannis VI, Breslow JL, SanGiacomo TR, Aden DP, Knowles BB. Characterization of the major apolipoproteins secreted by two human hepatoma cell lines. Biochemistry (1981) 20(25):7089–96. 10.1021/bi00528a006 [DOI] [PubMed] [Google Scholar]
- 9.Koch A, Denkhaus D, Albrecht S, Leuschner I, von Schweinitz D, Pietsch T. Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the beta-catenin gene. Cancer Res (1999) 59(2):269–73. [PubMed] [Google Scholar]
- 10.Tokiwa T, Doi I, Sato J. Preparation of single cell suspensions from hepatoma cells in culture. Acta Med Okayama (1975) 29(2):147–50. [PubMed] [Google Scholar]
- 11.Purcell R, Childs M, Maibach R, Miles C, Turner C, Zimmermann A, et al. HGF/c-Met related activation of beta-catenin in hepatoblastoma. J Exp Clin Cancer Res (2011) 30:96. 10.1186/1756-9966-30-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pietsch T, Fonatsch C, Albrecht S, Maschek H, Wolf HK, von Schweinitz D. Characterization of the continuous cell line HepT1 derived from a human hepatoblastoma. Lab Invest (1996) 74(4):809–18. [PubMed] [Google Scholar]
- 13.Ellerkamp V, Armeanu-Ebinger S, Wenz J, Warmann SW, Schafer J, Ruck P, et al. Successful establishment of an orthotopic hepatoblastoma in vivo model in NOD/LtSz-scid IL2Rgammanull mice. PLoS One (2011) 6(8):e23419. 10.1371/journal.pone.0023419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen TT, Rakheja D, Hung JY, Hornsby PJ, Tabaczewski P, Malogolowkin M, et al. Establishment and characterization of a cancer cell line derived from an aggressive childhood liver tumor. Pediatr Blood Cancer (2009) 53(6):1040–7. 10.1002/pbc.22187 [DOI] [PubMed] [Google Scholar]
- 15.Koch A, Waha A, Hartmann W, Hrychyk A, Schuller U, Waha A, et al. Elevated expression of Wnt antagonists is a common event in hepatoblastomas. Clin Cancer Res (2005) 11(12):4295–304. 10.1158/1078-0432.CCR-04-1162 [DOI] [PubMed] [Google Scholar]
- 16.Scheil S, Hagen S, Bruderlein S, Leuschner I, Behnisch W, Moller P. Two novel in vitro human hepatoblastoma models, HepU1 and HepU2, are highly characteristic of fetal-embryonal differentiation in hepatoblastoma. Int J Cancer (2003) 105(3):347–52. 10.1002/ijc.11082 [DOI] [PubMed] [Google Scholar]
- 17.Kanno S, Tsunoda Y, Shibusawa K, Ishikawa M, Okamoto S, Matsumura M, et al. [Establishment of a human hepatoblastoma (immature type) cell line OHR]. Hum Cell (1989) 2(2):211. [PubMed] [Google Scholar]
- 18.Ohnishi H, Kawamura M, Hanada R, Kaneko Y, Tsunoda Y, Hongo T, et al. Infrequent mutations of the TP53 gene and no amplification of the MDM2 gene in hepatoblastomas. Genes Chromosomes Cancer (1996) 15(3):187–90. [DOI] [PubMed] [Google Scholar]
- 19.Manchester KM, Warren DJ, Erlandson RA, Wheatley JM, La Quaglia MP. Establishment and characterization of a novel hepatoblastoma-derived cell line. J Pediatr Surg (1995) 30(4):553–8. 10.1016/0022-3468(95)90129-9 [DOI] [PubMed] [Google Scholar]
- 20.Hata Y, Uchino J, Sato K, Sasaki F, Une Y, Naito H, et al. Establishment of an experimental model of human hepatoblastoma. Cancer (1982) 50(1):97–101. [DOI] [PubMed] [Google Scholar]
- 21.Feng X, Tang Z, Zheng Z. [Preliminary studies on the effects of tumor necrosis factor gene transfer on the growth of human hepatocellular carcinoma cells in nude mice]. Zhonghua Zhong Liu Za Zhi (1995) 17(3):167–9. [PubMed] [Google Scholar]
- 22.Delgado ER, Yang J, So J, Leimgruber S, Kahn M, Ishitani T, et al. Identification and characterization of a novel small-molecule inhibitor of beta-catenin signaling. Am J Pathol (2014) 184(7):2111–22. 10.1016/j.ajpath.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res (1982) 42(9):3858–63. [PubMed] [Google Scholar]
- 24.Hsu IC, Tokiwa T, Bennett W, Metcalf RA, Welsh JA, Sun T, et al. p53 gene mutation and integrated hepatitis B viral DNA sequences in human liver cancer cell lines. Carcinogenesis (1993) 14(5):987–92. 10.1093/carcin/14.5.987 [DOI] [PubMed] [Google Scholar]
- 25.Falk PM, Sabater RT, Carballo DD, Jr. Response of the human hepatic tissue cultures Hep-G2 and WRL-68 to cocaine. J Pharmacol Toxicol Methods (1995) 33(2):113–20. 10.1016/1056-8719(94)00065-C [DOI] [PubMed] [Google Scholar]
- 26.Sakairi T, Kobayashi K, Goto K, Okada M, Kusakabe M, Tsuchiya T, et al. Greater expression of transforming growth factor alpha and proliferating cell nuclear antigen staining in mouse hepatoblastomas than hepatocellular carcinomas induced by a diethylnitrosamine-sodium phenobarbital regimen. Toxicol Pathol (2001) 29(4):479–82. 10.1080/01926230152499962 [DOI] [PubMed] [Google Scholar]
- 27.Mokkapati S, Niopek K, Huang L, Cunniff KJ, Ruteshouser EC, deCaestecker M, et al. Beta-catenin activation in a novel liver progenitor cell type is sufficient to cause hepatocellular carcinoma and hepatoblastoma. Cancer Res (2014) 74(16):4515–25. 10.1158/0008-5472.CAN-13-3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen LH, Robinton DA, Seligson MT, Wu L, Li L, Rakheja D, et al. Lin28b is sufficient to drive liver cancer and necessary for its maintenance in murine models. Cancer Cell (2014) 26(2):248–61. 10.1016/j.ccr.2014.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger M, Neth O, Ilmer M, Garnier A, Salinas-Martin MV, de Agustin Asencio JC, et al. Hepatoblastoma cells express truncated neurokinin-1 receptor and can be growth inhibited by aprepitant in vitro and in vivo. J Hepatol (2014) 60(5):985–94. 10.1016/j.jhep.2013.12.024 [DOI] [PubMed] [Google Scholar]
- 30.Wagner F, Henningsen B, Lederer C, Eichenmuller M, Godeke J, Muller-Hocker J, et al. Rapamycin blocks hepatoblastoma growth in vitro and in vivo implicating new treatment options in high-risk patients. Eur J Cancer (2012) 48(15):2442–50. 10.1016/j.ejca.2011.12.032 [DOI] [PubMed] [Google Scholar]
- 31.Cairo S, Wang Y, de Reynies A, Duroure K, Dahan J, Redon MJ, et al. Stem cell-like micro-RNA signature driven by Myc in aggressive liver cancer. Proc Natl Acad Sci U S A (2010) 107(47):20471–6. 10.1073/pnas.1009009107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eicher C, Dewerth A, Thomale J, Ellerkamp V, Hildenbrand S, Warmann SW, et al. Effect of sorafenib combined with cytostatic agents on hepatoblastoma cell lines and xenografts. Br J Cancer (2013) 108(2):334–41. 10.1038/bjc.2012.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lieber J, Eicher C, Wenz J, Kirchner B, Warmann SW, Fuchs J, et al. The BH3 mimetic ABT-737 increases treatment efficiency of paclitaxel against hepatoblastoma. BMC Cancer (2011) 11:362. 10.1186/1471-2407-11-362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marotta DE, Cao W, Wileyto EP, Li H, Corbin I, Rickter E, et al. Evaluation of bacteriochlorophyll-reconstituted low-density lipoprotein nanoparticles for photodynamic therapy efficacy in vivo. Nanomedicine (Lond) (2011) 6(3):475–87. 10.2217/nnm.11.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayashi S, Fujita K, Matsumoto S, Akita M, Satomi A. Isolation and identification of cancer stem cells from a side population of a human hepatoblastoma cell line, HuH-6 clone-5. Pediatr Surg Int (2011) 27(1):9–16. 10.1007/s00383-010-2719-x [DOI] [PubMed] [Google Scholar]
- 36.Mezzanotte L, Fazzina R, Michelini E, Tonelli R, Pession A, Branchini B, et al. In vivo bioluminescence imaging of murine xenograft cancer models with a red-shifted thermostable luciferase. Mol Imaging Biol (2010) 12(4):406–14. 10.1007/s11307-009-0291-3 [DOI] [PubMed] [Google Scholar]
- 37.Klein JL, Nguyen TH, Laroque P, Kopher KA, Williams JR, Wessels BW, et al. Yttrium-90 and iodine-131 radioimmunoglobulin therapy of an experimental human hepatoma. Cancer Res (1989) 49(22):6383–9. [PubMed] [Google Scholar]
- 38.Lieber J, Dewerth A, Wenz J, Kirchner B, Eicher C, Warmann SW, et al. Increased efficacy of CDDP in a xenograft model of hepatoblastoma using the apoptosis sensitizer ABT-737. Oncol Rep (2013) 29(2):646–52. 10.3892/or.2012.2150 [DOI] [PubMed] [Google Scholar]
- 39.Lieber J, Ellerkamp V, Vogt F, Wenz J, Warmann SW, Fuchs J, et al. BH3-mimetic drugs prevent tumour onset in an orthotopic mouse model of hepatoblastoma. Exp Cell Res (2014) 322(1):217–25. 10.1016/j.yexcr.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 40.Fabre M, Nicolle D, Gorse A, Déas O, Mussini C, Brugières L, et al. A panel of pediatric liver cancer patient-derived xenografts to improve stratification of children with hepatoblastoma. In: Pieters R, editor. European Journal of Cancer. ENA 2014: EORTC-NCI-AACR Symposium; 2014; Badalona, Spain. Oxford: Elsevier; (2014). 25 p. [Google Scholar]
- 41.Zhang Y, Zhang WL, Huang DS, Hong L, Wang YZ, Zhu X, et al. Clinical effectiveness of multimodality treatment on advanced pediatric hepatoblastoma. Eur Rev Med Pharmacol Sci (2014) 18(7):1018–26. [PubMed] [Google Scholar]
- 42.Lopez-Terrada D, Gunaratne PH, Adesina AM, Pulliam J, Hoang DM, Nguyen Y, et al. Histologic subtypes of hepatoblastoma are characterized by differential canonical Wnt and Notch pathway activation in DLK+ precursors. Hum Pathol (2009) 40(6):783–94. 10.1016/j.humpath.2008.07.022 [DOI] [PubMed] [Google Scholar]
- 43.Eichenmuller M, Trippel F, Kreuder M, Beck A, Schwarzmayr T, Haberle B, et al. The genomic landscape of hepatoblastoma and their progenies with HCC-like features. J Hepatol (2014) 61(6):1312–20. 10.1016/j.jhep.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 44.Ilmer M, Garnier A, Vykoukal J, Alt E, von Schweinitz D, Kappler R, et al. Targeting the neurokinin-1 receptor compromises canonical Wnt signaling in hepatoblastoma. Mol Cancer Ther (2015) 14(12):2712–21. 10.1158/1535-7163.MCT-15-0206 [DOI] [PubMed] [Google Scholar]
- 45.Li YC, Deng YH, Guo ZH, Zhang MM, Zhu J, Pu CL, et al. Prognostic value of hedgehog signal component expressions in hepatoblastoma patients. Eur J Med Res (2010) 15(11):468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chai H, Brown RE. Field effect in cancer-an update. Ann Clin Lab Sci (2009) 39(4):331–7. [PubMed] [Google Scholar]
- 47.Asada N, Tanaka Y, Hayashido Y, Toratani S, Kan M, Kitamoto M, et al. Expression of fibroblast growth factor receptor genes in human hepatoma-derived cell lines. In Vitro Cell Dev Biol Anim (2003) 39(7):321–8. [DOI] [PubMed] [Google Scholar]
- 48.Duncan AW, Hanlon Newell AE, Bi W, Finegold MJ, Olson SB, Beaudet AL, et al. Aneuploidy as a mechanism for stress-induced liver adaptation. J Clin Invest (2012) 122(9):3307–15. 10.1172/JCI64026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kotecha RS, Kees UR, Cole CH, Gottardo NG. Rare childhood cancers – an increasing entity requiring the need for global consensus and collaboration. Cancer Med (2015) 4(6):819–24. 10.1002/cam4.426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aronson DC, Czauderna P, Maibach R, Perilongo G, Morland B. The treatment of hepatoblastoma: its evolution and the current status as per the SIOPEL trials. J Indian Assoc Pediatr Surg (2014) 19(4):201–7. 10.4103/0971-9261.142001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grotzer M. History SIOPEL (2016). Available from: http://www.siopel.org/?q=node/47
- 52.Czauderna P, Haeberle B, Hiyama E, Rangaswami A, Krailo M, Maibach R, et al. The Children’s Hepatic tumors International Collaboration (CHIC): novel global rare tumor database yields new prognostic factors in hepatoblastoma and becomes a research model. Eur J Cancer (2016) 52:92–101. 10.1016/j.ejca.2015.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grasso CS, Tang Y, Truffaux N, Berlow NE, Liu L, Debily MA, et al. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med (2015) 21(6):555–9. 10.1038/nm.3855 [DOI] [PMC free article] [PubMed] [Google Scholar]


